Effects of 1,2-Dimethylhydrazine on Barrier Properties of Rat Large Intestine and IPEC-J2 Cells
Abstract
:1. Introduction
2. Results
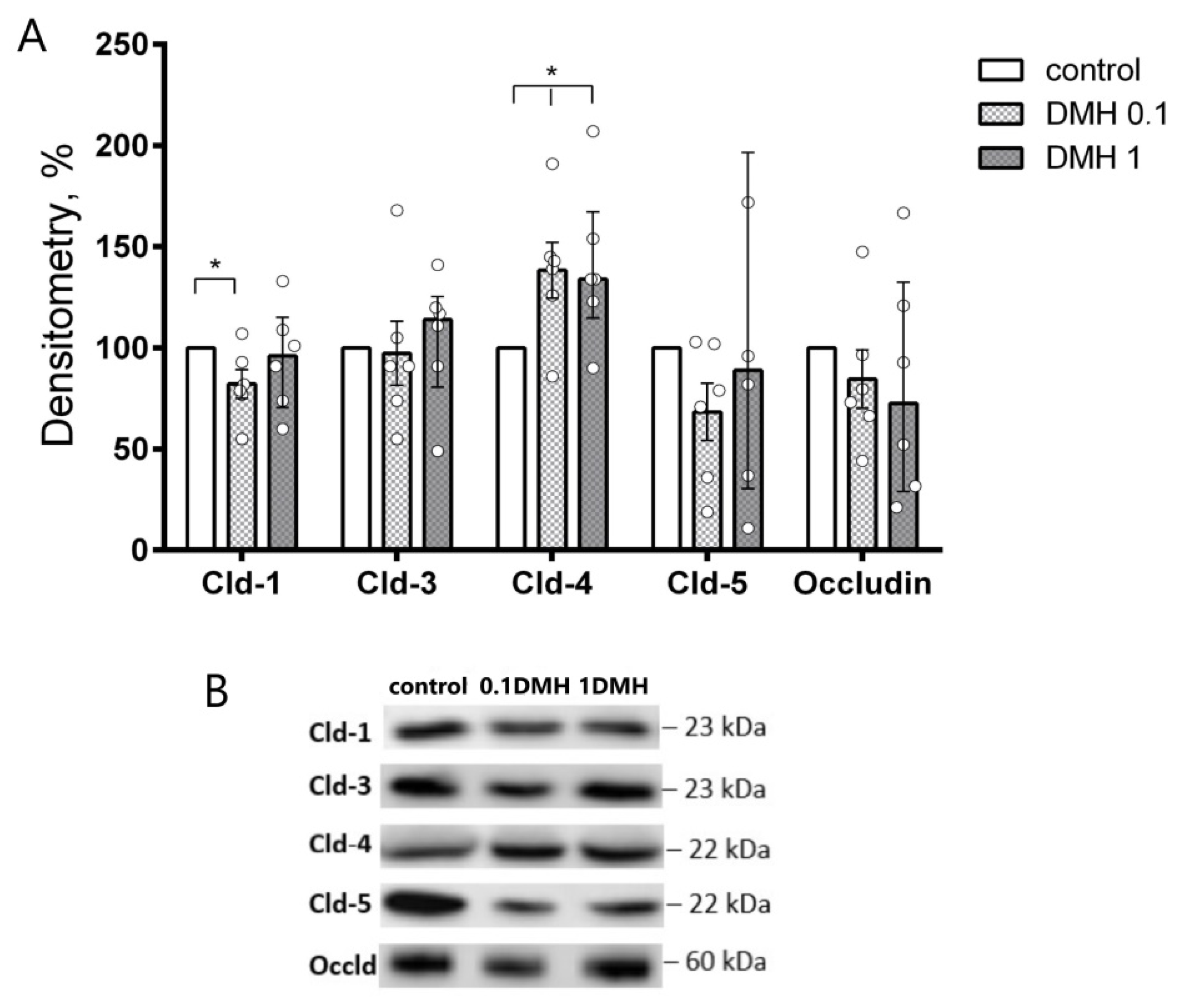
2.1. Western Blot Analysis of Colonic TJ Protein Expression
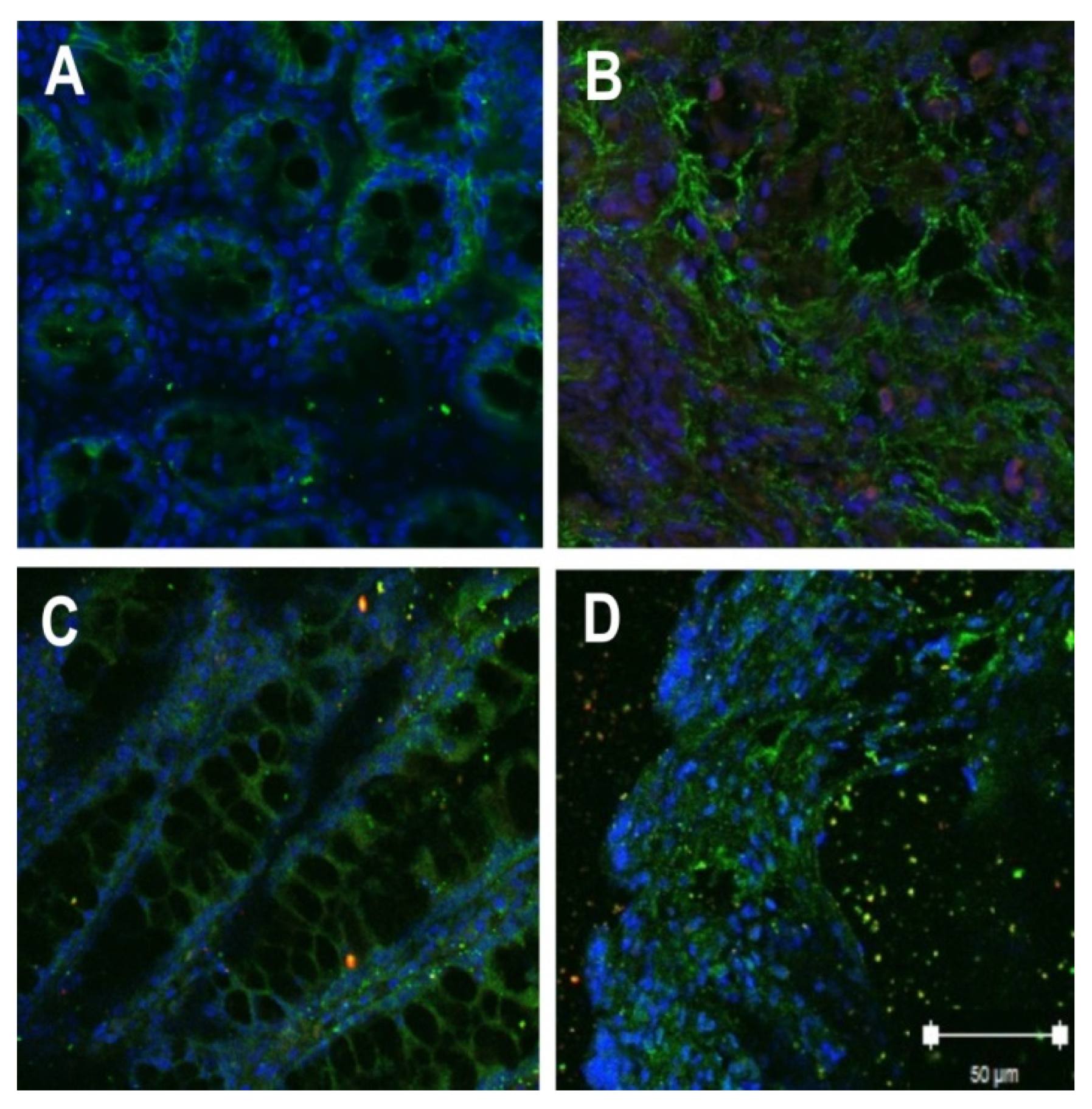
2.2. Localization of Tight Junction Proteins in Rat Colon Adenocarcinomas
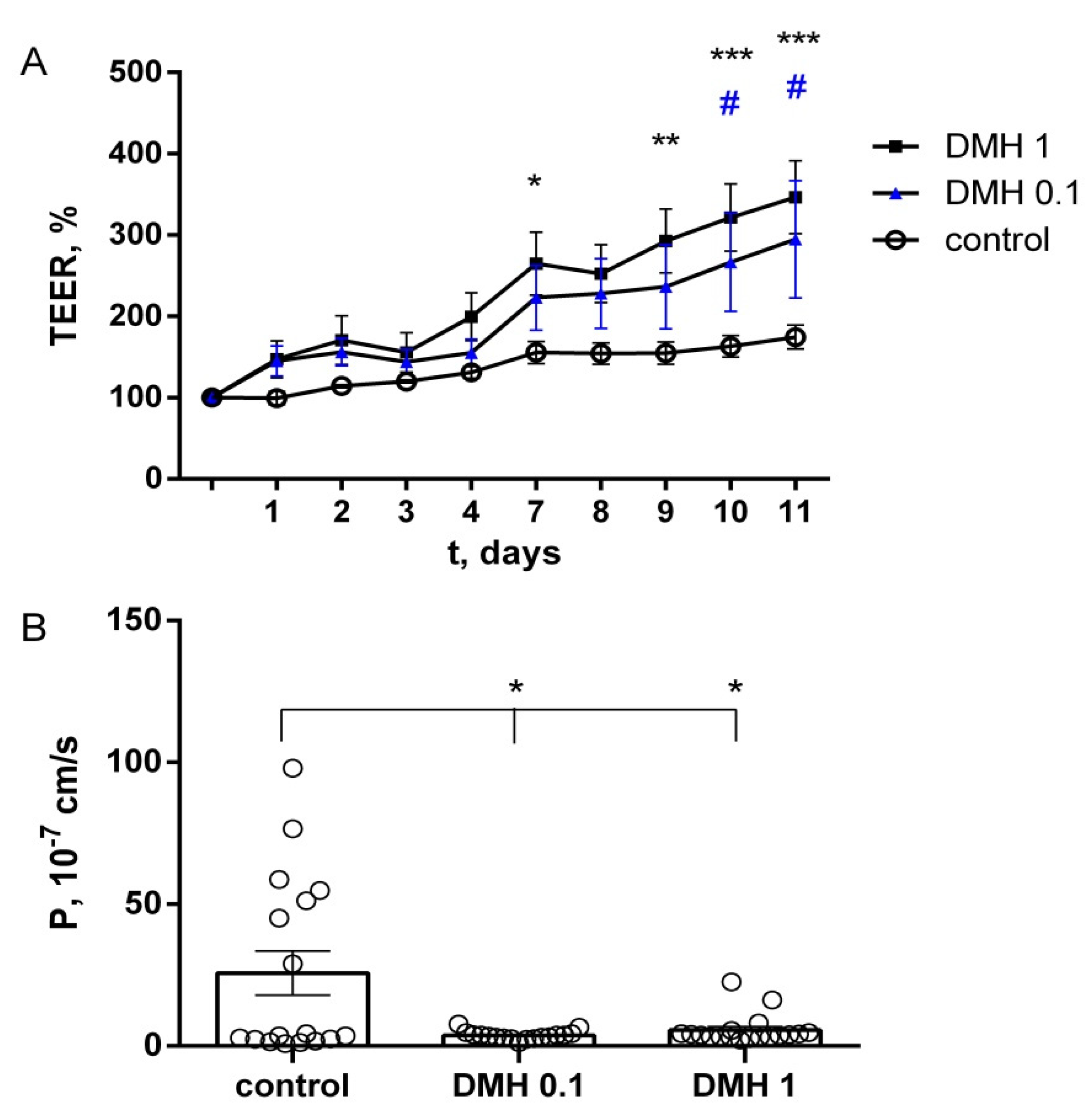
2.3. Study of TEER and Paracellular Permeability of Fluorescein in IPEC-J2 Cells
2.4. Western Blot Analysis of TJ Proteins in IPEC-J2 Cells
3. Discussion
4. Materials and Methods
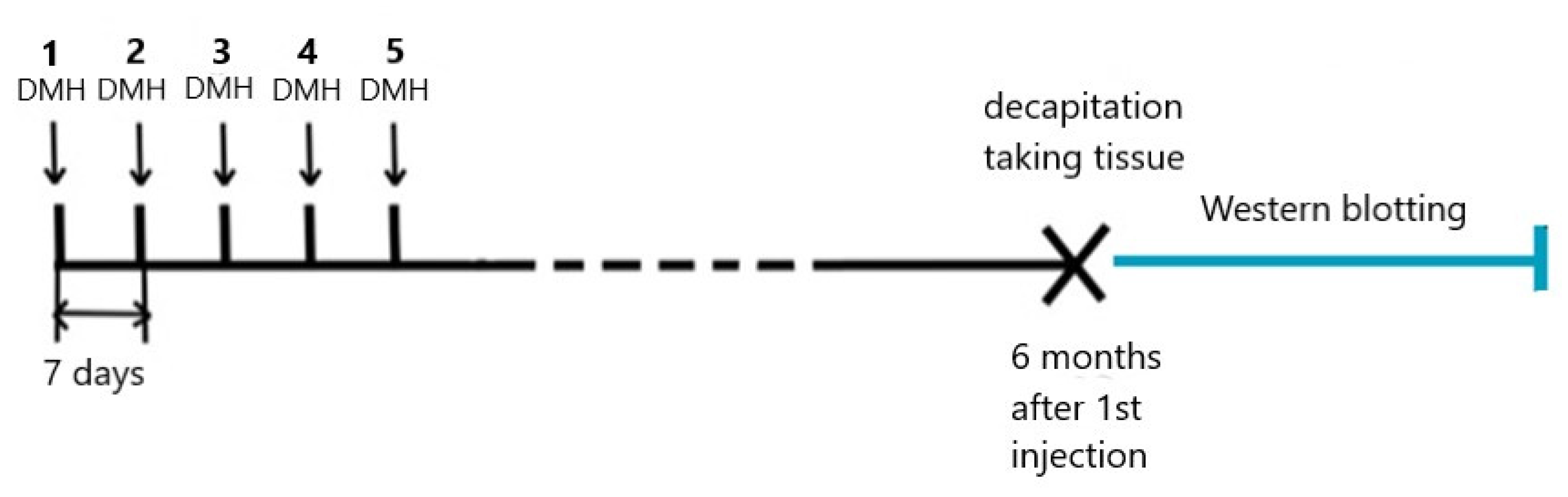
4.1. Animals and DMH Treatment
4.2. Colon Tissues
4.2.1. Western Blotting of the Colon Tissues
4.2.2. Immunohistochemistry
4.3. Cell Culturing and DMH Treatment
4.3.1. Electrophysiological Assay of the Cell Culture
4.3.2. Assessment of the Paracellular Permeability of the Cell Culture
4.3.3. Western Blotting of the Cell Culture
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kunzelmann, K.; Mall, M. Electrolyte transport in the mammalian colon: Mechanisms and implications for disease. Physiol. Rev. 2002, 82, 245–289. [Google Scholar] [CrossRef] [Green Version]
- Markov, A.G.; Veshnyakova, A.; Fromm, M.; Amasheh, M.; Amasheh, S. Segmental expression of claudin proteins correlates with tight junction barrier properties in rat intestine. J. Comp. Physiol. B 2010, 180, 591–598. [Google Scholar] [CrossRef]
- Mullin, J.M.; Laughlin, K.V.; Ginanni, N.; Marano, C.W.; Clarke, H.M.; Soler, A.P. Increased tight junction permeability can result from protein kinase C activation/translocation and act as a tumor promotional event in epithelial cancers. Ann. N. Y. Acad. Sci. 2006, 915, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.A.; Jiang, W. Tight junctions and their role in cancer metastasis. Histol. Histopathol. 2001, 1183–1195. [Google Scholar] [CrossRef]
