The Antagonism of the Prokineticin System Counteracts Bortezomib Induced Side Effects: Focus on Mood Alterations
Abstract
:1. Introduction
- verify if, in the experimental model of BTZ-induced neuropathic pain, the presence of hypersensitivity is related to the presence of emotional alterations such as anxiety and depression;
- evaluate the role of neuroinflammation and the involvement of the PK system in such alterations;
- verify whether the treatment with the PKRs antagonist PC1, at a protocol able to counteract pain, can prevent the development of mood alterations.
2. Results
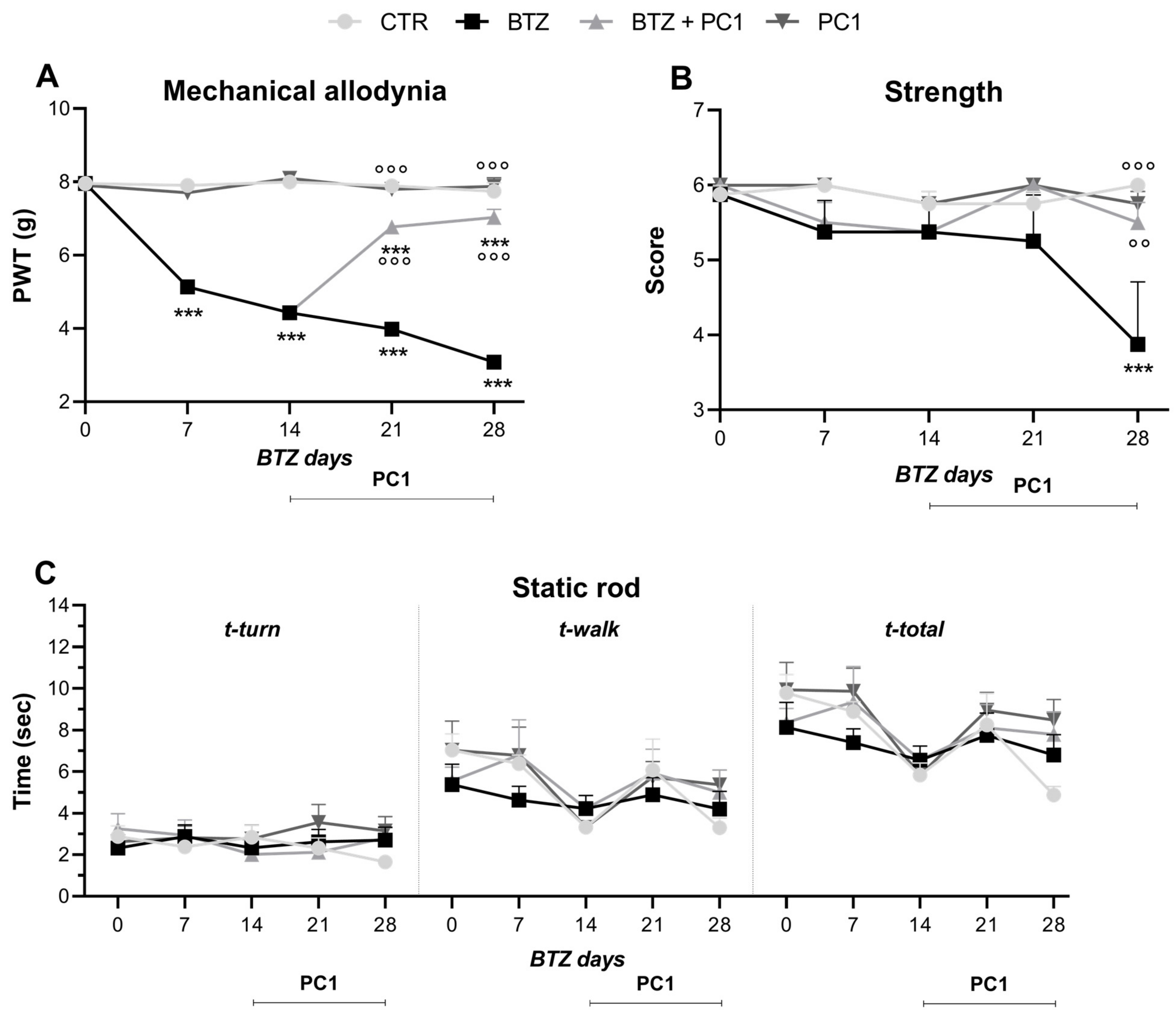
2.1. Effect of BTZ Treatment on Mechanical Allodynia, Strength and Locomotor Activity
2.2. Effect of BTZ Treatment on Mood
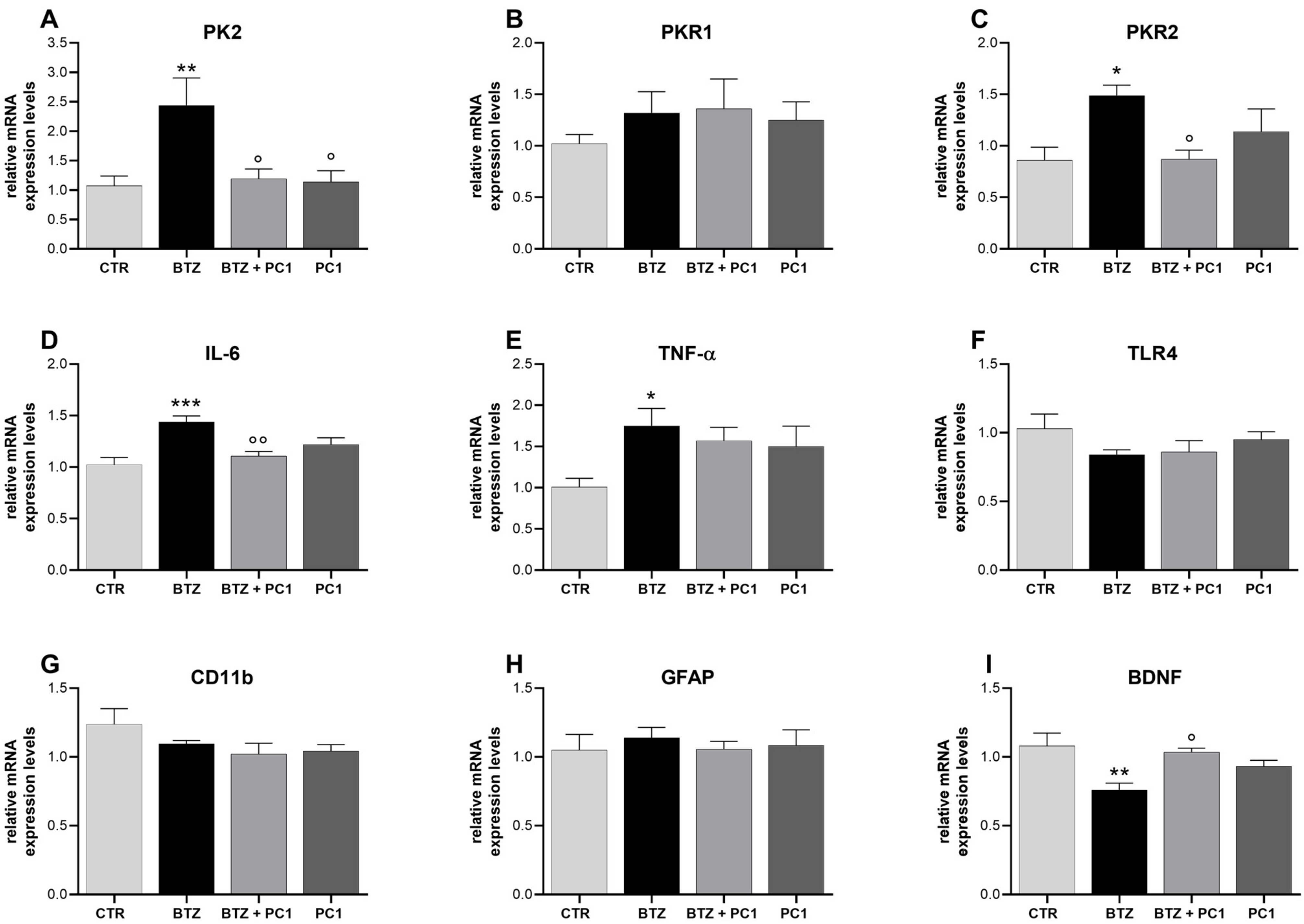
2.3. Effect of BTZ Treatment on Hippocampus (HPC) Neuroinflammation
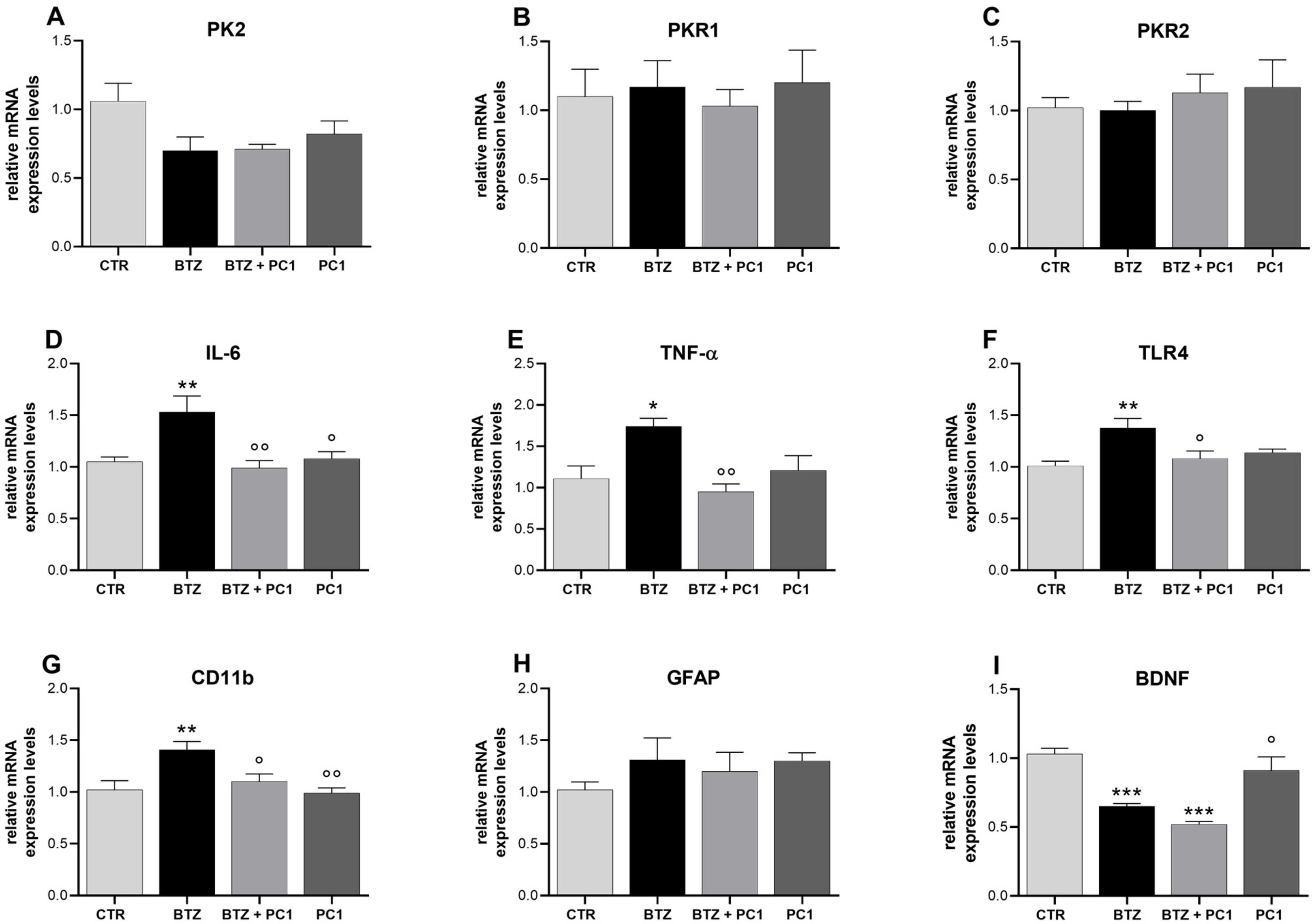
2.4. Effect of BTZ Treatment on Prefrontal Cortex (PFC) Neuroinflammation
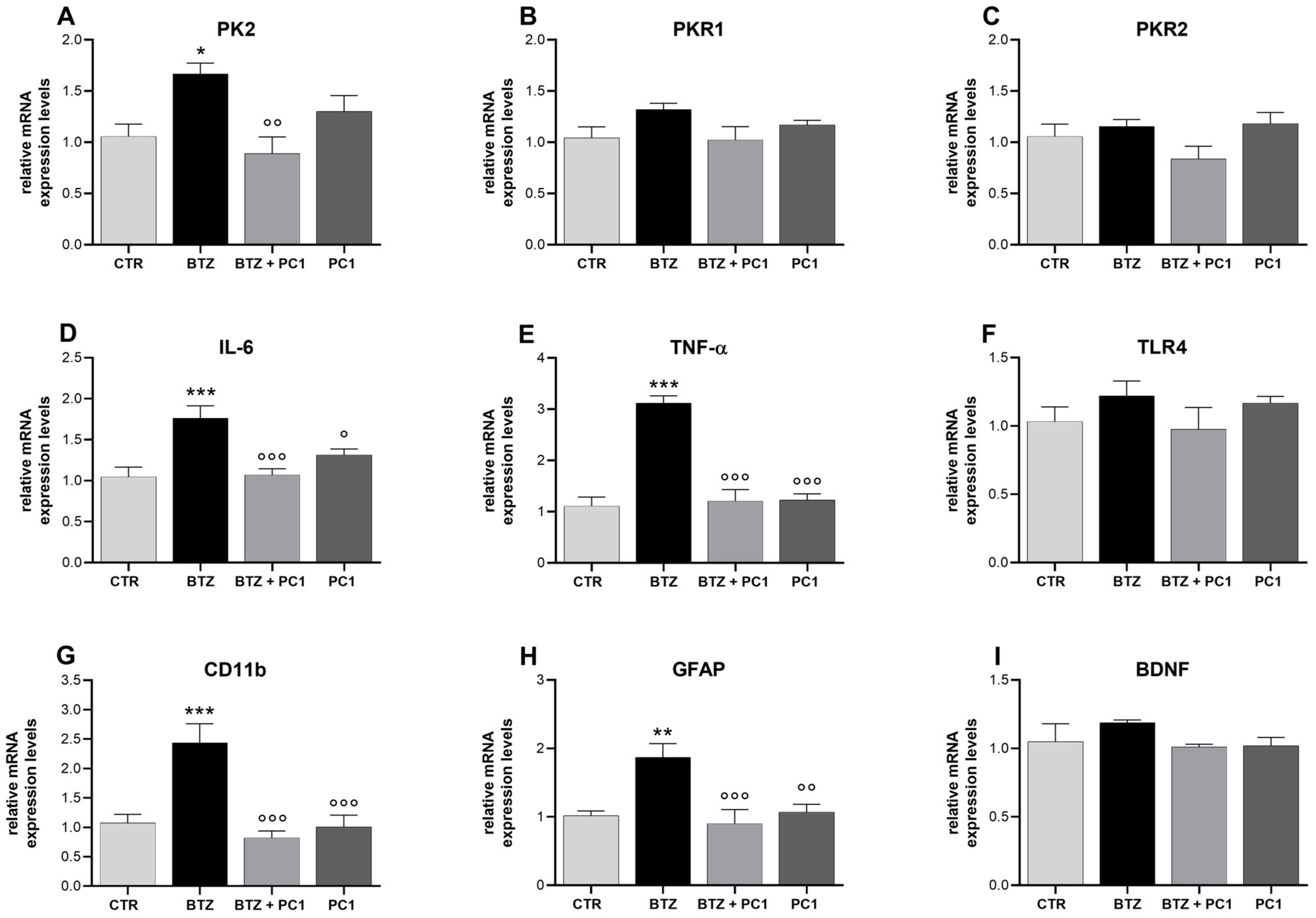
2.5. Effect of BTZ Treatment on Hypothalamus (HPT) Neuroinflammation
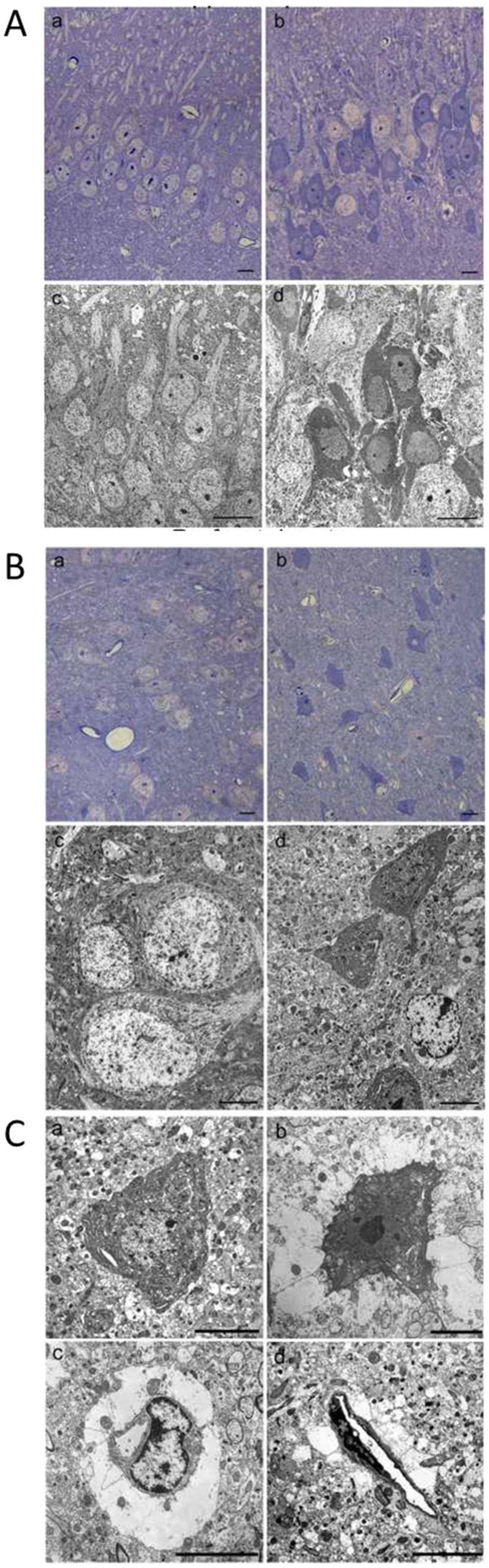
2.6. Morphological Changes of Hippocampus and Prefrontal Cerebral Cortex Observed by Light and Transmission Electron Microscopy
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Neuropathy Induction and Therapeutic Treatment with PC1
4.3. Behavioral Tests
4.4. Von Frey Test: Mechanical Allodynia
4.5. Static Rod Test: Coordination and Balance
4.6. Grip Strength Test: Muscle Force
4.7. Dark Light Test: Anxiety-Like Behavior
4.8. Marble Burying Test: Anxiety-Like Behavior
4.9. Swimming Forced Test: Depression-like Behavior
4.10. Sucrose Preference Test: Anhedonia/Depression-like Behavior
4.11. Brain Areas Collection and Real Time-qPCR
4.12. Light and Transmission Eelectron Microscopy
4.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kannarkat, G.; Lasher, E.E.; Schiff, D. Neurologic complications of chemotherapy agents. Curr. Opin. Neurol. 2007, 20, 719–725. [Google Scholar] [CrossRef]
- Vitali, M.; Ripamonti, C.I.; Roila, F.; Proto, C.; Signorelli, D.; Imbimbo, M.; Corrao, G.; Brissa, A.; Rosaria, G.; de Braud, F.; et al. Cognitive impairment and chemotherapy: A brief overview. Crit. Rev. Oncol. Hematol. 2017, 118, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Wefel, J.S.; Schagen, S.B. Chemotherapy-related cognitive dysfunction. Curr. Neurol. Neurosci. Rep. 2012, 12, 267–275. [Google Scholar] [CrossRef]
