Increased Potential of Bone Formation with the Intravenous Injection of a Parathyroid Hormone-Related Protein Minicircle DNA Vector
Abstract
:1. Introduction
2. Results
2.1. Generation of the mcPTHrP 1–34+107–139 Vector In Vitro
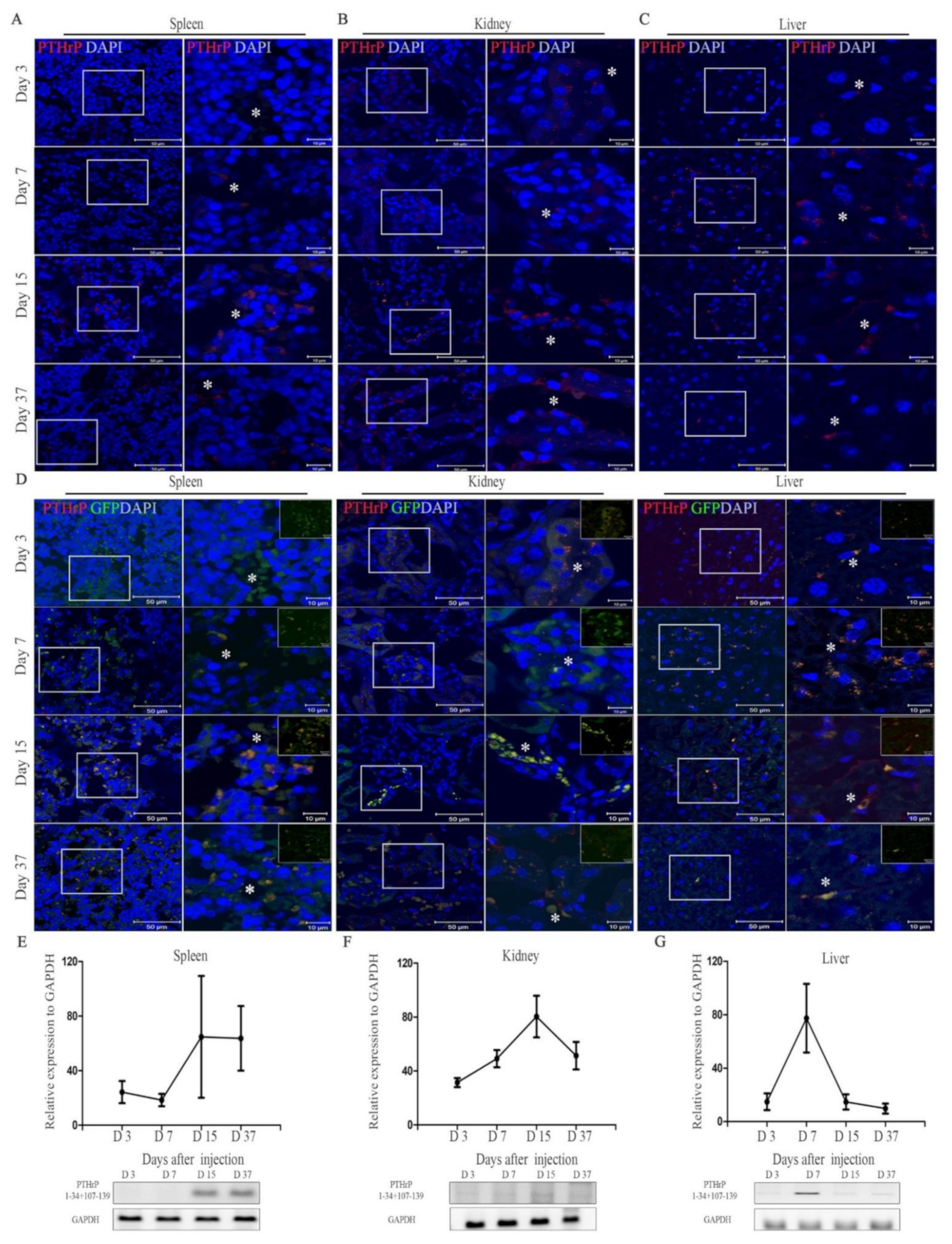
2.2. In Vivo Detection of mcPTHrP 1–34+107–139
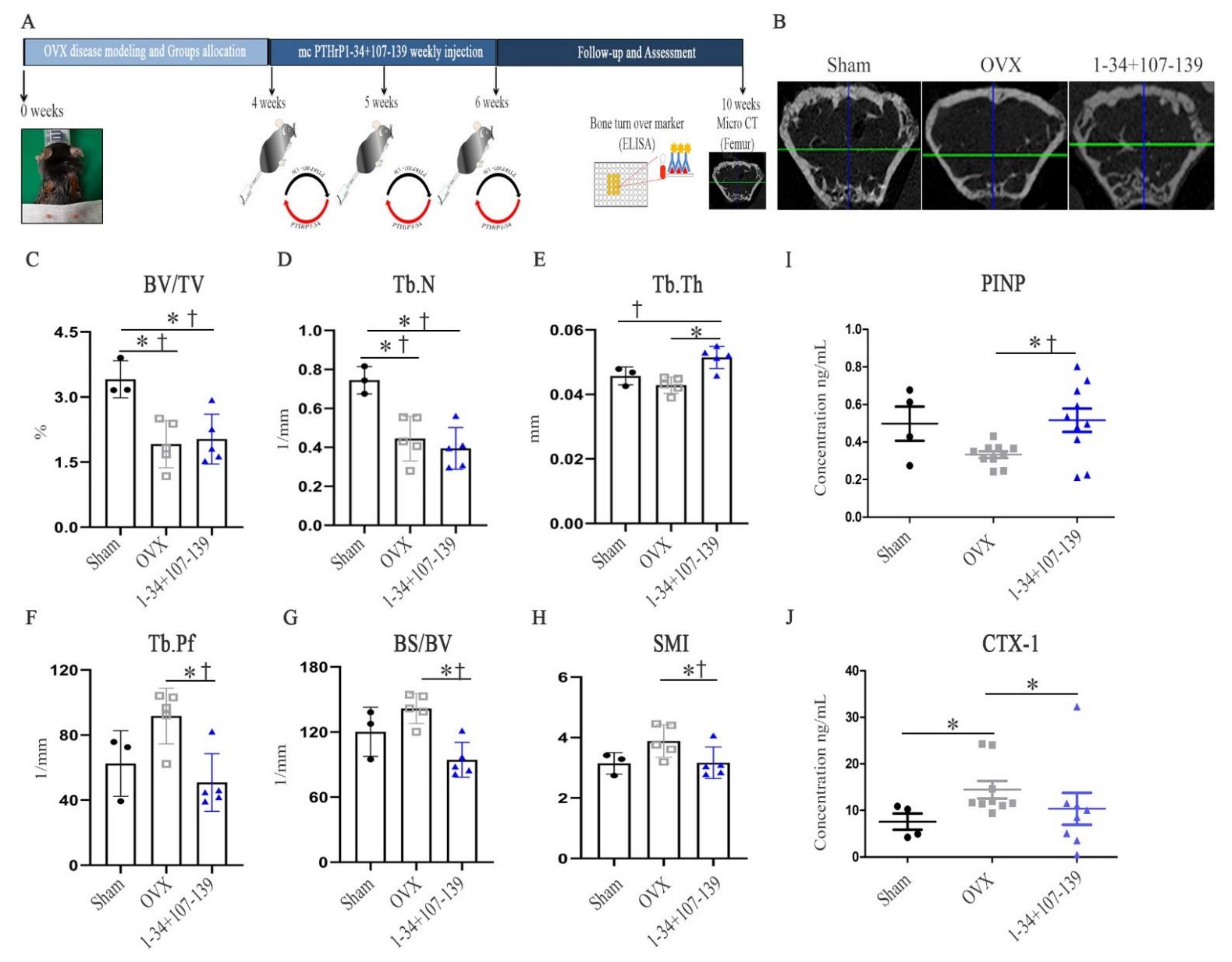
2.3. mcPTHrP 1–34+107–139 Promotes Bone Formation and Prevents Bone Resorption in OVX Mice with a Positive Impact on the Microarchitecture of Trabecular Bones
2.4. Therapeutic Application of mcPTHrP 1–34+107–139-Transfected MSCs in the Context of OVX Mice
2.5. Protein Expression of PTHrP and PTH/PTHrP-R, and Bone Formation/Resorption in mcPTHrP 1–34+107–139-Administered Mice
2.6. Protein Expression of PTHrP and PTH/PTHrP-R, and Bone Formation/Resorption in eMSCs-Treated Mice
3. Discussion
4. Materials and Methods
4.1. Production of the Mc PTHrP 1–34+107–139 Vector
4.2. HEK 293T Cell Culture and MC Vector Transfection
4.3. Immunocytochemistry
4.4. Transfection of Human MSCs with the Mc PTHrP 1–34+107–139 Vector
4.5. Characterization of eMSCs
4.6. Animal Model and Group Allocation
4.7. In Vivo Delivery of mcPTHrP 1–34+107–139 via Intravenous Injection
4.8. In Vivo Delivery of eMSCs
4.9. Reverse Transcription-Polymerase Chain Reaction
4.10. Western Blot
4.11. Immunofluorescence Staining
4.12. Micro CT Analysis
4.13. Quantification of the Serum Bone Turnover Markers
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Armas, L.A.; Recker, R.R. Pathophysiology of osteoporosis: New mechanistic insights. Endocrinol. Metab. Clin. N. Am. 2012, 41, 475–486. [Google Scholar] [CrossRef]
- Raisz, L.G. Pathogenesis of osteoporosis: Concepts, conflicts, and prospects. J. Clin. Investig. 2005, 115, 3318–3325. [Google Scholar] [CrossRef] [Green Version]
- Diab, D.L.; Watts, N.B. Postmenopausal osteoporosis. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 501–509. [Google Scholar] [CrossRef]
- Eriksen, E.F.; Diez-Perez, A.; Boonen, S. Update on long-term treatment with bisphosphonates for postmenopausal osteoporosis: A systematic review. Bone 2014, 58, 126–135. [Google Scholar] [CrossRef]
