Peripheral Blood-Based Biomarkers for Immune Checkpoint Inhibitors
Abstract
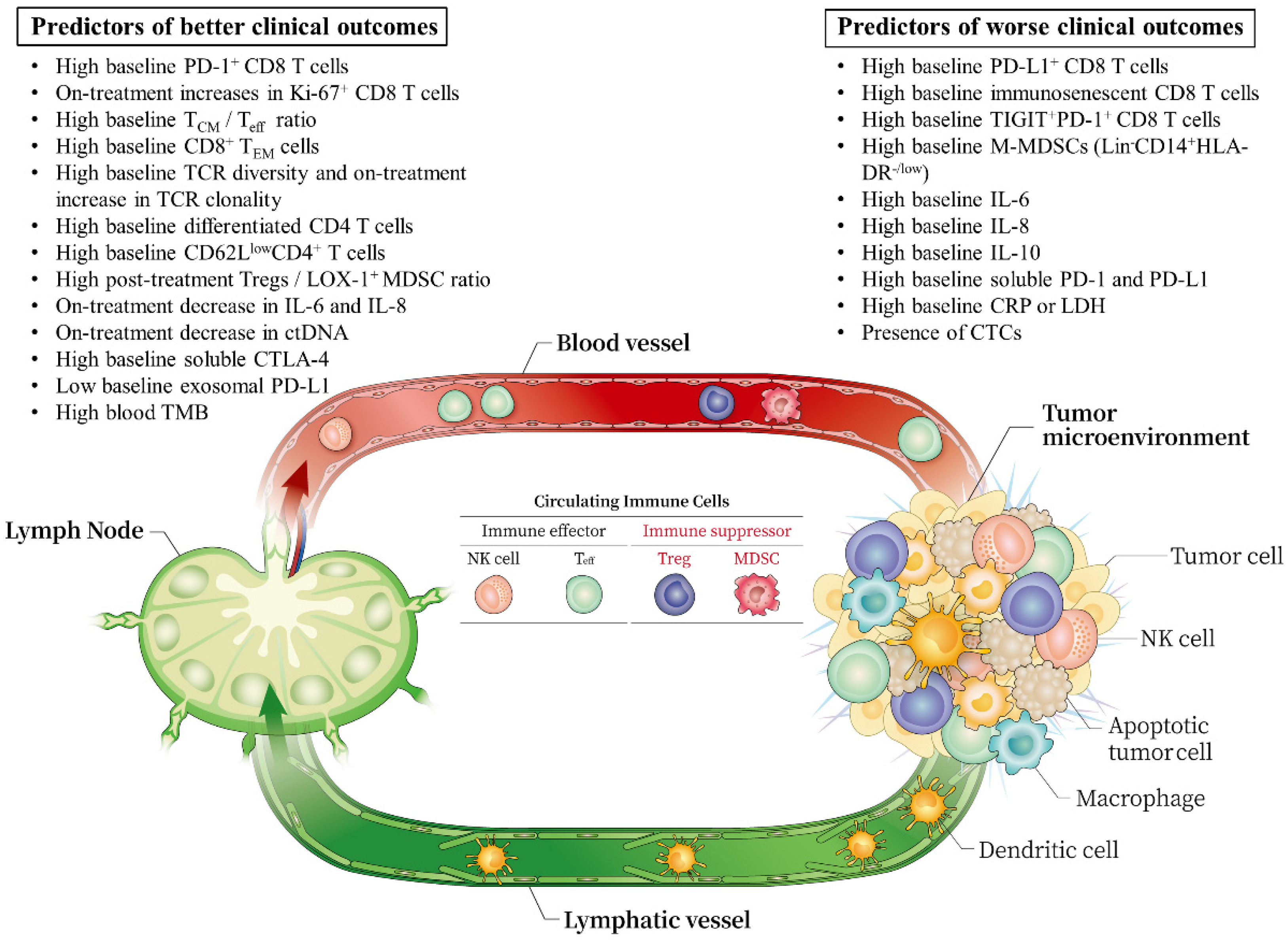
:1. Introduction
2. Circulating Immune Cells
2.1. CD8 T Cells
2.2. PD-1 or PD-L1 Expressing CD8 T Cells
2.3. TIM-3 Expressing T Cells
2.4. Immunosenescent CD8 T Cells
2.5. Memory T Cells
2.6. TCR Clonality and Diversity of PD-1+ CD8+ T Cells
2.7. CD4 T Cells
2.8. Immunosuppressive Cells: Myeloid-Derived Suppressive Cells (MDSCs) and Tregs
2.9. Natural Killer (NK) Cells
3. Cytokines and Soluble Proteins
3.1. IL-6
3.2. IL-8
3.3. IL-10
3.4. Soluble CTLA-4 (sCTLA-4)
3.5. Soluble PD-1 (sPD-1) or PD-L1 (sPD-L1)
3.6. CRP
3.7. LDH
4. Circulating Tumor Cells and Tumor Cell-Derived Factors
4.1. Circulating Tumor Cells (CTCs)
4.2. ctDNA
4.3. Blood TMB
4.4. Exosomes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [Green Version]
- Murciano-Goroff, Y.R.; Warner, A.B.; Wolchok, J.D. The future of cancer immunotherapy: Microenvironment-targeting combinations. Cell Res. 2020, 30, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Hegde, P.S.; Chen, D.S. Top 10 challenges in cancer immunotherapy. Immunity 2020, 52, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Yang, H.; Chon, H.J.; Kim, C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp. Mol. Med. 2020, 52, 1475–1485. [Google Scholar] [CrossRef]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.-X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef] [Green Version]
- Chon, H.J.; Lee, W.S.; Yang, H.; Kong, S.J.; Lee, N.K.; Moon, E.S.; Choi, J.; Han, E.C.; Kim, J.H.; Ahn, J.B.; et al. Tumor microenvironment remodeling by intratumoral oncolytic vaccinia virus enhances the efficacy of immune checkpoint blockade. Clin. Cancer Res. 2018, 25, 1612–1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.-S.; Kim, I.-K.; Han, S.; Park, I.; Kim, C.; Bae, J.; Oh, S.J.; Lee, S.; Kim, J.H.; Woo, D.-C.; et al. Normalization of tumor vessels by Tie2 activation and Ang2 inhibition enhances drug delivery and produces a favorable tumor microenvironment. Cancer Cell 2016, 30, 953–967. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.G.; Kim, C.; Yoon, S.E.; Kim, K.H.; Choi, S.J.; Kang, B.; Kim, H.R.; Park, S.-H.; Shin, E.-C.; Kim, Y.-Y.; et al. Hyperprogressive disease during PD-1 blockade in patients with advanced hepatocellular carcinoma. J. Hepatol. 2021, 74, 350–359. [Google Scholar] [CrossRef]
- Ren, D.; Hua, Y.; Yu, B.; Ye, X.; He, Z.; Li, C.; Wang, J.; Mo, Y.; Wei, X.; Chen, Y.; et al. Predictive biomarkers and mechanisms underlying resistance to PD1/PD-L1 blockade cancer immunotherapy. Mol. Cancer 2020, 19, 1–19. [Google Scholar] [CrossRef]
- Nixon, A.B.; Schalper, K.A.; Jacobs, I.; Potluri, S.; Wang, I.-M.; Fleener, C. Peripheral immune-based biomarkers in cancer immunotherapy: Can we realize their predictive potential? J. Immunother. Cancer 2019, 7, 325. [Google Scholar] [CrossRef]
- Tray, N.; Weber, J.; Adams, S. Predictive biomarkers for checkpoint immunotherapy: Current status and challenges for clinical application. Cancer Immunol. Res. 2018, 6, 1122–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, A.A.; Patel, V.G. The role of PD-L1 expression as a predictive biomarker: An analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Lv, Z.; Xu, D.; Cui, J. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark. Res. 2020, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Duchemann, B.; Remon, J.; Naigeon, M.; Mezquita, L.; Ferrara, R.; Cassard, L.; Jouniaux, J.M.; Boselli, L.; Grivel, J.; Auclin, E.; et al. Integrating circulating biomarkers in the immune checkpoint inhibitor treatment in lung cancer. Cancers 2020, 12, 3625. [Google Scholar] [CrossRef] [PubMed]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; Tarpey, P.; et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Kim, E.K.; Jung, H.; Chon, H.J.; Han, J.W.; Shin, K.-H.; Hu, H.; Kim, K.S.; Choi, Y.D.; Kim, S.; et al. Prognostic implications of PD-L1 expression in patients with soft tissue sarcoma. BMC Cancer 2016, 16, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Nabet, B.Y.; Esfahani, M.S.; Moding, E.J.; Hamilton, E.G.; Chabon, J.J.; Rizvi, H.; Steen, C.B.; Chaudhuri, A.A.; Liu, C.L.; Hui, A.B.; et al. Noninvasive early identification of therapeutic benefit from immune checkpoint inhibition. Cell 2020, 183, 363–376.e13. [Google Scholar] [CrossRef]
- Simonds, E.F.; Lu, E.D.; Badillo, O.; Karimi, S.; Liu, E.V.; Tamaki, W.; Rancan, C.; Downey, K.M.; Stultz, J.; Sinha, M.; et al. Deep immune profiling reveals targetable mechanisms of immune evasion in immune checkpoint inhibitor-refractory glioblastoma. J. Immunother. Cancer 2021, 9, e002181. [Google Scholar] [CrossRef] [PubMed]