- Landy, J.; Ronde, E.; English, N.; Clark, S.K.; Hart, A.L.; Knight, S.C.; Ciclitira, P.J.; Al-Hassi, H.O. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J. Gastroenterol. 2016, 22, 3117–3126. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.B.; Sharma, A.; Dhawan, P. Claudin Family of Proteins and Cancer: An Overview. J. Oncol. 2010, 2010, 541957. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tully, O.; Ngo, B.; Zitin, M.; Mullin, J.M. Epithelial Tight Junctional Changes in Colorectal Cancer Tissues. Sci. World J. 2011, 11, 826–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeisel, M.B.; Dhawan, P.; Baumert, T.F. Tight junction proteins in gastrointestinal and liver disease. Gut 2018, 68, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Turksen, K.; Troy, T.-C. Junctions gone bad: Claudins and loss of the barrier in cancer. Biochim. Biophys. Acta (BBA)-Bioenerg. 2011, 1816, 73–79. [Google Scholar] [CrossRef]
- Soler, A.P.; Miller, R.; Laughlin, K.V.; Carp, N.Z.; Klurfeld, D.; Mullin, J. Increased tight junctional permeability is associated with the development of colon cancer. Carcinogenesis 1999, 20, 1425–1432. [Google Scholar] [CrossRef] [Green Version]
- Bekusova, V.V.; Falchuk, E.L.; Okorokova, L.S.; Kruglova, N.M.; Nozdrachev, A.D.; Markov, A.G. Increased paracellular permeability of tumor-adjacent areas in 1,2-dimethylhydrazine-induced colon carcinogenesis in rats. Cancer Biol. Med. 2018, 15, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, S.S.; Richter, J.F.; Krug, S.; Jebautzke, B.; Lee, I.-F.M.; Rieger, J.; Sachtleben, M.; Bondzio, A.; Schulzke, J.D.; Fromm, M.; et al. Improved Cell Line IPEC-J2, Characterized as a Model for Porcine Jejunal Epithelium. PLoS ONE 2013, 8, e79643. [Google Scholar] [CrossRef] [Green Version]
- Perše, M.; Cerar, A. Morphological and Molecular Alterations in 1,2 Dimethylhydrazine and Azoxymethane Induced Colon Carcinogenesis in Rats. J. Biomed. Biotechnol. 2010, 2011, 473964. [Google Scholar] [CrossRef] [PubMed]