- Fumagalli, G.; Monza, L.; Cavaletti, G.; Rigolio, R.; Meregalli, C. Neuroinflammatory Process Involved in Different Preclinical Models of Chemotherapy-Induced Peripheral Neuropathy. Front. Immunol. 2020, 11, 626687. [Google Scholar] [CrossRef]
- Carozzi, V.A.; Canta, A.; Chiorazzi, A. Chemotherapy-induced peripheral neuropathy: What do we know about mechanisms? Neurosci. Lett. 2015, 596, 90–107. [Google Scholar] [CrossRef] [PubMed]
- Zajaczkowska, R.; Kocot-Kepska, M.; Leppert, W.; Wrzosek, A.; Mika, J.; Wordliczek, J. Mechanisms of Chemotherapy-Induced Peripheral Neuropathy. Int. J. Mol. Sci. 2019, 20, 1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, X.; Boriero, D.; Chaiswing, L.; Bondada, S.; St Clair, D.K.; Butterfield, D.A. Plausible biochemical mechanisms of chemotherapy-induced cognitive impairment (“chemobrain”), a condition that significantly impairs the quality of life of many cancer survivors. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Boyette-Davis, J.A.; Hou, S.; Abdi, S.; Dougherty, P.M. An updated understanding of the mechanisms involved in chemotherapy-induced neuropathy. Pain Manag. 2018, 8, 363–375. [Google Scholar] [CrossRef]
- Wang, X.M.; Walitt, B.; Saligan, L.; Tiwari, A.F.; Cheung, C.W.; Zhang, Z.J. Chemobrain: A critical review and causal hypothesis of link between cytokines and epigenetic reprogramming associated with chemotherapy. Cytokine 2015, 72, 86–96. [Google Scholar] [CrossRef] [Green Version]
- Flatters, S.J.L.; Dougherty, P.M.; Colvin, L.A. Clinical and preclinical perspectives on Chemotherapy-Induced Peripheral Neuropathy (CIPN): A narrative review. Br. J. Anaesth. 2017, 119, 737–749. [Google Scholar] [CrossRef] [Green Version]
- Bonhof, C.S.; Poll-Franse, L.V.; Vissers, P.A.; Wasowicz, D.K.; Wegdam, J.A.; Révész, D.; Vreugdenhil, G.; Mols, F. Anxiety and depression mediate the association between chemotherapy-induced peripheral neuropathy and fatigue: Results from the population-based PROFILES registry. Psychooncology 2019, 28, 1926–1933. [Google Scholar] [CrossRef] [Green Version]