- Reyes, M.; Vickers, D.; Billman, K.; Eisenhaure, T.; Hoover, P.; Browne, E.P.; Rao, D.A.; Hacohen, N.; Blainey, P.C. Multiplexed enrichment and genomic profiling of peripheral blood cells reveal subset-specific immune signatures. Sci. Adv. 2019, 5, eaau9223. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Paczkowski, P.; Mackay, S.; Ng, C.; Zhou, J. Single-cell multiplexed proteomics on the IsoLight resolves cellular functional heterogeneity to reveal clinical responses of cancer patients to immunotherapies. In Methods in Molecular Biology; Springer: New York, NY, USA, 2020; Volume 2055, pp. 413–431. [Google Scholar]
- Spitzer, M.H.; Nolan, G.P. Mass cytometry: Single cells, many features. Cell 2016, 165, 780–791. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Kim, C.G.; Shin, E.-C. Peripheral blood immune cell-based biomarkers in anti-PD-1/PD-L1 therapy. Immune Netw. 2020, 20, e8. [Google Scholar] [CrossRef] [PubMed]
- De Lima, V.A.B.; Hansen, M.; Spanggaard, I.; Rohrberg, K.; Hadrup, S.R.; Lassen, U.; Svane, I.M. Immune cell profiling of peripheral blood as signature for response during checkpoint inhibition across cancer types. Front. Oncol. 2021, 11, 11. [Google Scholar] [CrossRef]
- Griffiths, J.I.; Wallet, P.; Pflieger, L.T.; Stenehjem, D.; Liu, X.; Cosgrove, P.A.; Leggett, N.A.; McQuerry, J.A.; Shrestha, G.; Rossetti, M.; et al. Circulating immune cell phenotype dynamics reflect the strength of tumor—Immune cell interactions in patients during immunotherapy. Proc. Natl. Acad. Sci. USA 2020, 117, 16072–16082. [Google Scholar] [CrossRef] [PubMed]
- Jacquelot, N.; Roberti, M.P.; Enot, D.P.; Rusakiewicz, S.; Ternès, N.; Jegou, S.; Woods, D.M.; Sodré, A.L.; Hansen, M.; Meirow, Y.; et al. Predictors of responses to immune checkpoint blockade in advanced melanoma. Nat. Commun. 2017, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Mazzaschi, G.; Facchinetti, F.; Missale, G.; Canetti, D.; Madeddu, D.; Zecca, A.; Veneziani, M.; Gelsomino, F.; Goldoni, M.; Buti, S.; et al. The circulating pool of functionally competent NK and CD8+ cells predicts the outcome of anti-PD1 treatment in advanced NSCLC. Lung Cancer 2019, 127, 153–163. [Google Scholar] [CrossRef]
- Kwon, M.; An, M.; Klempner, S.J.; Lee, H.; Kim, K.-M.; Sa, J.K.; Cho, H.J.; Hong, J.Y.; Lee, T.; Min, Y.W.; et al. Determinants of response and intrinsic resistance to PD-1 blockade in microsatellite instability-high gastric cancer. Cancer Discov. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kamphorst, A.O.; Pillai, R.N.; Yang, S.; Nasti, T.H.; Akondy, R.S.; Wieland, A.; Sica, G.L.; Yu, K.; Koenig, L.; Patel, N.T.; et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc. Natl. Acad. Sci. USA 2017, 114, 4993–4998. [Google Scholar] [CrossRef] [Green Version]
- Huang, A.C.; Postow, M.A.; Orlowski, R.J.; Mick, R.; Bengsch, B.; Manne, S.; Xu, W.; Harmon, S.; Giles, J.R.; Wenz, B.; et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 2017, 545, 60–65. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Cho, J.; Ku, B.M.; Koh, J.; Sun, J.-M.; Lee, S.-H.; Ahn, J.S.; Cheon, J.; Min, Y.J.; Park, S.-H.; et al. The first-week proliferative response of peripheral blood PD-1+ CD8+ T cells predicts the response to anti-PD-1 therapy in solid tumors. Clin. Cancer Res. 2019, 25, 2144–2154. [Google Scholar] [CrossRef]
- Juliá, E.P.; Mandó, P.; Rizzo, M.M.; Cueto, G.R.; Tsou, F.; Luca, R.; Pupareli, C.; Bravo, A.I.; Astorino, W.; Mordoh, J.; et al. Peripheral changes in immune cell populations and soluble mediators after anti-PD-1 therapy in non-small cell lung cancer and renal cell carcinoma patients. Cancer Immunol. Immunother. 2019, 68, 1585–1596. [Google Scholar] [CrossRef]
- Kato, R.; Yamasaki, M.; Urakawa, S.; Nishida, K.; Makino, T.; Morimoto-Okazawa, A.; Kawashima, A.; Iwahori, K.; Suzuki, S.; Ueda, R.; et al. Increased Tim-3+ T cells in PBMCs during nivolumab therapy correlate with responses and prognosis of advanced esophageal squamous cell carcinoma patients. Cancer Immunol. Immunother. 2018, 67, 1673–1683. [Google Scholar] [CrossRef]
- Ferrara, R.; Naigeon, M.; Auclin, E.; Duchemann, B.; Cassard, L.; Jouniaux, J.-M.; Boselli, L.; Grivel, J.; Desnoyer, A.; Mezquita, L.; et al. Circulating T-cell immunosenescence in patients with advanced non-small cell lung cancer treated with single-agent PD-1/PD-L1 inhibitors or platinum-based chemotherapy. Clin. Cancer Res. 2021, 27, 492–503. [Google Scholar] [CrossRef]
- Manjarrez-Orduño, N.; Menard, L.C.; Kansal, S.; Fischer, P.; Kakrecha, B.; Jiang, C.; Cunningham, M.; Greenawalt, D.; Patel, V.; Yang, M.; et al. Circulating T cell subpopulations correlate with immune responses at the tumor site and clinical response to PD1 inhibition in non-small cell lung cancer. Front. Immunol. 2018, 9, 1613. [Google Scholar] [CrossRef] [Green Version]
