1. Introduction
Radiotherapy is a mainstay and an affordable treatment choice for head and neck squamous cell carcinoma (HNSCC). However, radiotherapy is also associated with side effects, particularly the unavoidable development of cancer cell resistance (extrinsic resistance) to radiation exposure [
1,
2]. Tumor radioresistance, even intrinsic- or extrinsic-resistance, is still the main obstacle to therapeutic success. These radioresistant cells lead to cancer relapse, metastasis, poor prognosis, and poor patient outcome. It is believed that a small subset of cancer stem cells (CSCs) emerge that are capable of self-renewal, differentiation, plasticity, therapy resistance, and high tumorigenicity. CSCs that exhibit such behavior are most likely to be responsible for tumor radioresistance [
3]. CSCs have been identified in many types of solid tumor including breast cancer, lung cancer, prostate cancer, and, especially, head and neck squamous cell carcinoma [
4,
5,
6]. Notably, recent studies revealed that anti-cancer therapy (radio/chemotherapy) induced the transformation of non-stem cancer cells to cancer stem cells, enabling them to re-establish the tumors and produce metastasis [
7,
8]. These findings suggest that anti-cancer therapy not only kills cancer cells but also has the side effect of directly inducing the dynamic transforming non-stem cells into cancer stem cells, possibly contributing to tumor recurrence and metastasis. Therefore, drugs targeting cancer stem cells or preventing reprogramming of non-stem cancer cells can be combined with anti-cancer therapy to improve its antitumor efficacy.
Signal transducer and activator of transcription 3 (STAT3), a transcription factor for survival and proliferation, is essential to survival and maintains tumorigenesis in many cancers [
9]. Aberrant activation of STAT3 has been well characterized in HNSCC mainly due to abnormal signaling of various growth factor receptors [
10]. The activation of STAT3 contributes to cancer cell proliferation, dedifferentiation, invasion, and angiogenesis [
11]. Therefore, targeting STAT3 activation has been proposed as a therapeutic target in cancer treatment. It has been reported that attenuated STAT3 activation results in inhibition of cell proliferation and apoptosis induction in many cancer cells. Furthermore, STAT3 has been validated to affect cancer cell sensitivity to radiation. Recent studies have reported that C225, an anti-epidermal growth factor receptor (EGFR) monoclonal antibody, can, in combination with radiation, suppress STAT3 activation results in radiosensitization in head and neck cancer [
12]. In addition, the activation of STAT3 is an essential factor in CSC formation, radioresistance, and metastasis in HNSCC cells [
13]. The signaling of cytokine IL-6 leading to STAT3 activation to promote CSC-associated
OCT-4 gene expression contributes to the conversion non-CSCs into CSCs [
14]. Due to the pivotal role of STAT3 in HNSCC, these cellular signals are potential molecular targets for effective therapy for HNSCC patients.
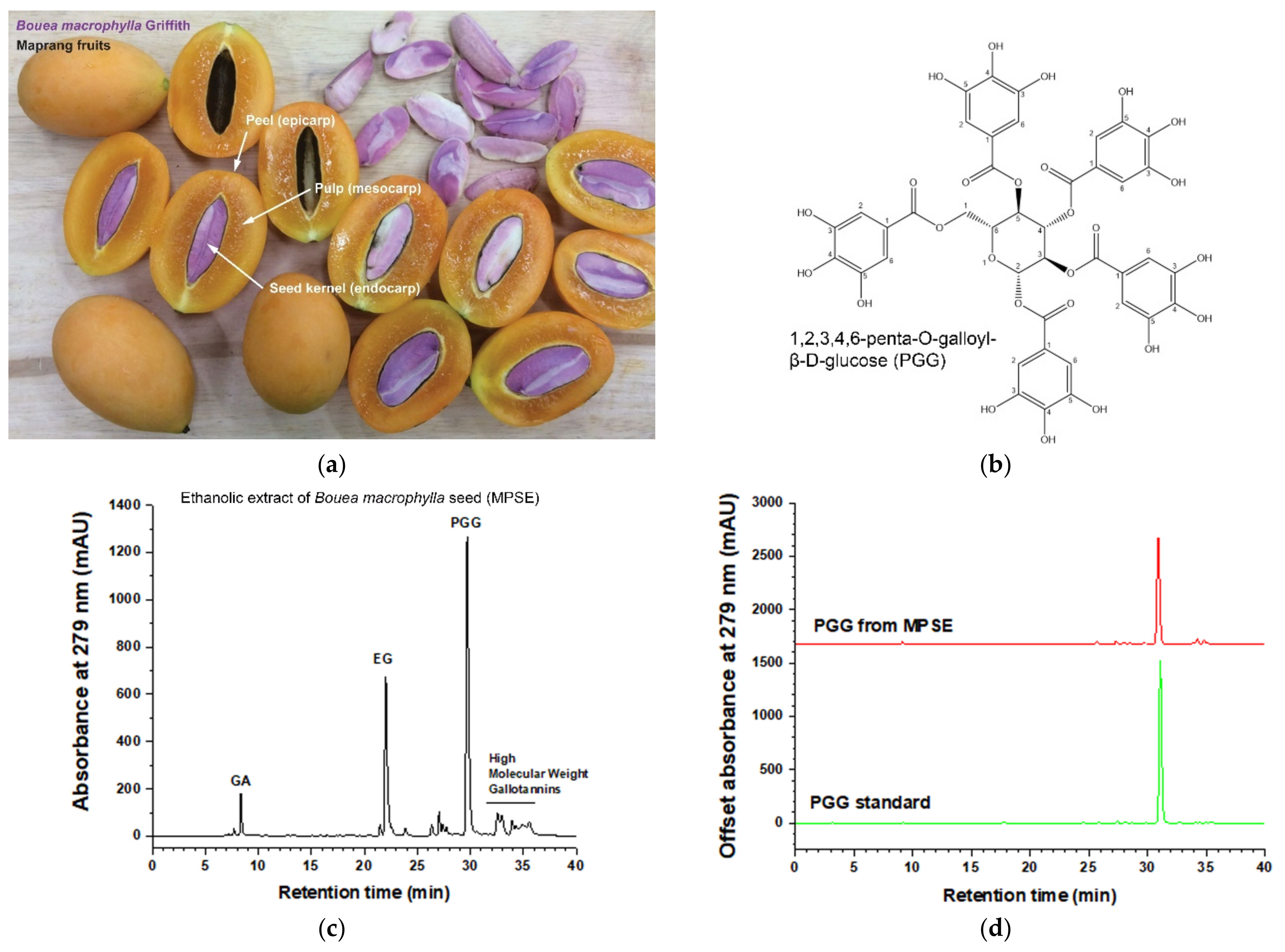
Maprang (
Bouea macrophylla Griffith) seed extract, so-called MPSE, a gallotannin with perfectly mixed constituents, has been shown to exert several pharmacological activities, especially anti-cancer activities [
15]. Our previous studies revealed that MPSE contains high amounts of active compounds such as 1,2,3,4-6-pentyl-
O-galloyl-β-
D-glucose (PGG), ethyl gallate (EG), and gallic acid (GA). MPSE induces increases in intracellular reactive oxygen species (ROS) and subsequently induces cell cycle arrest and apoptosis in human breast cancer cells [
16]. A main active compound found in MPSE, PGG has been extensively studied for its anti-cancer properties. Hyo-Jeong L et al. reported that orally administered PGG can inhibit triple-negative breast cancer (TNBC) growth and metastasis. The mechanism by which PGG exerted anti-tumorigenic effects is probably through the inhibition of STAT3 activation, which further inhibits angiogenesis, proliferation, and induced apoptosis in TNBC [
17]. Additionally, PGG inhibits STAT3 phosphorylation and induces caspase-mediated apoptosis in prostate cancer cells in vitro and decreases in vivo tumor xenograft growth. The reduced activation of STAT3 results in the downregulation of downstream target Bcl-XL and Mcl-1 and further induced apoptosis [
18]. A notable recent report by our team showed that MPSE pretreatment before irradiation in breast cancer cells inhibits the radiation induced epithelial to mesenchymal transition (EMT) process and confers radiosensitivity, probably by attenuating the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) and mitogen-activated protein kinase (MAPK) pathways [
19].