- Bonhof, C.S.; Trompetter, H.R.; Vreugdenhil, G.; van de Poll-Franse, L.V.; Mols, F. Painful and non-painful chemotherapy-induced peripheral neuropathy and quality of life in colorectal cancer survivors: Results from the population-based PROFILES registry. Support. Care Cancer 2020, 28, 5933–5941. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.C.; Pyter, L.M. Neuroimmunology of Behavioral Comorbidities Associated With Cancer and Cancer Treatments. Front. Immunol. 2018, 9, 1195. [Google Scholar] [CrossRef] [Green Version]
- Bravo, L.; Llorca-Torralba, M.; Suarez-Pereira, I.; Berrocoso, E. Pain in neuropsychiatry: Insights from animal models. Neurosci. Biobehav. Rev. 2020, 115, 96–115. [Google Scholar] [CrossRef]
- Bair, M.J.; Robinson, R.L.; Katon, W.; Kroenke, K. Depression and Pain Comorbidity: A Literature Review. Arch. Intern. Med. 2003, 163, 2433–2445. [Google Scholar] [CrossRef] [Green Version]
- Tofthagen, C. Patient perceptions associated with chemotherapy-induced peripheral neuropathy. Clin. J. Oncol. Nurs. 2010, 14, E22–E28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dellarole, A.; Morton, P.; Brambilla, R.; Walters, W.; Summers, S.; Bernardes, D.; Grilli, M.; Bethea, J.R. Neuropathic pain-induced depressive-like behavior and hippocampal neurogenesis and plasticity are dependent on TNFR1 signaling. Brain Behav. Immun. 2014, 41, 65–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Branca, J.J.V.; Maresca, M.; Morucci, G.; Becatti, M.; Paternostro, F.; Gulisano, M.; Ghelardini, C.; Salvemini, D.; Mannelli, L.D.C.; Pacini, A. Oxaliplatin-induced blood brain barrier loosening: A new point of view on chemotherapy-induced neurotoxicity. Oncotarget 2018, 9, 23426–23438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seigers, R.; Schagen, S.B.; Van Tellingen, O.; Dietrich, J. Chemotherapy-related cognitive dysfunction: Current animal studies and future directions. Brain Imaging Behav. 2013, 7, 453–459. [Google Scholar] [CrossRef]
- Levy, M.J.F.; Boulle, F.; Steinbusch, H.W.; van den Hove, D.L.A.; Kenis, G.; Lanfumey, L. Neurotrophic factors and neuroplasticity pathways in the pathophysiology and treatment of depression. Psychopharmacology 2018, 235, 2195–2220. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.R.; Geiger, L.; Wiborg, O.; Czeh, B. Stress-Induced Morphological, Cellular and Molecular Changes in the Brain-Lessons Learned from the Chronic Mild Stress Model of Depression. Cells 2020, 9, 1026. [Google Scholar] [CrossRef] [Green Version]