- Krieg, C.; Nowicka, M.; Guglietta, S.; Schindler, S.; Hartmann, F.J.; Weber, L.M.; Dummer, R.; Robinson, M.D.; Levesque, M.P.; Becher, B. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat. Med. 2018, 24, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Wistuba-Hamprecht, K.; Martens, A.; Heubach, F.; Romano, E.; Foppen, M.G.; Yuan, J.; Postow, M.; Wong, P.; Mallardo, D.; Schilling, B.; et al. Peripheral CD8 effector-memory type 1 T-cells correlate with outcome in ipilimumab-treated stage IV melanoma patients. Eur. J. Cancer 2017, 73, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.G.; Kim, K.H.; Lee, C.Y.; Park, S.-H.; Cho, B.C.; Shim, H.S.; Shin, E.-C.; Kim, H.R.; Pyo, K.-H.; Xin, C.-F.; et al. Hyperprogressive disease during PD-1/PD-L1 blockade in patients with non-small-cell lung cancer. Ann. Oncol. 2019, 30, 1104–1113. [Google Scholar] [CrossRef]
- Han, J.; Duan, J.; Bai, H.; Wang, Y.; Wan, R.; Wang, X.; Chen, S.; Tian, Y.; Wang, D.; Fei, K. TCR repertoire diversity of peripheral PD-1+ CD8+ T cells predicts clinical out-comes after immunotherapy in patients with non-small cell lung cancer. Cancer Immunol. Res. 2020, 8, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Zuazo, M.; Arasanz, H.; Fernández-Hinojal, G.; García-Granda, M.J.; Gato, M.; Bocanegra, A.; Martínez, M.; Hernández, B.; Teijeira, L.; Morilla, I.; et al. Functional systemic CD 4 immunity is required for clinical responses to PD-L1/PD-1 blockade therapy. EMBO Mol. Med. 2019, 11, e10293. [Google Scholar] [CrossRef]
- Kagamu, H.; Kitano, S.; Yamaguchi, O.; Yoshimura, K.; Horimoto, K.; Kitazawa, M.; Fukui, K.; Shiono, A.; Mouri, A.; Nishihara, F.; et al. CD4+ T-cell immunity in the peripheral blood correlates with response to anti-PD-1 therapy. Cancer Immunol. Res. 2020, 8, 334–344. [Google Scholar] [CrossRef] [Green Version]
- Kitano, S.; Postow, M.A.; Ziegler, C.; Kuk, D.; Panageas, K.S.; Cortez, C.; Rasalan, T.; Adamow, M.; Yuan, J.; Wong, P.; et al. Computational algorithm-driven evaluation of monocytic myeloid-derived suppressor cell frequency for prediction of clinical outcomes. Cancer Immunol. Res. 2014, 2, 812–821. [Google Scholar] [CrossRef] [Green Version]
- Martens, A.; Wistuba-Hamprecht, K.; Foppen, M.G.; Yuan, J.; Postow, M.A.; Wong, P.; Romano, E.; Khammari, A.; Dreno, B.; Capone, M.; et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin. Cancer Res. 2016, 22, 2908–2918. [Google Scholar] [CrossRef] [Green Version]
- Tarhini, A.A.; Edington, H.; Butterfield, L.; Lin, Y.; Shuai, Y.; Tawbi, H.; Sander, C.; Yin, Y.; Holtzman, M.; Johnson, J.; et al. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS ONE 2014, 9, e87705. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.R.; Park, S.-M.; Seo, S.-U.; Jung, I.; Yoon, H.I.; Gabrilovich, D.I.; Cho, B.C.; Seong, S.-Y.; Ha, S.-J.; Youn, J.-I. The ratio of peripheral regulatory T cells to Lox-1+ polymorphonuclear myeloid-derived suppressor cells predicts the early response to anti-PD-1 therapy in patients with non-small cell lung cancer. Am. J. Respir. Crit. Care Med. 2019, 199, 243–246. [Google Scholar] [CrossRef]
- Farhood, B.; Najafi, M.; Mortezaee, K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A review. J. Cell. Physiol. 2019, 234, 8509–8521. [Google Scholar] [CrossRef]
- Yang, H.; Lee, W.S.; Kong, S.J.; Kim, C.G.; Kim, J.H.; Chang, S.K.; Kim, S.; Kim, G.; Chon, H.J.; Kim, C. STING activation reprograms tumor vasculatures and synergizes with VEGFR2 blockade. J. Clin. Investig. 2019, 129, 4350–4364. [Google Scholar] [CrossRef] [Green Version]
- Chon, H.J.; Kim, H.; Noh, J.H.; Yang, H.; Lee, W.S.; Kong, S.J.; Lee, S.J.; Lee, Y.S.; Kim, W.R.; Kim, J.H.; et al. STING signaling is a potential immunotherapeutic target in colorectal cancer. J. Cancer 2019, 10, 4932–4938. [Google Scholar] [CrossRef] [PubMed]
- Simoni, Y.; Becht, E.; Fehlings, M.G.; Loh, C.Y.; Koo, S.-L.; Teng, K.W.W.; Yeong, J.P.S.; Nahar, R.; Zhang, T.; Kared, H.; et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 2018, 557, 575–579. [Google Scholar] [CrossRef]
- Gros, A.; Parkhurst, M.R.; Tran, E.; Pasetto, A.; Robbins, P.F.; Ilyas, S.; Prickett, T.D.; Gartner, J.J.; Crystal, J.S.; Roberts, I.M.; et al. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat. Med. 2016, 22, 433–438. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, X.; Zhang, J.; Rice, S.J.; Wagman, M.; Kong, Y.; Zhu, L.; Zhu, J.; Joshi, M.; Belani, C.P. Expression of PD-1 on CD4+ T cells in peripheral blood associates with poor clinical outcome in non-small cell lung cancer. Oncotarget 2016, 7, 56233–56240. [Google Scholar] [CrossRef] [Green Version]