- Miller, P.D.; Pannacciulli, N.; Malouf-Sierra, J.; Singer, A.; Czerwinski, E.; Bone, H.G.; Wang, C.; Huang, S.; Chines, A.; Lems, W.; et al. Efficacy and safety of denosumab vs. bisphosphonates in postmenopausal women previously treated with oral bisphosphonates. Osteoporos. Int. 2020, 31, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Min, H.K.; Ahn, J.H.; Ha, K.Y.; Kim, Y.H.; Kim, S.I.; Park, H.Y.; Rhyu, K.W.; Kim, Y.Y.; Oh, I.S.; Seo, J.Y.; et al. Effects of anti-osteoporosis medications on radiological and clinical results after acute osteoporotic spinal fractures: A retrospective analysis of prospectively designed study. Osteoporos. Int. 2019, 30, 2249–2256. [Google Scholar] [CrossRef] [PubMed]
- Ishtiaq, S.; Fogelman, I.; Hampson, G. Treatment of post-menopausal osteoporosis: Beyond bisphosphonates. J. Endocrinol. Investig. 2015, 38, 13–29. [Google Scholar] [CrossRef]
- Ha, K.Y.; Park, K.S.; Kim, S.I.; Kim, Y.H. Does bisphosphonate-based anti-osteoporosis medication affect osteoporotic spinal fracture healing? Osteoporos. Int. 2016, 27, 483–488. [Google Scholar] [CrossRef]
- Anagnostis, P.; Gkekas, N.K.; Potoupnis, M.; Kenanidis, E.; Tsiridis, E.; Goulis, D.G. New therapeutic targets for osteoporosis. Maturitas 2019, 120, 1–6. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Hattersley, G.; Fitzpatrick, L.A.; Harris, A.G.; Shevroja, E.; Banks, K.; Leder, B.Z.; Zanchetta, J.R.; Hans, D. Abaloparatide-SC improves trabecular microarchitecture as assessed by trabecular bone score (TBS): A 24-week randomized clinical trial. Osteoporos. Int. 2018, 29, 323–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chew, C.K.; Clarke, B.L. Abaloparatide: Recombinant human PTHrP (1-34) anabolic therapy for osteoporosis. Maturitas 2017, 97, 53–60. [Google Scholar] [CrossRef]
- Kaufman, J.M.; Orwoll, E.; Goemaere, S.; San Martin, J.; Hossain, A.; Dalsky, G.P.; Lindsay, R.; Mitlak, B.H. Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: Treatment and discontinuation of therapy. Osteoporos. Int. 2005, 16, 510–516. [Google Scholar] [CrossRef]