- Menard, C.; Hodes, G.E.; Russo, S.J. Pathogenesis of depression: Insights from human and rodent studies. Neuroscience 2016, 321, 138–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dantzer, R. Neuroimmune Interactions: From the Brain to the Immune System and Vice Versa. Physiol. Rev. 2018, 98, 477–504. [Google Scholar] [CrossRef] [PubMed]
- Pfau, M.L.; Menard, C.; Russo, S.J. Inflammatory Mediators in Mood Disorders: Therapeutic Opportunities. Annu. Rev. Pharm. Toxicol. 2018, 58, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Jones, D.N.C. Emerging role of the P2X7-NLRP3-IL1β pathway in mood disorders. Psychoneuroendocrinology 2018, 98, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.C.; Yao, W.; Hashimoto, K. Brain-derived Neurotrophic Factor (BDNF)-TrkB Signaling in Inflammation-related Depression and Potential Therapeutic Targets. Curr. Neuropharmacol. 2016, 14, 721–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.H.; Chen, P.S. Inflammatory Biomarkers for Mood Disorders—A Brief Narrative Review. Curr. Pharm. Des. 2020, 26, 236–243. [Google Scholar] [CrossRef]
- Moschetti, G.; Amodeo, G.; Maftei, D.; Lattanzi, R.; Procacci, P.; Sartori, P.; Balboni, G.; Onnis, V.; Conte, V.; Panerai, A.; et al. Targeting prokineticin system counteracts hypersensitivity, neuroinflammation, and tissue damage in a mouse model of bortezomib-induced peripheral neuropathy. J. Neuroinflamm. 2019, 16, 89. [Google Scholar] [CrossRef] [Green Version]
- Moschetti, G.; Amodeo, G.; Paladini, M.S.; Molteni, R.; Balboni, G.; Panerai, A.; Sacerdote, P.; Franchi, S. Prokineticin 2 promotes and sustains neuroinflammation in vincristine treated mice: Focus on pain and emotional like behavior. Brain Behav. Immun. 2019, 82, 422–431. [Google Scholar] [CrossRef]
- Li, J.D.; Hu, W.P.; Zhou, Q.Y. Disruption of the circadian output molecule prokineticin 2 results in anxiolytic and antidepressant-like effects in mice. Neuropsychopharmacology 2009, 34, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Staff, N.P.; Grisold, A.; Grisold, W.; Windebank, A.J. Chemotherapy-induced peripheral neuropathy: A current review. Ann. Neurol. 2017, 81, 772–871. [Google Scholar] [CrossRef]
