Metal and Metal Oxide Nanoparticle as a Novel Antibiotic Carrier for the Direct Delivery of Antibiotics
Abstract
:1. Introduction
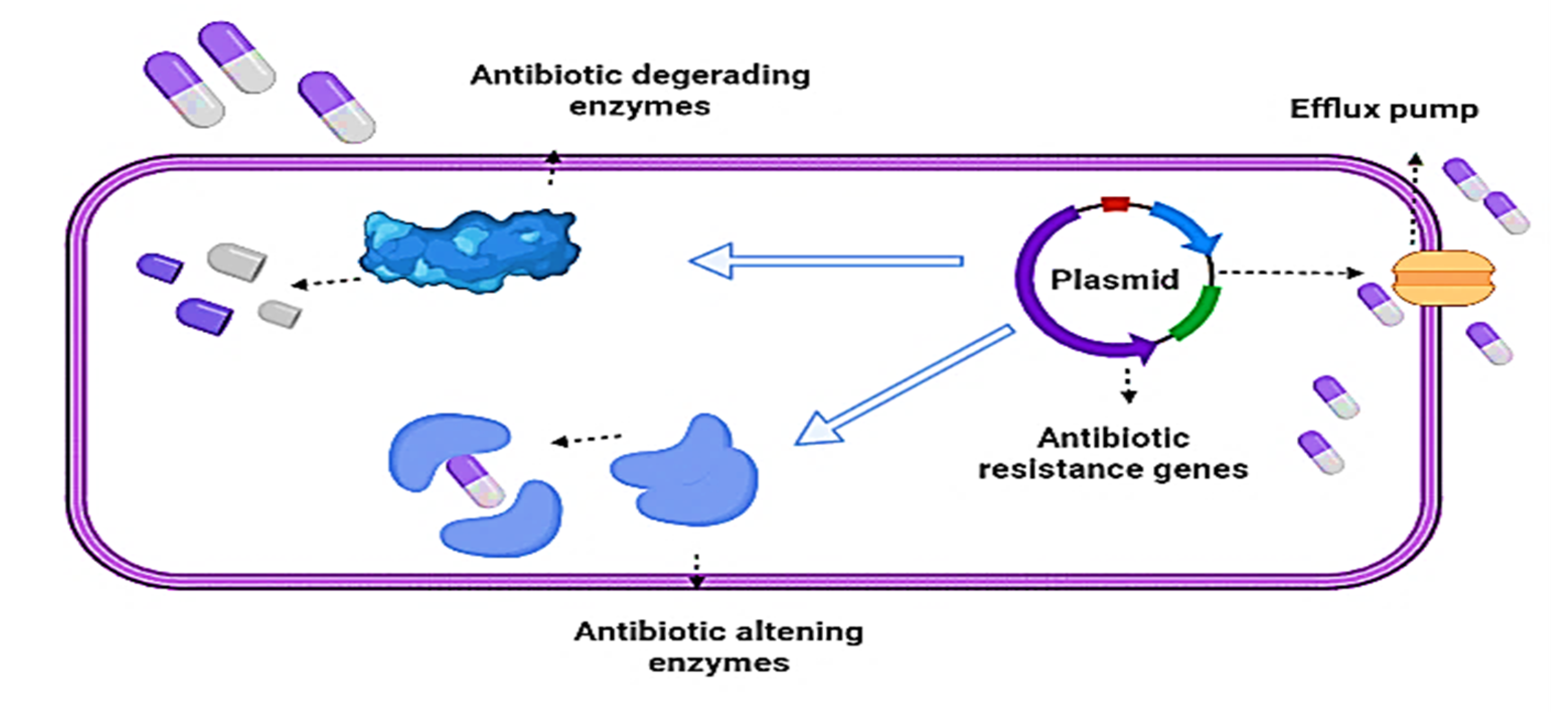
2. Bacterial Resistance against Antibiotics
2.1. Mechanisms of Bacterial Resistance againts Antibiotics
2.2. Enzymatic Mechanisms
β-Lactamases Production
2.3. Non-Enzymatic Mechanisms
2.3.1. Efflux Pump
2.3.2. Inhibitors of Bacterial Biofilm Formation
2.3.3. Modification of the Target Site
2.3.4. Modification/Elimination of Antibiotic Entry Points
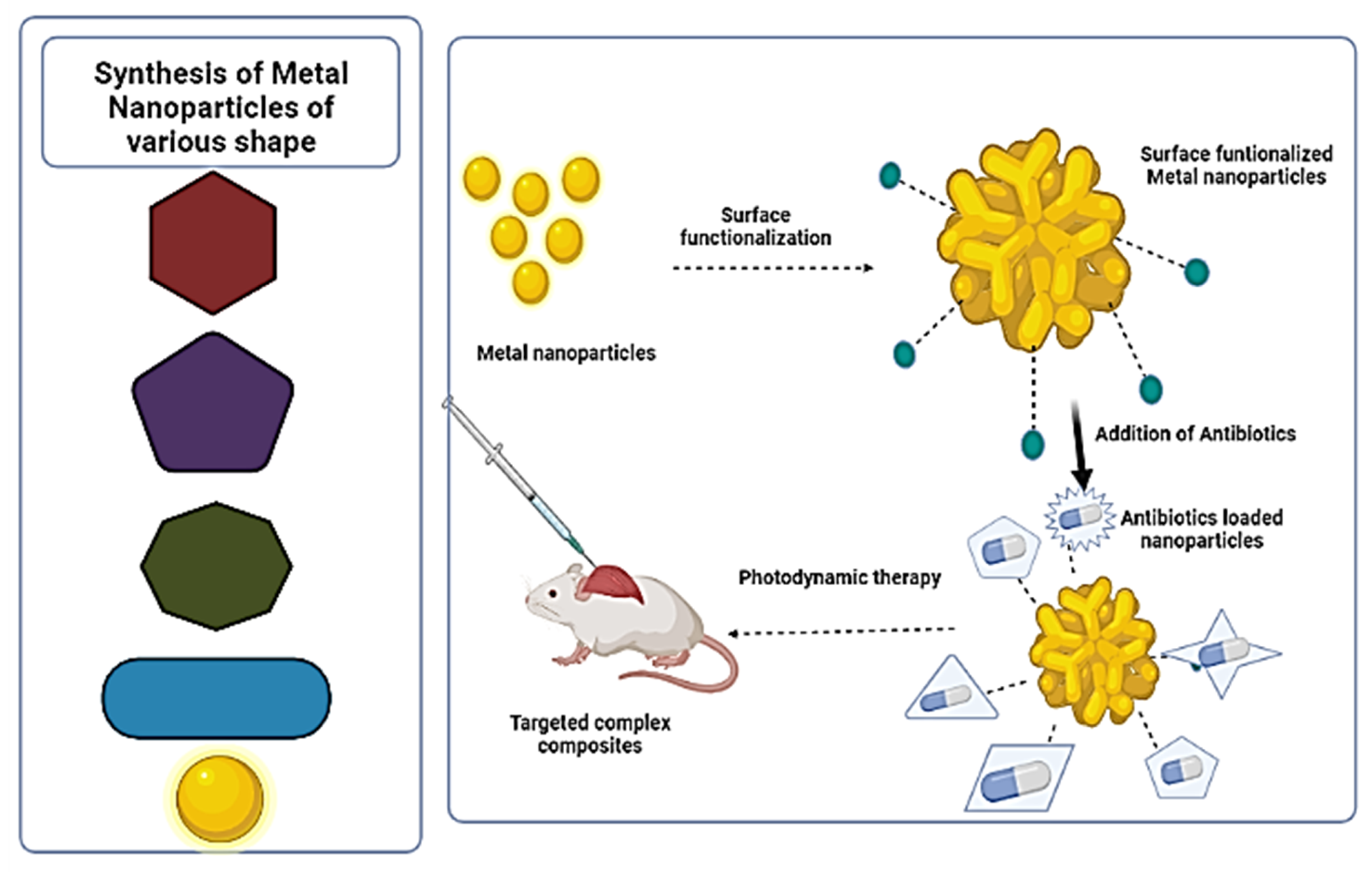
3. Synthesis of Metal Nanoparticles (MNPs) Complex for Delivery of Antibiotics
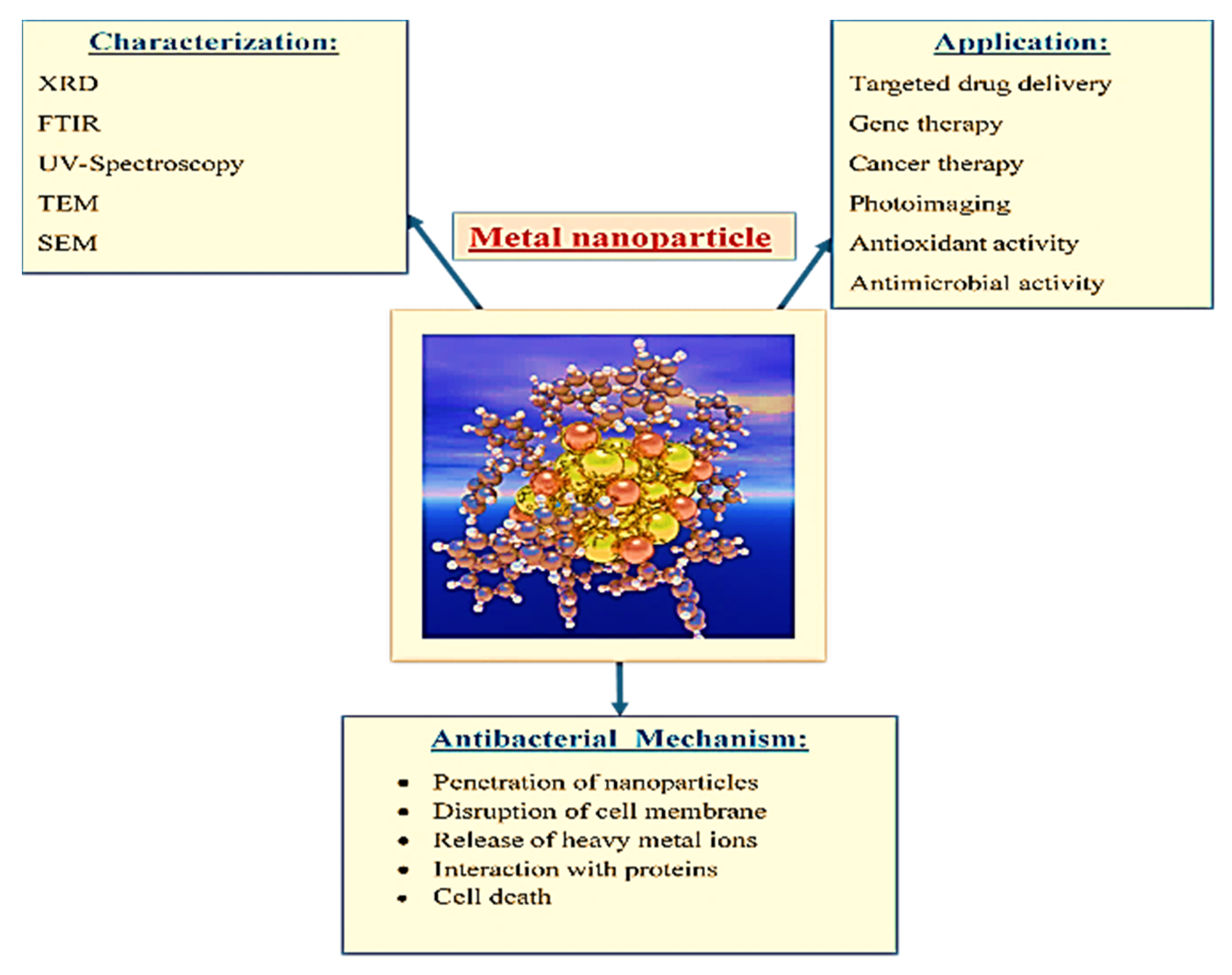
4. Characterization of Metal Nanoparticles (MNPs) Complex
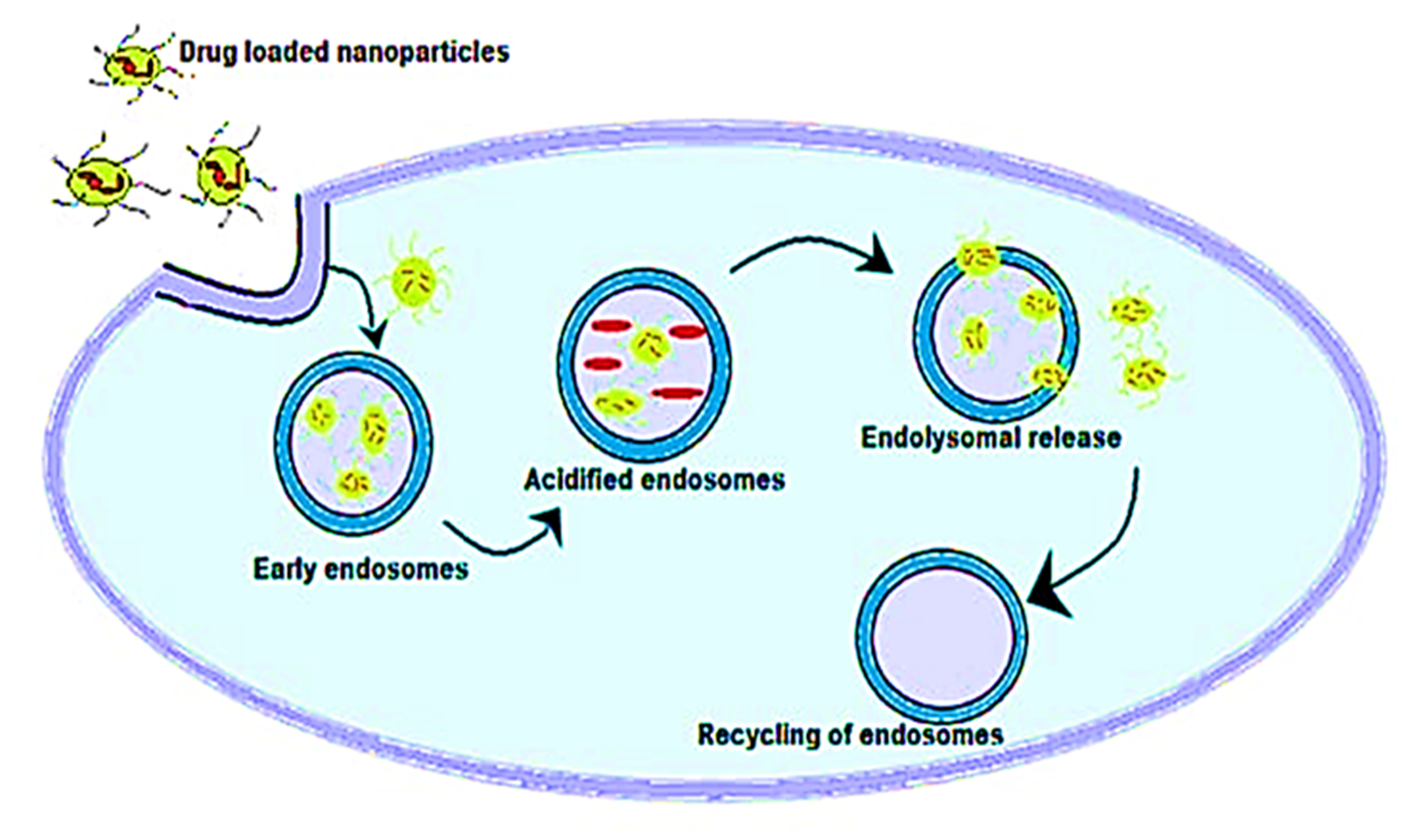
5. Antimicrobial Activity of Metal Nanoparticles and Antibiotics Complex
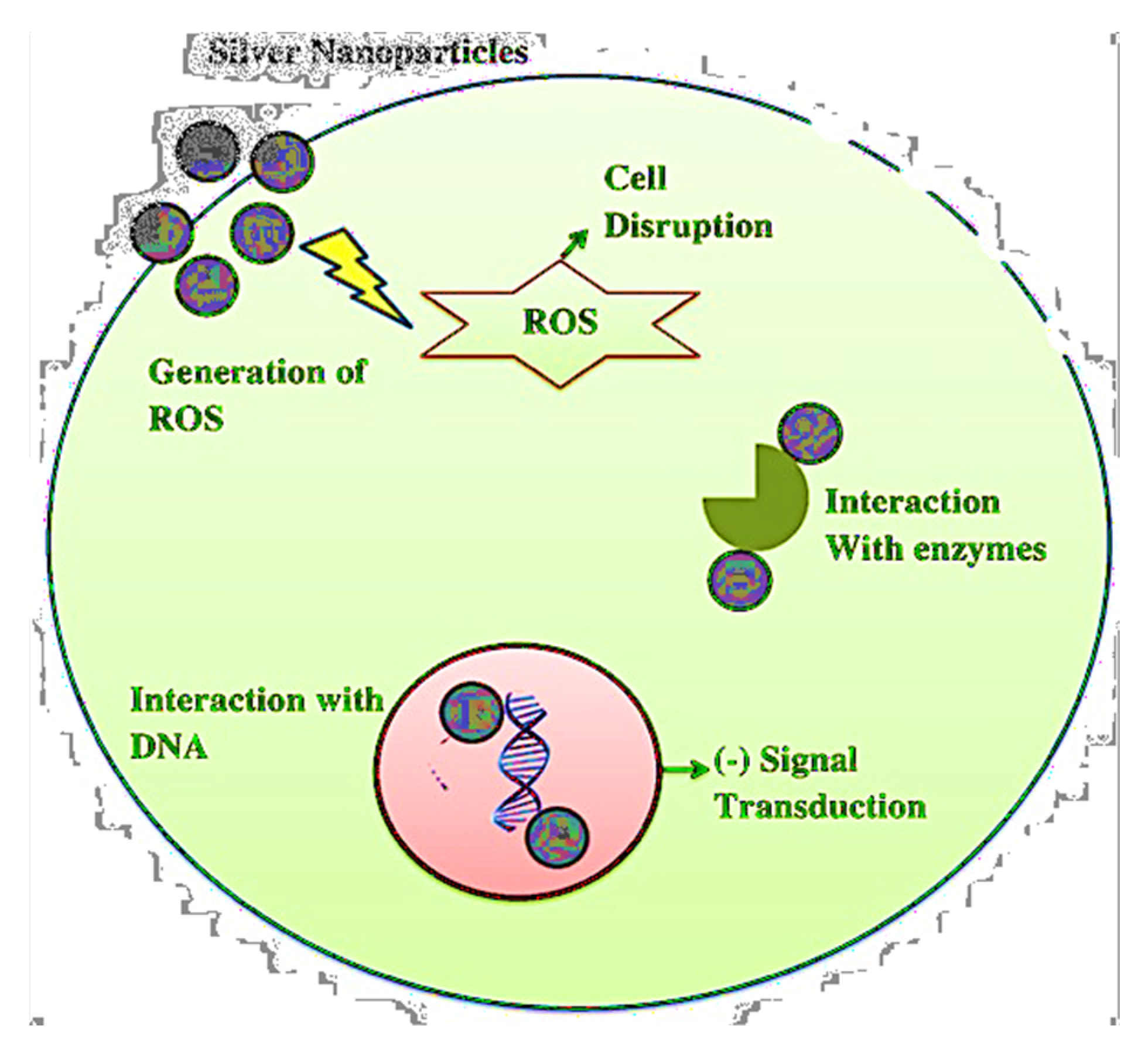
Mechanism of Antimicrobial Activity of Metal Nanoparticles and Antibiotics
6. Cytotoxic Activity of the Metal Nanoparticles and Antibiotics Complex
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vijayakumar, V.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int. J. Biol. Macromol. 2019, 122, 137–148. [Google Scholar] [CrossRef]
- Chawla, P.; Kumar, N.; Bains, A.; Dhull, S.B.; Kumar, M.; Kaushik, R.; Punia, S. Gum arabic capped copper nanoparticles: Synthesis, characterization, and applications. Int. J. Biol. Macromol. 2020, 146, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Aithal, P.S.; Aithal, S. Ideal Technology Concept & its Realization Opportunity using Nanotechnology. Int. J. Appl. Innov. Eng. Manag. 2015, 4, 153–164. [Google Scholar]
- Shobha, G.; Moses, V.; Ananda, S. Biological Synthesis of Copper Nanoparticles and its impact—A Review. Int. J. Pharm. Sci. Invent. 2014, 3, 29–30. [Google Scholar]
- Jelinkova, P.; Mazumdar, A.; Sur, V.P.; Kociova, S.; Dolezelikova, K.; Jimenez, A.M.J.; Koudelkova, Z.; Mishra, P.K.; Smerkova, K.; Heger, Z.; et al. Nanoparticle-drug conjugates treating bacterial infections. J. Control. Release 2019, 307, 166–185. [Google Scholar] [CrossRef]
- Gao, R.; Lin, Y.; Ying, Y.-L.; Long, Y.-T. Nanopore-based sensing interface for single molecule electrochemistry. Sci. China Chem. 2019, 62, 1576–1587. [Google Scholar] [CrossRef]
- Naveed, M.; Chaudhry, Z.; Bukhari, S.A.; Meer, B.; Ashraf, H. Antibiotics Resistance Mechanism; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128188828. [Google Scholar]
- Fan, Z.; Zhang, L.; Baumann, D.; Mei, L.; Yao, Y.; Duan, X.; Shi, Y.; Huang, J.; Huang, Y.; Duan, X. In Situ Transmission Electron Microscopy for Energy Materials and Devices. Adv. Mater. 2019, 31, 1–22. [Google Scholar] [CrossRef]
- Kalhapure, R.; Suleman, N.; Mocktar, C.; Seedat, N.; Govender, T. Nanoengineered Drug Delivery Systems for Enhancing Antibiotic Therapy. J. Pharm. Sci. 2015, 104, 872–905. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Thamphiwatana, S.; Angsantikul, P.; Zhang, L. Nanoparticle approaches against bacterial infections. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 532–547. [Google Scholar] [CrossRef]
