TNF-α and IFN-γ Participate in Improving the Immunoregulatory Capacity of Mesenchymal Stem/Stromal Cells: Importance of Cell–Cell Contact and Extracellular Vesicles
Abstract
1. Mesenchymal Stem/Stromal Cells (MSCs)
2. Immunoregulatory Properties of BM-MSCs
3. Immunoregulation Mediated by Secreted Factors
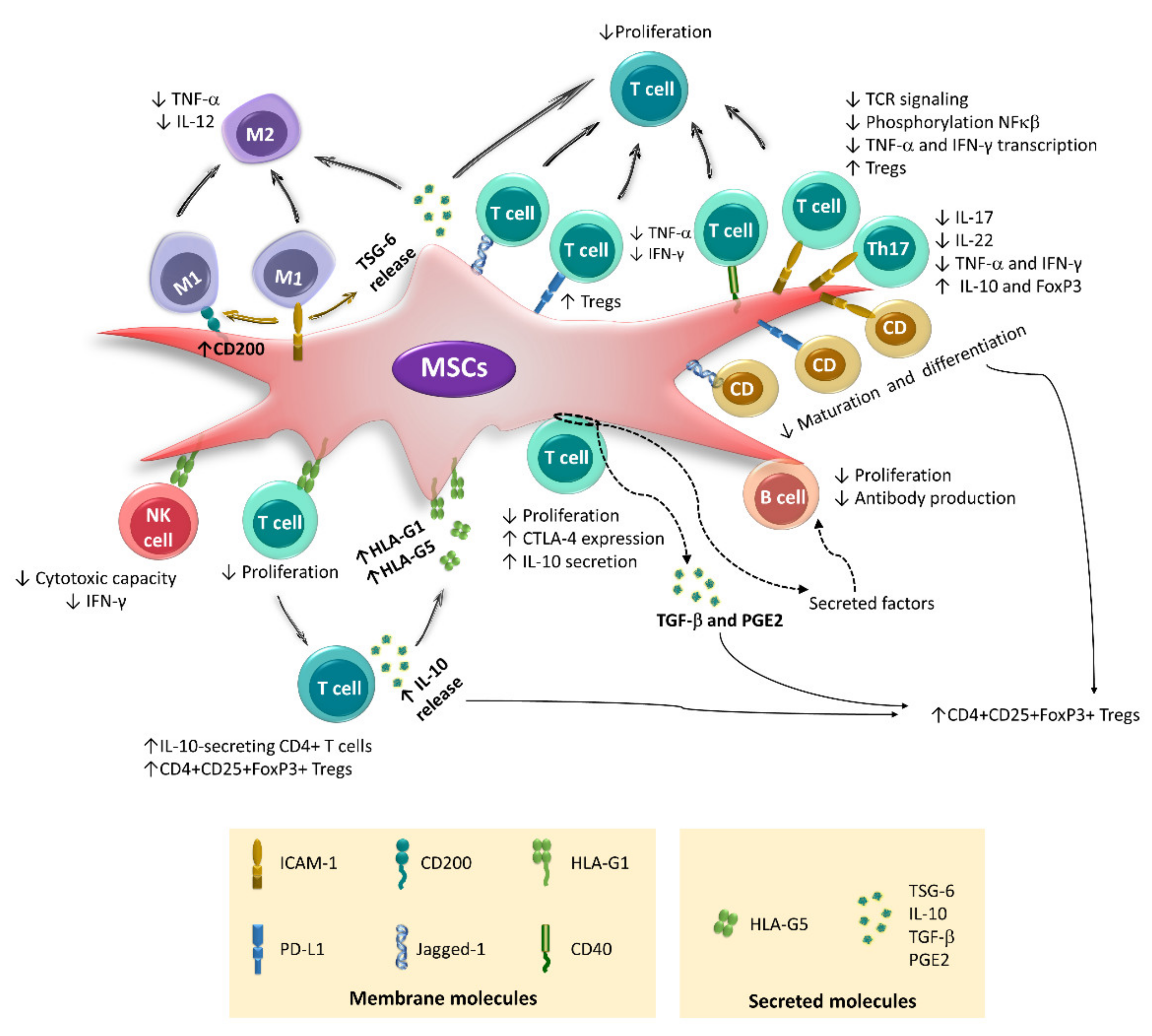
4. Immunoregulation Mediated by Cell–Cell Contact
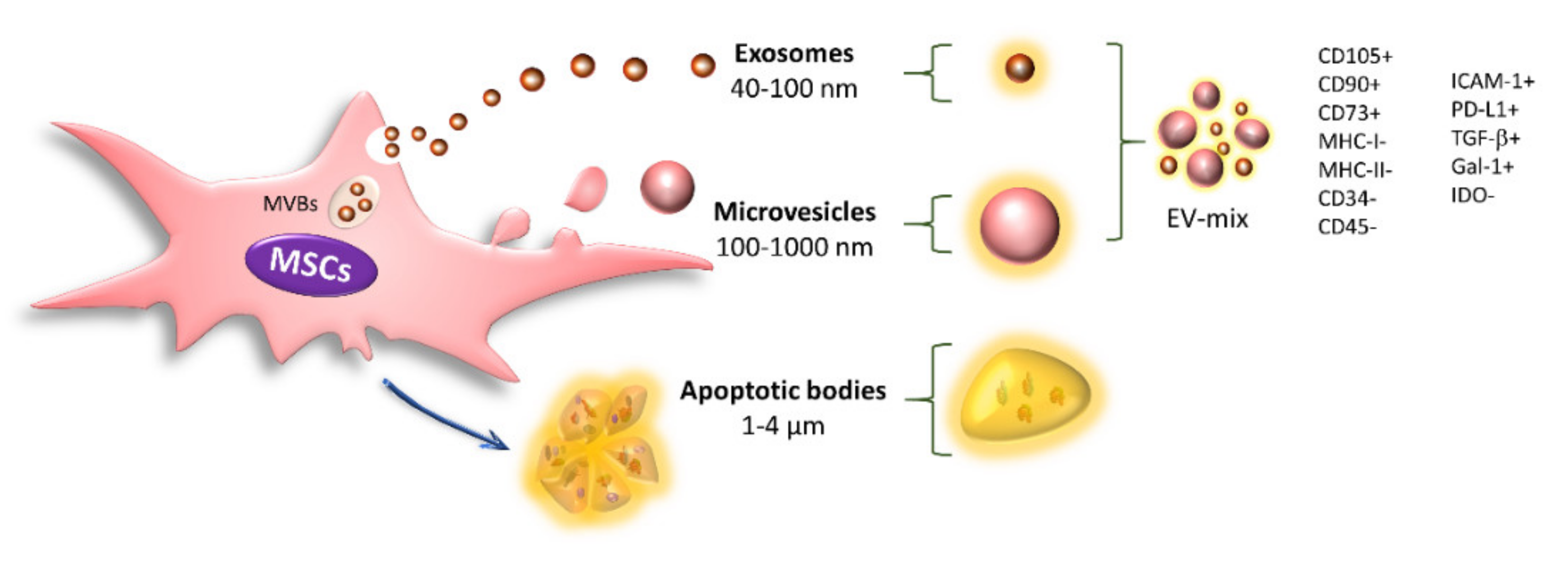
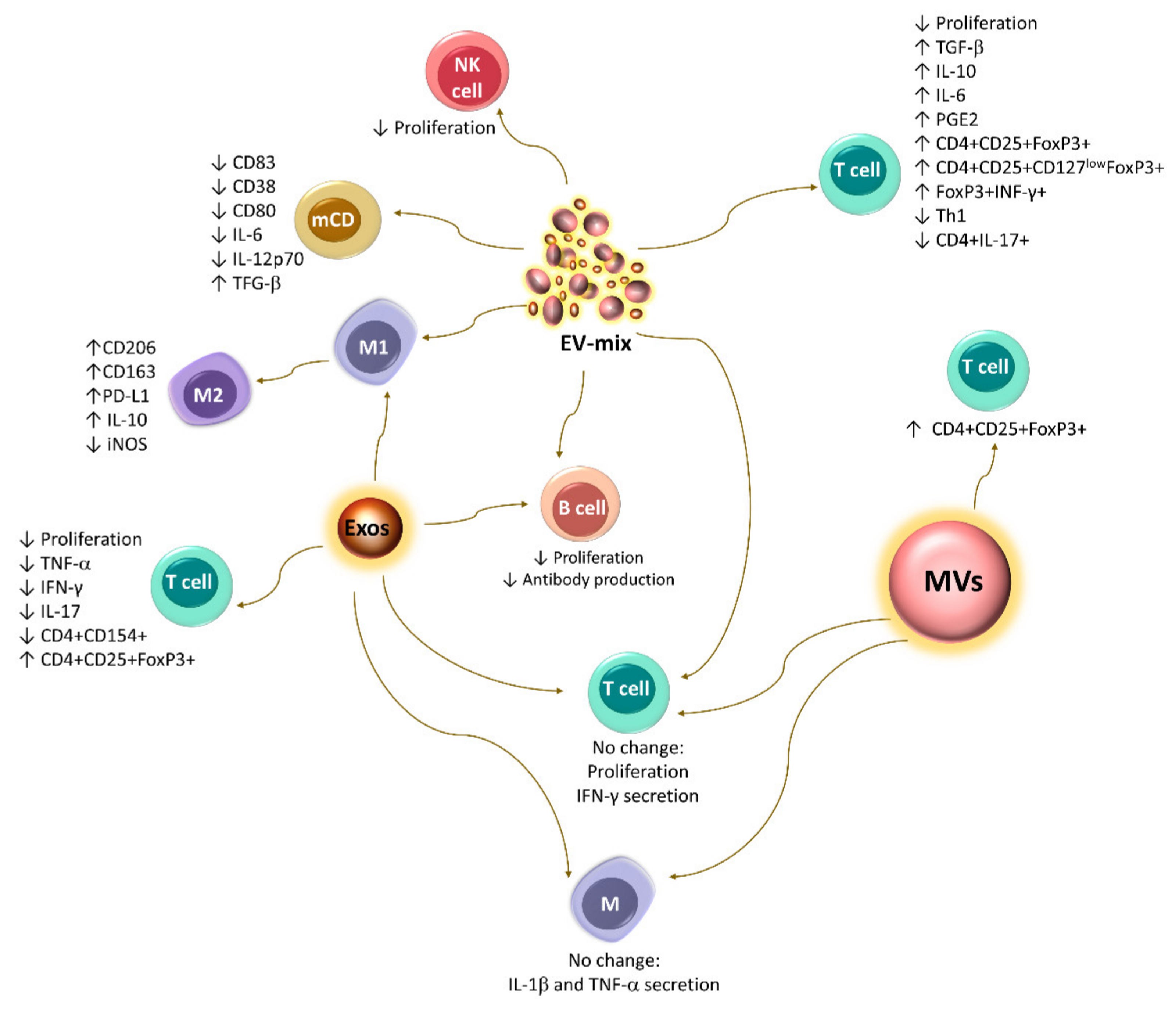
5. Immunoregulation Mediated by Extracellular Vesicles
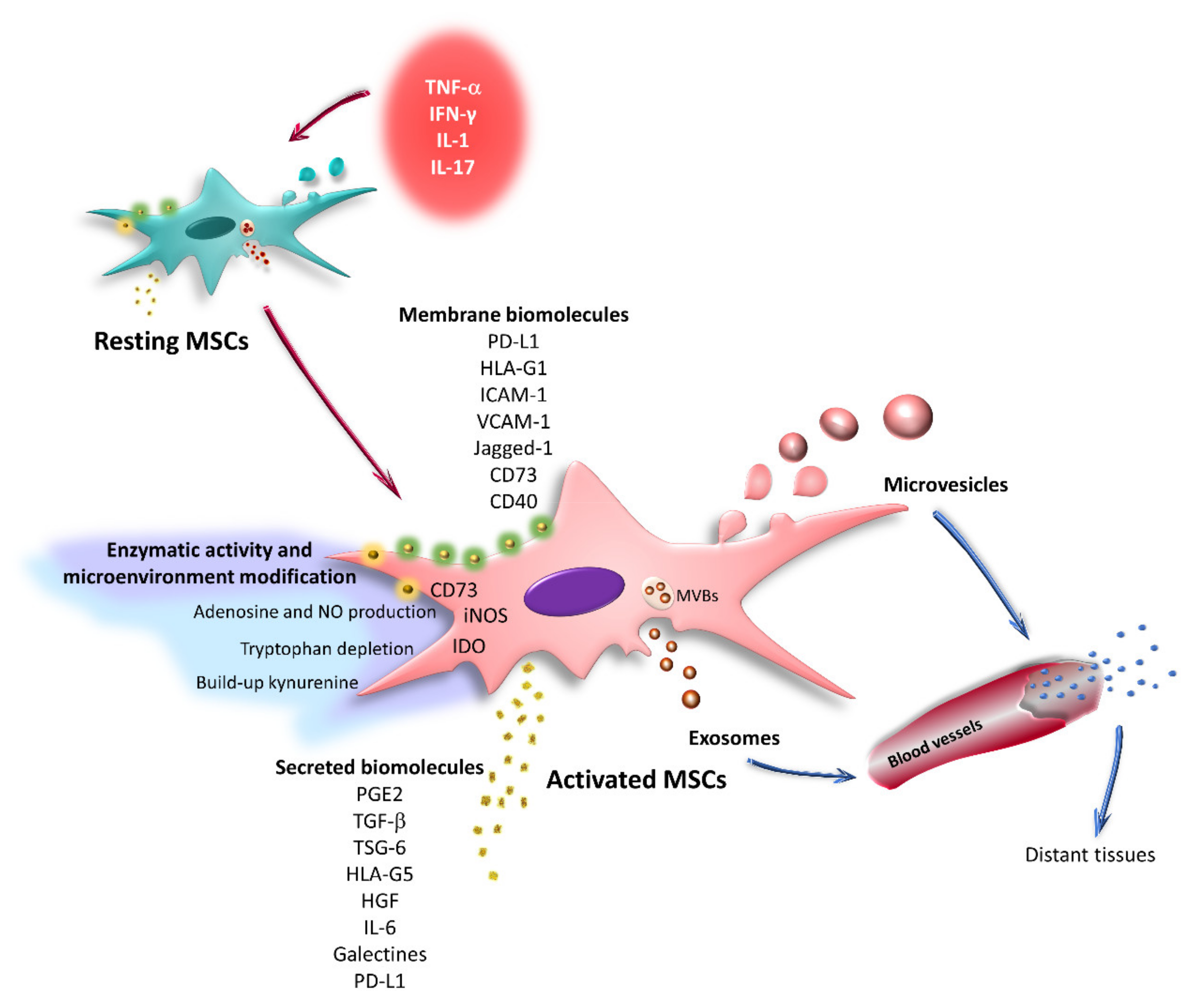
6. Effect of the Inflammatory Microenvironment on the Immunoregulatory Capacity of MSCs
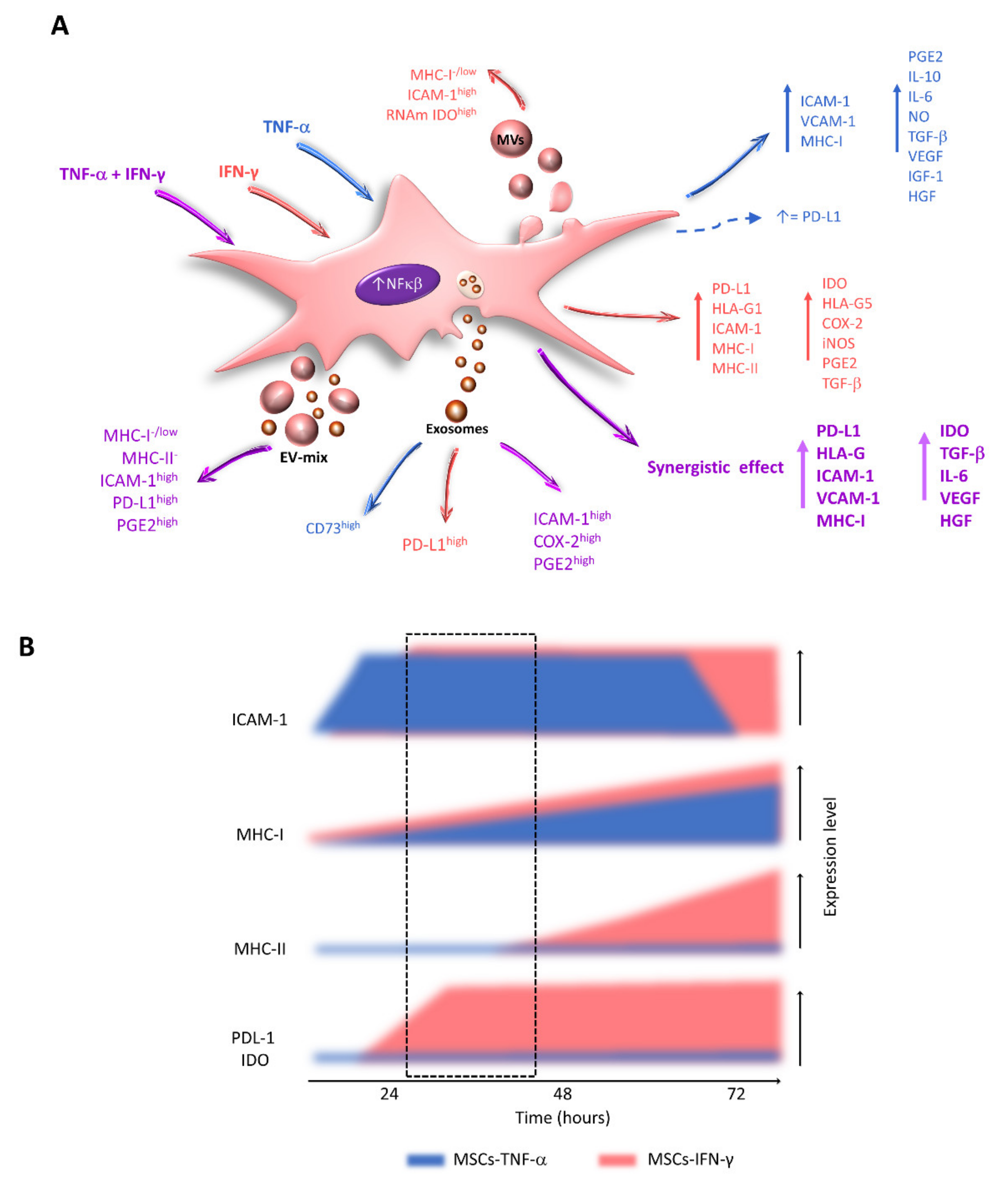
7. Effects of TNF-α and IFN-γ on the Expression of Immunoregulatory Molecules by MSCs
8. Effects of the Combination of TNF-α and IFN-γ on the Immunoregulatory Capacity of MSCs
9. Important Aspects to Consider for In Vitro Activation Protocols
10. Alterations in Cell Morphology and Proliferation
11. Increased Immunogenicity
12. Increased Expression of Immunoregulatory Molecules
13. Effect of Cytokines on EV-MSC Content
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedenstein, A.J.; Piatetzky, S., II; Petrakova, K.V. Osteogenesis in transplants of bone marrow cells. J. Embryol. Exp. Morphol. 1966, 16, 381–390. [Google Scholar]
- Friedenstein, A.J.; Deriglasova, U.F.; Kulagina, N.N.; Panasuk, A.F.; Rudakowa, S.F.; Luria, E.A.; Ruadkow, I.A. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp. Hematol. 1974, 2, 83–92. [Google Scholar]
- Castro-Manrreza, M.E.; Montesinos, J.J. Immunoregulation by mesenchymal stem cells: Biological aspects and clinical applications. J. Immunol. Res. 2015, 2015, 394917. [Google Scholar] [CrossRef]
- Naji, A.; Eitoku, M.; Favier, B.; Deschaseaux, F.; Rouas-Freiss, N.; Suganuma, N. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol. Life Sci. 2019, 76, 3323–3348. [Google Scholar] [CrossRef]
- Wang, M.; Yuan, Q.; Xie, L. Mesenchymal Stem Cell-Based Immunomodulation: Properties and Clinical Application. Stem Cells Int. 2018, 2018, 3057624. [Google Scholar] [CrossRef]
- Leyendecker, A., Jr.; Pinheiro, C.C.G.; Amano, M.T.; Bueno, D.F. The Use of Human Mesenchymal Stem Cells as Therapeutic Agents for the in vivo Treatment of Immune-Related Diseases: A Systematic Review. Front. Immunol. 2018, 9, 2056. [Google Scholar] [CrossRef]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Wang, Y.; Jin, Y.; Zhang, Q.; Zhang, Y.; Wang, L.; Shen, B.; Yin, S.; Liu, W.; Cui, L.; et al. A critical role of IFNgamma in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1. Cell Res. 2008, 18, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Sivanathan, K.N.; Rojas-Canales, D.M.; Hope, C.M.; Krishnan, R.; Carroll, R.P.; Gronthos, S.; Grey, S.T.; Coates, P.T. Interleukin-17A-Induced Human Mesenchymal Stem Cells Are Superior Modulators of Immunological Function. Stem Cells 2015, 33, 2850–2863. [Google Scholar] [CrossRef]
- Kim, D.S.; Jang, I.K.; Lee, M.W.; Ko, Y.J.; Lee, D.H.; Lee, J.W.; Sung, K.W.; Koo, H.H.; Yoo, K.H. Enhanced Immunosuppressive Properties of Human Mesenchymal Stem Cells Primed by Interferon-γ. EBioMedicine 2018, 28, 261–273. [Google Scholar] [CrossRef]
- Redondo-Castro, E.; Cunningham, C.; Miller, J.; Martuscelli, L.; Aoulad-Ali, S.; Rothwell, N.J.; Kielty, C.M.; Allan, S.M.; Pinteaux, E. Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res. Ther. 2017, 8, 79. [Google Scholar] [CrossRef]
- Bulati, M.; Miceli, V.; Gallo, A.; Amico, G.; Carcione, C.; Pampalone, M.; Conaldi, P.G. The Immunomodulatory Properties of the Human Amnion-Derived Mesenchymal Stromal/Stem Cells Are Induced by INF-γ Produced by Activated Lymphomonocytes and Are Mediated by Cell-To-Cell Contact and Soluble Factors. Front. Immunol. 2020, 11, 54. [Google Scholar] [CrossRef]
- Prockop, D.J.; Oh, J.Y. Mesenchymal stem/stromal cells (MSCs): Role as guardians of inflammation. Mol. Ther. 2012, 20, 14–20. [Google Scholar] [CrossRef]
- English, K.; Barry, F.P.; Field-Corbett, C.P.; Mahon, B.P. IFN-gamma and TNF-alpha differentially regulate immunomodulation by murine mesenchymal stem cells. Immunol. Lett. 2007, 110, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.R.R.; Dahlke, M.H. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front. Immunol. 2019, 10, 1191. [Google Scholar] [CrossRef]
- Dorronsoro, A.; Lang, V.; Ferrin, I.; Fernández-Rueda, J.; Zabaleta, L.; Pérez-Ruiz, E.; Sepúlveda, P.; Trigueros, C. Intracellular role of IL-6 in mesenchymal stromal cell immunosuppression and proliferation. Sci. Rep. 2020, 10, 21853. [Google Scholar] [CrossRef]
- Gieseke, F.; Kruchen, A.; Tzaribachev, N.; Bentzien, F.; Dominici, M.; Muller, I. Proinflammatory stimuli induce galectin-9 in human mesenchymal stromal cells to suppress T-cell proliferation. Eur. J. Immunol. 2013, 43, 2741–2749. [Google Scholar] [CrossRef]
- Ryan, J.M.; Barry, F.; Murphy, J.M.; Mahon, B.P. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin. Exp. Immunol. 2007, 149, 353–363. [Google Scholar] [CrossRef]
- Ren, G.; Roberts, A.I.; Shi, Y. Adhesion molecules: Key players in Mesenchymal stem cell-mediated immunosuppression. Cell Adhes. Migr. 2011, 5, 20–22. [Google Scholar] [CrossRef]
- Thaweesapphithak, S.; Tantrawatpan, C.; Kheolamai, P.; Tantikanlayaporn, D.; Roytrakul, S.; Manochantr, S. Human serum enhances the proliferative capacity and immunomodulatory property of MSCs derived from human placenta and umbilical cord. Stem Cell Res. Ther. 2019, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Kerkelä, E.; Laitinen, A.; Räbinä, J.; Valkonen, S.; Takatalo, M.; Larjo, A.; Veijola, J.; Lampinen, M.; Siljander, P.; Lehenkari, P.; et al. Adenosinergic Immunosuppression by Human Mesenchymal Stromal Cells Requires Co-Operation with T cells. Stem Cells 2016, 34, 781–790. [Google Scholar] [CrossRef]
- Selmani, Z.; Naji, A.; Zidi, I.; Favier, B.; Gaiffe, E.; Obert, L.; Borg, C.; Saas, P.; Tiberghien, P.; Rouas-Freiss, N.; et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells 2008, 26, 212–222. [Google Scholar] [CrossRef]
- Milosavljevic, N.; Gazdic, M.; Simovic Markovic, B.; Arsenijevic, A.; Nurkovic, J.; Dolicanin, Z.; Djonov, V.; Lukic, M.L.; Volarevic, V. Mesenchymal stem cells attenuate acute liver injury by altering ratio between interleukin 17 producing and regulatory natural killer T cells. Liver Transpl. 2017, 23, 1040–1050. [Google Scholar] [CrossRef]
- Moloudizargari, M.; Govahi, A.; Fallah, M.; Rezvanfar, M.A.; Asghari, M.H.; Abdollahi, M. The mechanisms of cellular crosstalk between mesenchymal stem cells and natural killer cells: Therapeutic implications. J. Cell Physiol. 2021, 236, 2413–2429. [Google Scholar] [CrossRef]
- Noone, C.; Kihm, A.; English, K.; O’Dea, S.; Mahon, B.P. IFN-γ stimulated human umbilical-tissue-derived cells potently suppress NK activation and resist NK-mediated cytotoxicity in vitro. Stem Cells Dev. 2013, 22, 3003–3014. [Google Scholar] [CrossRef]
- Holopainen, M.; Impola, U.; Lehenkari, P.; Laitinen, S.; Kerkelä, E. Human Mesenchymal Stromal Cell Secretome Promotes the Immunoregulatory Phenotype and Phagocytosis Activity in Human Macrophages. Cells 2020, 9, 2142. [Google Scholar] [CrossRef]
- Stevens, H.Y.; Bowles, A.C.; Yeago, C.; Roy, K. Molecular Crosstalk Between Macrophages and Mesenchymal Stromal Cells. Front. Cell Dev. Biol. 2020, 8, 600160. [Google Scholar] [CrossRef]
- Nemeth, K.; Leelahavanichkul, A.; Yuen, P.S.; Mayer, B.; Parmelee, A.; Doi, K.; Robey, P.G.; Leelahavanichkul, K.; Koller, B.H.; Brown, J.M.; et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009, 15, 42–49. [Google Scholar] [CrossRef]
- Hyvärinen, K.; Holopainen, M.; Skirdenko, V.; Ruhanen, H.; Lehenkari, P.; Korhonen, M.; Käkelä, R.; Laitinen, S.; Kerkelä, E. Mesenchymal Stromal Cells and Their Extracellular Vesicles Enhance the Anti-Inflammatory Phenotype of Regulatory Macrophages by Downregulating the Production of Interleukin (IL)-23 and IL-22. Front. Immunol. 2018, 9, 771. [Google Scholar] [CrossRef]