- Washington, M.K.; Powell, A.E.; Sullivan, R.; Sundberg, J.P.; Wright, N.; Coffey, R.J.; Dove, W.F. Pathology of Rodent Models of Intestinal Cancer: Progress Report and Recommendations. Gastroenterology 2013, 144, 705–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Ma, X.; Wang, L. Modulating effect of graphine oxide loaded hesperidin nanocomposite on the 1,2-dimethylhydrazine provoked colon carcinogenesis in rats via inhibiting the iNOS and COX-2 pathways. Arab. J. Chem. 2020, 13, 6708–6723. [Google Scholar] [CrossRef]
- Brosnahan, A.J.; Brown, D.R. Porcine IPEC-J2 intestinal epithelial cells in microbiological investigations. Vet. Microbiol. 2012, 156, 229–237. [Google Scholar] [CrossRef] [Green Version]
- De-Souza, A.S.C.; Costa-Casagrande, T.A. Animal models for colorectal cancer. Abcd-Arq. Bras. Cir. Dig. Braz. Arch. Dig. Surg. 2018, 31, e1369. [Google Scholar] [CrossRef]
- Mittal, V.K.; Bhullar, J.S.; Jayant, K. Animal models of human colorectal cancer: Current status, uses and limitations. World J. Gastroenterol. 2015, 21, 11854–11861. [Google Scholar] [CrossRef] [Green Version]
- Weisburger, J.H.; Fiala, E.S.; Sohn, O.S. Mechanisms of action of genotoxic carcinogens associated with colorectal cancer. Toxicology 2001, 168, 29–30. [Google Scholar]
- Umesalma, S.; Sudhandiran, G. Differential Inhibitory Effects of the Polyphenol Ellagic Acid on Inflammatory Mediators NF-kappa B, iNOS, COX-2, TNF-alpha, and IL-6 in 1,2-Dimethylhydrazine-Induced Rat Colon Carcinogenesis. Basic Clin. Pharmacol. Toxicol. 2010, 107, 650–655. [Google Scholar] [CrossRef]
- Yu, W.Y.; Liu, C.P.; Li, X.; Yang, F.G.; Cheng, L.X.; Liu, C.; Song, Y. Inositol hexaphosphate suppresses colorectal cancer cell proliferation via the Akt/GSK-3 beta/beta-catenin signaling cascade in a 1,2-dimethylhydrazine-induced rat model. Eur. J. Pharmacol. 