- Chawla, P.; Kumar, N.; Kaushik, R.; Dhull, S.B. Synthesis, characterization and cellular mineral absorption of nanoemulsions of Rhododendron arboreum flower extracts stabilized with gum arabic. J. Food Sci. Technol. 2019, 56, 5194–5203. [Google Scholar] [CrossRef]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Arias, M.; Boutinguiza, M.; Del Val, J.; Covarrubias, C.; Bastias, F.; Gómez, L.; Maureira, M.; Arias-González, F.; Riveiro, A.; Pou, J. Copper nanoparticles obtained by laser ablation in liquids as bactericidal agent for dental applications. Appl. Surf. Sci. 2020, 507, 145032. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The-antimicrobial-activity-of-nanoparticles—Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [Green Version]
- Pathania, R.; Sharma, B.; Chawla, P.; Kaushik, R.; Khan, M.A. Nanoemulsions Formulated with Cinnamon Oil and Their Antimicrobial Applications. In Nanotechnological Approaches in Food Microbiology; CRC Press: Boca Raton, FL, USA, 2020; pp. 249–266. [Google Scholar]
- Bhargav, E.; Bhargav, E.; Madhuri, N.; Ramesh, K.; Ravi, V. Targeted Drug Delivery—A Review. World J. Pharm. Pharm. Sci. 2014, 3, 150–169. [Google Scholar]
- Fair, R.J.; Tor, Y. Antibiotics and Bacterial Resistance in the 21st Century. Perspect. Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef] [Green Version]
- Anbarasu, A.; Ramaiah, S. Gene interaction network approach to elucidate the multidrug resistance mechanisms in the pathogenic bacterial strain Proteus mirabilis. J. Cell. Physiol. 2021, 236, 468–479. [Google Scholar] [CrossRef]
- Tshireletso, P.; Ateba, C.; Fayemi, O. Spectroscopic and Antibacterial Properties of CuONPs from Orange, Lemon and Tangerine Peel Extracts: Potential for Combating Bacterial Resistance. Molecules 2021, 26, 586. [Google Scholar] [CrossRef]
- Dziarski, R.; Gupta, D. How innate immunity proteins kill bacteria and why they are not prone to resistance. Curr. Genet. 2017, 64, 125–129. [Google Scholar] [CrossRef]
- Aghdam, E.M.; Hejazi, M.S.; Barzegar, A. Riboswitches: From living biosensors to novel targets of antibiotics. Gene 2016, 592, 244–259. [Google Scholar] [CrossRef]
- Li, T.; Teng, D.; Mao, R.; Hao, Y.; Wang, X.; Wang, J. A critical review of antibiotic resistance in probiotic bacteria. Food Res. Int. 2020, 136, 109571. [Google Scholar] [CrossRef] [PubMed]
- Arzanlou, M.; Chai, W.; Venter, H. Intrinsic, adaptive and acquired antimicrobial resistance in Gram-negative bacteria. Essays Biochem. 2017, 61, 49–59. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Wang, Y.; Lin, Z.; Wang, D.; Sun, H. Resistance risk induced by quorum sensing inhibitors and their combined use with antibiotics: Mechanism and its relationship with toxicity. Chemosphere 2021, 265, 129153. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Swick, M.C.; Ledesma, K.R.; Yang, Z.; Hu, M.; Zechiedrich, L.; Tam, V.H. Temporal Interplay between Efflux Pumps and Target Mutations in Development of Antibiotic Resistance in Escherichia coli. Antimicrob. Agents Chemother. 2012, 56, 1680–1685. [Google Scholar] [CrossRef] [Green Version]