- Arrieta, O.; Montes-Servín, E.; Hernandez-Martinez, J.-M.; Cardona, A.F.; Casas-Ruiz, E.; Crispin, J.; Motola, D.; Flores-Estrada, D.; Barrera, L. Expression of PD-1/PD-L1 and PD-L2 in peripheral T-cells from non-small cell lung cancer patients. Oncotarget 2017, 8, 101994–102005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gros, A.; Tran, E.; Parkhurst, M.R.; Ilyas, S.; Pasetto, A.; Groh, E.M.; Robbins, P.F.; Yossef, R.; Garcia-Garijo, A.; Fajardo, C.A.; et al. Recognition of human gastrointestinal cancer neoantigens by circulating PD-1+ lymphocytes. J. Clin. Investig. 2019, 129, 4992–5004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lian, J.; Yue, Y.; Yu, W.; Zhang, Y. Immunosenescence: A key player in cancer development. J. Hematol. Oncol. 2020, 13, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Bandres, E.; Merino, J.; Vázquez, B.; Inogés, S.; Moreno, C.; Subirá, M.; Sánchez-Ibarrola, A. The increase of IFN-γ production through aging correlates with the expanded CD8+ highCD28− CD57+ subpopulation. Clin. Immunol. 2000, 96, 230–235. [Google Scholar] [CrossRef]
- Anagnostou, V.; Forde, P.M.; White, J.R.; Niknafs, N.; Hruban, C.; Naidoo, J.; Marrone, K.; Sivakumar, I.A.; Bruhm, D.C.; Rosner, S.; et al. Dynamics of tumor and immune responses during immune checkpoint blockade in non-small cell lung cancer. Cancer Res. 2018, 79, 1214–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahnke, Y.D.; Brodie, T.M.; Sallusto, F.; Roederer, M.; Lugli, E. The who’s who of T-cell differentiation: Human memory T-cell subsets. Eur. J. Immunol. 2013, 43, 2797–2809. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Sun, Z.; Chen, L. Memory T cells: Strategies for optimizing tumor immunotherapy. Protein Cell 2020, 11, 549–564. [Google Scholar] [CrossRef] [Green Version]
- Olugbile, S.O.; Kiyotani, K.; Park, J.H.; Hoffman, P.C.; Szeto, L.; Patel, J.D.; Vokes, E.E.; Nakamura, Y. Sustained oligoclonal T cell expansion correlates with durable response to anti-PD1 therapy. J. Clin. Oncol. 2017, 35, 3061. [Google Scholar] [CrossRef]
- Walz, A.; Peveri, P.; Aschauer, H.; Baggiolini, M. Purification and amino acid sequencing of NAF, a novel neutrophil-activating factor produced by monocytes. Biochem. Biophys. Res. Commun. 1987, 149, 755–761. [Google Scholar] [CrossRef]
- Borst, J.; Ahrends, T.; Bąbała, N.; Melief, C.J.; Kastenmüller, W. CD4+ T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2018, 18, 635–647. [Google Scholar] [CrossRef]
- Weber, R.; Fleming, V.; Hu, X.; Nagibin, V.; Groth, C.; Altevogt, P.; Utikal, J.; Umansky, V. Myeloid-derived suppressor cells hinder the anti-cancer activity of immune checkpoint inhibitors. Front. Immunol. 2018, 9, 1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. 2019, 110, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T cells in cancer immunosuppression—Implications for anticancer therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371. [Google Scholar] [CrossRef] [PubMed]
- Condamine, T.; Dominguez, G.A.; Youn, J.-I.; Kossenkov, A.V.; Mony, S.; Alicea-Torres, K.; Tcyganov, E.; Hashimoto, A.; Nefedova, Y.; Lin, C.; et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci. Immunol. 2016, 1, aaf8943. [Google Scholar] [CrossRef] [Green Version]
- Shimasaki, N.; Jain, A.; Campana, D. NK cells for cancer immunotherapy. Nat. Rev. Drug Discov. 2020, 19, 200–218. [Google Scholar] [CrossRef]
- Cho, Y.-H.; Choi, M.G.; Kim, D.H.; Choi, Y.J.; Kim, S.Y.; Sung, K.J.; Lee, J.C.; Kim, S.-Y.; Rho, J.K.; Choi, C.-M. Natural killer cells as a potential biomarker for predicting immunotherapy efficacy in patients with non-small cell lung cancer. Target. Oncol. 2020, 15, 241–247. [Google Scholar] [CrossRef]
- Berraondo, P.; Sanmamed, M.F.; Ochoa, M.C.; Etxeberria, I.; Aznar, M.A.; Pérez-Gracia, J.L.; Rodríguez-Ruiz, M.E.; Ponz-Sarvise, M.; Castañón, E.; Melero, I. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 2019, 120, 6–15. [Google Scholar] [CrossRef] [Green Version]
- Arasanz, H.; Zuazo, M.; Bocanegra, A.; Gato, M.; Martínez-Aguillo, M.; Morilla, I.; Fernández, G.; Hernández, B.; López, P.; Alberdi, N.; et al. Early detection of hyperprogressive disease in non-small cell lung cancer by monitoring of systemic T cell dynamics. Cancers 2020, 12, 344. [Google Scholar] [CrossRef] [Green Version]
- Keegan, A.; Ricciuti, B.; Garden, P.; Cohen, L.; Nishihara, R.; Adeni, A.; Paweletz, C.; Supplee, J.; Jänne, P.A.; Severgnini, M.; et al. Plasma IL-6 changes correlate to PD-1 inhibitor responses in NSCLC. J. Immunother. Cancer 2020, 8, e000678. [Google Scholar] [CrossRef]
- Yuen, K.C.; Liu, L.-F.; Gupta, V.; Madireddi, S.; Keerthivasan, S.; Li, C.; Rishipathak, D.; Williams, P.; Kadel, E.E.; Koeppen, H.; et al. High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat. Med. 2020, 26, 693–698. [Google Scholar] [CrossRef]
