Evaluation of Epigenetic and Radiomodifying Effects during Radiotherapy Treatments in Zebrafish
Abstract
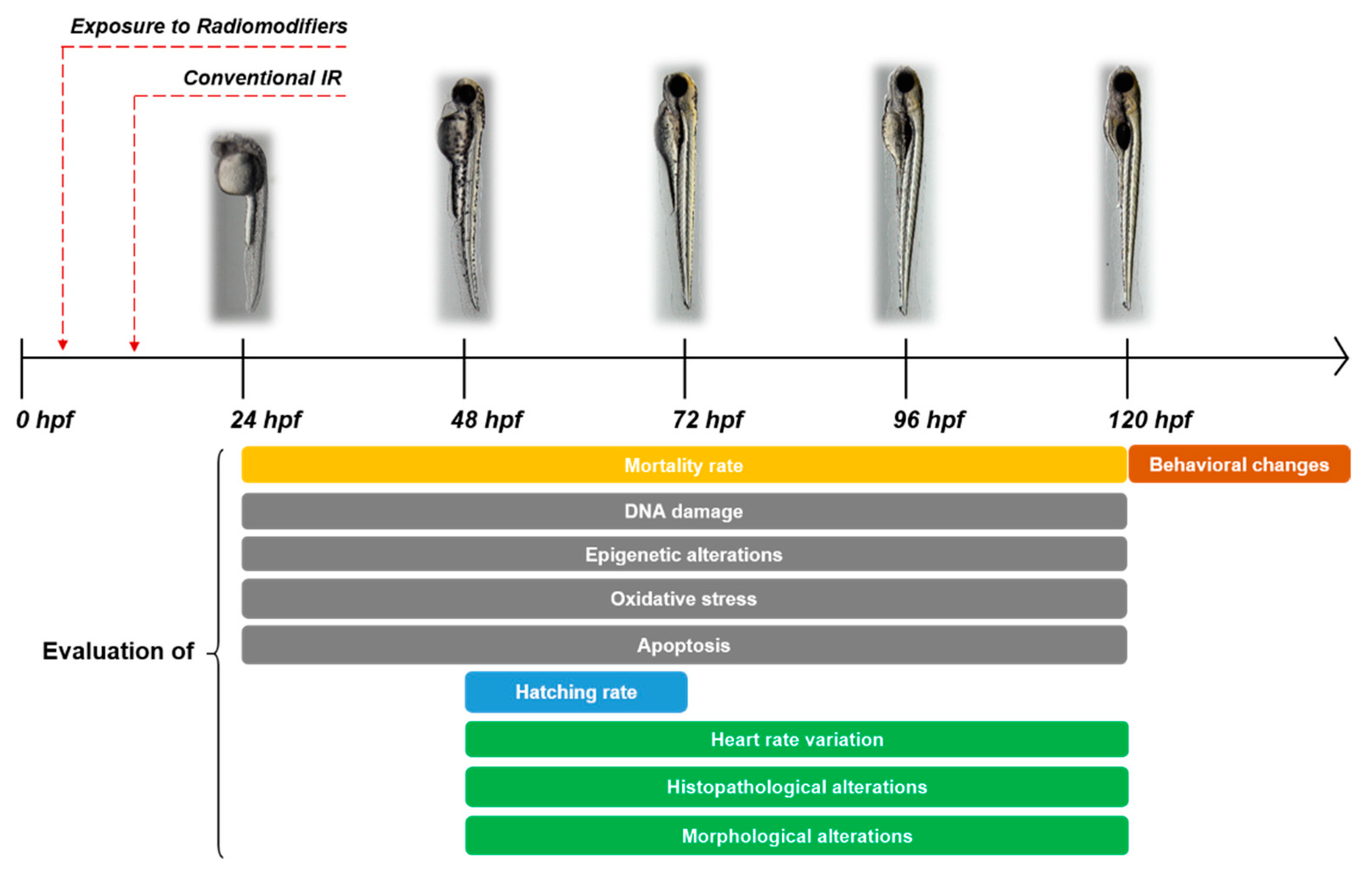
1. Introduction
1.1. The Role of Radiotherapy in Cancer Treatment
1.2. Zebrafish as a Model in Radiobiology
2. Epigenetic Changes Inflicted by Radiation during Zebrafish Embryogenesis
3. Radiation Modifiers in Zebrafish
3.1. Oxidative Stress
3.2. DNA Damage
3.3. Apoptosis
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAT | Catalase |
| Ccdc94 | Coiled-coil domain containing gene 94 |
| Ccnd1 | Cyclin D1 |
| CORM | CO-releasing molecule |
| DDR | DNA Damage Response |
| DMR | differentially methylated region |
| DNMT | DNA methyltransferases |
| dpi | days post irradiation |
| DSB | Double Strand Break |
| GPC | L-alpha glycerylphosphorylcholine |
| H3K27me3 | Histone H3 trimethylation at lysine 27 |
| H3K4me3 | Histone H3 trimethylation at lysine 4 |
| H3K9me3 | Histone H3 trimethylation at lysine 9 |
| hpf | hours post fertilization |
| HypNA-pPNA | Hydroxylprolyl-phosphono peptide nucleic acid |
| IRAK | Interleukin-1 Receptor-Associated Kinase |
| IR | Ionizing Radiation |
| LET | Linear Energy Transfer |
| MO | Morpholino Oligonucleotide |
| NHEJ | Non-Homologous End Joining |
| OMA | Octopus ocellatus Meat |
| PFT-α | Pifithrin-α |
| PTM | Post-translational modification |
| RBE | Relative Biological Effectiveness |
| RNS | Reactive Nitrogen Species |
| ROS | Reactive Oxygen Species |
| rs7 | radiosensitizing mutation 7 |
| RT | Radiation Therapy |
| SNAP | S-nitroso-N-acetylpenicillamine |
| SOD | Superoxide dismutase |
References
- Forte, G.I.; Minafra, L.; Bravatà, V.; Cammarata, F.P.; Lamia, D.; Pisciotta, P.; Cirrone, G.A.P.; Cuttone, G.; Gilardi, M.C.; Russo, G. Radiogenomics: The utility in patient selection. Transl. Cancer Res. 2017, 65, S852–S874. [Google Scholar] [CrossRef]
- Messa, C.; Di Muzio, N.; Picchio, M.; Gilardi, M.C.; Bettinardi, V.; Fazio, F. PET/CT and radiotherapy. Q. J. Nucl Med. Mol. Imaging. 2006, 50, 4–14. [Google Scholar] [PubMed]
- Bravatà, V.; Cammarata, F.P.; Minafra, L.; Pisciotta, P.; Scazzone, C.; Manti, L.; Savoca, G.; Petringa, G.; Cirrone, G.A.P.; Cuttone, G.; et al. Proton-irradiated breast cells: Molecular points of view. J. Radiat. Res. 2019, 60, 451–465. [Google Scholar] [CrossRef]
- Tang, L.; Wei, F.; Wu, Y.; He, Y.; Shi, L.; Xiong, F.; Gong, Z.; Guo, C.; Li, X.; Deng, H.; et al. Role of metabolism in cancer cell radioresistance and radiosensitization methods. J. Exp. Clin. Cancer Res. 2018, 37, 87. [Google Scholar] [CrossRef] [PubMed]