- Saldaña, L.; Bensiamar, F.; Vallés, G.; Mancebo, F.J.; García-Rey, E.; Vilaboa, N. Immunoregulatory potential of mesenchymal stem cells following activation by macrophage-derived soluble factors. Stem Cell Res. Ther. 2019, 10, 58. [Google Scholar] [CrossRef]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef]
- Spaggiari, G.M.; Abdelrazik, H.; Becchetti, F.; Moretta, L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: Central role of MSC-derived prostaglandin E2. Blood 2009, 113, 6576–6583. [Google Scholar] [CrossRef]
- Yu, Y.; Yoo, S.M.; Park, H.H.; Baek, S.Y.; Kim, Y.J.; Lee, S.; Kim, Y.L.; Seo, K.W.; Kang, K.S. Preconditioning with interleukin-1 beta and interferon-gamma enhances the efficacy of human umbilical cord blood-derived mesenchymal stem cells-based therapy via enhancing prostaglandin E2 secretion and indoleamine 2,3-dioxygenase activity in dextran sulfate sodium-induced colitis. J. Tissue Eng. Regen. Med. 2019, 13, 1792–1804. [Google Scholar]
- Ghannam, S.; Pene, J.; Moquet-Torcy, G.; Jorgensen, C.; Yssel, H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J. Immunol. 2010, 185, 302–312. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Q.; Wang, Z.; Tong, H.; Ma, L.; Zhang, Y.; Shan, F.; Meng, Y.; Yuan, Z. Comparative analysis of human mesenchymal stem cells from fetal-bone marrow, adipose tissue, and Warton’s jelly as sources of cell immunomodulatory therapy. Hum. Vaccin. Immunother. 2016, 12, 85–96. [Google Scholar] [CrossRef]
- DelaRosa, O.; Lombardo, E.; Beraza, A.; Mancheño-Corvo, P.; Ramirez, C.; Menta, R.; Rico, L.; Camarillo, E.; García, L.; Abad, J.L.; et al. Requirement of IFN-gamma-mediated indoleamine 2,3-dioxygenase expression in the modulation of lymphocyte proliferation by human adipose-derived stem cells. Tissue Eng. Part A 2009, 15, 2795–2806. [Google Scholar] [CrossRef] [PubMed]
- Serejo, T.R.T.; Silva-Carvalho, A.; Braga, L.; Neves, F.A.R.; Pereira, R.W.; Carvalho, J.L.; Saldanha-Araujo, F. Assessment of the Immunosuppressive Potential of INF-γ Licensed Adipose Mesenchymal Stem Cells, Their Secretome and Extracellular Vesicles. Cells 2019, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Corcione, A.; Benvenuto, F.; Ferretti, E.; Giunti, D.; Cappiello, V.; Cazzanti, F.; Risso, M.; Gualandi, F.; Mancardi, G.L.; Pistoia, V.; et al. Human mesenchymal stem cells modulate B-cell functions. Blood 2006, 107, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Hu, C.; Chen, J.; Cen, P.; Wang, J.; Li, L. Interaction between Mesenchymal Stem Cells and B-Cells. Int. J. Mol. Sci. 2016, 17, 650. [Google Scholar] [CrossRef] [PubMed]
- Luk, F.; Carreras-Planella, L.; Korevaar, S.S.; de Witte, S.F.H.; Borràs, F.E.; Betjes, M.G.H.; Baan, C.C.; Hoogduijn, M.J.; Franquesa, M. Inflammatory Conditions Dictate the Effect of Mesenchymal Stem or Stromal Cells on B Cell Function. Front. Immunol. 2017, 8, 1042. [Google Scholar] [CrossRef] [PubMed]
- Hermankova, B.; Zajicova, A.; Javorkova, E.; Chudickova, M.; Trosan, P.; Hajkova, M.; Krulova, M.; Holan, V. Suppression of IL-10 production by activated B cells via a cell contact-dependent cyclooxygenase-2 pathway upregulated in IFN-γ-treated mesenchymal stem cells. Immunobiology 2016, 221, 129–136. [Google Scholar] [CrossRef] [PubMed]
- English, K.; Ryan, J.M.; Tobin, L.; Murphy, M.J.; Barry, F.P.; Mahon, B.P. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin. Exp. Immunol. 2009, 156, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.J.; Wang, C.J.; Chang, C.J.; Hu, H.I.; Hsu, P.J.; Wu, Y.C.; Bai, C.H.; Sytwu, H.K.; Yen, B.L. Surface expression of HLA-G is involved in mediating immunomodulatory effects of placenta-derived multipotent cells (PDMCs) towards natural killer lymphocytes. Cell Transplant. 2011, 20, 1721–1730. [Google Scholar] [CrossRef]
- Yang, H.M.; Sung, J.H.; Choi, Y.S.; Lee, H.J.; Roh, C.R.; Kim, J.; Shin, M.; Song, S.; Kwon, C.H.; Joh, J.W.; et al. Enhancement of the immunosuppressive effect of human adipose tissue-derived mesenchymal stromal cells through HLA-G1 expression. Cytotherapy 2012, 14, 70–79. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Ding, L.; Wang, Y.X.; Zhao, Z.D.; Mao, N.; Wu, C.T.; Wang, H.; Zhu, H.; Ning, S.B. Intercellular adhesion molecule-1 enhances the therapeutic effects of MSCs in a dextran sulfate sodium-induced colitis models by promoting MSCs homing to murine colons and spleens. Stem Cell Res. Ther. 2019, 10, 267. [Google Scholar] [CrossRef]
- Liotta, F.; Angeli, R.; Cosmi, L.; Fili, L.; Manuelli, C.; Frosali, F.; Mazzinghi, B.; Maggi, L.; Pasini, A.; Lisi, V.; et al. Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling. Stem Cells 2008, 26, 279–289. [Google Scholar] [CrossRef]
- Lee, H.J.; Jung, H.; Kim, D.K. IDO and CD40 May Be Key Molecules for Immunomodulatory Capacity of the Primed Tonsil-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 5772. [Google Scholar] [CrossRef]
- Matula, Z.; Németh, A.; Lőrincz, P.; Szepesi, Á.; Brózik, A.; Buzás, E.I.; Lőw, P.; Német, K.; Uher, F.; Urbán, V.S. The Role of Extracellular Vesicle and Tunneling Nanotube-Mediated Intercellular Cross-Talk Between Mesenchymal Stem Cells and Human Peripheral T Cells. Stem Cells Dev. 2016, 25, 1818–1832. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, M.; Fleury, M.; Vernochet, A.; Ketroussi, F.; Clay, D.; Azzarone, B.; Lataillade, J.J.; Durrbach, A. Long-lasting inhibitory effects of fetal liver mesenchymal stem cells on T-lymphocyte proliferation. PLoS ONE 2011, 6, e19988. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, R.; Campioni, D.; Stignani, M.; Melchiorri, L.; Bagnara, G.P.; Bonsi, L.; Alviano, F.; Lanzoni, G.; Moretti, S.; Cuneo, A.; et al. A functional role for soluble HLA-G antigens in immune modulation mediated by mesenchymal stromal cells. Cytotherapy 2008, 10, 364–375. [Google Scholar] [CrossRef]
- Castro-Manrreza, M.E.; Mayani, H.; Monroy-Garcia, A.; Flores-Figueroa, E.; Chavez-Rueda, K.; Legorreta-Haquet, V.; Santiago-Osorio, E.; Montesinos, J.J. Human mesenchymal stromal cells from adult and neonatal sources: A comparative in vitro analysis of their immunosuppressive properties against T cells. Stem Cells Dev. 2014, 23, 1217–1232. [Google Scholar] [CrossRef]
- Najar, M.; Raicevic, G.; Fayyad-Kazan, H.; De Bruyn, C.; Bron, D.; Toungouz, M.; Lagneaux, L. Bone Marrow Mesenchymal Stromal Cells Induce Proliferative, Cytokinic and Molecular Changes During the T Cell Response: The Importance of the IL-10/CD210 Axis. Stem Cell Rev. 2015, 11, 442–452. [Google Scholar] [CrossRef]
- Yan, Z.; Zhuansun, Y.; Chen, R.; Li, J.; Ran, P. Immunomodulation of mesenchymal stromal cells on regulatory T cells and its possible mechanism. Exp. Cell Res. 2014, 324, 65–74. [Google Scholar] [CrossRef]
- Rosado, M.M.; Bernardo, M.E.; Scarsella, M.; Conforti, A.; Giorda, E.; Biagini, S.; Cascioli, S.; Rossi, F.; Guzzo, I.; Vivarelli, M.; et al. Inhibition of B-cell proliferation and antibody production by mesenchymal stromal cells is mediated by T cells. Stem Cells Dev. 2015, 24, 93–103. [Google Scholar] [CrossRef]
- Ren, G.; Zhao, X.; Zhang, L.; Zhang, J.; L’Huillier, A.; Ling, W.; Roberts, A.I.; Le, A.D.; Shi, S.; Shao, C.; et al. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J. Immunol. 2010, 184, 2321–2328. [Google Scholar] [CrossRef] [PubMed]
- Rubtsov, Y.; Goryunov, K.; Romanov, A.; Suzdaltseva, Y.; Sharonov, G.; Tkachuk, V. Molecular Mechanisms of Immunomodulation Properties of Mesenchymal Stromal Cells: A New Insight into the Role of ICAM-1. Stem Cells Int. 2017, 2017, 6516854. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.M.; Wiesolek, H.L.; Sumagin, R. ICAM-1: A master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J. Leukoc. Biol. 2020, 108, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, J.J.; Krummel, M.F. The importance of prolonged binding to antigen-presenting cells for T cell fate decisions. Immunity 2008, 28, 143–145. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schittenhelm, L.; Hilkens, C.M.; Morrison, V.L. β(2) Integrins As Regulators of Dendritic Cell, Monocyte, and Macrophage Function. Front. Immunol. 2017, 8, 1866. [Google Scholar] [CrossRef] [PubMed]