- Sanmamed, M.F.; Perez-Gracia, J.L.; Schalper, K.A.; Fusco, J.P.; Gonzalez, A.; Rodriguez-Ruiz, M.E.; Oñate, C.; Perez, G.; Alfaro, C.; Martín-Algarra, S.; et al. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann. Oncol. 2017, 28, 1988–1995. [Google Scholar] [CrossRef]
- Tarhini, A.A.; Zahoor, H.; Lin, Y.; Malhotra, U.; Sander, C.; Butterfield, L.H.; Kirkwood, J.M. Baseline circulating IL-17 predicts toxicity while TGF-β1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J. Immunother. Cancer 2015, 3, 39. [Google Scholar] [CrossRef] [Green Version]
- Giunta, E.F.; Barra, G.; de Falco, V.; Argenziano, G.; Napolitano, S.; Vitale, P.; Zanaletti, N.; Terminiello, M.; Martinelli, E.; Morgillo, F.; et al. Baseline IFN-γ and IL-10 expression in PBMCs could predict response to PD-1 checkpoint inhibitors in advanced melanoma patients. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Pistillo, M.P.; Fontana, V.; Morabito, A.; Dozin, B.; Laurent, S.; Carosio, R.; Banelli, B.; Ferrero, F.; Spano, L.; Tanda, E.; et al. Soluble CTLA-4 as a favorable predictive biomarker in metastatic melanoma patients treated with ipilimumab: An Italian melanoma intergroup study. Cancer Immunol. Immunother. 2019, 68, 97–107. [Google Scholar] [CrossRef]
- Ugurel, S.; Schadendorf, D.; Horny, K.; Sucker, A.; Schramm, S.; Utikal, J.; Pföhler, C.; Herbst, R.; Schilling, B.; Blank, C.; et al. Elevated baseline serum PD-1 or PD-L1 predicts poor outcome of PD-1 inhibition therapy in metastatic melanoma. Ann. Oncol. 2020, 31, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Iivanainen, S.; Ahvonen, J.; Knuuttila, A.; Tiainen, S.; Koivunen, J.P. Elevated CRP levels indicate poor progression-free and overall survival on cancer patients treated with PD-1 inhibitors. ESMO Open 2019, 4, e000531. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, S.; Saito, K.; Yasuda, Y.; Kijima, T.; Yoshida, S.; Yokoyama, M.; Ishioka, J.; Matsuoka, Y.; Kageyama, Y.; Fujii, Y. Impact of C-reactive protein flare-response on oncological outcomes in patients with metastatic renal cell carcinoma treated with nivolumab. J. Immunother. Cancer 2021, 9, e001564. [Google Scholar] [CrossRef]
- Bigot, F.; Castanon, E.; Baldini, C.; Hollebecque, A.; Carmona, A.; Postel-Vinay, S.; Angevin, E.; Armand, J.-P.; Ribrag, V.; Aspeslagh, S.; et al. Prospective validation of a prognostic score for patients in immunotherapy phase I trials: The Gustave Roussy Immune Score (GRIm-Score). Eur. J. Cancer 2017, 84, 212–218. [Google Scholar] [CrossRef]
- Weide, B.; Martens, A.; Hassel, J.C.; Berking, C.; Postow, M.A.; Bisschop, K.; Simeone, E.; Mangana, J.; Schilling, B.; di Giacomo, A.M.; et al. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin. Cancer Res. 2016, 22, 5487–5496. [Google Scholar] [CrossRef] [Green Version]
- Tamminga, M.; de Wit, S.; Hiltermann, T.J.N.; Timens, W.; Schuuring, E.; Terstappen, L.; Groen, H.J. Circulating tumor cells in advanced non-small cell lung cancer patients are associated with worse tumor response to checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 173. [Google Scholar] [CrossRef]
- Raja, R.; Kuziora, M.; Brohawn, P.Z.; Higgs, B.; Gupta, A.; Dennis, P.A.; Ranade, K. Early reduction in ctDNA predicts survival in patients with lung and bladder cancer treated with durvalumab. Clin. Cancer Res. 2018, 24, 6212–6222. [Google Scholar] [CrossRef] [Green Version]
- Ricciuti, B.; Jones, G.; Severgnini, M.; Alessi, J.V.; Recondo, G.; Lawrence, M.; Forshew, T.; Lydon, C.; Nishino, M.; Cheng, M.; et al. Early plasma circulating tumor DNA (ctDNA) changes predict response to first-line pembrolizumab-based therapy in non-small cell lung cancer (NSCLC). J. Immunother. Cancer 2021, 9, e001504. [Google Scholar] [CrossRef] [PubMed]
- Bratman, S.V.; Yang, S.Y.C.; Iafolla, M.A.J.; Liu, Z.; Hansen, A.R.; Bedard, P.L.; Lheureux, S.; Spreafico, A.; Razak, A.A.; Shchegrova, S.; et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat. Rev. Cancer 2020, 1, 873–881. [Google Scholar] [CrossRef]
- Gandara, D.R.; Paul, S.M.; Kowanetz, M.; Schleifman, E.; Zou, W.; Li, Y.; Rittmeyer, A.; Fehrenbacher, L.; Otto, G.; Malboeuf, C.; et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat. Med. 2018, 24, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Tucci, M.; Passarelli, A.; Mannavola, F.; Stucci, L.S.; Ascierto, P.A.; Capone, M.; Madonna, G.; Lopalco, P.; Silvestris, F. Serum exosomes as predictors of clinical response to ipilimumab in metastatic melanoma. OncoImmunology 2017, 7, e1387706. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xu, F.; Lu, T.; Duan, Z.; Zhang, Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat. Rev. 2012, 38, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Hoejberg, L.; Bastholt, L.; Johansen, J.S.; Christensen, I.J.; Gehl, J.; Schmidt, H. Serum interleukin-6 as a prognostic biomarker in patients with metastatic melanoma. Melanoma Res. 2012, 22, 287–293. [Google Scholar] [CrossRef]