- Neer, R.M.; Arnaud, C.D.; Zanchetta, J.R.; Prince, R.; Gaich, G.A.; Reginster, J.Y.; Hodsman, A.B.; Eriksen, E.F.; Ish-Shalom, S.; Genant, H.K.; et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med. 2001, 344, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Wysolmerski, J.J. Parathyroid hormone-related protein: An update. J. Clin. Endocrinol. Metab. 2012, 97, 2947–2956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, J.A.; Partridge, N.C. Physiological Bone Remodeling: Systemic Regulation and Growth Factor Involvement. Physiology 2016, 31, 233–245. [Google Scholar] [CrossRef]
- Bahar, H.; Gallacher, K.; Downall, J.; Nelson, C.A.; Shomali, M.; Hattersley, G. Six Weeks of Daily Abaloparatide Treatment Increased Vertebral and Femoral Bone Mineral Density, Microarchitecture and Strength in Ovariectomized Osteopenic Rats. Calcif. Tissue Int. 2016, 99, 489–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arlt, H.; Mullarkey, T.; Hu, D.; Baron, R.; Ominsky, M.S.; Mitlak, B.; Lanske, B.; Besschetnova, T. Effects of abaloparatide and teriparatide on bone resorption and bone formation in female mice. Bone Rep. 2020, 13, 100291. [Google Scholar] [CrossRef]
- De Castro, L.F.; Lozano, D.; Portal-Nunez, S.; Maycas, M.; De la Fuente, M.; Caeiro, J.R.; Esbrit, P. Comparison of the skeletal effects induced by daily administration of PTHrP (1-36) and PTHrP (107-139) to ovariectomized mice. J. Cell. Physiol. 2012, 227, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, J.; Yin, Y.; Wu, J.; Wang, Z.; Miao, D.; Sun, W. Recombinant human parathyroid hormone related protein 1-34 and 1-84 and their roles in osteoporosis treatment. PLoS ONE 2014, 9, e88237. [Google Scholar] [CrossRef] [Green Version]
- Khosla, S.; Hofbauer, L.C. Osteoporosis treatment: Recent developments and ongoing challenges. Lancet. Diabetes Endocrinol. 2017, 5, 898–907. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Zhang, P.; Zhang, X.; Lv, L.; Zhou, Y. Advances in mesenchymal stem cell transplantation for the treatment of osteoporosis. Cell Prolif 2021, 54, e12956. [Google Scholar] [CrossRef]
- Su, P.; Tian, Y.; Yang, C.; Ma, X.; Wang, X.; Pei, J.; Qian, A. Mesenchymal Stem Cell Migration during Bone Formation and Bone Diseases Therapy. Int. J. Mol. Sci. 2018, 19, 2343. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.; Chen, Z.; Hu, S.; Jia, F.; Li, Z.; Hoyt, G.; Robbins, R.C.; Kay, M.A.; Wu, J.C. Novel minicircle vector for gene therapy in murine myocardial infarction. Circulation 2009, 120, S230–S237. [Google Scholar] [CrossRef] [Green Version]
