Genetic Architecture and Genome-Wide Adaptive Signatures Underlying Stem Lenticel Traits in Populus tomentosa
Abstract
1. Introduction
2. Results
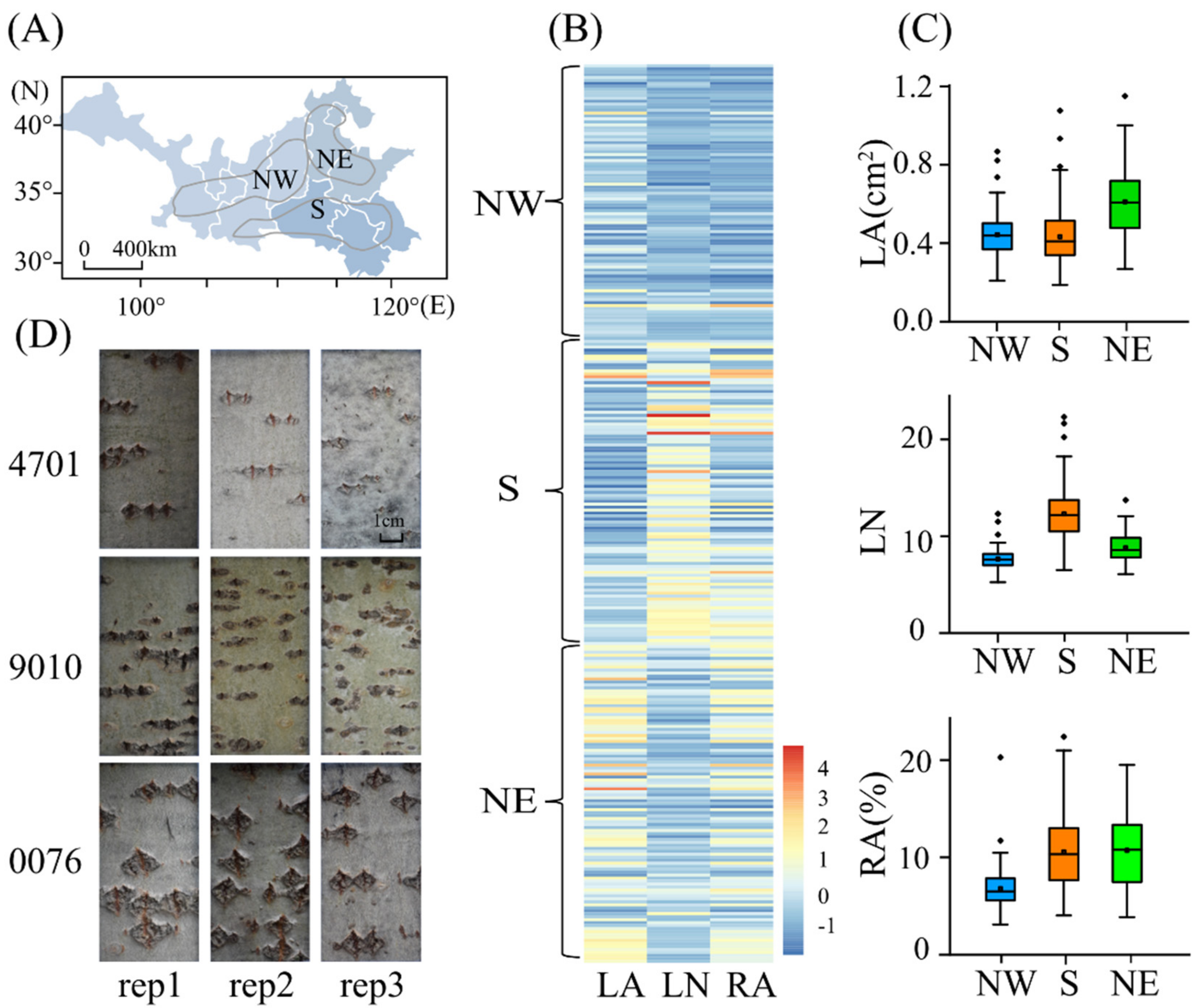
2.1. Phenotypic Variation in the Lenticel Traits of the Natural P. tomentosa Population
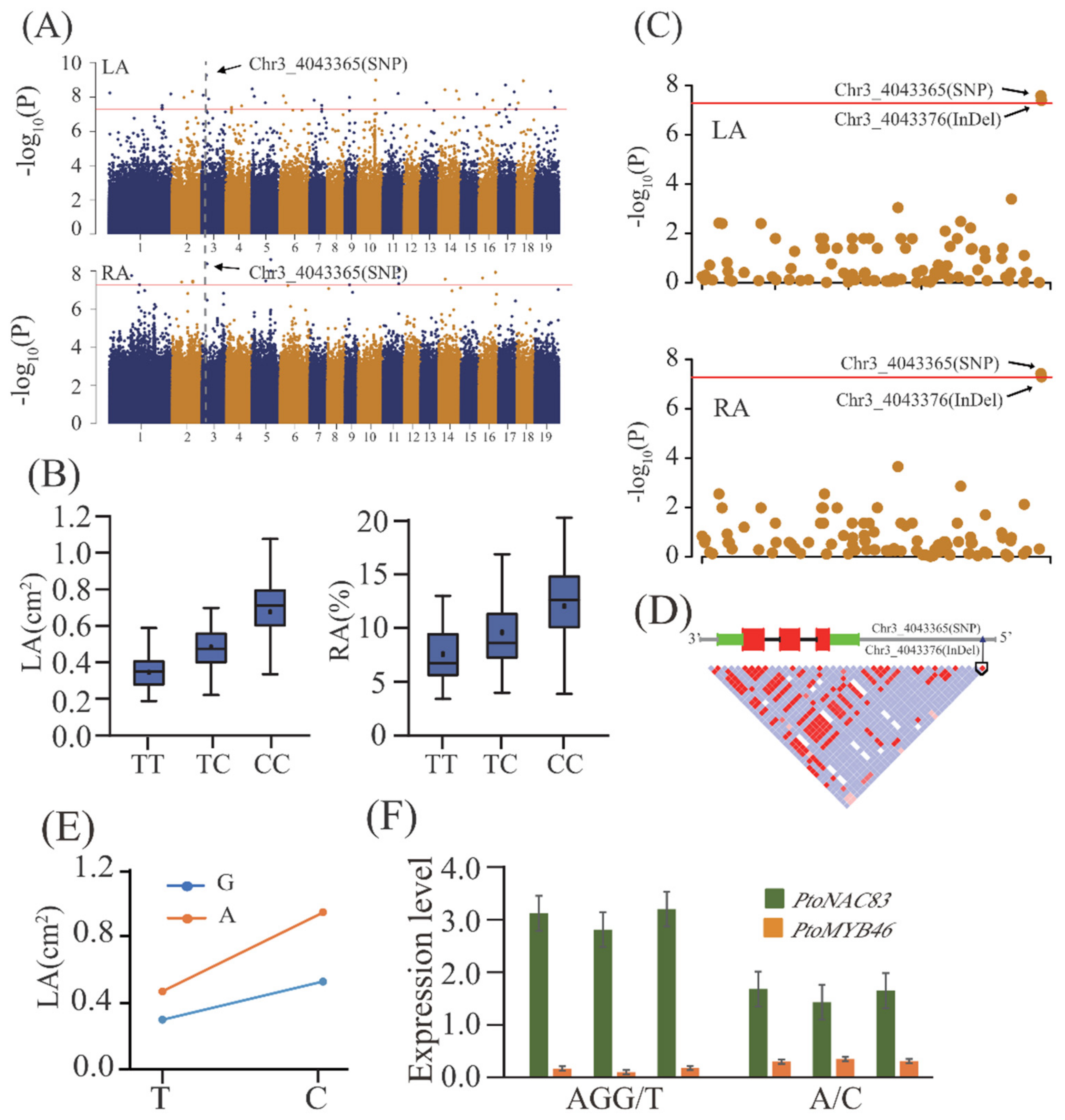
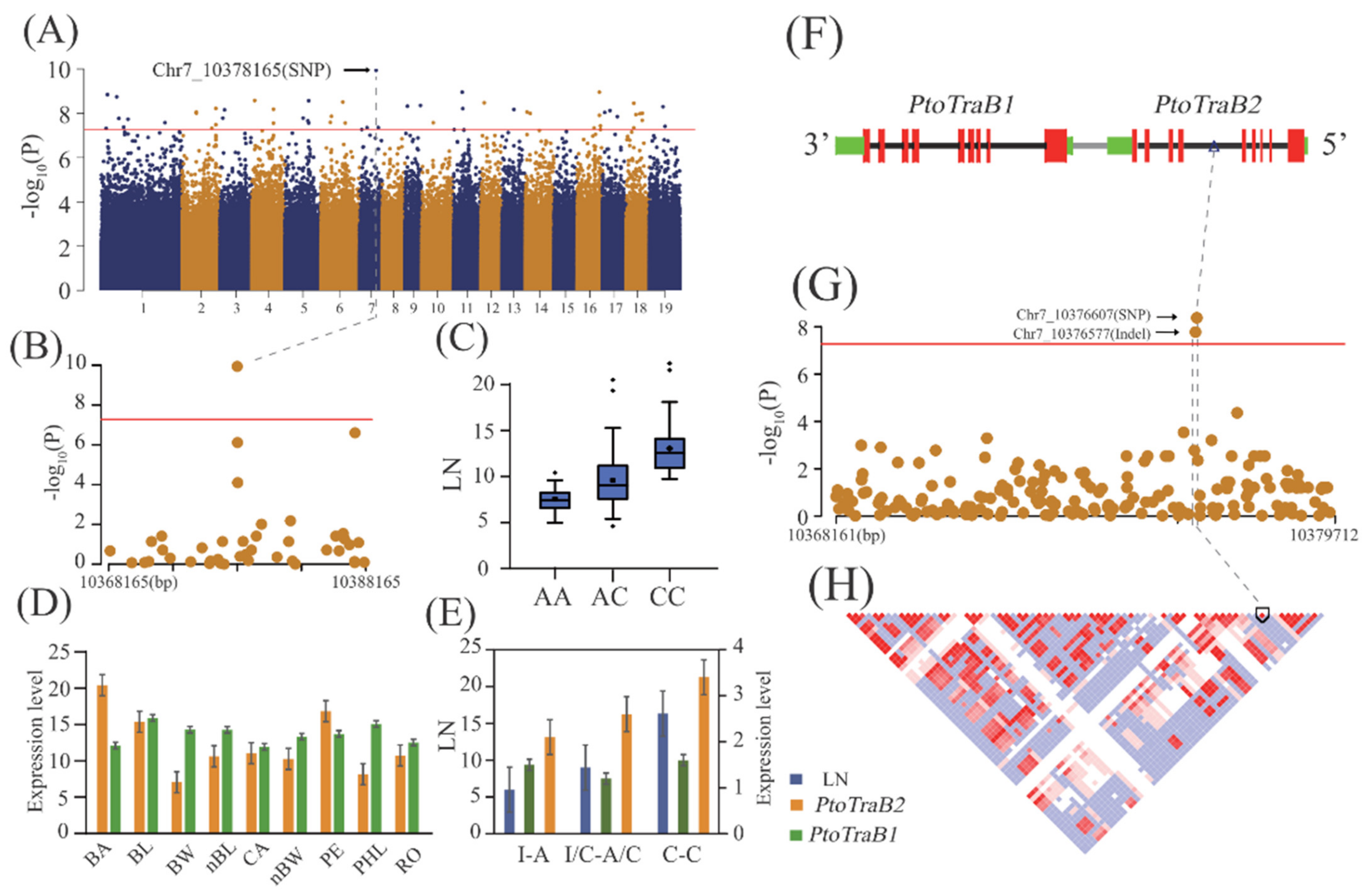
2.2. Additive and Dominant Effects of Natural Variants Underlying Lenticel Traits Determined by GWAS
2.3. Pairwise Epistatic Effects of Natural Variants Underlying Lenticel Traits
2.4. Differences in Allele Frequency of Associated Genes among P. tomentosa Subpopulations
2.5. Allelic Interpretation of the Potential Causal Genes Underlying Lenticel Variation
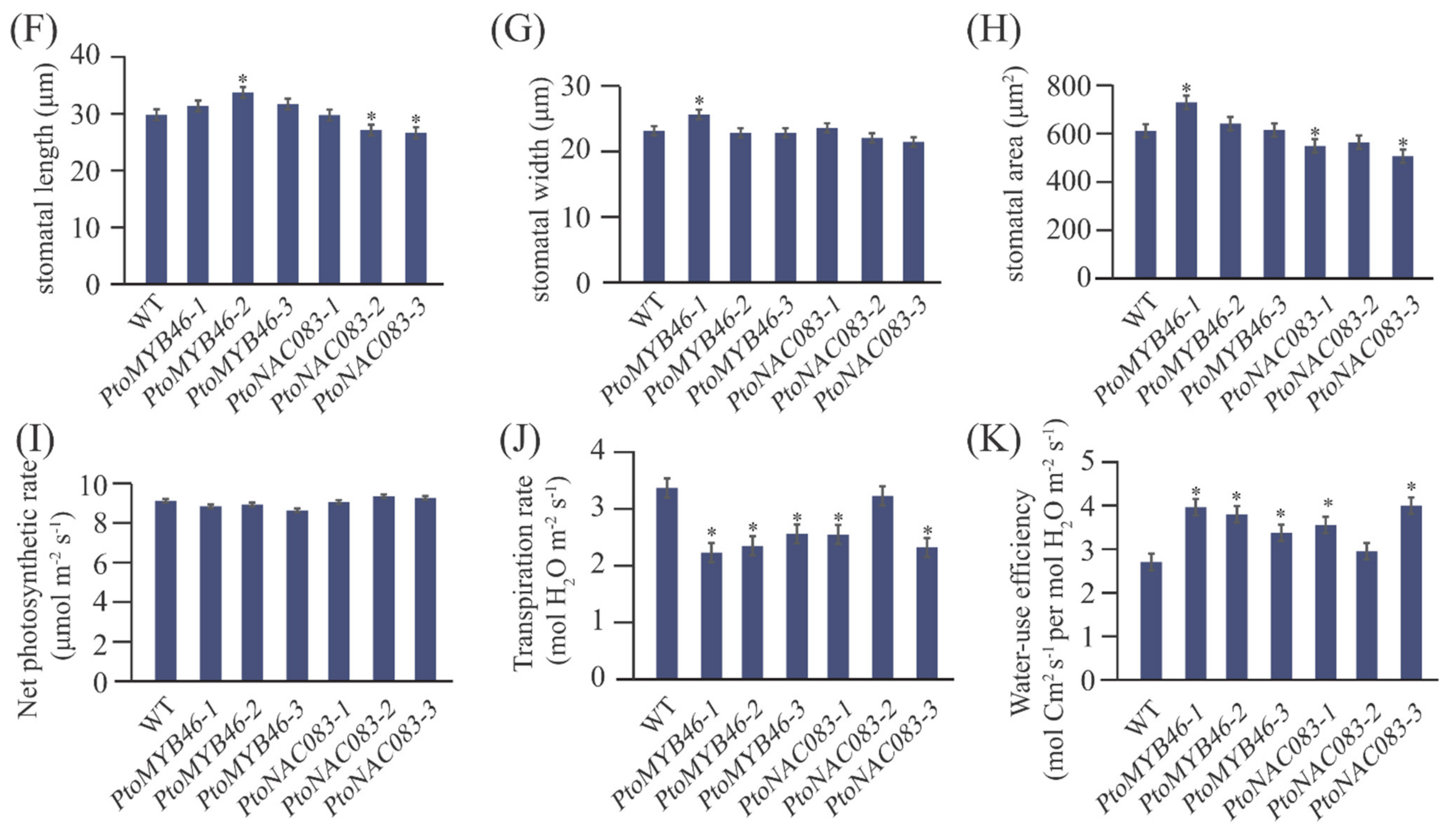
2.6. A NAC-Type Transcription Factor (PtoNAC83) Is an Important Genetic Regulator Contributing to Lenticel Variation
3. Discussion
3.1. GWAS Permits Comprehensive Understanding of the Genetic Basis of Lenticel in Populus
3.2. Local Adaptation of Candidate Genes with Overlapping Selection Signals and Their Associated Loci
3.3. PtoNAC083 Is a Causal Gene Underlying Lenticel Formation in P. tomentosa
4. Materials and Methods
4.1. Association Population and Phenotyping
4.2. Resequencing and Single Nucleotide Polymorphism (SNP) Calling
4.3. Population Genetics Analysis
4.4. GWAS Test and Candidate Gene Association Analyses
4.5. Gene Ontology Enrichment Analysis
4.6. Epistasis Analysis of Each SNP Pair
4.7. RNA Extraction and RNA Sequencing
4.8. Screening for Selective Sweeps
4.9. Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
4.10. PtoNAC083 and PtoMYB46 Vector Construction and Transformation in A. thaliana
4.11. Paraffin Sectioning and Histological Analysis
4.12. Physiological Measurements
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Savolainen, O.; Lascoux, M.; Merilä, J. Ecological genomics of local adaptation. Nat. Rev. Genet. 2013, 14, 807–820. [Google Scholar] [CrossRef]
- McKown, A.D.; Klápště, J.; Guy, R.; El-Kassaby, Y.; Mansfield, S. Ecological genomics of variation in bud-break phenology and mechanisms of response to climate warming in Populus trichocarpa. New Phytol. 2018, 220, 300–316. [Google Scholar] [CrossRef]
- Chamaillard, S.; Fichot, R.; Vincent-Barbaroux, C.; Bastien, C.; Depierreux, C.; Dreyer, E.; Villar, M.; Brignolas, F. Variations in bulk leaf carbon isotope discrimination.; growth and related leaf traits among three Populus nigra L. populations. Tree Physiol. 2011, 31, 1076–1087. [Google Scholar] [CrossRef]
- Eckert, A.J.; Bower, A.D.; Wegrzyn, J.L.; Pande, B.; Jermstad, K.D.; Krutovsky, K.V.; St Clair, J.B.; Neale, D.B. Association genetics of coastal Douglas fir (Pseudotsuga menziesii var. menziesii. Pinaceae). I. Cold-hardiness related traits. Genetics 2009, 182, 1289–1302. [Google Scholar] [CrossRef]