- Maier, P.; Hartmann, L.; Wenz, F.; Herskind, C. Cellular Pathways in Response to Ionizing Radiation and Their Targetability for Tumor Radiosensitization. Int. J. Mol. Sci. 2016, 17, 102. [Google Scholar] [CrossRef] [PubMed]
- Zindler, J.D.; Thomas, C.R., Jr.; Hahn, S.M.; Hoffmann, A.L.; Troost, E.G.; Lambin, P. Increasing the Therapeutic Ratio of Stereotactic Ablative Radiotherapy by Individualized Isotoxic Dose Prescription. J. Natl. Cancer Inst. 2015, 108, djv305. [Google Scholar] [CrossRef]
- Van Meer, P.; Raber, J. Mouse behavioural analysis in systems biology. Biochem. J. 2005, 389 (Pt. 3), 593–610. [Google Scholar] [CrossRef]
- Ackerman, S.D.; Monk, K.R. The scales and tales of myelination: Using zebrafish and mouse to study myelinating glia. Brain Res. 2016, 1641 Pt A, 79–91. [Google Scholar] [CrossRef]
- Barriuso, J.; Nagaraju, R.; Hurlstone, A. Howe Zebrafish: A new companion for translational research in oncology. Clin. Cancer Res. 2015, 21, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- McAleer, M.F.; Duffy, K.T.; Davidson, W.R.; Kari, G.; Dicker, A.P.; Rodeck, U.; Wickstrom, E. Antisense inhibition of cyclin d1 expression is equivalent to flavopiridol for radiosensitization of zebrafish embryos. Int. J. Radiat. Oncol. Biol. Phys. 2005, 66, 546–551. [Google Scholar] [CrossRef][Green Version]
- Lam, P.-Y.; Peterson, R.T. Developing zebrafish disease models for in vivo small molecule screens. Curr. Opin. Chem. Biol. 2019, 50, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, V.; Spinelli, G. Environmental epigenetics in zebrafish. Epigenet. Chromatin 2017, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Trede, N.S.; Langenau, D.M.; Traver, D.; Look, A.T.; Zon, L.I. The use of zebrafish to understand immunity. Immunity 2004, 20, 367–379. [Google Scholar] [CrossRef]
- Hwang, M.; Yong, C.; Moretti, L.; Lu, B. Zebrafish as a model system to screen radiation modifiers. Curr. Genom. 2007, 8, 360–369. [Google Scholar]
- Szabó, E.R.; Brand, M.; Hans, S.; Hideghéty, K.; Karsch, L.; Lessmann, E.; Pawelke, J.; Schürer, M.; Beyreuther, E. Radiobiological effects and proton RBE determined by wildtype zebrafish embryos. PLoS ONE 2018, 13, e0206879. [Google Scholar] [CrossRef] [PubMed]
- Bladen, C.L.; Lam, W.K.; Dynan, W.S.; Kozlowski, D.J. DNA damage response and Ku80 function in the vertebrate embryo. Nucleic Acids Res. 2005, 33, 3002–3010. [Google Scholar] [CrossRef] [PubMed]
- Gagnaire, B.; Cavalié, I.; Pereira, S.; Floriani, M.; Dubourg, N.; Camilleri, V.; Adam-Guillermin, C. External gamma irradiation-induced effects in early-life stages of zebrafish, Danio rerio. Aquat. Toxicol. 2015, 169, 69–78. [Google Scholar] [CrossRef]