- Bakouny, Z.; Choueiri, T.K. IL-8 and cancer prognosis on immunotherapy. Nat. Med. 2020, 26, 650–651. [Google Scholar] [CrossRef]
- Infanger, D.W.; Cho, Y.; Lopez, B.; Mohanan, S.; Liu, S.C.; Gursel, D.; Boockvar, J.A.; Fischbach, C. Glioblastoma stem cells are regulated by interleukin-8 signaling in a tumoral perivascular niche. Cancer Res. 2013, 73, 7079–7089. [Google Scholar] [CrossRef] [Green Version]
- Bellmunt, J.; Larriba, J.L.G.; Prior, C.; Maroto, P.; Carles, J.; Castellano, D.; Mellado, B.; Gallardo, E.; Perez-Gracia, J.L.; Aguilar, G.; et al. Phase II study of sunitinib as first-line treatment of urothelial cancer patients ineligible to receive cisplatin-based chemotherapy: Baseline interleukin-8 and tumor contrast enhancement as potential predictive factors of activity. Ann. Oncol. 2011, 22, 2646–2653. [Google Scholar] [CrossRef]
- Rallis, K.S.; Corrigan, A.E.; Dadah, H.; George, A.M.; Keshwara, S.M.; Sideris, M.; Szabados, B. Cytokine-based cancer immunotherapy: Challenges and opportunities for IL-10. Anticancer Res. 2021, 41, 3247–3252. [Google Scholar] [CrossRef]
- Mittal, S.K.; Cho, K.-J.; Ishido, S.; Roche, P.A. Interleukin 10 (IL-10)-mediated immunosuppression: March-I induction regulates antigen presentation by macrophages but not dendritic cells. J. Biol. Chem. 2015, 290, 27158–27167. [Google Scholar] [CrossRef] [Green Version]
- Qiao, J.; Liu, Z.; Dong, C.; Luan, Y.; Zhang, A.; Moore, C.; Fu, K.; Peng, J.; Wang, Y.; Ren, Z.; et al. Targeting tumors with IL-10 prevents dendritic cell-mediated CD8+ T cell apoptosis. Cancer Cell 2019, 35, 901–915.e4. [Google Scholar] [CrossRef]
- Zhao, S.; Wu, D.; Wu, P.; Wang, Z.; Huang, J. Serum IL-10 predicts worse outcome in cancer patients: A meta-analysis. PLoS ONE 2015, 10, e0139598. [Google Scholar] [CrossRef] [Green Version]
- Magistrelli, G.; Jeannin, P.; Herbault, N.; Benoit de Coignac, A.; Gauchat, J.F.; Bonnefoy, J.Y.; Delneste, Y. A soluble form of CTLA-4 generated by alternative splicing is expressed by nonstimulated human T cells. Eur. J. Immunol. 1999, 29, 3596–3602. [Google Scholar] [CrossRef]
- Frigola, X.; Inman, B.A.; Krco, C.J.; Liu, X.; Harrington, S.M.; Bulur, P.A.; Dietz, A.B.; Dong, H.; Kwon, E.D. Soluble B7-H1: Differences in production between dendritic cells and T cells. Immunol. Lett. 2012, 142, 78–82. [Google Scholar] [CrossRef] [Green Version]
- Okuma, Y.; Wakui, H.; Utsumi, H.; Sagawa, Y.; Hosomi, Y.; Kuwano, K.; Homma, S. Soluble programmed cell death ligand 1 as a novel biomarker for nivolumab therapy for non-small-cell lung cancer. Clin. Lung Cancer 2018, 19, 410–417.e1. [Google Scholar] [CrossRef]
- Zhou, J.; Mahoney, K.M.; Giobbie-Hurder, A.; Zhao, F.; Lee, S.; Liao, X.; Rodig, S.; Li, J.; Wu, X.; Butterfield, L.H.; et al. Soluble PD-L1 as a biomarker in malignant melanoma treated with checkpoint blockade. Cancer Immunol. Res. 2017, 5, 480–492. [Google Scholar] [CrossRef] [Green Version]
- Siemes, C.; Visser, L.E.; Coebergh, J.-W.W.; Splinter, T.A.; Witteman, J.C.; Uitterlinden, A.G.; Hofman, A.; Pols, H.A.; Stricker, B.H. C-reactive protein levels, variation in the C-reactive protein gene, and cancer risk: The Rotterdam study. J. Clin. Oncol. 2006, 24, 5216–5222. [Google Scholar] [CrossRef]
- Vasseur, A.; Kiavue, N.; Bidard, F.; Pierga, J.; Cabel, L. Clinical utility of circulating tumor cells: An update. Mol. Oncol. 2021, 15, 1647–1666. [Google Scholar] [CrossRef]
- Pantel, K.; Speicher, M. The biology of circulating tumor cells. Oncogene 2016, 35, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabières, C.; Pantel, K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016, 6, 479–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.; Tolani, B.; Nie, X.; Zhi, X.; Hu, M.; He, B. Review of the clinical applications and technological advances of circulating tumor DNA in cancer monitoring. Ther. Clin. Risk Manag. 2017, 13, 1363–1374. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Duan, J.; Cai, S.; Han, M.; Dong, H.; Zhao, J.; Zhu, B.; Wang, S.; Zhuo, M.; Sun, J.; et al. Assessment of blood tumor mutational burden as a potential biomarker for immunotherapy in patients with non-small cell lung cancer with use of a next-generation sequencing cancer gene panel. JAMA Oncol. 2019, 5, 696–702. [Google Scholar] [CrossRef]