- Evans, L.M.; Slavov, G.T.; Rodgers-Melnick, E.; Martin, J.; Ranjan, P.; Muchero, W.; Brunner, A.M.; Schackwitz, W.; Gunter, L.; Chen, J.G.; et al. Population genomics of Populus trichocarpa identifies signatures of selection and adaptive trait associations. Nature Genet. 2014, 46, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- González-Martínez, S.; Krutovsky, K.; Neale, D.B. Forest-tree population genomics and adaptive evolution. New Phytol. 2006, 170, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Neale, D.B.; Kremer, A. Forest tree genomics: Growing resources and applications. Nat. Rev. Genet. 2011, 12, 111–122. [Google Scholar] [CrossRef]
- Bdeir, R.; Muchero, W.; Yordanov, Y.; Tuskan, G.A.; Busov, V.; Gailing, O. Genome-wide association studies of bark texture in Populus trichocarpa. Tree Genet. Genomes 2019, 15, 14. [Google Scholar] [CrossRef]
- Drost, D.R.; Puranik, S.; Novaes, E.; Novaes, C.R.; Dervinis, C.; Gailing, O.; Kirst, M. Genetical genomics of Populus leaf shape variation. BMC Plant Biol. 2015, 15, 166. [Google Scholar] [CrossRef] [PubMed]
- Evert, R.F. Esau's Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body: Their Structure Function and Development; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2006; pp. 440–442. [Google Scholar]
- Lendzian, K. Survival strategies of plants during secondary growth: Barrier properties of phellems and lenticels towards water.; oxygen.; and carbon dioxide. J. Exp. Bot. 2006, 57, 2535–2546. [Google Scholar] [CrossRef] [PubMed]
- Manetas, Y.; Pfanz, H. Spatial heterogeneity of light penetration through periderm and lenticels and concomitant patchy acclimation of corticular photosynthesis. Trees 2005, 19, 409–414. [Google Scholar] [CrossRef]
- Schönherr, J.; Ziegler, H. Water permeability of Betula periderm. Planta 1980, 147, 345–354. [Google Scholar] [CrossRef]
- Nemesio-Gorriz, M.; McGuinness, B.; Grant, J.; Dowd, L.; Douglas, G.C. Lenticel infection in Fraxinus excelsior shoots in the context of ash dieback. iForest 2019, 12, 160–165. [Google Scholar] [CrossRef]
- Laue, B.; Steele, H.; Green, S. Survival, cold tolerance and seasonality of infection of European horse chestnut (Aesculus hippocastanum) by Pseudomonas syringae pv. aesculi. Plant Pathol. 2014, 63, 1417–1425. [Google Scholar] [CrossRef]
- Rosner, S.; Führer, E. The significance of lenticels for successful Pityogenes chalcographus (Coleoptera: Scolytidae) invasion of Norway spruce trees [Picea abies (Pinaceae)]. Trees 2002, 16, 497–503. [Google Scholar] [CrossRef]
- Alonso-Serra, J.; Safronov, O.; Lim, K.J.; Fraser-Miller, S.J.; Blokhina, O.B.; Campilho, A.; Chong, S.L.; Fagerstedt, K.; Haavikko, R.; Helariutta, Y.; et al. Tissue-specific study across the stem reveals the chemistry and transcriptome dynamics of birch bark. New Phytol. 2019, 222, 1816–1831. [Google Scholar] [CrossRef] [PubMed]