Herein, we extended the efficacy evaluation of MPSE and PGG radiosensitizing activity in the radioresistant cell lines, HPV-negative HNSCC. Our results provide the evidence of a molecular mechanism for the radiosensitizer effect of MPSE and PGG, demonstrating that MPSE and PGG combination with radiotherapy inhibit the radiation-induced CSCs trait, partially in the path, via attenuating the STAT3 activation and increasing therapeutic efficacy in HNSCC cell lines.
3. Discussion
Radiotherapy is a mainstay and an affordable treatment choice for head and neck squamous cell carcinoma (HNSCC). However, radioresistance is the main cause of treatment failure, and subsequently disease-related mortality. Therefore, the identification of molecules to strengthen therapeutic outcome is the main challenge in HNSCC treatment. In the present study, we provided insight into the mechanisms of gallotannin-rich extract compounds (which are naturally perfectly mixed constituents by 50%PGG, 36%EG, and 0.5%GA) from Maprang (Bouea macrophylla Griffith) seed (MPSE) and its major active compound, PGG, as a promising radiation sensitizer in HNSCC cell lines. MPSE or PGG could enhance the efficacy of radiotherapy in HNSCC via (a) inhibiting IR-induced pro-survival signaling and enhancing the effect of IR-induced DNA damage. (b) Inhibiting IR-induced enrichment of cancer stem cells population, that is responsible for radioresistance in cancer, and subsequently inhibiting anti-apoptotic pathways and increasing IR-induced cell death in HNSCC. In addition, the studies on molecular mechanisms revealed that MPSE or PGG could enhance the radiosensitivity of HNSCC via targeting cancer stem-like cells through attenuated STAT3 activation.