- Scholer, A.; Hugues, S.; Boissonnas, A.; Fetler, L.; Amigorena, S. Intercellular adhesion molecule-1-dependent stable interactions between T cells and dendritic cells determine CD8+ T cell memory. Immunity 2008, 28, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.A.; Barnum, S.R.; Bullard, D.C.; Zajac, A.J. ICAM-1-dependent tuning of memory CD8 T-cell responses following acute infection. Proc. Natl. Acad. Sci. USA 2013, 110, 1416–1421. [Google Scholar] [CrossRef]
- Podgrabinska, S.; Kamalu, O.; Mayer, L.; Shimaoka, M.; Snoeck, H.; Randolph, G.J.; Skobe, M. Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via Mac-1/ICAM-1-dependent mechanism. J. Immunol. 2009, 183, 1767–1779. [Google Scholar] [CrossRef]
- Tang, B.; Li, X.; Liu, Y.; Chen, X.; Chu, Y.; Zhu, H.; Liu, W.; Xu, F.; Zhou, F.; Zhang, Y. The Therapeutic Effect of ICAM-1-Overexpressing Mesenchymal Stem Cells on Acute Graft-Versus-Host Disease. Cell Physiol. Biochem. 2018, 46, 2624–2635. [Google Scholar] [CrossRef]
- Espagnolle, N.; Balguerie, A.; Arnaud, E.; Sensebé, L.; Varin, A. CD54-Mediated Interaction with Pro-inflammatory Macrophages Increases the Immunosuppressive Function of Human Mesenchymal Stromal Cells. Stem Cell Rep. 2017, 8, 961–976. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Xu, L.; Dong, L.; Zheng, J.; Lin, Y.; Huang, J.; Zhang, Y.; Tao, Y.; Zang, X.; et al. Cell-cell contact with proinflammatory macrophages enhances the immunotherapeutic effect of mesenchymal stem cells in two abortion models. Cell Mol. Immunol. 2019, 16, 908–920. [Google Scholar] [CrossRef]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.K.; Yoon, H.H.; Yang, M.S.; Lee, J.E.; Lee, D.H.; Lee, M.W.; Kim, D.S.; Park, J.E. B7-H1 inhibits T cell proliferation through MHC class II in human mesenchymal stem cells. Transplant. Proc. 2014, 46, 1638–1641. [Google Scholar] [CrossRef] [PubMed]
- Suva, D.; Passweg, J.; Arnaudeau, S.; Hoffmeyer, P.; Kindler, V. In vitro activated human T lymphocytes very efficiently attach to allogenic multipotent mesenchymal stromal cells and transmigrate under them. J. Cell Physiol. 2008, 214, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Ciccocioppo, R.; Cangemi, G.C.; Kruzliak, P.; Gallia, A.; Betti, E.; Badulli, C.; Martinetti, M.; Cervio, M.; Pecci, A.; Bozzi, V.; et al. Ex vivo immunosuppressive effects of mesenchymal stem cells on Crohn’s disease mucosal T cells are largely dependent on indoleamine 2,3-dioxygenase activity and cell-cell contact. Stem Cell Res. Ther. 2015, 6, 137. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, L.; Chi, Y.; Ren, X.; Gao, Y.; Song, B.; Li, C.; Han, Z. High-efficient generation of VCAM-1(+) mesenchymal stem cells with multidimensional superiorities in signatures and efficacy on aplastic anaemia mice. Cell Prolif. 2020, 53, e12862. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Huang, K.; Xia, W.; Shi, J.; Liu, Q.; Zhang, X.; Li, G.; Chen, J.; Wang, T.; Chen, X.; et al. Mesenchymal Stromal Cells Rapidly Suppress TCR Signaling-Mediated Cytokine Transcription in Activated T Cells Through the ICAM-1/CD43 Interaction. Front. Immunol. 2021, 12, 609544. [Google Scholar] [CrossRef]
- Cahill, E.F.; Tobin, L.M.; Carty, F.; Mahon, B.P.; English, K. Jagged-1 is required for the expansion of CD4+ CD25+ FoxP3+ regulatory T cells and tolerogenic dendritic cells by murine mesenchymal stromal cells. Stem Cell Res. Ther. 2015, 6, 19. [Google Scholar] [CrossRef]
- Tipnis, S.; Viswanathan, C.; Majumdar, A.S. Immunosuppressive properties of human umbilical cord-derived mesenchymal stem cells: Role of B7-H1 and IDO. Immunol. Cell Biol. 2010, 88, 795–806. [Google Scholar] [CrossRef]
- Chinnadurai, R.; Copland, I.B.; Patel, S.R.; Galipeau, J. IDO-independent suppression of T cell effector function by IFN-γ-licensed human mesenchymal stromal cells. J. Immunol. 2014, 192, 1491–1501. [Google Scholar] [CrossRef]
- Jin, P.; Zhao, Y.; Liu, H.; Chen, J.; Ren, J.; Jin, J.; Bedognetti, D.; Liu, S.; Wang, E.; Marincola, F.; et al. Interferon-gamma and Tumor Necrosis Factor-alpha Polarize Bone Marrow Stromal Cells Uniformly to a Th1 Phenotype. Sci. Rep. 2016, 6, 26345. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Ezzati, P.; Spicer, V.; Krokhin, O.; Wall, D.; Wilkins, J.A. Interferon γ induced compositional changes in human bone marrow derived mesenchymal stem/stromal cells. Clin. Proteom. 2017, 14, 26. [Google Scholar] [CrossRef]
- Loke, P.; Allison, J.P. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc. Natl. Acad. Sci. USA 2003, 100, 5336–5341. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.C.; Heldring, N.; Kadri, N.; Le Blanc, K. Mesenchymal Stromal Cell Secretion of Programmed Death-1 Ligands Regulates T Cell Mediated Immunosuppression. Stem Cells 2017, 35, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.E.; Liang, S.C.; Guleria, I.; Latchman, Y.E.; Qipo, A.; Albacker, L.A.; Koulmanda, M.; Freeman, G.J.; Sayegh, M.H.; Sharpe, A.H. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J. Exp. Med. 2006, 203, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Leibacher, J.; Henschler, R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res. Ther. 2016, 7, 7. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Tkach, M.; Thery, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; El Andaloussi, S.; Wood, M.J. Exosomes and microvesicles: Extracellular vesicles for genetic information transfer and gene therapy. Hum. Mol. Genet. 2012, 21, R125–R134. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Gilkes, D.M.; Takano, N.; Xiang, L.; Luo, W.; Bishop, C.J.; Chaturvedi, P.; Green, J.J.; Semenza, G.L. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc. Natl. Acad. Sci. USA 2014, 111, E3234–E3242. [Google Scholar] [CrossRef] [PubMed]
- Kilpinen, L.; Impola, U.; Sankkila, L.; Ritamo, I.; Aatonen, M.; Kilpinen, S.; Tuimala, J.; Valmu, L.; Levijoki, J.; Finckenberg, P.; et al. Extracellular membrane vesicles from umbilical cord blood-derived MSC protect against ischemic acute kidney injury, a feature that is lost after inflammatory conditioning. J. Extracell. Vesicles 2013, 2, 21927. [Google Scholar] [CrossRef]
- Collino, F.; Pomatto, M.; Bruno, S.; Lindoso, R.S.; Tapparo, M.; Sicheng, W.; Quesenberry, P.; Camussi, G. Exosome and Microvesicle-Enriched Fractions Isolated from Mesenchymal Stem Cells by Gradient Separation Showed Different Molecular Signatures and Functions on Renal Tubular Epithelial Cells. Stem Cell Rev. Rep. 2017, 13, 226–243. [Google Scholar] [CrossRef]
- Reis, M.; Mavin, E.; Nicholson, L.; Green, K.; Dickinson, A.M.; Wang, X.N. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Dendritic Cell Maturation and Function. Front. Immunol. 2018, 9, 2538. [Google Scholar] [CrossRef]
- Wen, S.; Dooner, M.; Cheng, Y.; Papa, E.; Del Tatto, M.; Pereira, M.; Deng, Y.; Goldberg, L.; Aliotta, J.; Chatterjee, D.; et al. Mesenchymal stromal cell-derived extracellular vesicles rescue radiation damage to murine marrow hematopoietic cells. Leukemia 2016, 30, 2221–2231. [Google Scholar] [CrossRef]
- Bruno, S.; Tapparo, M.; Collino, F.; Chiabotto, G.; Deregibus, M.C.; Soares Lindoso, R.; Neri, F.; Kholia, S.; Giunti, S.; Wen, S.; et al. Renal Regenerative Potential of Different Extracellular Vesicle Populations Derived from Bone Marrow Mesenchymal Stromal Cells. Tissue Eng. Part A 2017, 23, 1262–1273. [Google Scholar] [CrossRef]