- Miguel, A.; Milhinhos, A.; Novák, O.; Jones, B.; Miguel, C.M. The SHORT-ROOT-like gene PtSHR2B is involved in Populus phellogen activity. J. Exp. Bot. 2016, 67, 1545–1555. [Google Scholar] [CrossRef]
- Du, Q.; Lu, W.; Quan, M.; Xiao, L.; Song, F.; Li, P.; Zhou, D.; Xie, J.; Wang, L.; Zhang, D. Genome-wide association studies to improve wood properties: Challenges and prospects. Front. Plant Sci. 2018, 9, 1912. [Google Scholar] [CrossRef] [PubMed]
- Porth, I.; Klapšte, J.; Skyba, O.; Hannemann, J.; McKown, A.D.; Guy, R.D.; DiFazio, S.P.; Muchero, W.; Ranjan, P.; Tuskan, G.A.; et al. Genome-wide association mapping for wood characteristics in Populus identifies an array of candidate single nucleotide polymorphisms. New Phytol. 2013, 200, 710–726. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, S.; Huang, Z.; Zhang, S.; Liao, Q.; Zhang, C.; Lin, T.; Qin, M.; Peng, M.; Yang, C.; et al. Rewiring of the Fruit Metabolome in Tomato Breeding. Cell 2018, 172, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Yang, X.; Xie, J.; Quan, M.; Xiao, L.; Lu, W.; Tian, J.; Gong, C.; Chen, J.; Li, B.; et al. Time-specific and pleiotropic quantitative trait loci coordinately modulate stem growth in Populus. Plant Biotechnol. J. 2019, 17, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Li, D.; Jiao, Y.Q.; Schnable, J.C.; Li, Y.F.; Li, H.H.; Chen, H.Z.; Hong, H.L.; Zhang, T.; Liu, B.; et al. Identification of loci controlling adaptation in Chinese soya bean landraces via a combination of conventional and bioclimatic GWAS. Plant Biotechnol. J. 2019, 18, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Josephs, E.; Stinchcombe, J.; Wright, S. What can genome-wide association studies tell us about the evolutionary forces maintaining genetic variation for quantitative traits? New Phytol. 2017, 214, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Wang, B.; Wei, Z.; Zhang, D.; Li, B. Genetic Diversity and Population Structure of Chinese White Poplar (Populus tomentosa) Revealed by SSR Markers. J. Hered. 2012, 103, 853–862. [Google Scholar] [CrossRef]
- Jackson, M.D.; Duran-Nebreda, S.; Kierzkowski, D.; Strauss, S.; Xu, H.; Landrein, B.; Bassel, G.W. Global topological order emerges through local mechanical control of cell divisions in the Arabidopsis shoot apical meristem. Cell Syst. 2019, 8, 53–65. [Google Scholar] [CrossRef]
- Hu, X.G.; Jin, Y.; Wang, X.R.; Mao, J.F.; Li, Y. Predicting impacts of future climate change on the distribution of the widespread conifer Platycladus orientalis. PLoS ONE 2015, 10, e0132326. [Google Scholar] [CrossRef]