- Astaras, C.; de Micheli, R.; Moura, B.; Hundsberger, T.; Hottinger, A.F. Neurological Adverse Events Associated with Immune Checkpoint Inhibitors: Diagnosis and Management. Curr. Neurol. Neurosci. Rep. 2018, 18, 3. [Google Scholar] [CrossRef]
- Haugh, A.M.; Probasco, J.C.; Johnson, D.B. Neurologic complications of immune checkpoint inhibitors. Expert Opin. Drug Saf. 2020, 19, 479–488. [Google Scholar] [CrossRef]
- Meregalli, C.; Marjanovic, I.; Scali, C.; Monza, L.; Spinoni, N.; Galliani, C.; Brivio, R.; Chiorazzi, A.; Ballarini, E.; Rodriguez-Menendez, V.; et al. High-dose intravenous immunoglobulins reduce nerve macrophage infiltration and the severity of bortezomib-induced peripheral neurotoxicity in rats. J. Neuroinflamm. 2018, 15, 232. [Google Scholar] [CrossRef] [PubMed]
- Boehmerle, W.; Huehnchen, P.; Peruzzaro, S.; Balkaya, M.; Endres, M. Electrophysiological, behavioral and histological characterization of paclitaxel, cisplatin, vincristine and bortezomib-induced neuropathy in C57Bl/6 mice. Sci. Rep. 2014, 4, 6370. [Google Scholar] [CrossRef] [PubMed]
- Himanshu, D.; Deepa Sarkar, N. A Review of Behavioral Tests to Evaluate Different Types of Anxiety and Anti-anxiety Effects. Clin. Psychopharmacol. Neurosci. 2020, 18, 341–351. [Google Scholar] [CrossRef]
- Liu, M.Y.; Yin, C.Y.; Zhu, L.J.; Zhu, X.H.; Xu, C.; Luo, C.X.; Chen, H.; Zhu, D.Y.; Zhou, Q.G. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat. Protoc. 2018, 13, 1686–1698. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Egashira, N. Pathological Mechanisms of Bortezomib-Induced Peripheral Neuropathy. Int. J. Mol. Sci. 2021, 22, 888. [Google Scholar] [CrossRef] [PubMed]
- Huehnchen, P.; Springer, A.; Kern, J.; Kopp, U.; Kohler, S.; Alexander, T.; Hiepe, F.; Meisel, A.; Boehmerle, W.; Matthias Endres, M. Bortezomib at therapeutic doses poorly passes the blood-brain barrier and does not impair cognition. Brain Commun. 2020, 2, fcaa021. [Google Scholar] [CrossRef] [PubMed]
- Foran, E.; Kwon, D.Y.; Nofziger, J.H.; Arnold, E.S.; Hall, M.D.; Fischbeck, K.H.; Burnett, B.G. CNS uptake of bortezomib is enhanced by P-glycoprotein inhibition: Implications for spinal muscular atrophy. Neurobiol. Dis. 2016, 88, 118–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gozzetti, A.; Cerase, A. Novel Agents in CNS Myeloma Treatment. Cent. Nerv. Syst. Agents Med. Chem. 2014, 14, 23–27. [Google Scholar] [CrossRef]