We have recently demonstrated that MPSE possessed promising anti-cancer activity and radiosensitization in breast cancer cells by inhibiting IR-induced pro-survival signaling [
19]. This study extends this observation to HNSCC, where poor patient prognosis and treatment failure were found due to intrinsic tumor radioresistance in HNSCC. In this study, we tested the activity of either MPSE or PGG in two radioresistant HPV-negative HNSCC (FaDu and CAL27) cells [
24]. Here, we demonstrated that MPSE or PGG suppresses the proliferation of HNSCC cells. In a comparison between two HNSCC cell lines, we also observed that FaDu cells are more responsive to MPSE than CAL27 cells (
Figure 2). Since HPV-negative HNSCC cells are incredibly resistant to IR [
25], we tested whether MPSE or PGG radiosensitizes CAL27 or FaDu cells. In line with the above finding, strongly decreased clonogenicity and increased cell death were seen after combined treatment with either MPSE or PGG and IR found in FaDu compared with CAL27 cell lines (
Figure 4 and
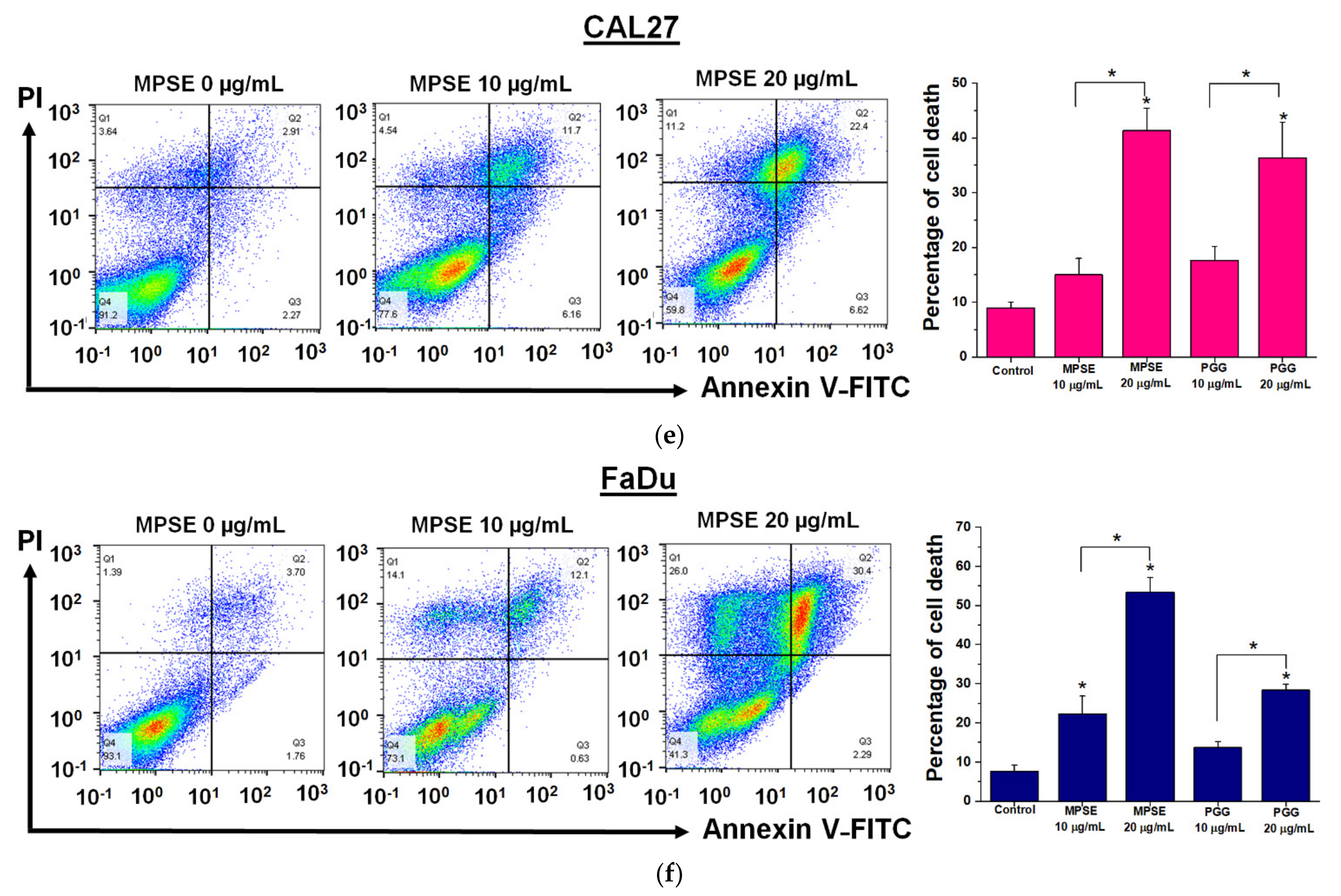
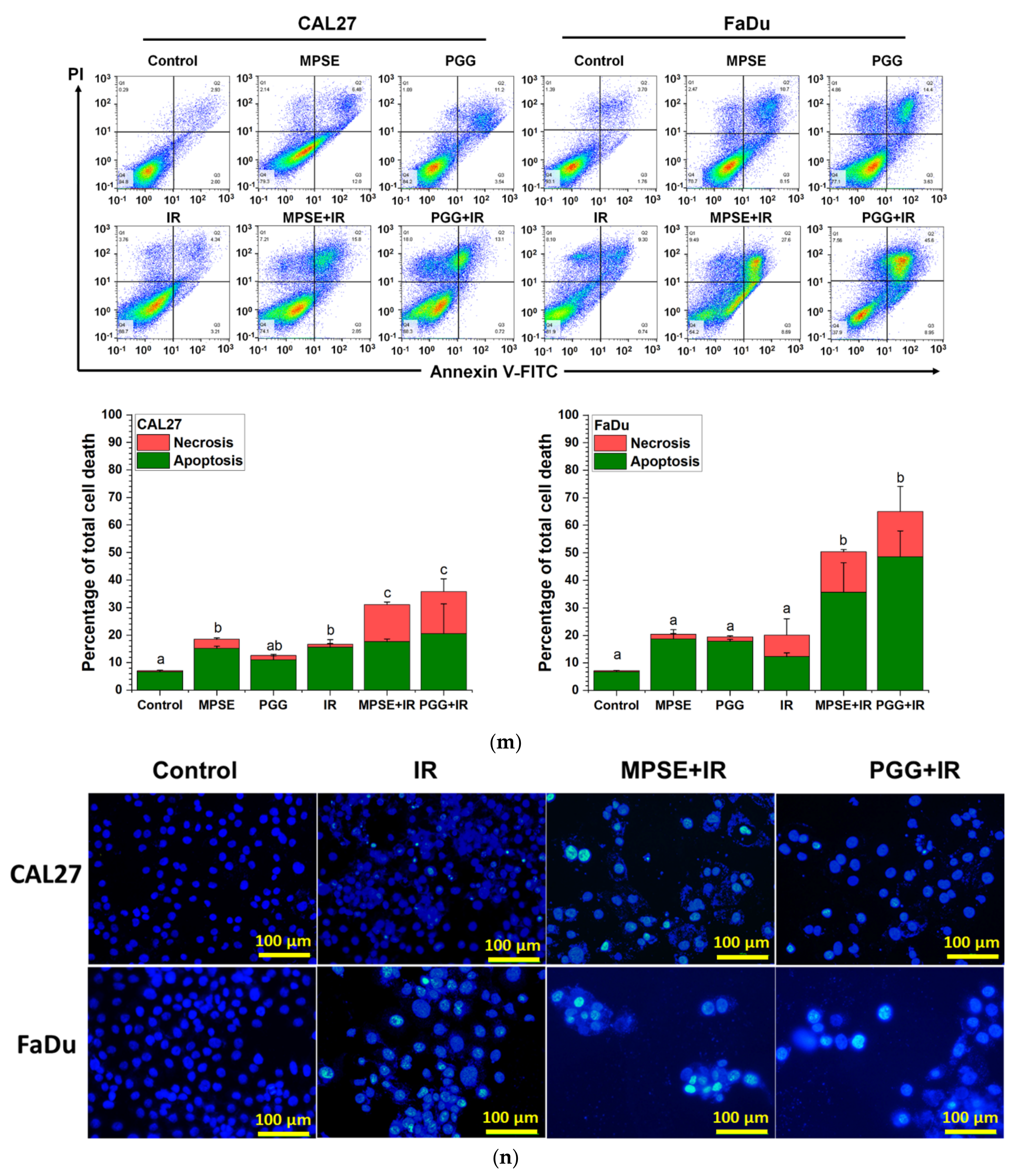
Figure 5). The combined treatment with either MPSE or PGG and IR exerts a negligible effect on enhanced apoptosis cell death in CAL27 cells (
Figure 5m). However, the significant complete loss of clonogenic potential was observed in combination treated CAL27 cells (
Figure 4b) through a possible induction of non-apoptotic programmed cell death. Thus, the exact nature of the programmed cell death, other than apoptosis, after the combination treatment of MPSE and IR in CAL27 cell line remains unclear and requires further investigation.