- Lu, W.; Du, Q.; Xiao, L.; Lv, C.; Quan, M.Y.; Li, P.; Yao, L.C.; Song, F.Y.; Zhang, D. Multi-omics analysis provides insights into genetic architecture of flavonoid metabolites in Populus. Ind. Crops Prod. 2021, 168, 113612. [Google Scholar] [CrossRef]
- Pangala, S.; Gowing, D.; Hornibrook, E.; Gauci, V. Controls on methane emissions from Alnus glutinosa saplings. New Phytol. 2014, 201, 887–896. [Google Scholar] [CrossRef]
- Groh, B.; Hübner, C.; Lendzian, K. Water and oxygen permeance of phellems isolated from trees: The role of waxes and lenticels. Planta 2002, 215, 794–801. [Google Scholar] [CrossRef]
- Fahrenkrog, A.M.; Neves, L.G.; Resende, M.F.; Vazquez, A.I.; de Los Campos, G.; Dervinis, C.; Sykes, R.; Davis, M.; Davenport, R.; Barbazuk, W.B.; et al. Genome-wide association study reveals putative regulators of bioenergy traits in Populus deltoides. New Phytol. 2017, 213, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Peng, Z.; Yang, X.; Wang, W.; Fu, J.; Wang, J.; Han, Y.; Chai, Y.; Guo, T.; Yang, N.; et al. Genome-wide association study dissects the genetic architecture of oil biosynthesis in maize kernels. Nat. Genet. 2013, 45, 43–50. [Google Scholar] [CrossRef]
- Müller, B.S.F.; Almeida, J.E.; Lima, B.M.; Garcia, C.C.; Missiaggia, A.; Aguiar, A.M.; Takahashi, E.; Kirst, M.; Gezan, S.A.; Silva-Junior, O.B. Independent and Joint-GWAS for growth traits in Eucalyptus by assembling genome-wide data for 3373 individuals across four breeding populations. New Phytol. 2019, 221, 818–833. [Google Scholar] [CrossRef]
- Plomion, C.; Leprovost, G.; Stokes, A. Wood Formation in Trees. Plant Physiol. 2001, 127, 1513–1523. [Google Scholar] [CrossRef]
- Hou, J.; Wu, Q.; Zuo, T.; Guo, L.; Chang, J.; Chen, J.; Wang, Y.; He, W. Genome-wide transcriptomic profiles reveal multiple regulatory responses of poplar to Lonsdalea quercina infection. Trees 2016, 30, 1389–1402. [Google Scholar] [CrossRef]
- Islam, N.; Li, G.; Garrett, W.M.; Lin, R.; Sriram, G.; Cooper, B.; Coleman, G.D. Proteomics of Nitrogen Remobilization in Poplar Bark. J. Proteome Res. 2014, 14, 1112–1126. [Google Scholar] [CrossRef]
- Piotrowski, M.; Schönfelder, S.; Weiler, E. The Arabidopsis thaliana Isogene NIT4 and Its Orthologs in Tobacco Encode β-Cyano-l-alanine Hydratase/Nitrilase. J. Biol. Chem. 2001, 276, 2616–2621. [Google Scholar] [CrossRef] [PubMed]
- Phillips, P. Epistasis—The essential role of gene interactions in the structure and evolution of genetic systems. Nat. Rev. Genet. 2008, 9, 855–867. [Google Scholar]
- Yamaguchi, M.; Ohtani, M.; Mitsuda, N.; Kubo, M.; Ohme-Takagi, M.; Fukuda, H.; Demura, T. VND-INTERACTING2, a NAC Domain Transcription Factor.; Negatively Regulates Xylem Vessel Formation in Arabidopsis. Plant Cell 2010, 22, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T. Why epistasis is important for selection and adaptation. Evolution 2013, 67, 3501–3511. [Google Scholar] [PubMed]