- Morita-Takemura, S.; Wanaka, A. Blood-to-brain communication in the hypothalamus for energy intake regulation. Neurochem. Int. 2019, 128, 135–142. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Pacini, A.; Bonaccini, L.; Zanardelli, M.; Mello, T.; Ghelardini, C. Morphologic features and glial activation in rat oxaliplatin-dependent neuropathic pain. J. Pain 2013, 14, 1585–1600. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare Mannelli, L.; Pacini, A.; Matera, C.; Zanardelli, M.; Mello, T.; De Amici, M.; Dallanoce, C.; Ghelardini, C. Involvement of α7 nAChR subtype in rat oxaliplatin-induced neuropathy: Effects of selective activation. Neuropharmacology 2014, 79, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, Y. Brain-derived neurotrophic factor: Role in depression and suicide. Neuropsychiatr. Dis. Treat. 2009, 5, 433–449. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Nie, Z.; Shu, H.; Kuang, Y.; Chen, X.; Cheng, J.; Cheng, J.; Yu, S.; Liu, H. The Role of BDNF on Neural Plasticity in Depression. Front. Cell Neurosci. 2020, 14, 82. [Google Scholar] [CrossRef]
- Cheng, M.Y.; Leslie, F.M.; Zhou, Q.Y. Expression of Prokineticins and Their Receptors in the Adult Mouse Brain. J. Comp. Neurol. 2006, 498, 796–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negri, L.; Ferrara, N. The Prokineticins: Neuromodulators and Mediators of Inflammation and Myeloid Cell-Dependent Angiogenesis. Physiol. Rev. 2018, 98, 1055–1082. [Google Scholar] [CrossRef] [Green Version]
- Cheng, M.Y.; Lee, A.G.; Culbertson, C.; Sun, G.; Talati, R.K.; Manley, N.C.; Li, X.; Zhao, H.; Lyons, D.M.; Zhou, Q.Y.; et al. Prokineticin 2 is an endangering mediator of cerebral ischemic injury. Proc. Natl. Acad. Sci. USA 2012, 109, 5475–5480. [Google Scholar] [CrossRef] [Green Version]
- Maftei, D.; Ratano, P.; Fusco, I.; Marconi, V.; Squillace, S.; Negri, L.; Severini, C.; Balboni, G.; Steardo, L.; Bronzuoli, M.R.; et al. The prokineticin receptor antagonist PC1 rescues memory impairment induced by beta amyloid administration through the modulation of prokineticin system. Neuropharmacology 2019, 158, 107739. [Google Scholar] [CrossRef]