- Peterson, E.; Kaur, P. Antibiotic Resistance Mechanisms in Bacteria: Relationships Between Resistance Determinants of Antibiotic Producers, Environmental Bacteria, and Clinical Pathogens. Front. Microbiol. 2018, 9, 1–21. [Google Scholar] [CrossRef]
- Bonomo, R.A. β-Lactamases: A Focus on Current Challenges. Cold Spring Harb. Perspect. Med. 2017, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Escandón-Vargas, K.; Reyes, S.; Gutiérrez, S.; Villegas, M.V. The epidemiology of carbapenemases in Latin America and the Caribbean. Expert Rev. Anti-Infect. Ther. 2016, 15, 277–297. [Google Scholar] [CrossRef]
- Vrancianu, C.O.; Gheorghe, I.; Czobor, I.B.; Chifiriuc, M.C. Antibiotic Resistance Profiles, Molecular Mechanisms and Innovative Treatment Strategies of Acinetobacter baumannii. Microorganisms 2020, 8, 935. [Google Scholar] [CrossRef] [PubMed]
- Blanco, P.; Hernando-Amado, S.; Reales-Calderon, J.A.; Corona, F.; Lira, F.; Alcalde-Rico, M.; Bernardini, A.; Sanchez, M.B.; Martinez, J.L. Bacterial Multidrug Efflux Pumps: Much More Than Antibiotic Resistance Determinants. Microorganisms 2016, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Handzlik, J.; Matys, A.; Kieć-Kononowicz, K. Recent Advances in Multi-Drug Resistance (MDR) Efflux Pump Inhibitors of Gram-Positive Bacteria S. aureus. Antibiotics 2013, 2, 28–45. [Google Scholar] [CrossRef] [Green Version]
- Schillaci, D.; Spanò, V.; Parrino, B.; Carbone, A.; Montalbano, A.; Barraja, P.; Diana, P.; Cirrincione, G.; Cascioferro, S. Pharmaceutical Approaches to Target Antibiotic Resistance Mechanisms. J. Med. Chem. 2017, 60, 8268–8297. [Google Scholar] [CrossRef]
- Abushaheen, M.A.; Fatani, A.J.; Alosaimi, M.; Mansy, W.; George, M.; Acharya, S.; Rathod, S.; Divakar, D.D.; Jhugroo, C.; Vellappally, S.; et al. Antimicrobial resistance, mechanisms and its clinical significance. Dis. Mon. 2020, 66, 100971. [Google Scholar] [CrossRef] [PubMed]
- Yelin, I.; Kishony, R. Antibiotic Resistance. Cell 2018, 172, 1136.e1. [Google Scholar] [CrossRef]
- Papaneophytou, C.; Giannenas, I.; Dragomir, C. Resistance of Bacteria, Fungi, and Parasites to Antibiotics or Natural Substances of Botanical Origin; Elsevier: Amsterdam, The Netherlands, 2020; pp. 339–354. [Google Scholar]
- Serpi, M.; Ferrari, V.; Pertusati, F. Nucleoside Derived Antibiotics to Fight Microbial Drug Resistance: New Utilities for an Established Class of Drugs? J. Med. Chem. 2016, 59, 10343–10382. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D. Something old, something new: Revisiting natural products in antibiotic drug discovery. Can. J. Microbiol. 2014, 60, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Sur, S.; Rathore, A.; Dave, V.; Reddy, K.R.; Chouhan, R.S.; Sadhu, V. Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano-Struct. Nano-Objects 2019, 20, 100397. [Google Scholar] [CrossRef]