- Mackay, T. Epistasis and quantitative traits: Using model organisms to study gene–gene interactions. Nat. Rev. Genet. 2014, 15, 22–33. [Google Scholar] [CrossRef]
- McKown, A.D.; Klápště, J.; Guy, R.D.; Geraldes, A.; Porth, I.; Hannemann, J.; Friedmann, M.; Muchero, W.; Tuskan, G.A.; Ehlting, J. Genome-wide association implicates numerous genes underlying ecological trait variation in natural populations of Populus trichocarpa. New Phytol. 2014, 203, 535–553. [Google Scholar] [CrossRef]
- Ma, L.; Runesha, H.B.; Dvorkin, D.; Garbe, J.R.; Da, Y. Parallel and serial computing tools for testing single-locus and epistatic SNP effects of quantitative traits in genome-wide association studies. BMC Bioinform. 2008, 9, 315. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, B. Flooding responses and water-use efficiency of subtropical and tropical fruit trees in an environmentally-sensitive wetland. Ann. Bot. 1998, 81, 475–481. [Google Scholar] [CrossRef]
- Huang, Z. The study on the climatic regionalization of the distributional region of Populus tomentosa. J. Beijing For. Univ. 1992, 14, 26–32. [Google Scholar]
- Steppe, K.; Saveyn, A.; McGuire, M.A.; Lemeur, R.; Teskey, R.O. Resistance to radial CO2 diffusion contributes to between-tree variation in CO2 efflux of Populus deltoides stems. Funct. Plant Biol. 2007, 34, 785–792. [Google Scholar] [CrossRef]
- Cao, F.L.; Conner, W.H. Selection of flood-tolerant Populus deltoides clones for reforestation projects in China. For. Ecol. Manag. 1999, 117, 211–220. [Google Scholar] [CrossRef]
- Collevatti, R.G.; Novaes, E.; Silva-Junior, O.B.; Vieira, L.D.; Lima-Ribeiro, M.S.; Grattapaglia, D.A. genome-wide scan shows evidence for local adaptation in a widespread keystone Neotropical forest tree. Heredity 2019, 123, 117–137. [Google Scholar] [CrossRef]
- Kunz, S.; Pesquet, E.; Kleczkowski, L.A. Functional dissection of sugar signals affecting gene expression in Arabidopsis thaliana. PLoS ONE 2014, 9, e100312. [Google Scholar] [CrossRef]
- Cornelius, S.; Witz, S.; Rolletschek, H.; Möhlmann, T. Pyrimidine degradation influences germination seedling growth and production of Arabidopsis seeds. J. Exp. Bot. 2011, 62, 5623–5632. [Google Scholar] [CrossRef]
- John, R.; Raja, V.; Ahmad, M.; Jan, N.; Majeed, U.; Ahmad, S.; Yaqoob, U.; Kaul, T. Trehalose: Metabolism and role in stress signaling in plants. In Stress Signaling in Plants: Genomics and Proteomics Perspective; Sarwat, M., Ahmad, A., Abdin, M.Z., Ibrahim, M.M., Eds.; Springer: New York, NY, USA, 2017; Volume 3, pp. 261–275. [Google Scholar]