- Zuena, A.R.; Casolini, P.; Lattanzi, R.; Maftei, D. Chemokines in Alzheimer’s Disease: New Insights Into Prokineticins, Chemokine-Like Proteins. Front. Pharm. 2019, 10, 622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maftei, D.; Marconi, V.; Florenzano, F.; Giancotti, L.A.; Castelli, M.; Moretti, S.; Borsani, E.; Rodella, L.F.; Balboni, G.; Luongo, L.; et al. Controlling the activation of the Bv8/prokineticin system reduces neuroinflammation and abolishes thermal and tactile hyperalgesia in neuropathic animals. Br. J. Pharm. 2014, 171, 4850–4865. [Google Scholar] [CrossRef] [Green Version]
- Mahakizadeh, S.; Mokhtari, T.; Navaee, F.; Poorhassan, M.; Tajik, A.; Hassanzadeh, G. Effects of chronic hypoxia on the expression of seladin-1/Tuj1 and the number of dark neurons of hippocampus. J. Chem. Neuroanat. 2020, 104, 101744. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Shimizu, H.; Hida, H.; Urakawa, S.; Ida, K.; Nishino, H. Argyrophilic dark neurons represent various states of neuronal damage in brain insults: Some come to die and others survive. Neuroscience 2004, 125, 633–644. [Google Scholar] [CrossRef]
- Castelli, M.; Amodeo, G.; Negri, L.; Lattanzi, R.; Maftei, D.; Gotti, C.; Pistillo, F.; Onnis, V.; Congu, C.; Panerai, A.E.; et al. Antagonism of the Prokineticin System Prevents and Reverses Allodynia and Inflammation in a Mouse Model of Diabetes. PLoS ONE 2016, 11, e0146259. [Google Scholar] [CrossRef] [PubMed]
- Fourrier, C.; Bosch-Bouju, C.; Boursereau, R.; Sauvant, J.; Aubert, A.; Capuron, L.; Ferreira, G.; Layé, S.; Castanon, N. Brain tumor necrosis factor-alpha mediates anxiety-like behavior in a mouse model of severe obesity. Brain Behav. Immun. 2019, 77, 25–36. [Google Scholar] [CrossRef]
- Li, X.J.; Ma, Q.Y.; Jiang, Y.M.; Bai, X.H.; Yan, Z.Y.; Liu, Q.; Pan, Q.X.; Liu, Y.Y.; Chen, J.X. Xiaoyaosan exerts anxiolytic-like effects by down-regulating the TNF-α/JAK2-STAT3 pathway in the rat hippocampus. Sci. Rep. 2017, 7, 353. [Google Scholar] [CrossRef] [Green Version]
- Monnier, J.; Samson, M. Prokineticins in angiogenesis and cancer. Cancer Lett. 2010, 296, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Corlan, A.S.; Cimpean, A.M.; Jitariu, A.A.; Melnic, E.; Raica, M. Endocrine Gland-Derived Vascular Endothelial Growth Factor/Prokineticin-1 in Cancer Development and Tumor Angiogenesis. Int. J. Endocrinol. 2017, 2017, 3232905. [Google Scholar] [CrossRef]