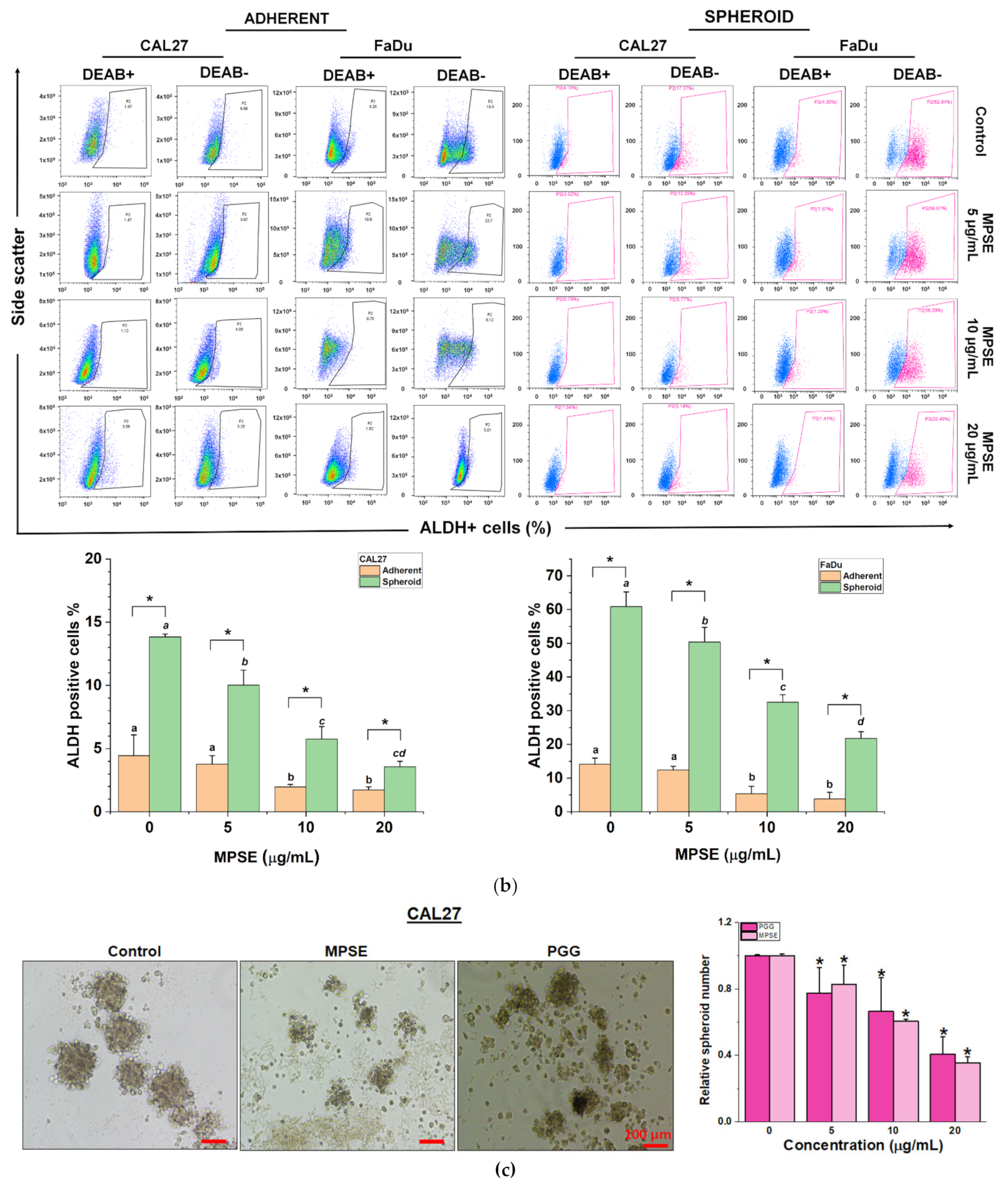
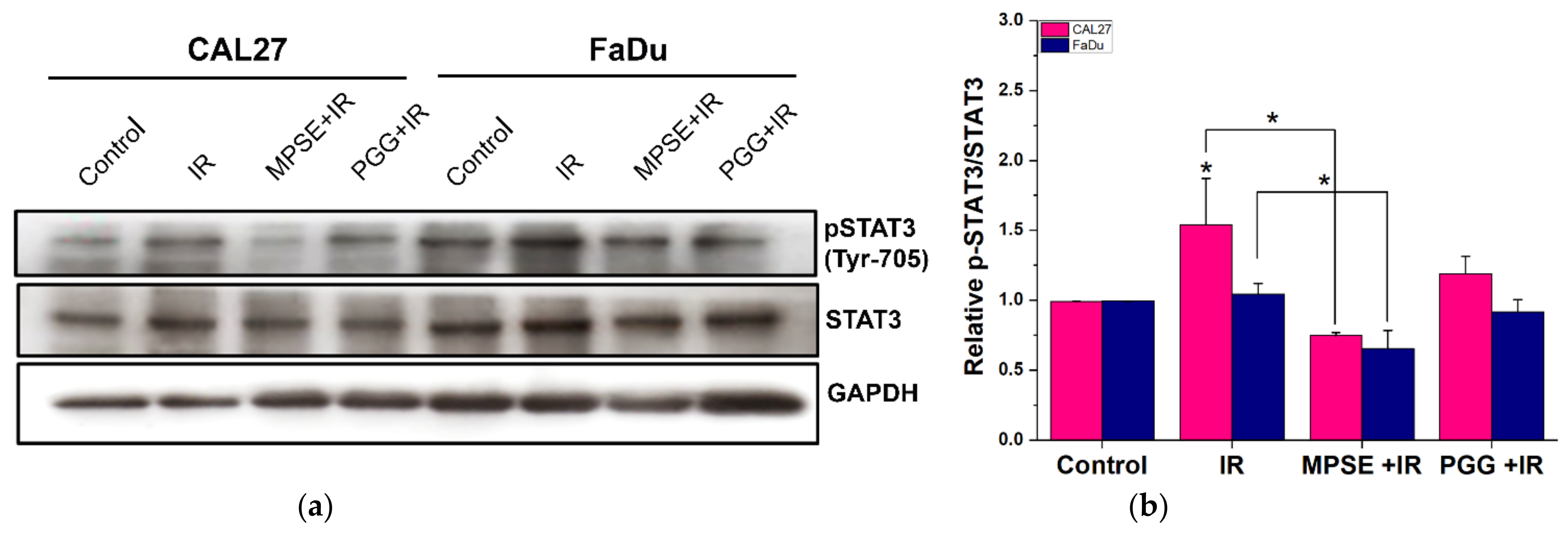
It has been proposed that the presence of a small population of CSCs in tumors results in resistance and recurrence of cancer after therapy [
3]. Therefore, potential compounds that can eliminate CSCs are imperative to overcome therapeutic resistance and improve the clinical outcomes of patients. The activation of STAT3, which is known to increase survival and proliferate signals, has been shown to play a critical role in regulating stemness in many cells, especially in HNSCC [
10]. To maintain CSC characteristics, STAT3 regulates the expression of several downstream target proteins associated with the stemness properties. Many CSC proteins and markers downstream of STAT3 have been identified, such as CD44, CD133, ALDH1, and the transcription factor regulates CSC (Oct4 and Sox2). Moreover, CSC self-renewal properties were shown to depend on STAT3 activation [
26]. Highly activated STAT3 is positively correlated with high-grade HNSCC tissue and promotes CSCs’ self-renewal, and the radioresistance ability of HNSCC. Blocking STAT3 activation by a specific inhibitor effectively blocked the tumor spheroid formation and decreased the number of CD44+ ALDH+ cells, and further induced apoptosis in HNSCC [
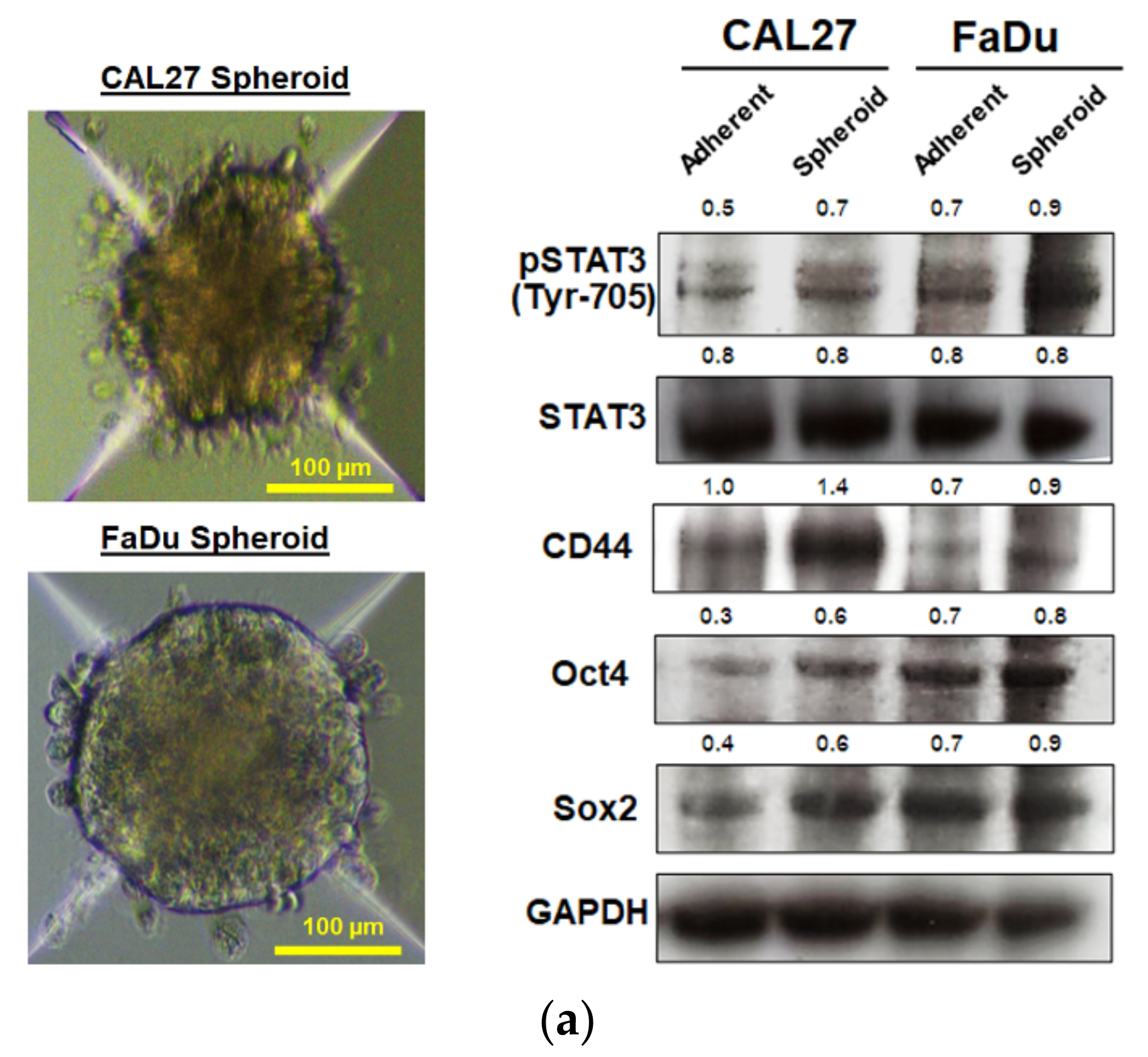
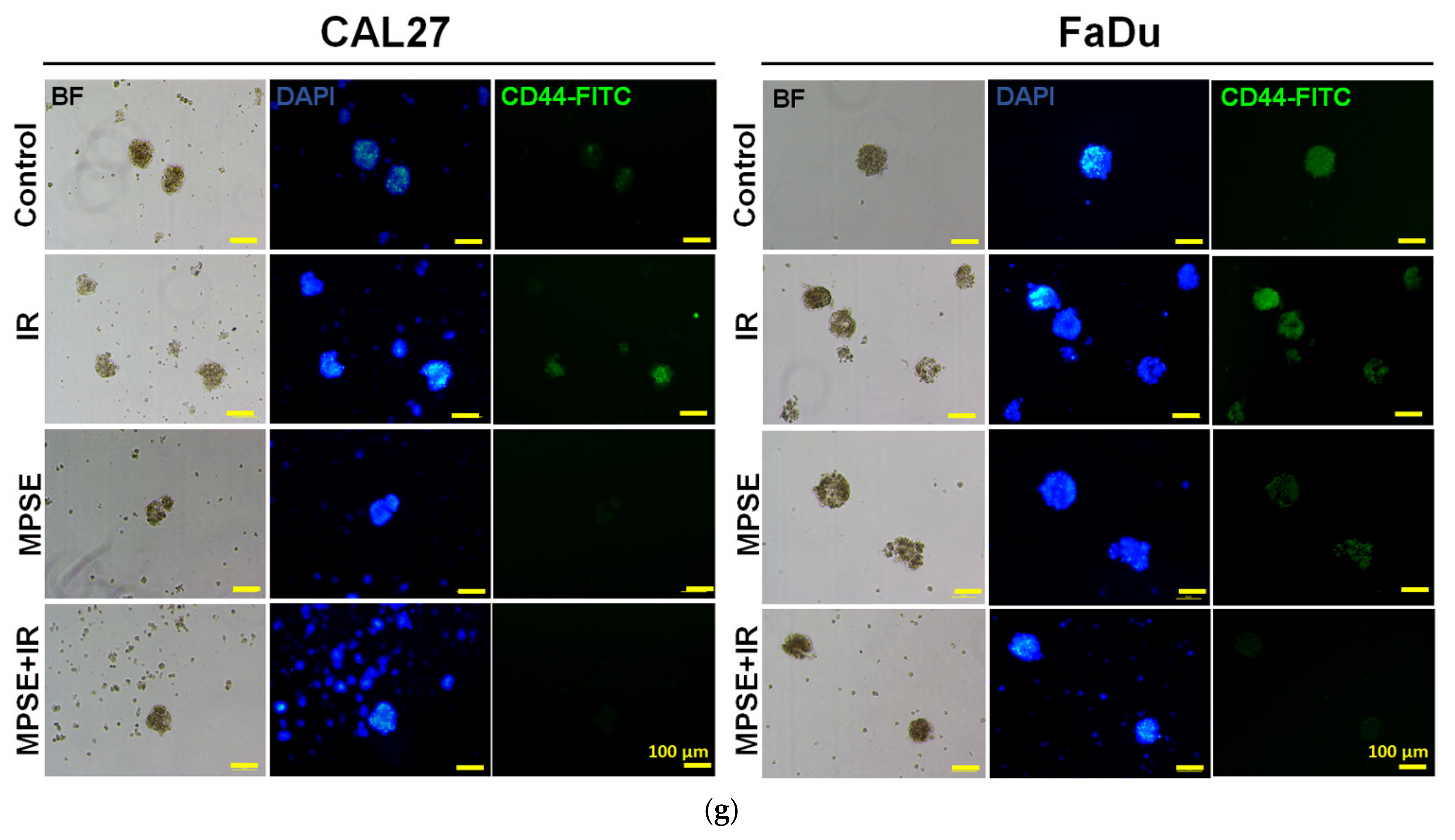