- Rains, M.; Gardiyehewa, D.; Silva, N.; Molina, I. Reconstructing the suberin pathway in poplar by chemical and transcriptomic analysis of bark tissues. Tree Physiol. 2018, 38, 340–361. [Google Scholar] [CrossRef]
- Johnsson, C.; Jin, X.; Xue, W.; Dubreuil, C.; Lezhneva, L.; Fischer, U. The plant hormone auxin directs timing of xylem development by inhibition of secondary cell wall deposition through repression of secondary wall NAC-domain transcription factors. Physiol. Plant. 2018, 165, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.J.; Chen, H.; Li, Q.; Li, W.; Wang, J.P.; Shi, R.; Tunlaya-Anukit, S.; Shuai, P.; Wang, Z.; Ma, H. Reciprocal cross-regulation of VND and SND multigene TF families for wood formation in Populus trichocarpa. Proc. Natl. Acad. Sci. USA 2017, 114, E9722–E9729. [Google Scholar] [CrossRef] [PubMed]
- Shuai, P.; Su, Y.; Liang, D.; Zhang, Z.; Xia, X.; Yin, W. Identification of phasiRNAs and their drought-responsiveness in Populus trichocarpa. FEBS Lett. 2016, 590, 3616–3627. [Google Scholar] [CrossRef] [PubMed]
- Seyfferth, C.; Wessels, B.; Jokipii-Lukkari, S.; Sundberg, B.; Delhomme, N.; Felten, J.; Tuominen, H. Ethylene-Related Gene Expression Networks in Wood Formation. Front. Plant Sci. 2018, 9, 272. [Google Scholar] [CrossRef]
- Zhong, R.; Richardson, E.; Ye, Z. The MYB46 Transcription factor is a direct target of SND1 and regulates secondary wall biosynthesis in Arabidopsis. Plant Cell 2007, 19, 2776–2792. [Google Scholar] [CrossRef] [PubMed]
- Seo, P.J.; Park, C.M. Signaling linkage between environmental stress resistance and leaf senescence in Arabidopsis. Plant Signal. Behav. 2011, 6, 1564–1566. [Google Scholar] [CrossRef]
- Yin, H.; Li, M.; Li, D.; Khan, S.A.; Hepworth, S.R.; Wang, S.M. Transcriptome analysis reveals regulatory framework for salt and osmotic tolerance in a succulent xerophyte. BMC Plant Biol. 2019, 19, 88. [Google Scholar] [CrossRef]
- Chen, K.; Song, M.; Guo, Y.; Liu, L.; Xue, H.; Dai, H.; Zhang, Z. MdMYB46 could enhance salt and osmotic stress tolerance in apple by directly activating stress-responsive signals. Plant Biotechnol. J. 2019, 17, 2341–2355. [Google Scholar] [CrossRef]
- Du, Q.; Tian, J.; Yang, X.; Pan, W.; Xu, B.; Li, B.; Ingvarsson, P.K.; Zhang, D. Identification of additive.; dominant.; and epistatic variation conferred by key genes in cellulose biosynthesis pathway in Populus tomentosa. DNA Res. 2015, 22, 53–67. [Google Scholar] [CrossRef][Green Version]
- Schindelin, J.; Rueden, C.; Hiner, M.; Eliceiri, K. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol. Reprod. Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef]
- Quan, M.; Du, Q.; Xiao, L.; Lu, W.; Wang, L.; Xie, J.; Song, Y.; Xu, B.; Zhang, D. Genetic architecture underlying the lignin biosynthesis pathway involves noncoding RNAs and transcription factors for growth and wood properties in Populus. Plant Biotechnol. J. 2019, 17, 302–315. [Google Scholar] [CrossRef]