- Yin, Z.; Yu, M.; Ma, T.; Zhang, C.; Huang, S.; Karimzadeh, M.R.; Momtazi-Borojeni, A.A.; Chen, S. Mechanisms underlying low-clinical responses to PD-1/PD-L1 blocking antibodies in immunotherapy of cancer: A key role of exosomal PD-L1. J. Immunother. Cancer 2021, 9, e001698. [Google Scholar] [CrossRef] [PubMed]
| Marker | Cancer Type | Treatment | N | Findings Associated with Clinical Response | Reference |
|---|---|---|---|---|---|
| CD8 T cells | NSCLC | PD-(L)1 inhibitor | 94 | Lower number of baseline CD8 T cells were associated with DCB and longer PFS (AUC 0.68 with combined modeling of pretreatment tumor PD-L1, bTMB, and circulating CD8 T cells). | Nabet et al., 2020 [18] |
| PD-L1+ CD8 T cells | Melanoma | Ipilimumab ±Nivolumab | 190 | Higher baseline levels of PD-L1+ CD8 T cells were associated with poor OS (AUC 0.76). | Jacquelot et al., 2017 [26] |
| PD-1+ CD8 T cells, NK cells | NSCLC | Nivolumab | 31 | Higher frequencies of PD-1+ CD8 T cells and active NK cells at baseline were associated with clinical benefit, PFS, and OS (AUC 0.85, 79% sensitivity, and 83% specificity). | Mazzaschi et al., 2019 [27] |
| PD-1+ CD8 T cells | MSI-high gastric cancer | Pembrolizumab | 19 | On-treatment increases in the frequencies of PD-1+ CD8 T cells were associated with DCB. | Kwon et al., 2021 [28] |
| Ki-67+ PD-1+ CD8 T cells | NSCLC | PD-1 inhibitor | 29 | Early proliferation (Ki-67+) of PD-1+ CD8 T cells within 4 weeks of treatment correlated with good response. | Kamphorst et al., 2018 [29] |
| Ki-67+ CD8 T cells | Melanoma | Pembrolizumab | 47 | Higher frequencies of Ki-67+ CD8 T cells to tumor burden at week 6 (> 1.94) were associated with better clinical outcomes. | Huang et al., 2017 [30] |
| Ki-67+ CD8 T cells | Thymic epithelial tumor/ NSCLC | Pembrolizumab or nivolumab | 64/ 46 | A fold change of Ki-67+ among PD-1+ CD8 T cells at baseline and day 7 (Ki-67 D7/D0) ≥2.8 correlated with DCB, PFS, and OS (AUC 0.89/0.81). | Kim et al., 2019 [31] |
| TIM-3+ T cells | NSCLC, RCC | PD-1 inhibitor | 43 | On-treatment increases in the frequencies of TIM-3-expressing CD4 or CD8 T cells were negatively associated with clinical responses and PFS. CD4+ TCM cells at baseline were associated with good response and prognosis. | Julia et al., 2019 [32] |
| TIM-3+ T cells | Esophageal cancer | Nivolumab | 20 | On-treatment increases in the frequencies of TIM-3 expressing CD4 or CD8 T cells were correlated with better responses and OS. | Kato et al., 2018 [33] |
| Immunosenescent CD8 T cells | NSCLC | PD-(L)1 inhibitors | 83 | Higher percentage of pretreatment immunosenescent CD8 T cells (CD28− CD57+ KLRG1+) was correlated with lower RR, DCB, worse PFS or OS (35% sensitivity, 100% specificity). | Ferrara et al., 2021 [34] |
| TCM/Teff ratio | Melanoma NSCLC | Nivolumab | 43/ 40 | Higher pretreatment TCM/Teff ratio was correlated with longer PFS. | Manjarrez-Orduno et al., 2018 [35] |
| CD8+ TEM cells | Melanoma | PD-1 inhibitor | 51 | Higher frequency of CD8+ TEM cells within 4 weeks of treatment initiation was correlated with the clinical benefit. | Krieg et al., 2018 [36] |
| CD8+ TEM type 1 cells | Melanoma | Ipilimumab | 137 | High frequencies of CD8+ TEM type 1 T cells (CD45RA− CCR7− CD27+ CD28+) at baseline were correlated with higher RR and longer OS. | Wistuba-Hamprecht et al., 2017 [37] |
| CD8+ TEM cells, TIGIT+ PD-1+ CD8 T cells | NSCLC | PD-(L)1 inhibitors | 263 | Lower frequency of CD8+ TEM cells and higher frequency of severely exhausted T cells (TIGIT+ PD-1+ CD8+) at baseline were associated with HPD and shorter OS. | Kim et al., 2019 [38] |
| TCR diversity and clonality of PD-1+ CD8 T cells | NSCLC | PD-(L)1 inhibitors | 40 | Pretreatment high PD-1+ CD8+ TCR diversity and increasing PD-1+ CD8+ TCR clonality after treatment were related to longer PFS and OS (87% sensitivity, 94% specificity). | Han et al., 2020 [39] |
| Highly differentiated CD4 T cells, Tregs | NSCLC | PD-(L)1 inhibitors | 83 | High proportion of highly differentiated CD4 T cells (CD27− CD28 low/−) and low percentage of CD25+ FOXP3+ CD4+ Tregs at baseline were associated with higher RR, longer PFS and OS (70% sensitivity, 100% specificity). | Zuazo et al., 2019 [40] |
| CD62 LlowCD4+ T cells, Tregs | NSCLC | Nivolumab | 126 | Higher CD62Llow CD4+ T cell level at baseline was associated with higher RR, longer PFS, or OS. Conversely, the percentage of CD25+ FOXP3+ CD4+ Tregs was lower in responders (85.7% sensitivity, 100% specificity). | Kagamu et al., 2020 [41] |
| M-MDSC | Melanoma | Ipilimumab | 68 | Lower frequency of M-MDSCs (Lin− CD14+ CD11b+ HLA-DRlow/−) at baseline or at week 6 was related to ICI response and OS. | Kitano et al., 2014 [42] |