13], this was consistent with our study. We found that the inhibition of STAT3 by MPSE or PGG could suppress CSC in FaDu and CAL27 cells. For HNSCC cells, either adherent or spheroid-derived CSC-rich population treatment with MPSE or PGG was shown to downregulate the expression of phosphorylated STAT3. Subsequently, it downregulated CSC-related proteins, Oct4, SOX2, and CD44. Moreover, MPSE or PGG treatment dramatically suppressed their spheroid formation ability (
Figure 3). This is linked to the effect of MPSE or PGG in suppressing activities on key stem cell-related and oncogenic pathway STAT3 signaling.
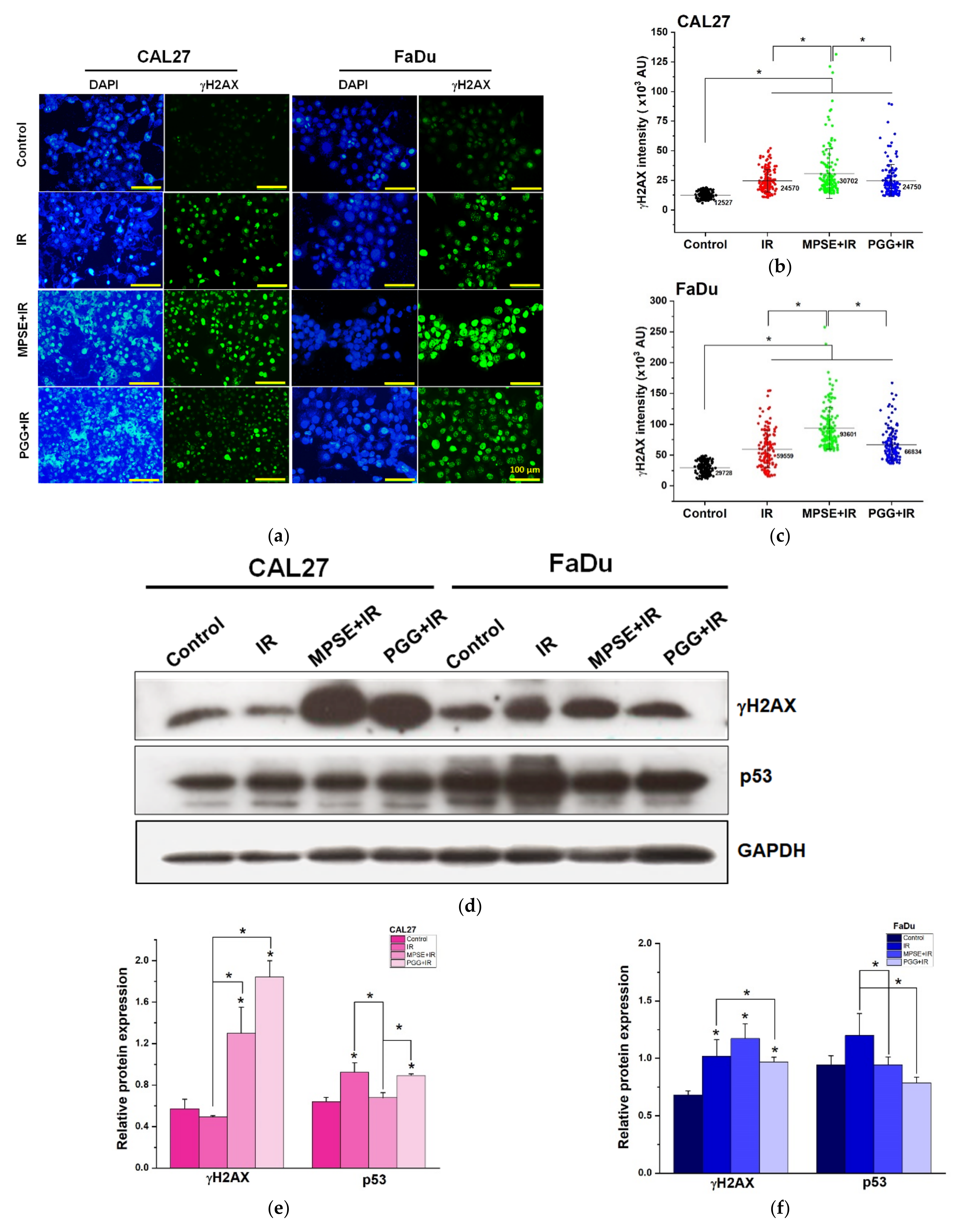
Following radiation treatment, the occurrence of DNA damage, especially to double-strand breaks (DSBs), has resulted in cell cycle arrest, apoptosis, and tumor regression. However, to survive, tumor cells always activate the pro-survival pathway and DNA repair mechanisms such as PI3K/Akt or RAS/Raf/Erk1/2 [
27]. Thus, tumor cells with highly efficient DNA repair are radioresistant [
28], whereas impaired repair DSBs are particularly detrimental to the cells. Recently, we have shown that MPSE increases IR-induced H2AX foci. Moreover, MPSE radiosensitized breast cancer cell lines via the inhibition of the pro-survival PI3K/Akt pathway. Similarly, in this study, we noticed that radiosensitization induced by MPSE or PGG was associated with impaired DNA repair, as evidenced by the marked decrease in the IR-induced phosphorylation of Akt and phosphorylation of ERK1/2, and increase in DNA damage response protein, γH2AX in HNSCC cells (
Figure 5). This implies that the DNA repair pathway, in the path, plays a critical role in the regulation of MPSE- or PGG-mediated radiosensitization in HNSCC cell lines.