- Li, H. Aligning sequence reads.; clone sequences and assembly contigs with BWA-MEM. arXiv 2013, arXiv:1303.3997. [Google Scholar]
- Mckenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, G.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Hardy, O.; Vekemans, X. SPAGeDi: A versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol. Ecol. Notes 2002, 2, 618–620. [Google Scholar] [CrossRef]
- Li, M.; Yeung, J.; Cherny, S.; Sham, P. Evaluating the effective numbers of independent tests and significant p-value thresholds in commercial genotyping arrays and public imputation reference datasets. Hum. Genet. 2012, 131, 747–756. [Google Scholar] [CrossRef]
- Barrett, J.; Fry, B.; Maller, J.; Daly, M. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef]
- Turner, S.D. qqman: An R package for visualizing GWAS results using QQ and manhattan plots. bioRxiv 2014. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, B.; Yang, X.; Zhang, Z.; Li, B. The sucrose synthase gene family in Populus: Structure, expression, and evolution. Tree Genet. Genomes 2011, 7, 443–456. [Google Scholar] [CrossRef]


| Phenotype | SNP | Alleles | SNP p-Value | Gene_Id | Regions of Selective Sweep | π_nw/π_s | Fst | Description |
|---|---|---|---|---|---|---|---|---|
| LA | Chr1_738204 | T/C | 5.76134 × 10−9 | Ptom.001G.00083 | Chr1: 725001–745000 | 6.958347576 | 0.40446225 | |
| Ptom.001G.00082 | Chr1: 725001–745000 | 6.958347576 | 0.40446225 | myb domain protein 5 | ||||
| LA | Chr3_5725284 | G/T | 1.28839 × 10−8 | Ptom.003G.00730 | Chr3: 5725001–5730000 | 2.835296259 | 0.662955 | trehalase 1 |
| LA | Chr10_13623192 | A/C | 1.49898 × 10−8 | Ptom.010G.01894 | Chr10: 13625001–13630000 | 2.504975728 | 0.293226 | |
| LN | Chr2_18582313 | C/G | 4.69554 × 10−8 | Ptom.002G.02263 | Chr2: 18580001–18600000 | 3.568314954 | 0.36376425 | NPL4-like protein 1 |
| LN | Chr6_6559040 | A/T | 1.37452 × 10−8 | Ptom.006G.00835 | Chr6: 6535001–6540000 | 3.304231993 | 0.384925 | Pentatricopeptide repeat (PPR) superfamily protein |
| Ptom.006G.00836 | Chr6: 6555001–6560000 | 3.304231993 | 0.384925 | uracil phosphoribosyltransferase | ||||
| LN | Chr10_7228249 | A/T | 2.67429 × 10−8 | Ptom.010G.01098 | Chr10: 7225001–7230000 | 3.587991883 | 0.253253 | CytoChrome P450 superfamily protein |
| LN | Chr17_4717361 | A/G | 7.59074 × 10−9 | Ptom.017G.00466 | Chr17: 4670001–4685000 | 4.793217649 | 0.485404 | P-loop containing nucleoside triphosphate hydrolases superfamily protein |
| Trait | SNP | Allele | Location | Marker_R2 | SNP p-Value | Populus tomentosa Gene | ATG (Arabidopsis thaliana Gene) | ATG Synonyms (Abbreviation) in Plants |
|---|---|---|---|---|---|---|---|---|