- Häffner, S.M.; Malmsten, M. Interplay between amphiphilic peptides and nanoparticles for selective membrane destabilization and antimicrobial effects. Curr. Opin. Colloid Interface Sci. 2019, 44, 59–71. [Google Scholar] [CrossRef]
- Liyanage, P.Y.; Hettiarachchi, S.D.; Zhou, Y.; Ouhtit, A.; Seven, E.S.; Oztan, C.Y.; Celik, E.; Leblanc, R.M. Nanoparticle-mediated targeted drug delivery for breast cancer treatment. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Seyed-Talebi, S.M.; Kazeminezhad, I.; Motamedi, H. TiO2 hollow spheres as a novel antibiotic carrier for the direct delivery of gentamicin. Ceram. Int. 2018, 44, 13457–13462. [Google Scholar] [CrossRef]
- Bellio, P.; Luzi, C.; Mancini, A.; Cracchiolo, S.; Passacantando, M.; Di Pietro, L.; Perilli, M.; Amicosante, G.; Santucci, S.; Celenza, G. Cerium oxide nanoparticles as potential antibiotic adjuvant. Effects of CeO2 nanoparticles on bacterial outer membrane permeability. Biochim. Biophys. Acta Biomembr. 2018, 1860, 2428–2435. [Google Scholar] [CrossRef]
- Serhan, M.; Sprowls, M.; Jackemeyer, D.; Long, M.; Perez, I.D.; Maret, W.; Tao, N.; Forzani, E. Total iron measurement in human serum with a smartphone. In Proceedings of the 2019 AIChE Annual Meeting, Orlando, FL, USA, 10–15 November 2019. [Google Scholar] [CrossRef]
- Gautam, S.; Agrawal, H.; Thakur, M.; Akbari, A.; Sharda, H.; Kaur, R.; Amini, M. Metal oxides and metal organic frameworks for the photocatalytic degradation: A review. J. Environ. Chem. Eng. 2020, 8, 103726. [Google Scholar] [CrossRef]
- Singh, A.; Gautam, P.K.; Verma, A.; Singh, V.; Shivapriya, P.M.; Shivalkar, S.; Sahoo, A.K.; Samanta, S.K. Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: A review. Biotechnol. Rep. 2020, 25, e00427. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, H.; Khan, S. Biological Synthesis of Silver Nanoparticles and Its Antibacterial Activity. J. Nanomed. Nanotechnol. 2016, 7, 1000366. [Google Scholar] [CrossRef] [Green Version]
- Sasidharan, D.; Namitha, T.; Johnson, S.P.; Jose, V.; Mathew, P. Synthesis of silver and copper oxide nanoparticles using Myristica fragrans fruit extract: Antimicrobial and catalytic applications. Sustain. Chem. Pharm. 2020, 16, 100255. [Google Scholar] [CrossRef]
- Długosz, O.; Sochocka, M.; Ochnik, M.; Banach, M. Metal and bimetallic nanoparticles: Flow synthesis, bioactivity and toxicity. J. Colloid Interface Sci. 2021, 586, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Hemeg, H.A. Nanomaterials for alternative antibacterial therapy. Int. J. Nanomed. 2017, 12, 8211–8225. [Google Scholar] [CrossRef] [Green Version]
- Khurana, C.; Sharma, P.; Pandey, O.; Chudasama, B. Synergistic Effect of Metal Nanoparticles on the Antimicrobial Activities of Antibiotics against Biorecycling Microbes. J. Mater. Sci. Technol. 2016, 32, 524–532. [Google Scholar] [CrossRef]