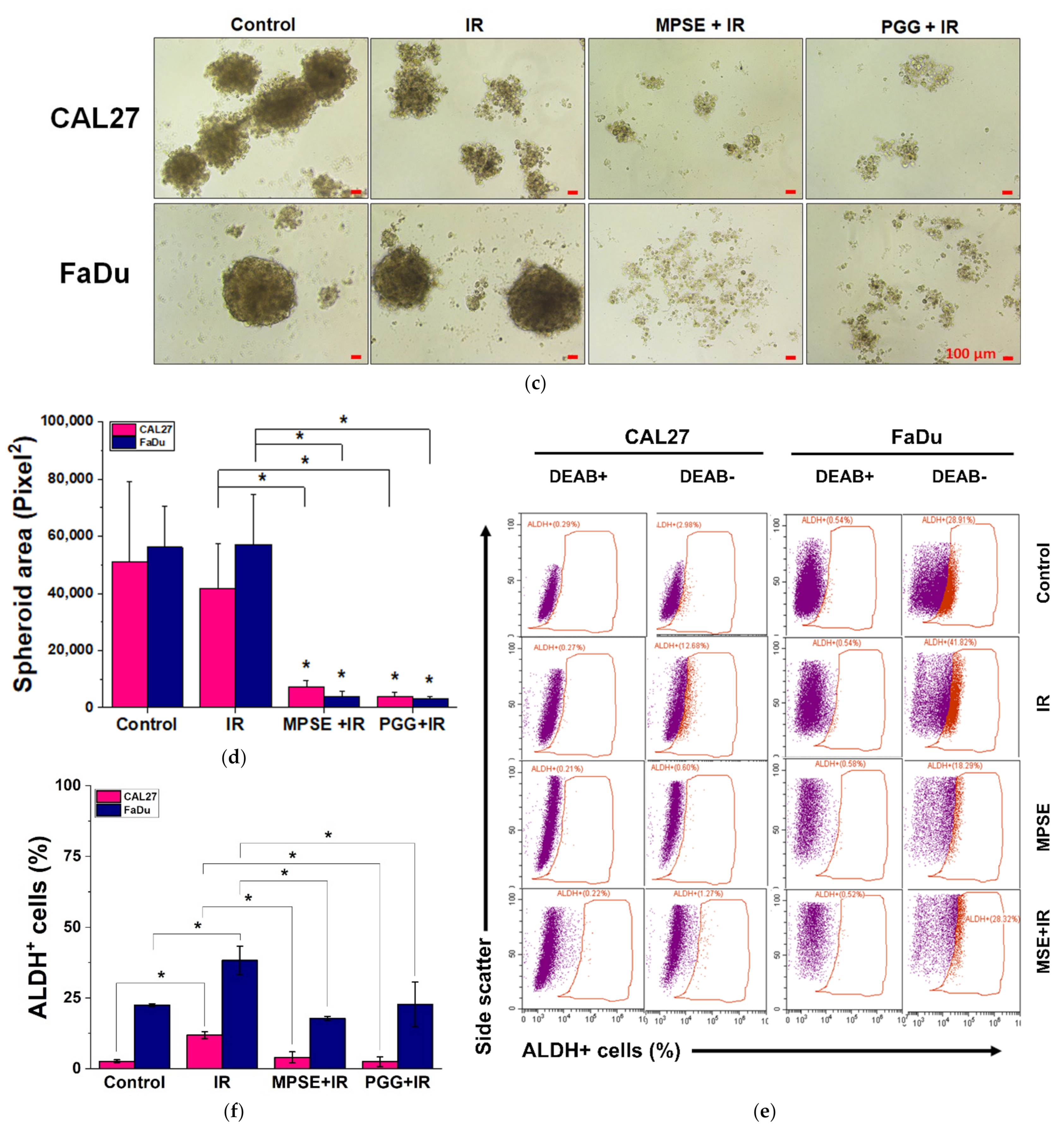
Recent evidence indicates that IR may induce the generation of a new CSCs population during a radiotherapy course [
29]. As previously mentioned, a critical event that determines the cancer radioresistance is CSCs [
3]. Suppression of IR-induced CSCs could enhance the efficacy of radiation therapy in human cancer cells. Our studies demonstrate that MPSE or PGG effectively suppress the expression of CSCs in HNSCC cells. We further analyzed the effect of IR on CSCs. As expected, we found that IR increased the number of CSCs in both FaDu and CAL27 cells. It worth nothing that pretreatment with MPSE or PGG significantly reduced the proportion of CSCs in comparison to either IR alone or untreated control (
Figure 6). These results suggest that pretreatment of MPSE or PGG before IR might eradicate both CSC and non-CSC populations, and this could prevent tumor recurrence.
4. Materials and Methods
4.1. Chemicals and Reagents
Eagle’s Minimum Essential Medium (EMEM) and Dulbecco’s Modified Eagle’s Medium/Nutrient Mixture F-12 (DMEM/F-12) were purchased from Caisson Lab (Smithfield, UT, USA). Trypsin-EDTA, fetal bovine serum, penicillin, and streptomycin were purchased from Gibco Thermo Fisher Scientific (Waltham, MA, USA). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), bovine serum albumin (BSA), human insulin, epidermal growth factor, basic fibroblast growth factor, hydrocortisone, penta-O-galloyl-β-D-glucose hydrate (PGG), ethyl gallate (EG), and gallic acid (GA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The annexin V-FITC/PI apoptosis detection kit and AldeRed ALDH detection assay were also purchased from Sigma-Aldrich (St. Louis, MO, USA). PMSF and cocktail protease inhibitors were purchased from Hi Media Laboratories (Marg, Mumbai, India). Additionally, 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI) was obtained from Thermo Fisher Scientific (Waltham, MA, USA). Primary antibodies against total Akt, phosphorylated Akt (Ser473), total ERK1/2, phosphorylated ERK1/2(Thr202/Tyr204, Thr185/Tyr187)) total STAT3, phosphorylated STAT3 (Tyr705), cleaved caspase 3, Bcl2, cleaved PARP, CD44 and GAPDH, as well as horseradish-peroxidase-labeled secondary antibodies, were purchased from Merck (Merck KGaA, Darmstadt, Germany). Anti Oct4 monoclonal antibody was obtained from Invitrogen (Waltham, MA, USA). Anti Sox2 antibody was from Thermo Fisher Scientific (Waltham, MA, USA). Primary rabbit polyclonal antibodies against γH2AX and Alexa Fluor 488 conjugated rabbit anti-human IgG antibodies were from Thermo Fisher Scientific (Waltham, MA, USA).