| LA | Chr3.4043365 | T/C | Promoter | 0.10964 | 5.41161 × 10−10 | Ptom.003G.00501 | AT5G13180 | NAC domain containing protein 83 |
| LA | Chr14.5078112 | C/T | Exon | 0.15034 | 3.76948 × 10−9 | Ptom.014G.00612 | AT3G61870 | |
| Ptom.014G.00614 | ||||||||
| LA | Chr2.16529660 | T/G | Downstream | 0.07527 | 4.70312 × 10−9 | Ptom.002G.02153 | AT1G02730 | cellulose synthase-like D5 |
| LA | Chr5.11035663 | A/T | Upstream | 0.09349 | 2.17057 × 10−8 | Ptom.005G.01280 | AT5G67080 | mitogen-activated protein kinase kinase kinase 19 |
| LN | Chr7.10376607 | A/C | Intron | 0.09408 | 1.15327 × 10−10 | Ptom.007G.00997 | AT1G05270 | TraB family protein |
| Ptom.007G.00998 | AT1G05270 | TraB family protein | ||||||
| RA | Chr5.15162313 | G/A | Downstream | 0.09193 | 2.47672 × 10−9 | Ptom.005G.01558 | ||
| Ptom.005G.01557 | AT5G20240 | K-box region and MADS-box transcription factor family protein | ||||||
| RA | Chr3.4043365 | T/C | Downstream | 0.14604 | 4.18145 × 10−9 | Ptom.003G.00501 | AT5G13180 | NAC domain containing protein 83 |
| RA | Chr14.5078112 | C/T | Exon | 0.13649 | 2.54129 × 10−8 | Ptom.014G.00612 | AT3G61870 | |
| Ptom.014G.00614 | ||||||||
| RA | Chr5.11035663 | A/T | Upstream | 0.09769 | 3.17383 × 10−8 | Ptom.005G.01280 | AT5G67080 | mitogen-activated protein kinase kinase kinase 19 |
| RA | Chr2.16529613 | C/T | Downstream | 0.16756 | 3.22066 × 10−8 | Ptom.002G.02153 | AT1G02730 | cellulose synthase-like D5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Zhou, J.; Wang, D.; Li, L.; Xiao, L.; Quan, M.; Lu, W.; Yao, L.; Fang, Y.; Lv, C.; et al. Genetic Architecture and Genome-Wide Adaptive Signatures Underlying Stem Lenticel Traits in Populus tomentosa. Int. J. Mol. Sci. 2021, 22, 9249. https://doi.org/10.3390/ijms22179249
Li P, Zhou J, Wang D, Li L, Xiao L, Quan M, Lu W, Yao L, Fang Y, Lv C, et al. Genetic Architecture and Genome-Wide Adaptive Signatures Underlying Stem Lenticel Traits in Populus tomentosa. International Journal of Molecular Sciences. 2021; 22(17):9249. https://doi.org/10.3390/ijms22179249
Chicago/Turabian StyleLi, Peng, Jiaxuan Zhou, Dan Wang, Lianzheng Li, Liang Xiao, Mingyang Quan, Wenjie Lu, Liangchen Yao, Yuanyuan Fang, Chenfei Lv, and et al. 2021. "Genetic Architecture and Genome-Wide Adaptive Signatures Underlying Stem Lenticel Traits in Populus tomentosa" International Journal of Molecular Sciences 22, no. 17: 9249. https://doi.org/10.3390/ijms22179249
APA StyleLi, P., Zhou, J., Wang, D., Li, L., Xiao, L., Quan, M., Lu, W., Yao, L., Fang, Y., Lv, C., Song, F., Du, Q., & Zhang, D. (2021). Genetic Architecture and Genome-Wide Adaptive Signatures Underlying Stem Lenticel Traits in Populus tomentosa. International Journal of Molecular Sciences, 22(17), 9249. https://doi.org/10.3390/ijms22179249