- Mokarizadeh, A.; Delirezh, N.; Morshedi, A.; Mosayebi, G.; Farshid, A.A.; Mardani, K. Microvesicles derived from mesenchymal stem cells: Potent organelles for induction of tolerogenic signaling. Immunol. Lett. 2012, 147, 47–54. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, G.; Cheng, Z.; Yin, D.; Du, T.; Ju, G.; Miao, S.; Liu, G.; Lu, M.; Zhu, Y. Microvesicles derived from human Wharton’s Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res. Ther. 2014, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Favaro, E.; Carpanetto, A.; Lamorte, S.; Fusco, A.; Caorsi, C.; Deregibus, M.C.; Bruno, S.; Amoroso, A.; Giovarelli, M.; Porta, M.; et al. Human mesenchymal stem cell-derived microvesicles modulate T cell response to islet antigen glutamic acid decarboxylase in patients with type 1 diabetes. Diabetologia 2014, 57, 1664–1673. [Google Scholar] [CrossRef] [PubMed]
- Harting, M.T.; Srivastava, A.K.; Zhaorigetu, S.; Bair, H.; Prabhakara, K.S.; Toledano Furman, N.E.; Vykoukal, J.V.; Ruppert, K.A.; Cox, C.S., Jr.; Olson, S.D. Inflammation-Stimulated Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Inflammation. Stem Cells 2018, 36, 79–90. [Google Scholar] [CrossRef]
- Franco da Cunha, F.; Andrade-Oliveira, V.; Candido de Almeida, D.; Borges da Silva, T.; Naffah de Souza Breda, C.; Costa Cruz, M.; Faquim-Mauro, E.L.; Antonio Cenedeze, M.; Ioshie Hiyane, M.; Pacheco-Silva, A.; et al. Extracellular Vesicles isolated from Mesenchymal Stromal Cells Modulate CD4(+) T Lymphocytes Toward a Regulatory Profile. Cells 2020, 9, 1059. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kuwabara, A.; Kamio, Y.; Hu, S.; Park, J.; Hashimoto, T.; Lee, J.W. Human Mesenchymal Stem Cell-Derived Microvesicles Prevent the Rupture of Intracranial Aneurysm in Part by Suppression of Mast Cell Activation via a PGE2-Dependent Mechanism. Stem Cells 2016, 34, 2943–2955. [Google Scholar] [CrossRef]
- Gennai, S.; Monsel, A.; Hao, Q.; Park, J.; Matthay, M.A.; Lee, J.W. Microvesicles Derived From Human Mesenchymal Stem Cells Restore Alveolar Fluid Clearance in Human Lungs Rejected for Transplantation. Am. J. Transplant. 2015, 15, 2404–2412. [Google Scholar] [CrossRef]
- Wei, X.; Liu, C.; Wang, H.; Wang, L.; Xiao, F.; Guo, Z.; Zhang, H. Surface Phosphatidylserine Is Responsible for the Internalization on Microvesicles Derived from Hypoxia-Induced Human Bone Marrow Mesenchymal Stem Cells into Human Endothelial Cells. PLoS ONE 2016, 11, e0147360. [Google Scholar] [CrossRef]
- Di Trapani, M.; Bassi, G.; Midolo, M.; Gatti, A.; Kamga, P.T.; Cassaro, A.; Carusone, R.; Adamo, A.; Krampera, M. Differential and transferable modulatory effects of mesenchymal stromal cell-derived extracellular vesicles on T, B and NK cell functions. Sci. Rep. 2016, 6, 24120. [Google Scholar] [CrossRef]
- Khare, D.; Or, R.; Resnick, I.; Barkatz, C.; Almogi-Hazan, O.; Avni, B. Mesenchymal Stromal Cell-Derived Exosomes Affect mRNA Expression and Function of B-Lymphocytes. Front. Immunol. 2018, 9, 3053. [Google Scholar] [CrossRef]
- Chamberlain, C.S.; Clements, A.E.B.; Kink, J.A.; Choi, U.; Baer, G.S.; Halanski, M.A.; Hematti, P.; Vanderby, R. Extracellular Vesicle-Educated Macrophages Promote Early Achilles Tendon Healing. Stem Cells 2019, 37, 652–662. [Google Scholar] [CrossRef]
- Biswas, S.; Mandal, G.; Roy Chowdhury, S.; Purohit, S.; Payne, K.K.; Anadon, C.; Gupta, A.; Swanson, P.; Yu, X.; Conejo-Garcia, J.R.; et al. Exosomes Produced by Mesenchymal Stem Cells Drive Differentiation of Myeloid Cells into Immunosuppressive M2-Polarized Macrophages in Breast Cancer. J. Immunol. 2019, 203, 3447–3460. [Google Scholar] [CrossRef] [PubMed]
- Dal Collo, G.; Adamo, A.; Gatti, A.; Tamellini, E.; Bazzoni, R.; Takam Kamga, P.; Tecchio, C.; Quaglia, F.M.; Krampera, M. Functional dosing of mesenchymal stromal cell-derived extracellular vesicles for the prevention of acute graft-versus-host-disease. Stem Cells 2020, 38, 698–711. [Google Scholar] [CrossRef]
- Guo, L.; Lai, P.; Wang, Y.; Huang, T.; Chen, X.; Geng, S.; Huang, X.; Luo, C.; Wu, S.; Ling, W.; et al. Extracellular vesicles derived from mesenchymal stem cells prevent skin fibrosis in the cGVHD mouse model by suppressing the activation of macrophages and B cells immune response. Int. Immunopharmacol. 2020, 84, 106541. [Google Scholar] [CrossRef] [PubMed]
- Stone, M.L.; Zhao, Y.; Robert Smith, J.; Weiss, M.L.; Kron, I.L.; Laubach, V.E.; Sharma, A.K. Mesenchymal stromal cell-derived extracellular vesicles attenuate lung ischemia-reperfusion injury and enhance reconditioning of donor lungs after circulatory death. Respir. Res. 2017, 18, 212. [Google Scholar] [CrossRef] [PubMed]
- Nassar, W.; El-Ansary, M.; Sabry, D.; Mostafa, M.A.; Fayad, T.; Kotb, E.; Temraz, M.; Saad, A.N.; Essa, W.; Adel, H. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater. Res. 2016, 20, 21. [Google Scholar] [CrossRef]
- Li, M.; Soder, R.; Abhyankar, S.; Abdelhakim, H.; Braun, M.W.; Trinidad, C.V.; Pathak, H.B.; Pessetto, Z.; Deighan, C.; Ganguly, S.; et al. WJMSC-derived small extracellular vesicle enhance T cell suppression through PD-L1. J. Extracell. Vesicles 2021, 10, e12067. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, J.J.; López-García, L.; Cortés-Morales, V.A.; Arriaga-Pizano, L.; Valle-Ríos, R.; Fajardo-Orduña, G.R.; Castro-Manrreza, M.E. Human Bone Marrow Mesenchymal Stem/Stromal Cells Exposed to an Inflammatory Environment Increase the Expression of ICAM-1 and Release Microvesicles Enriched in This Adhesive Molecule: Analysis of the Participation of TNF-α and IFN-γ. J. Immunol. Res. 2020, 2020, 8839625. [Google Scholar] [CrossRef]
- Domenis, R.; Cifù, A.; Quaglia, S.; Pistis, C.; Moretti, M.; Vicario, A.; Parodi, P.C.; Fabris, M.; Niazi, K.R.; Soon-Shiong, P.; et al. Pro inflammatory stimuli enhance the immunosuppressive functions of adipose mesenchymal stem cells-derived exosomes. Sci. Rep. 2018, 8, 13325. [Google Scholar] [CrossRef]
- Mohammadi, M.R.; Riazifar, M.; Pone, E.J.; Yeri, A.; Van Keuren-Jensen, K.; Lässer, C.; Lotvall, J.; Zhao, W. Isolation and characterization of microvesicles from mesenchymal stem cells. Methods 2020, 177, 50–57. [Google Scholar] [CrossRef]
- Zhang, Q.; Fu, L.; Liang, Y.; Guo, Z.; Wang, L.; Ma, C.; Wang, H. Exosomes originating from MSCs stimulated with TGF-β and IFN-γ promote Treg differentiation. J. Cell Physiol. 2018, 233, 6832–6840. [Google Scholar] [CrossRef]
- Cha, J.M.; Shin, E.K.; Sung, J.H.; Moon, G.J.; Kim, E.H.; Cho, Y.H.; Park, H.D.; Bae, H.; Kim, J.; Bang, O.Y. Efficient scalable production of therapeutic microvesicles derived from human mesenchymal stem cells. Sci. Rep. 2018, 8, 1171. [Google Scholar] [CrossRef]
- Carceller, M.C.; Guillén, M.I.; Gil, M.L.; Alcaraz, M.J. Extracellular Vesicles Do Not Mediate the Anti-Inflammatory Actions of Mouse-Derived Adipose Tissue Mesenchymal Stem Cells Secretome. Int. J. Mol. Sci. 2021, 22, 1375. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Lu, T.; Zhou, C.; Cai, J.; Zhang, X.; Liang, J.; Sui, X.; Chen, X.; Chen, L.; Sun, Y.; et al. Extracellular Vesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells Protect Liver Ischemia/Reperfusion Injury by Reducing CD154 Expression on CD4+ T Cells via CCT2. Adv. Sci. 2020, 7, 1903746. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Ma, D.; Zhang, G.; Gao, J.; Su, Y.; Liu, S.; Liu, Y.; Han, J.; Tian, M.; Wei, C.; et al. Human umbilical cord mesenchymal stem cell-derived small extracellular vesicles ameliorate collagen-induced arthritis via immunomodulatory T lymphocytes. Mol. Immunol. 2021, 135, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Altemus, J.; Lightner, A.L. Mesenchymal stem cells and acellular products attenuate murine induced colitis. Stem Cell Res. Ther. 2020, 11, 515. [Google Scholar] [CrossRef]
- Duan, L.; Huang, H.; Zhao, X.; Zhou, M.; Chen, S.; Wang, C.; Han, Z.; Han, Z.C.; Guo, Z.; Li, Z.; et al. Extracellular vesicles derived from human placental mesenchymal stem cells alleviate experimental colitis in mice by inhibiting inflammation and oxidative stress. Int. J. Mol. Med. 2020, 46, 1551–1561. [Google Scholar] [CrossRef]