- Patil, S.; Gao, Y.G.; Lin, X.; Li, Y.; Dang, K.; Tian, Y.; Zhang, W.J.; Jiang, S.F.; Qadir, A.; Qian, A.R. The Development of Functional Non-Viral Vectors for Gene Delivery. Int. J. Mol. Sci. 2019, 20, 5491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rim, Y.A.; Nam, Y.; Park, N.; Jung, H.; Lee, K.; Lee, J.; Ju, J.H. Chondrogenic Differentiation from Induced Pluripotent Stem Cells Using Non-Viral Minicircle Vectors. Cells 2020, 9, 582. [Google Scholar] [CrossRef] [PubMed]
- Kay, M.A.; He, C.Y.; Chen, Z.Y. A robust system for production of minicircle DNA vectors. Nat. Biotechnol. 2010, 28, 1287–1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, N.; Rim, Y.A.; Jung, H.; Kim, J.; Yi, H.; Kim, Y.; Jang, Y.; Jung, S.M.; Lee, J.; Kwok, S.K.; et al. Etanercept-Synthesising Mesenchymal Stem Cells Efficiently Ameliorate Collagen-Induced Arthritis. Sci. Rep. 2017, 7, 39593. [Google Scholar] [CrossRef] [Green Version]
- Yi, H.; Kim, Y.; Kim, J.; Jung, H.; Rim, Y.A.; Jung, S.M.; Park, S.H.; Ju, J.H. A new strategy to deliver synthetic protein drugs: Self-reproducible biologics using minicircles. Sci. Rep. 2014, 4, 5961. [Google Scholar] [CrossRef] [Green Version]
- Rim, Y.A.; Yi, H.; Kim, Y.; Park, N.; Jung, H.; Kim, J.; Jung, S.M.; Park, S.H.; Ju, J.H. Self in vivo production of a synthetic biological drug CTLA4Ig using a minicircle vector. Sci. Rep. 2014, 4, 6935. [Google Scholar] [CrossRef] [Green Version]
- Klinck, J.; Boyd, S.K. The magnitude and rate of bone loss in ovariectomized mice differs among inbred strains as determined by longitudinal in vivo micro-computed tomography. Calcif. Tissue Int. 2008, 83, 70–79. [Google Scholar] [CrossRef]
- Greenwood, C.; Clement, J.G.; Dicken, A.J.; Evans, J.P.; Lyburn, I.D.; Martin, R.M.; Rogers, K.D.; Stone, N.; Adams, G.; Zioupos, P. The micro-architecture of human cancellous bone from fracture neck of femur patients in relation to the structural integrity and fracture toughness of the tissue. Bone Rep. 2015, 3, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Muller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Eastell, R.; Szulc, P. Use of bone turnover markers in postmenopausal osteoporosis. Lancet Diabetes Endocrinol. 2017, 5, 908–923. [Google Scholar] [CrossRef]