- Roshmi, T.; Soumya, K.R.; Jyothis, M.; Radhakrishnan, E.K. Effect of biofabricated gold nanoparticle-based antibiotic conjugates on minimum inhibitory concentration of bacterial isolates of clinical origin. Gold Bull. 2015, 48, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Assadi, Z.; Emtiazi, G.; Zarrabi, A. Hyperbranched polyglycerol coated on copper oxide nanoparticles as a novel core-shell nano-carrier hydrophilic drug delivery model. J. Mol. Liq. 2018, 250, 375–380. [Google Scholar] [CrossRef]
- Gatadi, S.; Madhavi, Y.V.; Nanduri, S. Nanoparticle drug conjugates treating microbial and viral infections: A review. J. Mol. Struct. 2021, 1228, 129750. [Google Scholar] [CrossRef]
- Kalita, S.; Kandimalla, R.; Sharma, K.K.; Kataki, A.C.; Deka, M.; Kotoky, J. Amoxicillin functionalized gold nanoparticles reverts MRSA resistance. Mater. Sci. Eng. C 2016, 61, 720–727. [Google Scholar] [CrossRef]
- Ashfaq, M.; Verma, N.; Khan, S. Copper/zinc bimetal nanoparticles-dispersed carbon nanofibers: A novel potential antibiotic material. Mater. Sci. Eng. C 2016, 59, 938–947. [Google Scholar] [CrossRef]
- Alabresm, A.; Chen, Y.P.; Wichter-Chandler, S.; Lead, J.; Benicewicz, B.C.; Decho, A.W. Nanoparticles as antibiotic-delivery vehicles (ADVs) overcome resistance by MRSA and other MDR bacterial pathogens: The grenade hypothesis. J. Glob. Antimicrob. Resist. 2020, 22, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Bello-Vieda, N.J.; Pastrana, H.F.; Garavito, M.F.; Ávila, A.G.; Celis, A.M.; Muñoz-Castro, A.; Restrepo, S.; Hurtado, J.J. Antibacterial Activities of Azole Complexes Combined with Silver Nanoparticles. Molecules 2018, 23, 361. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Nguyen, T.T.H.; Wang, S.-L.; Vo, T.P.K.; Nguyen, A.D. Preparation of chitosan nanoparticles by TPP ionic gelation combined with spray drying, and the antibacterial activity of chitosan nanoparticles and a chitosan nanoparticle–amoxicillin complex. Res. Chem. Intermed. 2016, 43, 3527–3537. [Google Scholar] [CrossRef]
- Kalhapure, R.S.; Sonawane, S.J.; Sikwal, D.R.; Jadhav, M.; Rambharose, S.; Mocktar, C.; Govender, T. Solid lipid nanoparticles of clotrimazole silver complex: An efficient nano antibacterial against Staphylococcus aureus and MRSA. Colloids Surf. B Biointerfaces 2015, 136, 651–658. [Google Scholar] [CrossRef]
- Bolla, P.K.; Kalhapure, R.; Rodriguez, V.A.; Ramos, D.V.; Dahl, A.; Renukuntla, J. Preparation of solid lipid nanoparticles of furosemide-silver complex and evaluation of antibacterial activity. J. Drug Deliv. Sci. Technol. 2019, 49, 6–13. [Google Scholar] [CrossRef]
- Shumatbaeva, A.M.; Morozova, J.E.; Shalaeva, Y.V.; Saifina, A.F.; Gubaidullin, A.T.; Syakaev, V.V.; Sapunova, A.S.; Voloshina, A.D.; Nizameev, I.R.; Kadirov, M.K.; et al. Synthesis of Ag-AgCl nanoparticles capped by calix[4]resorcinarene-mPEG conjugate and their antimicrobial activity. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125124. [Google Scholar] [CrossRef]