- Tolomeo, A.M.; Castagliuolo, I.; Piccoli, M.; Grassi, M.; Magarotto, F.; De Lazzari, G.; Malvicini, R.; Caicci, F.; Franzin, C.; Scarpa, M.; et al. Extracellular Vesicles Secreted by Mesenchymal Stromal Cells Exert Opposite Effects to Their Cells of Origin in Murine Sodium Dextran Sulfate-Induced Colitis. Front. Immunol. 2021, 12, 627605. [Google Scholar] [CrossRef]
- Nakao, Y.; Fukuda, T.; Zhang, Q.; Sanui, T.; Shinjo, T.; Kou, X.; Chen, C.; Liu, D.; Watanabe, Y.; Hayashi, C.; et al. Exosomes from TNF-α-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomater. 2021, 122, 306–324. [Google Scholar] [CrossRef]
- Cheng, A.; Choi, D.; Lora, M.; Shum-Tim, D.; Rak, J.; Colmegna, I. Human multipotent mesenchymal stromal cells cytokine priming promotes RAB27B-regulated secretion of small extracellular vesicles with immunomodulatory cargo. Stem Cell Res. Ther. 2020, 11, 539. [Google Scholar] [CrossRef]
- Gómez-Ferrer, M.; Villanueva-Badenas, E.; Sánchez-Sánchez, R.; Sánchez-López, C.M.; Baquero, M.C.; Sepúlveda, P.; Dorronsoro, A. HIF-1α and Pro-Inflammatory Signaling Improves the Immunomodulatory Activity of MSC-Derived Extracellular Vesicles. Int. J. Mol. Sci. 2021, 22, 3416. [Google Scholar] [CrossRef]
- Losurdo, M.; Pedrazzoli, M.; D’Agostino, C.; Elia, C.A.; Massenzio, F.; Lonati, E.; Mauri, M.; Rizzi, L.; Molteni, L.; Bresciani, E.; et al. Intranasal delivery of mesenchymal stem cell-derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3xTg model of Alzheimer’s disease. Stem Cells Transl. Med. 2020, 9, 1068–1084. [Google Scholar] [CrossRef]
- Lei, Q.; Liu, T.; Gao, F.; Xie, H.; Sun, L.; Zhao, A.; Ren, W.; Guo, H.; Zhang, L.; Wang, H.; et al. Microvesicles as Potential Biomarkers for the Identification of Senescence in Human Mesenchymal Stem Cells. Theranostics 2017, 7, 2673–2689. [Google Scholar] [CrossRef]
- Krawczenko, A.; Bielawska-Pohl, A.; Paprocka, M.; Kraskiewicz, H.; Szyposzynska, A.; Wojdat, E.; Klimczak, A. Microvesicles from Human Immortalized Cell Lines of Endothelial Progenitor Cells and Mesenchymal Stem/Stromal Cells of Adipose Tissue Origin as Carriers of Bioactive Factors Facilitating Angiogenesis. Stem Cells Int. 2020, 2020, 1289380. [Google Scholar] [CrossRef]
- Wang, Y.; Han, B.; Wang, C.; Zhang, H.; Xue, J.; Wang, X.; Niu, T.; Niu, Z.; Chen, Y. Mesenchymal stem cell-secreted extracellular vesicles carrying TGF-β1 up-regulate miR-132 and promote mouse M2 macrophage polarization. J. Cell Mol. Med. 2020, 24, 12750–12764. [Google Scholar] [CrossRef] [PubMed]
- Budoni, M.; Fierabracci, A.; Luciano, R.; Petrini, S.; Di Ciommo, V.; Muraca, M. The immunosuppressive effect of mesenchymal stromal cells on B lymphocytes is mediated by membrane vesicles. Cell Transplant. 2013, 22, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Huang, S.; Yuan, X.; Liang, J.; Xu, R.; Yao, G.; Feng, X.; Sun, L. The regulation of the Treg/Th17 balance by mesenchymal stem cells in human systemic lupus erythematosus. Cell Mol. Immunol. 2017, 14, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, S.; Yang, P.; Cao, H.; Li, L. The role of mesenchymal stem cells in hematopoietic stem cell transplantation: Prevention and treatment of graft-versus-host disease. Stem Cell Res. Ther. 2019, 10, 182. [Google Scholar] [CrossRef]
- Sayegh, S.; El Atat, O.; Diallo, K.; Rauwel, B.; Degboé, Y.; Cavaignac, E.; Constantin, A.; Cantagrel, A.; Trak-Smayra, V.; Alaaeddine, N.; et al. Rheumatoid Synovial Fluids Regulate the Immunomodulatory Potential of Adipose-Derived Mesenchymal Stem Cells Through a TNF/NF-κB-Dependent Mechanism. Front. Immunol. 2019, 10, 1482. [Google Scholar] [CrossRef] [PubMed]
- Duijvestein, M.; Wildenberg, M.E.; Welling, M.M.; Hennink, S.; Molendijk, I.; van Zuylen, V.L.; Bosse, T.; Vos, A.C.; de Jonge-Muller, E.S.; Roelofs, H.; et al. Pretreatment with interferon-γ enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells 2011, 29, 1549–1558. [Google Scholar] [CrossRef]
- Burks, S.R.; Nagle, M.E.; Bresler, M.N.; Kim, S.J.; Star, R.A.; Frank, J.A. Mesenchymal stromal cell potency to treat acute kidney injury increased by ultrasound-activated interferon-γ/interleukin-10 axis. J. Cell Mol. Med. 2018, 22, 6015–6025. [Google Scholar] [CrossRef]
- Polchert, D.; Sobinsky, J.; Douglas, G.; Kidd, M.; Moadsiri, A.; Reina, E.; Genrich, K.; Mehrotra, S.; Setty, S.; Smith, B.; et al. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur. J. Immunol. 2008, 38, 1745–1755. [Google Scholar] [CrossRef]
- Takeshita, K.; Motoike, S.; Kajiya, M.; Komatsu, N.; Takewaki, M.; Ouhara, K.; Iwata, T.; Takeda, K.; Mizuno, N.; Fujita, T.; et al. Xenotransplantation of interferon-gamma-pretreated clumps of a human mesenchymal stem cell/extracellular matrix complex induces mouse calvarial bone regeneration. Stem Cell Res. Ther. 2017, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Min, X.H.; Wang, Q.Y.; Leung, F.W.; Shi, L.; Zhou, Y.; Yu, T.; Wang, C.M.; An, G.; Sha, W.H.; et al. Pre-activation of mesenchymal stem cells with TNF-α, IL-1β and nitric oxide enhances its paracrine effects on radiation-induced intestinal injury. Sci. Rep. 2015, 5, 8718. [Google Scholar] [CrossRef] [PubMed]
- Silva-Carvalho, A.; Rodrigues, L.P.; Schiavinato, J.L.; Alborghetti, M.R.; Bettarello, G.; Simões, B.P.; Neves, F.A.R.; Panepucci, R.A.; de Carvalho, J.L.; Saldanha-Araujo, F. GVHD-derived plasma as a priming strategy of mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 156. [Google Scholar] [CrossRef]
- Kalliolias, G.D.; Ivashkiv, L.B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2016, 12, 49–62. [Google Scholar] [CrossRef]
- Sohn, C.; Lee, A.; Qiao, Y.; Loupasakis, K.; Ivashkiv, L.B.; Kalliolias, G.D. Prolonged tumor necrosis factor α primes fibroblast-like synoviocytes in a gene-specific manner by altering chromatin. Arthritis Rheumatol. 2015, 67, 86–95. [Google Scholar] [CrossRef]
- Pierini, A.; Strober, W.; Moffett, C.; Baker, J.; Nishikii, H.; Alvarez, M.; Pan, Y.; Schneidawind, D.; Meyer, E.; Negrin, R.S. TNF-α priming enhances CD4+FoxP3+ regulatory T-cell suppressive function in murine GVHD prevention and treatment. Blood 2016, 128, 866–871. [Google Scholar] [CrossRef]
- Dorronsoro, A.; Ferrin, I.; Salcedo, J.M.; Jakobsson, E.; Fernández-Rueda, J.; Lang, V.; Sepulveda, P.; Fechter, K.; Pennington, D.; Trigueros, C. Human mesenchymal stromal cells modulate T-cell responses through TNF-α-mediated activation of NF-κB. Eur. J. Immunol. 2014, 44, 480–488. [Google Scholar] [CrossRef]
- Nogueira-Pedro, A.; Makiyama, E.N.; Segreto, H.R.C.; Fock, R.A. The Role of Low-Dose Radiation in Association with TNF-α on Immunomodulatory Properties of Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2020, 17, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Han, B.; Cai, S.; Lei, Y.; Sun, T.; Sheng, Z. Migration of bone marrow-derived mesenchymal stem cells induced by tumor necrosis factor-alpha and its possible role in wound healing. Wound Repair Regen. 2009, 17, 185–191. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Chen, W.C.; Li, Y.H.; Li, L.; Zhang, H.; Pang, Y.; Xiao, Z.F.; Xiao, H.W.; Xiao, Y. TNF-α enhances vascular cell adhesion molecule-1 expression in human bone marrow mesenchymal stem cells via the NF-κB, ERK and JNK signaling pathways. Mol. Med. Rep. 2016, 14, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Crisostomo, P.R.; Herring, C.; Meldrum, K.K.; Meldrum, D.R. Human progenitor cells from bone marrow or adipose tissue produce VEGF, HGF, and IGF-I in response to TNF by a p38 MAPK-dependent mechanism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R880–R884. [Google Scholar] [CrossRef]
- Crisostomo, P.R.; Wang, Y.; Markel, T.A.; Wang, M.; Lahm, T.; Meldrum, D.R. Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. Am. J. Physiol. Cell Physiol. 2008, 294, C675–C682. [Google Scholar] [CrossRef] [PubMed]
- Putra, A.; Ridwan, F.B.; Putridewi, A.I.; Kustiyah, A.R.; Wirastuti, K.; Sadyah, N.A.C.; Rosdiana, I.; Munir, D. The Role of TNF-α induced MSCs on Suppressive Inflammation by Increasing TGF-β and IL-10. Open Access Maced. J. Med. Sci. 2018, 6, 1779–1783. [Google Scholar] [CrossRef] [PubMed]