4.2. MPSE Preparation, Isolation of PGG, and High-Performance Liquid Chromatography (HPLC) Quantification of Major Active Ingredients
Bouea macrophylla Griffith (Maprang) seeds were collected with permission from private landowners from the Maprang Plantation located in Nakhon Nayok Province. The plant was authenticated by Dr. Tanawat Chaowasku, and the voucher specimens (CMUB39942) of these plants have been deposited at the CMUB herbarium at Chiang Mai University, Thailand. Dried seed kernel was prepared as described by previous work [
15,
16] with a modification. In brief, 50 g of the minced dried seed was immersed in 500 mL of 75% ethanol to macerate them for 7 days with daily shaking. Then, the extracts were filtered, and ethanol was evaporated in a rotatory evaporator system (Buchi Rotavapor R-100, BÜCHI Labortechnik AG, Flawil, Switzerland). After lyophilization, a total of 20 g of extract powder (MPSE) was obtained (40% yield). MPSE was collected and stored at room temperature in a desiccator for further study. To prepare the stock solution, MPSE were solubilized with deionized water (Pure Lap Option-Q, ELGA LabWater, UK) at a final concentration of 1 mg/mL. The solution was then filtered with a 0.22 µm syringe filter and the extract active gradients, penta-
O-galloyl-β-
D-glucose hydrate (PGG), ethyl gallate (EG), and gallic acid (GA) were quantified by using Shimadzu LC-20AD Prominence Liquid Chromatograph system equipped with a SPD-M20A Prominence Diode Array Detector (Shimadzu, Nakagyo-ku, Kyoto, Japan) according to a previously well established and validated method [
15]. To isolate PGG, 1 g/L MPSE in deionized water was prepared and kept at −20 °C in a freezer for 3 h and then cooled to 4 °C by air-exposure at room temperature for 3 h. After that, the cold solution was collected in a 50 mL centrifuge tube and centrifuged at 2500×
g for 20 min (NUVE NF400; Nuve Sanayi Malzemeleri Imalat TAS, Akyurt, Ankara, Turkey). The pellets were washed with 50 mL cold water and centrifuged at 2500×
g for 20 min for 5 cycle times. Next, the PGG pellets were collected and lyophilized to powder (weight = 0.1322 g; yield = 13.22%) and kept in a desiccator at room temperature for further study. PGG purity was 97% determined by HPLC.
4.3. Cell Lines and Cell Culture Conditions
Human head and neck squamous cell carcinoma CAL27 and FaDu cell lines were obtained from American Type Culture Collection (Manassas, VA, USA). CAL27 and FaDu were maintained in Eagle’s Minimum Essential medium (EMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, and 100 U/mL penicillin and streptomycin. Cell cultures were maintained at 37 °C and with a 95% air humidified incubator with 5% CO2.
4.4. Cell Proliferation Assay
Cells were seeded onto 96-well plates at a density of 5 × 103 cells/well and allowed to grow overnight in an incubator. Cells were then treated with various concentrations of MPSE or PGG (0–100 µg/mL) for an appropriate time (24, 48, and 72 h). Cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, in brief, cells were incubated with MTT solution (0.1 mg/mL) for 4 h in a 37 °C incubator and then the formazan product was solubilized using 100 µL of DMSO. The intensity of the formazan solution was determined by measurement of the absorbance at 560 nm using a microplate reader (BioTekTM EonTM microplate reader, Winooski, VT, USA). Relative cell viability was calculated by dividing the absorbance of the treated cells by that of the control cells. The half-maximal inhibition concentration (IC50) was determined from three independent experiments using OriginPro version 2018 software (Origin Lab, MA, USA).
4.5. Irradiation Treatment
Cells were grown in a T75 cm2 cell culture flask and treated with or without either MPSE or PGG for 24 h and then subjected to a single dose of irradiation (IR). IR was performed using a linear 6 MV X-ray accelerator at a dose rate of 200 MU/minute (Primus, Siemens Healthineers, Malvern, PA, USA) at room temperature.
4.6. Clonogenic Assay
Cancer cells were seeded in 6-well plates and allowed to grow overnight, then treated with or without MPSE or PGG (pretreatment) for 24 h and irradiated with various doses of radiation (2–8 Gy). Cells were maintained for 14 days to allow colonies to form. After that, colonies were fixed in fixation solution (3:1 of methanol/acetic acid) for 30 min at room temperature and stained with 1% crystal violet. Cell colonies with more than 50 cells were counted using an inverted microscope (ECLIPSE Ts2, Nikon, Tokyo, Japan). Plating efficiency was calculated by dividing the average number of colonies per well by the number of cells plated. Survival fractions were calculated by normalization to the plating efficiency of appropriate control groups. These data were used for survival curve plotting and fitting as a function of X-ray dosage using a linear-quadratic model via OriginPro version 2018 software (Origin Lab, MA, USA).
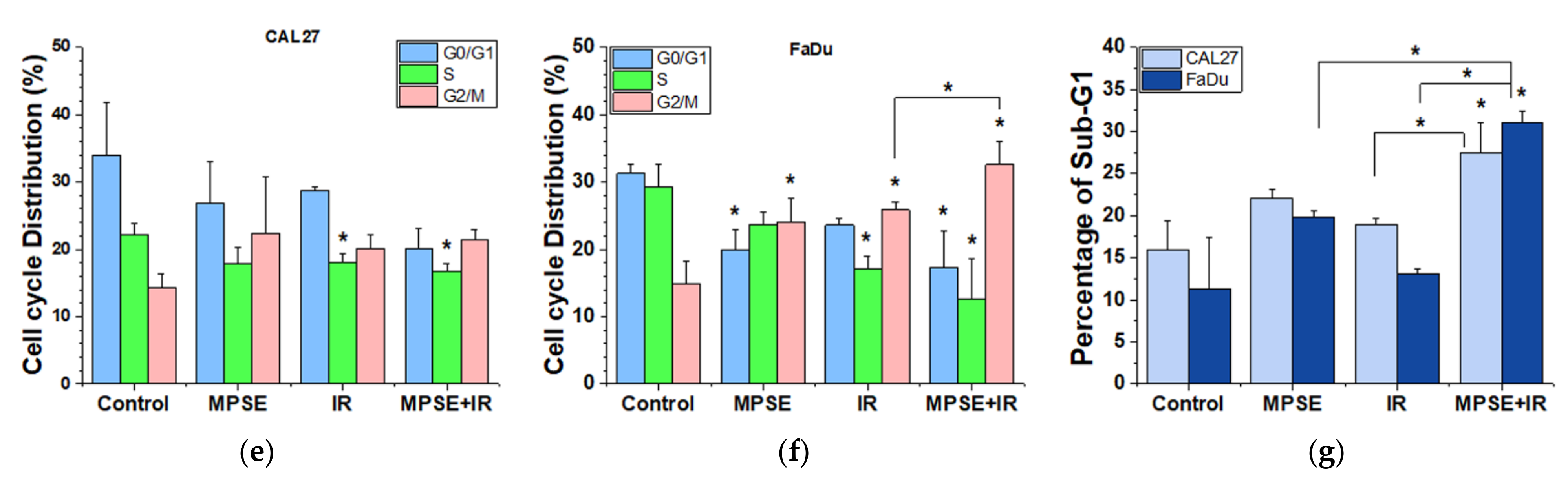
4.7. Cell Cycle Analysis
Changes in cell cycle distribution induced by either IR or MPSE (15 µg/mL) combined with IR were analyzed using propidium iodide (PI) staining. In brief, cells were seeded into 6-well plates and treated as indicated then cells were incubated for 24 h. After completion of the incubation phase, both the suspension cells and the adherent cells were harvested, washed with PBS, and centrifuged at 7000 rpm for 1 min. The supernatant was discarded, and the pellets were collected and then fixed with 70% ethanol overnight at 4 °C. Subsequently, the fixed cells were washed with ice-cold PBS before being stained with the binding buffer containing 0.1% Triton X-100, 0.2 mg/mL of RNase, and 10 µg/mL of PI. Stained cells were then incubated at 37 °C for 30 min in the dark. Finally, the stained cells were analyzed by flow cytometry (Epics XL-MCL, Beckman Coulter, Brea, CA, USA). Flow cytometric data were then analyzed by FlowJo™ software version 10 (Becton, Dickinson & Company, NJ, USA;
https://www.flowjo.com/solutions/flowjo/ accessed on 16 January 2021).