2017, 805, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.A.L.; Huang, S.Y. Crosstalk of the Wnt/beta-catenin pathway with other pathways in cancer cells. Genes Dis. 2016, 3, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Shojaei-Zarghani, S.; Rafraf, M.; Khosroushahi, A.Y.; Sheikh-Najafi, S. Effectiveness of theobromine on inhibition of 1,2-dimethylhydrazine-induced rat colon cancer by suppression of the Akt/GSK3 beta/beta-catenin signaling pathway. J. Funct. Foods 2020, 75, 104293. [Google Scholar] [CrossRef]
- Hewitt, K.J.; Agarwal, R.; Morin, P.J. The claudin gene family: Expression in normal and neoplastic tissues. BMC Cancer 2006, 6, 186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miwa, N.; Furuse, M.; Tsukita, S.; Niikawa, N.; Nakamura, Y.; Furukawa, Y. Involvement of claudin-1 in the beta-catenin/Tcf signaling pathway and its frequent upregulation in human colorectal cancers. Oncol. Res. 2001, 12, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Holczbauer, A.; Gyöngyösi, B.; Lotz, G.; Szijártó, A.; Kupcsulik, P.; Schaff, Z.; Kiss, A. Distinct Claudin Expression Profiles of Hepatocellular Carcinoma and Metastatic Colorectal and Pancreatic Carcinomas. J. Histochem. Cytochem. 2013, 61, 294–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhawan, P.; Singh, A.B.; Deane, N.G.; No, Y.; Shiou, S.-R.; Schmidt, C.; Neff, J.; Washington, M.K.; Beauchamp, R.D. Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J. Clin. Investig. 2005, 115, 1765–1776. [Google Scholar] [CrossRef] [Green Version]
- Mees, S.T.; Mennigen, R.; Spieker, T.; Rijcken, E.; Senninger, N.; Haier, J.; Bruewer, M. Expression of tight and adherens junction proteins in ulcerative colitis associated colorectal carcinoma: Upregulation of claudin-1, claudin-3, claudin-4, and β-catenin. Int. J. Colorectal Dis. 2009, 24, 361–368. [Google Scholar] [CrossRef]