- Schoenborn, J.R.; Wilson, C.B. Regulation of interferon-gamma during innate and adaptive immune responses. Adv. Immunol. 2007, 96, 41–101. [Google Scholar] [PubMed]
- Chan, W.K.; Lau, A.S.; Li, J.C.; Law, H.K.; Lau, Y.L.; Chan, G.C. MHC expression kinetics and immunogenicity of mesenchymal stromal cells after short-term IFN-gamma challenge. Exp. Hematol. 2008, 36, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- Bocelli-Tyndall, C.; Trella, E.; Frachet, A.; Zajac, P.; Pfaff, D.; Geurts, J.; Heiler, S.; Barbero, A.; Mumme, M.; Resink, T.J.; et al. FGF2 induces RANKL gene expression as well as IL1β regulated MHC class II in human bone marrow-derived mesenchymal progenitor stromal cells. Ann. Rheum. Dis. 2015, 74, 260–266. [Google Scholar] [CrossRef] [PubMed]
- De Witte, S.F.H.; Merino, A.M.; Franquesa, M.; Strini, T.; van Zoggel, J.A.A.; Korevaar, S.S.; Luk, F.; Gargesha, M.; O’Flynn, L.; Roy, D.; et al. Cytokine treatment optimises the immunotherapeutic effects of umbilical cord-derived MSC for treatment of inflammatory liver disease. Stem Cell Res. Ther. 2017, 8, 140. [Google Scholar] [CrossRef]
- Yen, B.L.; Hwa, H.L.; Hsu, P.J.; Chen, P.M.; Wang, L.T.; Jiang, S.S.; Liu, K.J.; Sytwu, H.K.; Yen, M.L. HLA-G Expression in Human Mesenchymal Stem Cells (MSCs) Is Related to Unique Methylation Pattern in the Proximal Promoter as well as Gene Body DNA. Int. J. Mol. Sci. 2020, 21, 5075. [Google Scholar] [CrossRef]
- Zimmermann, J.A.; McDevitt, T.C. Pre-conditioning mesenchymal stromal cell spheroids for immunomodulatory paracrine factor secretion. Cytotherapy 2014, 16, 331–345. [Google Scholar] [CrossRef]
- Li, C.; Li, G.; Liu, M.; Zhou, T.; Zhou, H. Paracrine effect of inflammatory cytokine-activated bone marrow mesenchymal stem cells and its role in osteoblast function. J. Biosci. Bioeng. 2016, 121, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Tong, Y.; Li, Y.; Yuan, J.; Hu, S.; Hu, T.; Song, G. Mesenchymal stem cells in inflammatory microenvironment potently promote metastatic growth of cholangiocarcinoma via activating Akt/NF-κB signaling by paracrine CCL5. Oncotarget 2017, 8, 73693–73704. [Google Scholar] [CrossRef]
- Jha, K.A.; Pentecost, M.; Lenin, R.; Klaic, L.; Elshaer, S.L.; Gentry, J.; Russell, J.M.; Beland, A.; Reiner, A.; Jotterand, V.; et al. Concentrated Conditioned Media from Adipose Tissue Derived Mesenchymal Stem Cells Mitigates Visual Deficits and Retinal Inflammation Following Mild Traumatic Brain Injury. Int. J. Mol. Sci. 2018, 19, 2016. [Google Scholar] [CrossRef] [PubMed]
- Montespan, F.; Deschaseaux, F.; Sensébé, L.; Carosella, E.D.; Rouas-Freiss, N. Osteodifferentiated mesenchymal stem cells from bone marrow and adipose tissue express HLA-G and display immunomodulatory properties in HLA-mismatched settings: Implications in bone repair therapy. J. Immunol. Res. 2014, 2014, 230346. [Google Scholar] [CrossRef]
- Jing, Y.; Han, Z.; Liu, Y.; Sun, K.; Zhang, S.; Jiang, G.; Li, R.; Gao, L.; Zhao, X.; Wu, D.; et al. Mesenchymal stem cells in inflammation microenvironment accelerates hepatocellular carcinoma metastasis by inducing epithelial-mesenchymal transition. PLoS ONE 2012, 7, e43272. [Google Scholar] [CrossRef]
- Kouroupis, D.; Bowles, A.C.; Willman, M.A.; Perucca Orfei, C.; Colombini, A.; Best, T.M.; Kaplan, L.D.; Correa, D. Infrapatellar fat pad-derived MSC response to inflammation and fibrosis induces an immunomodulatory phenotype involving CD10-mediated Substance P degradation. Sci. Rep. 2019, 9, 10864. [Google Scholar] [CrossRef] [PubMed]
- François, M.; Romieu-Mourez, R.; Li, M.; Galipeau, J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol. Ther. 2012, 20, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Rovira Gonzalez, Y.I.; Lynch, P.J.; Thompson, E.E.; Stultz, B.G.; Hursh, D.A. In vitro cytokine licensing induces persistent permissive chromatin at the Indoleamine 2,3-dioxygenase promoter. Cytotherapy 2016, 18, 1114–1128. [Google Scholar] [CrossRef]
- Tu, Z.; Li, Q.; Bu, H.; Lin, F. Mesenchymal stem cells inhibit complement activation by secreting factor H. Stem Cells Dev. 2010, 19, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Crop, M.J.; Baan, C.C.; Korevaar, S.S.; Ijzermans, J.N.; Pescatori, M.; Stubbs, A.P.; van Ijcken, W.F.; Dahlke, M.H.; Eggenhofer, E.; Weimar, W.; et al. Inflammatory conditions affect gene expression and function of human adipose tissue-derived mesenchymal stem cells. Clin. Exp. Immunol. 2010, 162, 474–486. [Google Scholar] [CrossRef]
- Goedhart, M.; Cornelissen, A.S.; Kuijk, C.; Geerman, S.; Kleijer, M.; van Buul, J.D.; Huveneers, S.; Raaijmakers, M.; Young, H.A.; Wolkers, M.C.; et al. Interferon-Gamma Impairs Maintenance and Alters Hematopoietic Support of Bone Marrow Mesenchymal Stromal Cells. Stem Cells Dev. 2018, 27, 579–589. [Google Scholar] [CrossRef]
- Oja, S.; Komulainen, P.; Penttilä, A.; Nystedt, J.; Korhonen, M. Automated image analysis detects aging in clinical-grade mesenchymal stromal cell cultures. Stem Cell Res. Ther. 2018, 9, 6. [Google Scholar] [CrossRef]
- Castro-Manrreza, M.E.; Bonifaz, L.; Castro-Escamilla, O.; Monroy-García, A.; Cortés-Morales, A.; Hernández-Estévez, E.; Hernández-Cristino, J.; Mayani, H.; Montesinos, J.J. Mesenchymal Stromal Cells from the Epidermis and Dermis of Psoriasis Patients: Morphology, Immunophenotype, Differentiation Patterns, and Regulation of T Cell Proliferation. Stem Cells Int. 2019, 2019, 4541797. [Google Scholar] [CrossRef] [PubMed]
- Klinker, M.W.; Marklein, R.A.; Lo Surdo, J.L.; Wei, C.H.; Bauer, S.R. Morphological features of IFN-γ-stimulated mesenchymal stromal cells predict overall immunosuppressive capacity. Proc. Natl. Acad. Sci. USA 2017, 114, E2598–E2607. [Google Scholar] [CrossRef]
- Fu, S.; Wei, J.; Wang, G.; Wang, B.; Wang, Y.; Lai, X.; Huang, H. The key role of PML in IFN-α induced cellular senescence of human mesenchymal stromal cells. Int. J. Oncol. 2015, 46, 351–359. [Google Scholar] [CrossRef][Green Version]
- Li, X.; Du, W.; Ma, F.X.; Feng, X.; Bayard, F.; Han, Z.C. High Concentrations of TNF-α Induce Cell Death during Interactions between Human Umbilical Cord Mesenchymal Stem Cells and Peripheral Blood Mononuclear Cells. PLoS ONE 2015, 10, e0128647. [Google Scholar] [CrossRef]
- Fawzy El-Sayed, K.M.; Hein, D.; Dörfer, C.E. Retinol/inflammation affect stemness and differentiation potential of gingival stem/progenitor cells via Wnt/β-catenin. J. Periodontal Res. 2019, 54, 413–423. [Google Scholar] [CrossRef]
- Fajardo-Orduna, G.; Mayani, H.; Castro-Manrreza, M.; Flores-Figueroa, E.; Flores, P.; Arriaga, L.; Pina-Sanchez, P.; Hernandez-Estevez, E.; Castell-Rodriguez, A.E.; Chavez-Rueda, K.; et al. Bone Marrow Mesenchymal Stromal Cells From Clinical Scale Culture: In Vitro Evaluation Of Their Differentiation, Hematopoietic Support And Immunosuppressive Capacities. Stem Cells Dev. 2016, 25, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Ratushnyy, A.; Ezdakova, M.; Buravkova, L. Secretome of Senescent Adipose-Derived Mesenchymal Stem Cells Negatively Regulates Angiogenesis. Int. J. Mol. Sci. 2020, 21, 1802. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Wang, Y.; Wang, J.; Zhai, J.; He, F.; Zhu, G. Irradiation-induced senescence of bone marrow mesenchymal stem cells aggravates osteogenic differentiation dysfunction via paracrine signaling. Am. J. Physiol. Cell Physiol. 2020, 318, C1005–C1017. [Google Scholar] [CrossRef]
- Sugihara, H.; Teramoto, N.; Yamanouchi, K.; Matsuwaki, T.; Nishihara, M. Oxidative stress-mediated senescence in mesenchymal progenitor cells causes the loss of their fibro/adipogenic potential and abrogates myoblast fusion. Aging 2018, 10, 747–763. [Google Scholar] [CrossRef]
- Suvakov, S.; Cubro, H.; White, W.M.; Butler Tobah, Y.S.; Weissgerber, T.L.; Jordan, K.L.; Zhu, X.Y.; Woollard, J.R.; Chebib, F.T.; Milic, N.M.; et al. Targeting senescence improves angiogenic potential of adipose-derived mesenchymal stem cells in patients with preeclampsia. Biol. Sex Differ. 2019, 10, 49. [Google Scholar] [CrossRef]