- Murali, M.; Mahendra, C.; Rajashekar, N.; Sudarshana, M.; Raveesha, K.; Amruthesh, K. Antibacterial and antioxidant properties of biosynthesized zinc oxide nanoparticles from Ceropegia candelabrum L.—An endemic species. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 179, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Pillai, A.M.; Sivasankarapillai, V.S.; Rahdar, A.; Joseph, J.; Sadeghfar, F.; Rajesh, K.; Kyzas, G.Z. Green synthesis and characterization of zinc oxide nanoparticles with antibacterial and antifungal activity. J. Mol. Struct. 2020, 1211, 128107. [Google Scholar] [CrossRef]
- Agarwal, H.; Menon, S.; Kumar, S.V.; Rajeshkumar, S. Mechanistic study on antibacterial action of zinc oxide nanoparticles synthesized using green route. Chem. Biol. Interact. 2018, 286, 60–70. [Google Scholar] [CrossRef]
- Soren, S.; Kumar, S.; Mishra, S.; Jena, P.K.; Verma, S.K.; Parhi, P. Evaluation of antibacterial and antioxidant potential of the zinc oxide nanoparticles synthesized by aqueous and polyol method. Microb. Pathog. 2018, 119, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Priya, P.V.; Balavijayalakshmi, J. Impact of Aluminium on “Structural, Optical and Morphological Properties of Copper Oxide Nanoparticles. ” Mater. Today Proc. 2019, 18, 1686–1695. [Google Scholar] [CrossRef]
- Padma, P.N.; Kumari, S.C.; Banu, S.T. Effect of Diverse Factors on Green Synthesis of Copper Nanoparticles. Int. J. Life Sci. 2018, 6, 659–664. [Google Scholar]
- Ojha, N.K.; Zyryanov, G.V.; Majee, A.; Charushin, V.N.; Chupakhin, O.N.; Santra, S. Copper nanoparticles as inexpensive and efficient catalyst: A valuable contribution in organic synthesis. Coord. Chem. Rev. 2017, 353, 1–57. [Google Scholar] [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef]
- Ahmed, K.B.A.; Raman, T.; Veerappan, A. Future prospects of antibacterial metal nanoparticles as enzyme inhibitor. Mater. Sci. Eng. C 2016, 68, 939–947. [Google Scholar] [CrossRef]
- Ding, F.; Songkiatisak, P.; Cherukuri, P.K.; Huang, T.; Xu, X.-H.N. Size-Dependent Inhibitory Effects of Antibiotic Drug Nanocarriers against Pseudomonas aeruginosa. ACS Omega 2018, 3, 1231–1243. [Google Scholar] [CrossRef] [Green Version]
- Djafari, J.; Marinho, C.; Santos, T.; Igrejas, G.; Torres, C.; Capelo, J.L.; Poeta, P.; Lodeiro, C.; Fernández-Lodeiro, J. New Synthesis of Gold- and Silver-Based Nano-Tetracycline Composites. ChemistryOpen 2016, 5, 206–212. [Google Scholar] [CrossRef] [Green Version]
- Thapa, R.; Bhagat, C.; Shrestha, P.; Awal, S.; Dudhagara, P. Enzyme-mediated formulation of stable elliptical silver nanoparticles tested against clinical pathogens and MDR bacteria and development of antimicrobial surgical thread. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lara, H.H.; Ayala-Núñez, N.V.; Turrent, L.D.; Padilla, C.R. Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J. Microbiol. Biotechnol. 2010, 26, 615–621. [Google Scholar] [CrossRef]