- Sui, B.; Hu, C.; Zhang, X.; Zhao, P.; He, T.; Zhou, C.; Qiu, X.; Chen, N.; Zhao, X.; Jin, Y. Allogeneic Mesenchymal Stem Cell Therapy Promotes Osteoblastogenesis and Prevents Glucocorticoid-Induced Osteoporosis. Stem Cells Transl. Med. 2016, 5, 1238–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scandella, V.; Paolicelli, R.C.; Knobloch, M. A novel protocol to detect green fluorescent protein in unfixed, snap-frozen tissue. Sci. Rep. 2020, 10, 14642. [Google Scholar] [CrossRef] [PubMed]
- Morris, L.M.; Klanke, C.A.; Lang, S.A.; Lim, F.Y.; Crombleholme, T.M. TdTomato and EGFP identification in histological sections: Insight and alternatives. Biotech. Histochem. Off. Publ. Biol. Stain. Comm. 2010, 85, 379–387. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-W.; Park, N.; Kang, J.; Kim, Y.; Jung, H.; Rim, Y.A.; Ju, J.H. Increased Potential of Bone Formation with the Intravenous Injection of a Parathyroid Hormone-Related Protein Minicircle DNA Vector. Int. J. Mol. Sci. 2021, 22, 9069. https://doi.org/10.3390/ijms22169069
Kim J-W, Park N, Kang J, Kim Y, Jung H, Rim YA, Ju JH. Increased Potential of Bone Formation with the Intravenous Injection of a Parathyroid Hormone-Related Protein Minicircle DNA Vector. International Journal of Molecular Sciences. 2021; 22(16):9069. https://doi.org/10.3390/ijms22169069
Chicago/Turabian StyleKim, Jang-Woon, Narae Park, Jaewoo Kang, Yena Kim, Hyerin Jung, Yeri Alice Rim, and Ji Hyeon Ju. 2021. "Increased Potential of Bone Formation with the Intravenous Injection of a Parathyroid Hormone-Related Protein Minicircle DNA Vector" International Journal of Molecular Sciences 22, no. 16: 9069. https://doi.org/10.3390/ijms22169069
APA StyleKim, J.-W., Park, N., Kang, J., Kim, Y., Jung, H., Rim, Y. A., & Ju, J. H. (2021). Increased Potential of Bone Formation with the Intravenous Injection of a Parathyroid Hormone-Related Protein Minicircle DNA Vector. International Journal of Molecular Sciences, 22(16), 9069. https://doi.org/10.3390/ijms22169069