- Kwon, J.H.; Kim, M.; Um, S.; Lee, H.J.; Bae, Y.K.; Choi, S.J.; Hwang, H.H.; Oh, W.; Jin, H.J. Senescence-Associated Secretory Phenotype Suppression Mediated by Small-Sized Mesenchymal Stem Cells Delays Cellular Senescence through TLR2 and TLR5 Signaling. Cells 2021, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ravikumar, M.; Ling, L.; Nurcombe, V.; Cool, S.M. Age-Related Changes in the Inflammatory Status of Human Mesenchymal Stem Cells: Implications for Cell Therapy. Stem Cell Rep. 2021, 16, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Lunyak, V.V.; Amaro-Ortiz, A.; Gaur, M. Mesenchymal Stem Cells Secretory Responses: Senescence Messaging Secretome and Immunomodulation Perspective. Front. Genet. 2017, 8, 220. [Google Scholar] [CrossRef]
- Gnani, D.; Crippa, S.; Della Volpe, L.; Rossella, V.; Conti, A.; Lettera, E.; Rivis, S.; Ometti, M.; Fraschini, G.; Bernardo, M.E.; et al. An early-senescence state in aged mesenchymal stromal cells contributes to hematopoietic stem and progenitor cell clonogenic impairment through the activation of a pro-inflammatory program. Aging Cell 2019, 18, e12933. [Google Scholar] [CrossRef] [PubMed]
- Mato-Basalo, R.; Morente-López, M.; Arntz, O.J.; van de Loo, F.A.J.; Fafián-Labora, J.; Arufe, M.C. Therapeutic Potential for Regulation of the Nuclear Factor Kappa-B Transcription Factor p65 to Prevent Cellular Senescence and Activation of Pro-Inflammatory in Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 3367. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, S.; Huang, S.; Zhang, Z.; Yuan, X.; Feng, X.; Lu, L.; Sun, L. Serum IFN-γ Predicts the Therapeutic Effect of Mesenchymal Stem Cells Transplantation in Systemic Lupus Erythematosus Patients. Stem Cells Transl. Med. 2017, 6, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.J.; Wan, J.; Lu, D.P. Serum TNFalpha levels in patients with acute graft-versus-host disease after bone marrow transplantation. Leukemia 2001, 15, 1089–1091. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yosefifard, M.; Vaezi, G.; Malekirad, A.A.; Faraji, F.; Hojati, V. A Randomized Control Trial Study to Determine the Effect of Melatonin on Serum Levels of IL-1β and TNF-α in Patients with Multiple Sclerosis. Iran. J. Allergy Asthma Immunol. 2019, 18, 649–654. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, J.; Gong, L.; Yu, D.; An, C.; Bunpetch, V.; Dai, J.; Huang, H.; Zou, X.; Ouyang, H.; et al. The Plasticity of Mesenchymal Stem Cells in Regulating Surface HLA-I. iScience 2019, 15, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, S.J.; Gopalakrishnan, D.; Shankar, S.R.; Vasandan, A.B. Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PLoS ONE 2010, 5, e9016. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.L.; Tang, K.C.; Patel, A.P.; Bonilla, L.M.; Pierobon, N.; Ponzio, N.M.; Rameshwar, P. Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-gamma. Blood 2006, 107, 4817–4824. [Google Scholar] [CrossRef] [PubMed]
- Guess, A.J.; Daneault, B.; Wang, R.; Bradbury, H.; La Perle, K.M.D.; Fitch, J.; Hedrick, S.L.; Hamelberg, E.; Astbury, C.; White, P.; et al. Safety Profile of Good Manufacturing Practice Manufactured Interferon γ-Primed Mesenchymal Stem/Stromal Cells for Clinical Trials. Stem Cells Transl. Med. 2017, 6, 1868–1879. [Google Scholar] [CrossRef]
- Kuca-Warnawin, E.; Skalska, U.; Janicka, I.; Musiałowicz, U.; Bonek, K.; Głuszko, P.; Szczęsny, P.; Olesińska, M.; Kontny, E. The Phenotype and Secretory Activity of Adipose-Derived Mesenchymal Stem Cells (ASCs) of Patients with Rheumatic Diseases. Cells 2019, 8, 1659. [Google Scholar] [CrossRef]
- Beldi, G.; Bahiraii, S.; Lezin, C.; Nouri Barkestani, M.; Abdelgawad, M.E.; Uzan, G.; Naserian, S. TNFR2 Is a Crucial Hub Controlling Mesenchymal Stem Cell Biological and Functional Properties. Front. Cell Dev. Biol. 2020, 8, 596831. [Google Scholar] [CrossRef]
- Opitz, C.A.; Litzenburger, U.M.; Lutz, C.; Lanz, T.V.; Tritschler, I.; Koppel, A.; Tolosa, E.; Hoberg, M.; Anderl, J.; Aicher, W.K.; et al. Toll-like receptor engagement enhances the immunosuppressive properties of human bone marrow-derived mesenchymal stem cells by inducing indoleamine-2,3-dioxygenase-1 via interferon-beta and protein kinase R. Stem Cells 2009, 27, 909–919. [Google Scholar] [CrossRef]
- Ungerer, C.; Quade-Lyssy, P.; Radeke, H.H.; Henschler, R.; Konigs, C.; Kohl, U.; Seifried, E.; Schuttrumpf, J. Galectin-9 is a suppressor of T and B cells and predicts the immune modulatory potential of mesenchymal stromal cell preparations. Stem Cells Dev. 2014, 23, 755–766. [Google Scholar] [CrossRef]
- Kim, S.N.; Lee, H.J.; Jeon, M.S.; Yi, T.; Song, S.U. Galectin-9 is Involved in Immunosuppression Mediated by Human Bone Marrow-derived Clonal Mesenchymal Stem Cells. Immune Netw. 2015, 15, 241–251. [Google Scholar] [CrossRef]
| Name Used in the Original Report Cell Source and In Vitro Conditioning Method | Isolation Method | Structures Obtained and Size Range | Study Model | Ref. |
|---|---|---|---|---|
| Extracellular vesicles Human TA-MSCs IFN-γ (50 ng/mL) for 48 h. | Exosome isolation reagent (Invitrogen). | Microvesicles ≈150–500 nm Mean: 262.4 nm | In vitro: T lymphocyte proliferation and differentiation. | [37] |
| Extracellular vesicles Human and murine TA-MSCs Resting MSCs | MVs and Exo preparations: (a) 300× g for 10 min; (b) 2000× g for 10 min; (c) 0.8 µm membrane filtration; (d) 12,500× g for 20 min at room temperature (pure MV isolates); (e) Removing the residual MVs by centrifugation at 20,500× g for 40 min; (f) 0.22 µm membrane filtration; (g) 100,000× g for 70 min (pure Exo isolates). | MVs: Mean: 400–500 nm Exosomes: Mean: 80–100 nm | In vitro: Proliferation and secretion of cytokines by T lymphocytes. | [48] |
| Extracellular membrane vesicles Human UCB-MSCs IFN-γ (100 ng/mL) for 24 and 48 h. | (a) 2000× g for 20 min; (b) 100,000× g for 1–2 h. | EV-mix 20–700 nm | In vitro: T lymphocyte proliferation and induction of regulatory T cells. In vivo:Ischemia-reperfusion-induced acute kidney injury rat model. | [85] |
| Extracellular Vesicles Human BM-MSCs Resting MSCs | (a) 400× g for 5 min; (b) 2000× g for 20 min; (c)10,000× g for 45 min; (d) 100,000× g for 90 min. | EV-mix 152 ± 23 nm | In vitro: Maturation and secretion of cytokines by CDs. | [87] |
| Microvesicles Murine BM-MSCs Resting MSCs | (a) 300× g for 10 min; (b) 1000× g for 20 min; (c) 10,000× g for 30 min; (d) 100,000× g for 2 h. | EV-mix 50–200 nm | In vitro: Proliferation and secretion of cytokines by T lymphocytes. Induction of regulatory T cells. | [90] |
| Microvesicles Human WJ-MSCs Resting MSCs | (a) 2000× g for 20 min; (b) 100,000× g for 1 h. | EV-mix 30–500 nm | In vivo: Ischemia-reperfusion-induced acute kidney injury rat model. | [91] |
| Microvesicles Human BM-MSCs Resting MSCs | (a) 1500× g for 20 min; (b) 10,000× g for 20 min; (c) 100,000× g for 1 h. | EV-mix 60–160 nm | In vitro: Proliferation and secretion of cytokines by T lymphocytes. Induction of regulatory T cells. | [92] |
| Extracellular vesicles Human BM-MSCs TNF-α (20 ng/mL) plus IFN-γ (20 ng/mL) during the night | (a) 0.2-µm membrane filtration; (b) Millipore Lab-scale TFF system equipped with a Biomax 500 kDa Pellicon filter. | Exosomes 75–165 nm | In vitro: Cytokine secretion by activated primary rat splenocytes. | [93] |
| Extracellular vesicles Murine TA-MSCsResting MSCs | (a) 2000× g for 20 min; (b) 100,000× g for 2 h. | EV-mix 100–1000 nm | In vitro: T lymphocyte proliferation and induction of regulatory T cells. | [94] |
| Microvesicles Human BM-MSCs Resting MSCs | 100,000 g for 1 h at 4°C twice. | EV-mix ≈200 nm | In vitro: Activation of murine mast cells. | [95] |
| Microvesicles Human BM-MSCs Resting MSCs | (a) 300× g for 20 min; (b) 100,000× g for 1 h. | EV-mix 50–200 nm | In vitro: Human type II alveolar cells. | [96] |