- Reddy, L.S.; Nisha, M.M.; Joice, M.; Shilpa, P.N. Antimicrobial activity of zinc oxide (ZnO) nanoparticle againstKlebsiella pneumoniae. Pharm. Biol. 2014, 52, 1388–1397. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.-H.; Hong, J.; McGuffie, M.; Yeom, B.; Vanepps, J.S.; Kotov, N. Shape-Dependent Biomimetic Inhibition of Enzyme by Nanoparticles and Their Antibacterial Activity. ACS Nano 2015, 9, 9097–9105. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Mechanism of Photogenerated Reactive Oxygen Species and Correlation with the Antibacterial Properties of Engineered Metal-Oxide Nanoparticles. ACS Nano 2012, 6, 1–22. [Google Scholar] [CrossRef]
- Ocsoy, I.; Yusufbeyoglu, S.; Yılmaz, V.; McLamore, E.S.; Ildız, N.; Ülgen, A. DNA aptamer functionalized gold nanostructures for molecular recognition and photothermal inactivation of methicillin-Resistant Staphylococcus aureus. Colloids Surf. B Biointerfaces 2017, 159, 16–22. [Google Scholar] [CrossRef]
- Roy, A.S.; Parveen, A.; Koppalkar, A.R.; Prasad, M.A. Effect of Nano—Titanium Dioxide with Different Antibiotics against Methicillin-Resistant Staphylococcus Aureus. J. Biomater. Nanobiotechnology 2010, 1, 37–41. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Zhao, S.; Wang, Z.; Wu, J.; Wang, J.; Wang, S. In situ immobilization of silver nanoparticles for improving permeability, antifouling and anti-bacterial properties of ultrafiltration membrane. J. Membr. Sci. 2016, 499, 269–281. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, H.; Pandey, M.; Lim, Y.Q.; Low, C.Y.; Lee, C.T.; Marilyn, T.C.L.; Loh, H.S.; Lim, Y.P.; Lee, C.F.; Bhattamishra, S.K.; et al. Silver nanoparticles: Advanced and promising technology in diabetic wound therapy. Mater. Sci. Eng. C 2020, 112, 110925. [Google Scholar] [CrossRef]
- Shaikh, S.; Nazam, N.; Rizvi, S.M.D.; Ahmad, K.; Baig, M.H.; Lee, E.J.; Choi, I. Mechanistic Insights into the Antimicrobial Actions of Metallic Nanoparticles and Their Implications for Multidrug Resistance. Int. J. Mol. Sci. 2019, 20, 2468. [Google Scholar] [CrossRef] [Green Version]
- Hasanzadeh, M.; Shadjou, N. Pharmacogenomic study using bio- and nanobioelectrochemistry: Drug–DNA interaction. Mater. Sci. Eng. C 2016, 61, 1002–1017. [Google Scholar] [CrossRef]
- Zaidi, S.; Misba, L.; Khan, A.U. Nano-therapeutics: A revolution in infection control in post antibiotic era. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2281–2301. [Google Scholar] [CrossRef]
- Ruddaraju, L.K.; Pammi, S.V.N.; Pallela, P.N.V.K.; Padavala, V.S.; Kolapalli, V.R.M. Antibiotic potentiation and anti-cancer competence through bio-mediated ZnO nanoparticles. Mater. Sci. Eng. C 2019, 103, 109756. [Google Scholar] [CrossRef]