- de Oliveira, S.S.; de Oliveira, I.M.; De Souza, W.; Morgado-Diaz, J.A. Claudins upregulation in human colorectal cancer. FEBS Lett. 2005, 579, 6179–6185. [Google Scholar] [CrossRef] [Green Version]
- Davies, R.; Asbun, H.; Thompson, S.; Goller, D.; Sandle, G. Uncoupling of sodium chloride transport in premalignant mouse colon. Gastroenterology 1990, 98, 1502–1508. [Google Scholar] [CrossRef]
- Bleich, M.; Ecke, D.; Schwartz, B.; Fraser, G.; Greger, R. Effects of the carcinogen dimethylhydrazine (DMH) on the function of rat colonic crypts. Pflüg. Arch. 1996, 433, 254–259. [Google Scholar] [CrossRef]
- Fraser, G.M.; Portnoy, M.; Bleich, M.; Ecke, D.; Niv, Y.; Greger, R.; Schwartz, B. Characterization of sodium and chloride conductances in preneoplastic and neoplastic murine colonocytes. Pflüg. Arch. 1997, 434, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.J.; Weidema, W.F.; Sandle, G.I.; Palmer, L.; Deschner, E.E.; Decosse, J.J. Sodium transport in a mouse model of colonic carcinogenesis. Cancer Res. 1987, 47, 4646–4650. [Google Scholar] [PubMed]
- Zahraoui, A.; Louvard, D.; Galli, T. Tight Junction, a Platform for Trafficking and Signaling Protein Complexes. J. Cell Biol. 2000, 151, F31–F36. [Google Scholar] [CrossRef]
- Markov, A.G.; Aschenbach, J.R.; Amasheh, S. Claudin clusters as determinants of epithelial barrier function. IUBMB Life 2015, 67, 29–35. [Google Scholar] [CrossRef]
- Citi, S. Intestinal barriers protect against disease. Science 2018, 359, 1097–1098. [Google Scholar] [CrossRef]
- Ouban, A. Claudin-1 role in colon cancer: An update and a review. Histol. Histopathol. 2018, 33, 1013–1019. [Google Scholar]
- Kinugasa, T.; Akagi, Y.; Shirouzu, K. S2048 Claudin-1 Protein, Which Is Tight Junction Specific Molecule, Is a Major Factor Involved in the Tumorigenesis of Colorectal Cancer. Gastroenterology 2009, 136, 320. [Google Scholar] [CrossRef]