| Microvesicles Human BM-MSCs Hypoxia-induced MSCs | a) 1500× g for 15 min; b) 0.22 μm membrane filtration; c) 170,000× g for 5 h. | Exosomes 50–100 nm | In vitro: Uptake by human umbilical cord endothelial cells. | [97] |
| Extracellular vesicles Human BM-MSCs IFN-γ (10 ng/mL) plus TNF-α (15 ng/mL) for 40 to 48 h. | (a) 300× g for 10 min; (b) 2000× g for 30 min; (c) 100,000× g for 90 min. | EV-mix ≈60–400 nm | In vitro: Proliferation and secretion of cytokines by NK, T, and B cells. | [98] |
| Exosomes Human BM-MSCs Resting MSCs | (a) 300× g for 10 min; (b) 10,000× g for 20 min; (c) 0.2 µm membrane filtration; (d) 100,000× g for 60 min. | Exosomes 65–100 nm | In vitro: Proliferation and differentiation of T and B lymphocytes. | [99] |
| Extracellular vesicles Human BM-MSCs Resting MSCs | (a) 2000× g for 20 min; (b) 100,000× g for 2 h. | EV-mix 61–121 nm | In vitro: Polarization of macrophages. | [100] |
| Exosomes MSCs from carcinoma and healthy breast tissue Resting MSCs | Exosome Isolation Kit (Invitrogen). | Exosomes Size not reported | In vitro: Polarization of macrophages. | [101] |
| Extracellular vesicles Human BM-MSCs IFN-γ (10 ng/mL) plus TNF-α (15 ng / ml) for 4 h. | (a) 300× g for 10 min; (b) 2000× g for 30 min; (c) 100,000× g for 90 min. | EV-mix ≈25–500 nm | In vitro: Induction of regulatory T cells. In vivo: A xenograft mouse model with steroid-refractory acute graft-versus-host disease. | [102] |
| Extracellular vesicles Human UC-MSCs Resting MSCs | (a) 300× g for 10 min; (b) 2000× g for 20 min; (c) 10,000× g for 30 min; (d) 0.2 µm membrane filtration; (e) 100,000× g for 90 min. | Exosomes 105.1–181.1 nm Mean: 139.2 nm | In vivo: Murine model of chronic graft-versus-host disease. Infiltration and activation of macrophages. | [103] |
| Extracellular vesicles Human WJ-MSCs Resting MSCs | a) 10,000× g for 20 min; b) 100,000× g for 1 h. | EV-mix 164 ± 10.4 nm | In vivo: Murine model of lung ischemia-reperfusion injury. Cytokine expression levels. | [104] |
| Extracellular vesicles Human UC-MSCs Resting MSCs | (a) 2000× g for 20 min; (b) 100,000× g for 60 min. | EV-mix 80–1000 nm | Single-center, randomized, placebo-controlled, phase II/III clinical pilot study. Patients with grade III-IV chronic kidney disease. | [105] |
| Small extracellular vesicle Human WJ-MSCs IFN-γ (2.5 ng/mL) Time is not reported | (a) 400× g for 10 min; (b) 2000× g for 30 min; (c) 10,000× g for 1.5 h; (d) 100,000× g for 90 min. | Exosomes 30–150 nm | In vitro: Activation of T lymphocytes. In vivo: Patients with acute graft-versus-host disease. | [106] |
| Microvesicles Human BM-MSCs IFN-γ (10 ng/mL) for 72 h | a) 500× g for 15 min; b) 2000× g for 20 min; c) 17,000× g for 60 min. | Microvesicles 130–1000 nm | In vitro: Analysis of changes in the transport of HLA-I and ICAM-1. | [107] |
| Exosomes Human TA-MSCs IFN-γ plus TNF-α (10, 20 and 40 ng/mL) for 48 h. | ExoQuick-TC System Biosciences. | Exosomes 115 ± 11.5 nm | In vitro: Polarization of macrophages. | [108] |
| Microvesicles Human BM-MSCs IFN-γ (10 ng/mL) for 48 h, 74 h, or 4 days | (a) 300× g for 30 min; (b) 16,500× g for 20 min. | MVs ≈150 nm | In vitro: Induction of regulatory T cells. | [109] |
| Exosomes UC-MSCs TGF-β (10 ng/mL) IFN-γ (1000 IU/mL) for 72 h. Alone or combined | (a) 3000× g for 30 min; (b) 0.22 μm membrane filtration;PEG6000 was added; (c) 3000 rpm for 30 min. | Exosomes 141.6 ± 23.3 nm | In vitro: Induction of regulatory T cells. | [110] |
| Microvesicles Human BM-MSCs Ischemic brain extract for 24 h. | (a) 2500× g for 10 min; (b) 14,000× g for 45 min at 10 °C. | MVs 150–450 nm | In vitro: Analysis of the content of immunoregulatory molecules. | [111] |
| Extracellular vesicles Murine TA-MSC Resting MSCs | MVs and Exo preparations: (a) 300× g for 10 min; (b) 0.8 µm membrane filtration; (c) 12,600× g for 30 min (MVs); (d) 0.22 µm membrane filtration; (g) 100,000× g for 70 min (Exo). | MVs: Mean: 271 nm Exosome: Mean: 90 nm | In vitro: Mouse-derived peritoneal macrophages. | [112] |
| Extracellular Vesicles Human UC-MSCs Resting MSCs | (a) 3200× g for 10 min; (b) 10,000× g for 30 min; (c) 100,000× g for 2 h. | EV-mix ≈100–300 nm | In vitro: Activation of CD4 + cells. In vivo: Mouse liver ischemia/reperfusion injury model. | [113] |
| Smallextracellular vesicles Human UC-MSCs Resting MSCs | (a) 300× g for 10 min; (b) 2000× g for 10 min; (c) 10,000× g for 30 min; (d) 100,000× g for 70 min. | EV-mix 40–200 nm | In vivo: Rat model of rheumatoid arthritis T lymphocyte proliferation, apoptosis, and differentiation. | [114] |
| Extracellular Vesicles Human TA-MSCs Resting MSCs | (a) 0.22 μm membrane filtration; (b) 30,000× g for 20 min; (c) 120,000× g for 3 h. | Exosomes 90–120 nm | In vivo: Mouse model of dextran sodium sulfate-induced colitis. | [115] |
| Extracellular vesicles Human PL-MSC Resting MSCs | (a) 500× g for 10 min; (b) 2000× g for 20 min; (c) 5000× g for 30 min; (d) 0.2 µm membrane filtration; (e) 130,000× g for 2 h. | Exosomes 85–125 nm | In vivo: Mouse model of colitis-induced with trinitrobenzene sulfonic acid. | [116] |
| Extracellular vesicles Murine BM-MSCs IL-6 (20 ng/mL), TNF-α (25 ng/mL) plus IL-1β (25 ng/mL) for 24 h | (a) 1200 rpm for 6 min; (b) 0.22 mm filter; (c) Ami-Con filters Ultra-15, regenerate cellulose 100,000 NMWL; Merck Millipore); (d) 3200× g at 4 °C for 15 min. | EV-mix ≈30–300 nm | In vivo: Murine model of sodium dextran. Sulfate-induced colitis. Polarization of intestinal macrophages. Regulatory T cell differentiation. | [117] |
| Exosomes Human G- MSCs TNF-α (100 ng/mL) for 48 h | (a) 300× g for 10 min; (b) 3000× g for 10 min; (c) 20,000× g for 30 min; (d) 120,000× g for 70 min; (e) Sucrose gradient; (f) 110,000× g for 3 h at 4 °C. | Exosomes Control: 123 ± 3.1 nm TNF-α: 164 ± 7.3 nm | In vitro: Macrophage polarization. In vivo: Ligature-induced periodontitis model in mice. | [118] |
| Small extracellular vesicles human TA-MSCs IFN-γ (10 ng/mL) plus TNF-α (15 ng/mL) for 72 h | (a) 800× g for 5 min; (b) 2000× g for 10 min; (c) 0.22 mm pore filters; (d) 110,000× g for 2 h. | EV-mix ≈80–300 nm | In vitro: CD4 T cell proliferation. | [119] |
| Extracellular vesicles DP-MSCs IFN-γ (50 ng/mL), TNF-α (10 ng/mL) plus IL-1β (10 ng/mL) for 48 h | (a) 2000× g for 20 min; (b) 10,000× g for 70 min; (c) 0.22 μm membrane filtration; (d) 110,000× g for 120 min. | EV-mix ≈100–350 nm | In vitro: T cell proliferation. In vivo: Delayed-type hypersensitivity mouse model. | [120] |
| Extracellular vesicles Human BM-MSCs IFN-γ (25 ng/mL) plus TNF-α (20 ng/mL) for 24 or 48 h | (a) Centrifugation to remove cells and cell debris; (b) 110,000× g for 70 min. | EV-mix ≈100–500 nm | In vitro: Murine primary microglia. In vivo: Triple-transgenic model of Alzheimer’s disease. | [121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-García, L.; Castro-Manrreza, M.E. TNF-α and IFN-γ Participate in Improving the Immunoregulatory Capacity of Mesenchymal Stem/Stromal Cells: Importance of Cell–Cell Contact and Extracellular Vesicles. Int. J. Mol. Sci. 2021, 22, 9531. https://doi.org/10.3390/ijms22179531
López-García L, Castro-Manrreza ME. TNF-α and IFN-γ Participate in Improving the Immunoregulatory Capacity of Mesenchymal Stem/Stromal Cells: Importance of Cell–Cell Contact and Extracellular Vesicles. International Journal of Molecular Sciences. 2021; 22(17):9531. https://doi.org/10.3390/ijms22179531
Chicago/Turabian StyleLópez-García, Lucero, and Marta E. Castro-Manrreza. 2021. "TNF-α and IFN-γ Participate in Improving the Immunoregulatory Capacity of Mesenchymal Stem/Stromal Cells: Importance of Cell–Cell Contact and Extracellular Vesicles" International Journal of Molecular Sciences 22, no. 17: 9531. https://doi.org/10.3390/ijms22179531
APA StyleLópez-García, L., & Castro-Manrreza, M. E. (2021). TNF-α and IFN-γ Participate in Improving the Immunoregulatory Capacity of Mesenchymal Stem/Stromal Cells: Importance of Cell–Cell Contact and Extracellular Vesicles. International Journal of Molecular Sciences, 22(17), 9531. https://doi.org/10.3390/ijms22179531