- Eichenberger, E.; Thaden, J.T. Epidemiology and Mechanisms of Resistance of Extensively Drug Resistant Gram-Negative Bacteria. Antibiotics 2019, 8, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yew, Y.P.; Shameli, K.; Miyake, M.; Khairudin, N.B.B.A.; Mohamad, S.E.B.; Naiki, T.; Lee, K.X. Green biosynthesis of superparamagnetic magnetite Fe3O4 nanoparticles and biomedical applications in targeted anticancer drug delivery system: A review. Arab. J. Chem. 2020, 13, 2287–2308. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Gupta, I.; Brandelli, A. Bioactivity of noble metal nanoparticles decorated with biopolymers and their application in drug delivery. Int. J. Pharm. 2015, 496, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Chawla, P.; Najda, A.; Bains, A.; Nurzyńska-Wierdak, R.; Kaushik, R.; Tosif, M.M. Potential of Gum Arabic Functionalized Iron Hydroxide Nanoparticles Embedded Cellulose Paper for Packaging of Paneer. Nanomaterials 2021, 11, 1308. [Google Scholar] [CrossRef]
- Brooks, B.D.; Brooks, A.E. Therapeutic strategies to combat antibiotic resistance. Adv. Drug Deliv. Rev. 2014, 78, 14–27. [Google Scholar] [CrossRef]
| Source of Nanoparticle | Antibiotic | Resistant Bacteria | Mode of Action | References |
|---|---|---|---|---|
| Ag NPs | Ofloxacin | Pseudomonas aeruginosa | Blocking of efflux pump | [72] |
| Ag NPs | Tetracycline | Staphylococcus aureus and Escherichia coli | Synergistic reaction with exanimated antibiotic | [73] |
| Ag NPs | Teicoplanin | Streptococcus pneumoniae | ROS death of mediated cell | [74] |
| Ag NPs | Ampicillin | Pseudomonas aeruginosa and Escherichia coli | Synergistic action with antibiotic | [75] |
| ZnO NPs | Ampicillin | Klebsiella pneumoniae | ROS rupture of mediated cell wall | [76] |
| ZnO NPs | Methicillin | Staphylococcus aureus | Enzymatic inhibition | [77] |
| Au NPs | Vancomycin | Staphylococcus aureus | Synergistic action with exanimated antibiotic | [78] |
| Au NPs | Methicillin | Staphylococcus aureus | Photoinactivation and generation of ROS | [79] |
| TiO2 NPs | Methicillin | Staphylococcus aureus | Degradation of protein | [80] |
| TiO2 NPs | Multidrug resistant | Escherichia coli | Cell wall rupture and generation of ROS | [81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotrange, H.; Najda, A.; Bains, A.; Gruszecki, R.; Chawla, P.; Tosif, M.M. Metal and Metal Oxide Nanoparticle as a Novel Antibiotic Carrier for the Direct Delivery of Antibiotics. Int. J. Mol. Sci. 2021, 22, 9596. https://doi.org/10.3390/ijms22179596
Kotrange H, Najda A, Bains A, Gruszecki R, Chawla P, Tosif MM. Metal and Metal Oxide Nanoparticle as a Novel Antibiotic Carrier for the Direct Delivery of Antibiotics. International Journal of Molecular Sciences. 2021; 22(17):9596. https://doi.org/10.3390/ijms22179596
Chicago/Turabian StyleKotrange, Harshada, Agnieszka Najda, Aarti Bains, Robert Gruszecki, Prince Chawla, and Mansuri M. Tosif. 2021. "Metal and Metal Oxide Nanoparticle as a Novel Antibiotic Carrier for the Direct Delivery of Antibiotics" International Journal of Molecular Sciences 22, no. 17: 9596. https://doi.org/10.3390/ijms22179596
APA StyleKotrange, H., Najda, A., Bains, A., Gruszecki, R., Chawla, P., & Tosif, M. M. (2021). Metal and Metal Oxide Nanoparticle as a Novel Antibiotic Carrier for the Direct Delivery of Antibiotics. International Journal of Molecular Sciences, 22(17), 9596. https://doi.org/10.3390/ijms22179596