- Ahmad, R.; Kumar, B.; Chen, Z.; Chen, X.; Muller, D.; Lele, S.M.; Washington, M.K.; Batra, S.K.; Dhawan, P.; Singh, A.B. Loss of claudin-3 expression induces IL6/gp130/Stat3 signaling to promote colon cancer malignancy by hyperactivating Wnt/beta-catenin signaling. Oncogene 2017, 36, 6592–6604. [Google Scholar] [CrossRef] [PubMed]
- De Souza, W.F.; Ferreira, M.D.S.; Boroni, M.; De Oliveira, I.M.; Freire-Neto, C.A.; Fernandes, P.V.; Souza-Santos, P.T.; De-Freitas-Junior, J.C.M. N-glycosylation and receptor tyrosine kinase signaling affect claudin-3 levels in colorectal cancer cells. Oncol. Rep. 2020, 44, 1649–1661. [Google Scholar] [CrossRef]
- Radloff, J.; Falchuk, E.L.; Markov, A.G.; Amasheh, S. Molecular Characterization of Barrier Properties in Follicle-Associated Epithelium of Porcine Peyer’s Patches Reveals Major Sealing Function of Claudin-4. Front. Physiol. 2017, 8, 579. [Google Scholar] [CrossRef] [Green Version]
- Breed, C.; Hicks, D.A.; Webb, P.G.; Galimanis, C.E.; Bitler, B.G.; Behbakht, K.; Baumgartner, H.K. Ovarian Tumor Cell Expression of Claudin-4 Reduces Apoptotic Response to Paclitaxel. Mol. Cancer Res. 2019, 17, 741–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanigan, F.; McKiernan, E.; Brennan, D.J.; Hegarty, S.; Millikan, R.C.; McBryan, J.; Jirstrom, K.; Landberg, G.; Martin, F.; Duffy, M.J.; et al. Increased claudin-4 expression is associated with poor prognosis and high tumour grade in breast cancer. Int. J. Cancer 2009, 124, 2088–2097. [Google Scholar] [CrossRef]
- Agarwal, R.; D’Souza, T.; Morin, P.J. Claudin-3 and Claudin-4 Expression in Ovarian Epithelial Cells Enhances Invasion and Is Associated with Increased Matrix Metalloproteinase-2 Activity. Cancer Res. 2005, 65, 7378–7385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.X.; Sun, J.X.; Zhao, J.H.; Yang, Y.C.; Shi, J.X.; Wu, Z.H.; Chen, X.W.; Gao, P.; Miao, Z.F.; Wang, Z.N. Non-coding RNAs participate in the regulatory network of CLDN4 via ceRNA mediated miRNA evasion. Nat. Commun. 2017, 8, 289. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.T.; Miao, H.L.; Jing, B.G.; Pan, Q.W.; Zhang, H.T.; Chen, Y.F.; Zhang, D.; Liang, Z.Z.; Wen, Z.L.; Li, M.Y. Claudin-4 controls the proliferation, apoptosis, migration and in vivo growth of MCF-7 breast cancer cells. Oncol. Rep. 2015, 34, 681–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Gao, Y.; Tang, L.; Ning, K.; Geng, N.; Zhang, H.; Li, Y.; Li, Y.; Liu, F.; Li, F. A novel PAK4-CEBPB-CLDN4 axis involving in breast cancer cell migration and invasion. Biochem. Biophys. Res. Commun. 2019, 511, 404–408. [Google Scholar] [CrossRef]
- Matsuoka, T.; Mitomi, H.; Fukui, N.; Kanazawa, H.; Saito, T.; Hayashi, T.; Yao, T. Cluster Analysis of Claudin-1 and-4, E-Cadherin, and beta-Catenin Expression in Colorectal Cancers. J. Surg. Oncol. 2011, 103, 674–686. [Google Scholar] [CrossRef]
- Fujita, K.; Katahira, J.; Horiguchi, Y.; Sonoda, N.; Furuse, M.; Tsukita, S. Clostridium perfringens enterotoxin binds to the second extracellular loop of claudin-3, a tight junction integral membrane protein. FEBS Lett. 2000, 476, 258–261. [Google Scholar] [CrossRef] [Green Version]
- Robertson, S.L.; Smedley, J.G.; McClane, B.A. Identification of a Claudin-4 Residue Important for Mediating the Host Cell Binding and Action of Clostridium perfringens Enterotoxin. Infect. Immun. 2010, 78, 505–517. [Google Scholar] [CrossRef] [Green Version]
- Markov, A.G.; Falchuk, E.L.; Kruglova, N.M.; Rybalchenko, O.V.; Fromm, M.; Amasheh, S. Comparative analysis of theophylline and cholera toxin in rat colon reveals an induction of sealing tight junction proteins. Pflug. Arch.-Eur. J. Physiol. 2014, 466, 2059–2065. [Google Scholar] [CrossRef] [PubMed]
- Mahler, M.; Berard, M.; Feinstein, R.; Gallagher, A.; Illgen-Wilcke, B.; Pritchett-Corning, K.; Raspa, M. FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units (vol 48, pg 178, 2014). Lab. Anim. 2015, 49, 88. [Google Scholar]