4.8. Spheroid Formation Assay
Cells were treated as indicated and then were dissociated into single cells with 0.5 % trypsin-EDTA. Cells 5 × 10
3 cells/well were seeded onto a 24-well ultralow attachment plate in serum-free DMEM/F-12 (1:1 ratio) media supplemented with 1% BSA, 5 µg/mL insulin, 25 ng/mL basic fibroblast growth factor, 25 ng/mL epidermal growth factor, and 0.5 µg/mL hydrocortisone. Cells were allowed to form tumorspheres for 10 days. Spheroid formation was observed and imaged using a phase-contrast microscope (ECLIPSE Ts2, Nikon, Tokyo, Japan). The area and number of tumorspheres larger than 50 µm in diameter were quantified using ImageJ 1.52v software (
https://imagej.nih.gov/ij/download/ accessed on 10 March 2021).
4.9. Detection of Cancer Stem Cell Marker via AldeRed ALDH Assay
Cells were seeded into a 6-well plate for 24 h to allow attachment before being treated with either MPSE or PGG, IR alone, or MPSE or PGG combined with 6 Gy IR X-ray. Cancer stem cell markers were determined by AldeRed ALDH detection assay following the manufacturer’s protocol with only a few modifications. In brief, 5 × 105 cells were resuspended in 1 mL AldeRed assay buffer. After the addition of 5 µL AldeRed reagent and mild mixing, 500 µL of the cell suspension was immediately transferred to another tube supplemented with 5 µL DEAB and pipetted to mix evenly. Then, both test and control tubes were incubated in a 37 °C incubator for 30 min to allow the reaction to occur. After 30 min, cells were washed twice with 2 mL AldeRed assay buffer and eventually resuspended in 500 µL AldeRed assay buffer before being analyzed by flow cytometry (CytoFLEX, Beckman Coulter, Brea, CA, USA).
4.10. Apoptosis Assay
Cells were seeded into a 6-well plate for 24 h to allow attachment before being treated with varying concentrations of either MPSE or PGG. Cells were harvested by trypsinization at 48 h after treatment. The harvested cells were stained using annexin V-FITC/PI apoptosis detection kit following the manufacturer’s instructions. Briefly, the harvested cells were washed by 1× binding buffer, then centrifuged at 7000 rpm for one minute and the supernatant was removed. The pellets were stained by adding 1× binding buffer, annexin V-FITC, and propidium iodide (PI) for 20 min at room temperature in total darkness. The stained cells were immediately analyzed by flow cytometry (CytoFLEX, Beckman Coulter, Brea, CA, USA). Flow cytometric data were then analyzed by FlowJo™ software version 10 (Becton, Dickinson & Company, NJ, USA;
https://www.flowjo.com/solutions/flowjo/ accessed on 16 January 2021).
4.11. Morphological Detection of Apoptosis by DAPI Staining
Morphological characteristics of apoptotic cells were determined using 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI) staining. In brief, 2 × 105 cells were seeded in 6-well plates and cultured for 24 h to allow cell attachment to occur. Cells were treated with either IR alone, or MPSE or PGG combined with 6 Gy IR X-ray for 48 h. Following treatment, the cells were washed with PBS, and then fixed with 4% formaldehyde in PBS for 15 min at room temperature and then washed twice with PBS. Cells were permeabilized with permeabilization buffer containing 1% BSA, 0.2% Triton-X100 in PBS for 1 h before being incubated with 300 nM DAPI stain solution for 5 min. Cells were protected from light throughout the procedure. The cells were washed twice with PBS and photographed with a fluorescence microscope (ECLIPSE Ts2, Nikon, Tokyo, Japan).
4.12. γH2AX Immunostaining Assay
Cells were plated in chamber slides and pretreated with MPSE or PGG for 24 h before irradiation with 6 Gy X-ray. At 48 h post-irradiation, the cells were fixed with 4% paraformaldehyde for 15 min at room temperature. Then, cells were permeabilized with 0.3% TritonX-100 and blocked with 5% BSA in PBS solution at room temperature for 30 min. The cells were then incubated overnight at 4 °C with primary rabbit polyclonal antibodies against γH2AX (1:500). After primary antibody incubation, the cells were washed with PBS with Tween 20 (PBST) and incubated with 1:200 diluted Alexa Fluor 488 conjugated rabbit anti-human IgG antibodies (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) for 1 h at room temperature. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI, 1 µg/mL). Pictures were captured with an inverted fluorescence microscope (ECLIPSE Ts2, Nikon, Tokyo, Japan). γH2AX intensity was quantified using an in-house software code developed in the MATLAB (MathWorks, MA, USA) programming kindly generated by Dr. Kittichai Wantanajittikul.
4.13. Immunostaining of Cancer Stem Cells Markers (CD44)
After treatment, cells were allowed to form tumor spheroids as described above, and then tumorspheres were fixed with 4% paraformaldehyde for 30 min and permeabilized with 0.1% Triton X-100 for 20 min. The tumorspheres were then blocked by incubating with 3% bovine serum albumin for 30 min and further washed twice with phosphate-buffered saline (PBS) and incubated with antiCD44-FITC-conjugated dyes for 1 h, at 4°C in the dark. The cells were washed with phosphate-buffered saline and co-stained with 4′,6-diamidino-2-phenylindole (DAPI) dye and were then visualized and imaged by fluorescence microscopy (ECLIPSE Ts2, Nikon, Tokyo, Japan).
4.14. Western Blot Analysis
Following treatment, cells or spheroids were lysed for protein extraction by CelLytic M lysis buffer (Sigma-Aldrich, St. Louis, MO, USA) in the presence of 1% protease inhibitor cocktail for 30 min on ice. The protein contents were evaluated by Bradford assay (Sigma-Aldrich). Denatured protein samples with equal amounts of protein (15 µg) were resolved by either 10% SDS-PAGE or NUPAGETM 4–12% Bis-Tris Gels (Thermo Fisher Scientific, Waltham, MA, USA) and then the resolved proteins were transferred into PVDF membrane (Merck KGaA, Darmstadt, Germany). Further, the whole transferred membranes were cut at the appropriate molecular weight range of protein interested and blocked for 1 h in 5% non-fat dry milk in Tris-buffered saline with Tween and incubated overnight with specific primary antibodies against total AKT, phosphorylated AKT, total ERK1/2, phosphorylated ERK1/2, total STAT3, phosphorylated STAT3, SOX2, Oct4, CD44, γH2AX, as well as GAPDH. Then, membranes were washed, followed by incubation with appropriate horseradish-peroxidase-labeled secondary antibodies (at dilution 1:10,000) for 1 h at room temperature. Signals were developed using the enhanced chemiluminescence assay (Millipore Corporation) and exposed to film. The images were scanned, and the intensity of each band was captured using an Image master 2D platinum version 5.0 (GE Healthcare Amersham Bioscience, Chicago, IL, USA). The protein content was normalized to GAPDH.
4.15. Statistical Analysis
Data are expressed as mean ± standard deviations (SDs) of at least three independent experiments. The differences in the mean values were determined by one-way ANOVA followed by Tukey’s HSD test or Student’s t-test using IBM® SPSS® Statistics Subscription software (IBM Corp. in Armonk, NY, USA). A value of p < 0.05 was considered statistically significant.