- Li, Q.F.; Zhu, H.Y.; Yang, Y.F.; Liu, J.; Xiao, F.J.; Zhang, Q.W.; Wu, C.T.; Wang, H.; Wang, L.S. Prokineticin-1/endocrine gland-derived vascular endothelial growth factor is a survival factor for human multiple myeloma cells. Leuk. Lymphoma 2010, 51, 1902–1912. [Google Scholar] [CrossRef]
- Shojaei, F.; Wu, X.; Zhong, C.; Yu, L.; Liang, X.H.; Yao, J.; Blanchard, D.; Bais, C.; Peale, F.V.; van Bruggen, N.; et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature 2007, 450, 825–831. [Google Scholar] [CrossRef]
- Balboni, G.; Lazzari, I.; Trapella, C.; Negri, L.; Lattanzi, R.; Giannini, E.; Nicotra, A.; Melchiorri, P.; Visentin, S.; De Nuccio, C.; et al. Triazine compounds as antagonists at Bv8-prokineticin receptors. J. Med. Chem. 2008, 51, 7635–7639. [Google Scholar] [CrossRef]
- Deacon, R.M.J. Measuring Motor Coordination in Mice. J. Vis. Exp. 2013, 75, e2609. [Google Scholar] [CrossRef] [PubMed]
- Deacon, R.M. Digging and marble burying in mice: Simple methods for in vivo identification of biological impacts. Nat. Protoc. 2006, 1, 122–124. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amodeo, G.; Verduci, B.; Sartori, P.; Procacci, P.; Conte, V.; Balboni, G.; Sacerdote, P.; Franchi, S. The Antagonism of the Prokineticin System Counteracts Bortezomib Induced Side Effects: Focus on Mood Alterations. Int. J. Mol. Sci. 2021, 22, 10256. https://doi.org/10.3390/ijms221910256
Amodeo G, Verduci B, Sartori P, Procacci P, Conte V, Balboni G, Sacerdote P, Franchi S. The Antagonism of the Prokineticin System Counteracts Bortezomib Induced Side Effects: Focus on Mood Alterations. International Journal of Molecular Sciences. 2021; 22(19):10256. https://doi.org/10.3390/ijms221910256
Chicago/Turabian StyleAmodeo, Giada, Benedetta Verduci, Patrizia Sartori, Patrizia Procacci, Vincenzo Conte, Gianfranco Balboni, Paola Sacerdote, and Silvia Franchi. 2021. "The Antagonism of the Prokineticin System Counteracts Bortezomib Induced Side Effects: Focus on Mood Alterations" International Journal of Molecular Sciences 22, no. 19: 10256. https://doi.org/10.3390/ijms221910256
APA StyleAmodeo, G., Verduci, B., Sartori, P., Procacci, P., Conte, V., Balboni, G., Sacerdote, P., & Franchi, S. (2021). The Antagonism of the Prokineticin System Counteracts Bortezomib Induced Side Effects: Focus on Mood Alterations. International Journal of Molecular Sciences, 22(19), 10256. https://doi.org/10.3390/ijms221910256