- Cima, G. AVMA Guidelines for the Euthanasia of Animal: 2013 Edition. JAVMA J. Am. Vet. Med. Assoc. 2013, 242, 715–716. [Google Scholar]
- Amasheh, S.; Milatz, S.; Krug, S.; Bergs, M.; Amasheh, M.; Schulzke, J.-D.; Fromm, M. Na+ absorption defends from paracellular back-leakage by claudin-8 upregulation. Biochem. Biophys. Res. Commun. 2009, 378, 45–50. [Google Scholar] [CrossRef]
- Amasheh, S.; Dullat, S.; Fromm, M.; Schulzke, J.D.; Buhr, H.J.; Kroesen, A.J. Inflamed pouch mucosa possesses altered tight junctions indicating recurrence of inflammatory bowel disease. Int. J. Colorectal Dis. 2009, 24, 1149–1156. [Google Scholar] [CrossRef]
- Droessler, L.; Cornelius, V.; Markov, A.G.; Amasheh, S. Tumor Necrosis Factor Alpha Effects on the Porcine Intestinal Epithelial Barrier Include Enhanced Expression of TNF Receptor. Int. J. Mol. Sci. 2021, 22, 8746. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekusova, V.; Droessler, L.; Amasheh, S.; Markov, A.G. Effects of 1,2-Dimethylhydrazine on Barrier Properties of Rat Large Intestine and IPEC-J2 Cells. Int. J. Mol. Sci. 2021, 22, 10278. https://doi.org/10.3390/ijms221910278
Bekusova V, Droessler L, Amasheh S, Markov AG. Effects of 1,2-Dimethylhydrazine on Barrier Properties of Rat Large Intestine and IPEC-J2 Cells. International Journal of Molecular Sciences. 2021; 22(19):10278. https://doi.org/10.3390/ijms221910278
Chicago/Turabian StyleBekusova, Viktoria, Linda Droessler, Salah Amasheh, and Alexander G. Markov. 2021. "Effects of 1,2-Dimethylhydrazine on Barrier Properties of Rat Large Intestine and IPEC-J2 Cells" International Journal of Molecular Sciences 22, no. 19: 10278. https://doi.org/10.3390/ijms221910278
APA StyleBekusova, V., Droessler, L., Amasheh, S., & Markov, A. G. (2021). Effects of 1,2-Dimethylhydrazine on Barrier Properties of Rat Large Intestine and IPEC-J2 Cells. International Journal of Molecular Sciences, 22(19), 10278. https://doi.org/10.3390/ijms221910278