| M-MDSCs, Tregs | Melanoma | Ipilimumab | 209 | Pretreatment M-MDSCs (Lin−CD14+ HLA-DR−/low) were negatively correlated with OS, while Tregs (CD4+ CD25+ FoxP3+) were positively correlated with OS. | Martens et al., 2016 [43] |
| M-MDSCs, Tregs | Melanoma | Neoadjuvant ipilimumab | 35 | Early on-treatment decrease in M-MDSCs (Lin1− HLA-DR− CD33+ CD11b+) and increase in Tregs at 6 weeks were associated with longer PFS. | Tarhini et al., 2014 [44] |
| LOX-1+ PMN-MDSCs, Tregs | NSCLC | Nivolumab | 63 | High ratio of Treg to LOX-1+ PMN-MDSCs ≥ 0.39 after treatment was correlated with longer PFS (87.5% sensitivity, 72.2% specificity). | Kim et al., 2019 [45] |
| Marker | Cancer Type | Treatment | N | Findings Associated with Clinical Response | Reference |
|---|---|---|---|---|---|
| IL-6 | NSCLC | PD-(L)1 inhibitors | 47 | On-treatment decrease in IL-6 level was associated with improved PFS. | Keegan et al., 2020 [70] |
| IL-8 | Melanoma, NSCLC | PD-1 inhibitors ± Ipilimumab | 44 /19 | On-treatment decrease in serum IL-8 level could be used to monitor and predict clinical benefit from ICIs (AUC 0.97 among three different patient groups). | Sanmamed et al., 2017 [72] |
| UC, RCC | Atezolizumab | 1445 | High baseline levels of IL-8 were associated with decreased efficacy of atezolizumab. On-treatment decrease in IL-8 was correlated with improved OS. | Yuen et al., 2020 [71] | |
| IL-10 | Melanoma | Ipilimumab | 35 | Combination of IL-10 and TGF-β was associated with PFS. | Tarhini et al. 2015 [73] |
| Melanoma | PD-1 inhibitors | 18 | Higher baseline IFN-γ/IL-10 ratio in PBMCs predicted longer PFS (AUC 0.96). | Giunta et al., 2020 [74] | |
| Soluble CTLA-4 | Melanoma | Ipilimumab | 113 | Higher serum levels of soluble CTLA-4 at baseline were associated with better ORR and OS. | Pistillo et al., 2018 [75] |
| Soluble PD-1/ PD-L1 | Melanoma | PD-1 inhibitors | 222 | Elevated baseline serum PD-1 or PD-L1 levels predicted poor outcome (AUC 0.61 for sPD-L1 and 0.53 for sPD-1 in OS). | Ugurel et al., 2020 [76] |
| CRP | Various solid tumors | PD-1 inhibitors | 326 | Elevated baseline CRP was an indicator of poor RFS and OS. | Livanainen et al., 2019 [77] |
| RCC | Nivolumab | 42 | The early on-treatment CRP flare-response was associated with tumor shrinkage and improved survival outcomes. | Fukuda et al., 2021 [78] | |
| LDH | Various solid tumors | Immunotherapy | 155 | High baseline LDH levels were correlated with poor OS. | Bigot et al., 2017 [79] |
| Melanoma | Pembrolizumab | 616 | Low pretreatment values of LDH were associated with favorable OS. | Weide et al., 2016 [80] | |
| CTCs | NSCLC | PD-(L)1 inhibitors | 104 | The presence of CTC is a predictive factor for a worse durable response rate to ICI. | Tamminga et al., 2019 [81] |
| ctDNA | NSCLC | Durvalumab | 28 | A drop in the ctDNA level is an early marker of therapeutic efficacy and predicts prolonged survival in patients treated with ICIs. | Raja et al., 2018 [82] |
| NSCLC | Pembrolizumab + chemotherapy | 62 | Decreases in ctDNA levels were related with clinical benefit. | Ricciuti et al., 2021 [83] | |
| Various solid tumors | Pembrolizumab | 94 | Baseline ctDNA levels were correlated with PFS, OS, and clinical response. | Bratman et al., 2020 [84] | |
| bTMB | NSCLC | PD-(L)1 inhibitors | 98 | ctDNA-based bTMB could be used as a potential biomarker for anti-PD-1 and anti-PD-L1 treatment in patients with NSCLC. | Wang et al., 2019 [60] |
| NSCLC | Atezolizumab | 216 | High bTMB is a clinically actionable biomarker for atezolizumab. | Gandara et al., 2018 [85] | |
| Exosome | Melanoma | Pembrolizumab | 44 | Lower baseline levels of exosomal PD-L1 and their increase during treatment were correlated with tumor response (AUC 0.91 for exosomal PD-L1, 0.70 for total PD-L1). | Chen et al., 2018 [86] |
| Melanoma | Ipilimumab | 59 | Increased exosomal PD-1, and the CD28 levels in T cells were associated with longer PFS and OS. | Tucci et al., 2018 [87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, H.J.; Chon, H.J.; Kim, C. Peripheral Blood-Based Biomarkers for Immune Checkpoint Inhibitors. Int. J. Mol. Sci. 2021, 22, 9414. https://doi.org/10.3390/ijms22179414
An HJ, Chon HJ, Kim C. Peripheral Blood-Based Biomarkers for Immune Checkpoint Inhibitors. International Journal of Molecular Sciences. 2021; 22(17):9414. https://doi.org/10.3390/ijms22179414
Chicago/Turabian StyleAn, Ho Jung, Hong Jae Chon, and Chan Kim. 2021. "Peripheral Blood-Based Biomarkers for Immune Checkpoint Inhibitors" International Journal of Molecular Sciences 22, no. 17: 9414. https://doi.org/10.3390/ijms22179414
APA StyleAn, H. J., Chon, H. J., & Kim, C. (2021). Peripheral Blood-Based Biomarkers for Immune Checkpoint Inhibitors. International Journal of Molecular Sciences, 22(17), 9414. https://doi.org/10.3390/ijms22179414