- Ching, S.; Gillette, S.; Powers, B.; Roberts, S.; Gillette, E.; Withrow, S. Radiation-induced ocular injury in the dog: A histological study. Int. J. Radiat. Oncol. 1990, 19, 321–328. [Google Scholar] [CrossRef]
- Sun, X.Z.; Inouye, M.; Hayasaka, S.; Takagishi, Y.; Yamamura, H. Effects of different doses of g-radiation on the developing brain of mice. Environ. Med. 1995, 39, 113–116. [Google Scholar] [PubMed]
- Heeran, A.B.; Berrigan, H.P.; O’Sullivan, J. The Radiation-Induced Bystander Effect (RIBE) and its Connections with the Hallmarks of Cancer. Radiat Res. 2019, 192, 668–679. [Google Scholar] [CrossRef]
- Shaughnessy, D.T.; McAllister, K.; Worth, L.; Haugen, A.C.; Meyer, J.; Domann, F.; Van Houten, B.; Mostoslavsky, R.; Bultman, S.J.; Baccarelli, A.; et al. Mitochondria, Energetics, Epigenetics, and Cellular Responses to Stress. Environ. Health Perspect. 2014, 122, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, V. Histones, Their Variants and Post-translational Modifications in Zebrafish Development. Front. Cell Dev. Biol. 2020, 8, 456. [Google Scholar] [CrossRef]
- Ha, K.; Lee, G.E.; Palii, S.S.; Brown, K.D.; Takeda, Y.; Liu, K.; Bhalla, K.N.; Robertson, K.D. Rapid and transient recruitment of DNMT1 to DNA double-strand breaks is mediated by its interaction with multiple components of the DNA damage response machinery. Hum. Mol. Genet. 2011, 20, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Malard, V.; Ravanat, J.L.; Davin, A.H.; Armengaud, J.; Foray, N.; Adam-Guillermin, C. Low doses of gamma-irradiation induce an early bystander effect in zebrafish cells which is sufficient to radioprotect cells. PLoS ONE 2014, 9, e92974. [Google Scholar] [CrossRef]
- Geiger, G.A.; Fu, W.; Kao, G.D. Temozolomide-mediated Radiosensitization of Human Glioma Cells in a Zebrafish Embryonic System. Cancer Res. 2008, 68, 3396–3404. [Google Scholar] [CrossRef]
- Cavalieri, V.; Spinelli, G. Ectopic hbox12 Expression Evoked by Histone Deacetylase Inhibition Disrupts Axial Specification of the Sea Urchin Embryo. PLoS ONE 2015, 10, e0143860. [Google Scholar] [CrossRef]
- Cavalieri, V. Model organisms and their application in environmental epigenetics. In Environmental Epigenetics in Toxicology and Public Health; Fry, R., Ed.; Translational Epigenetics; Elsevier, Academic Press: Cambridge, MA, USA, 2020; Volume 22, Chapter 3; pp. 67–87. [Google Scholar]
- Lindeman, L.C.; Kamstra, J.H.; Ballangby, J.; Hurem, S.; Martín, L.M.; Brede, D.A.; Teien, H.C.; Oughton, D.H.; Salbu, B.; Lyche, J.L.; et al. Gamma radiation induces locus specific changes to histone modification enrichment in zebrafish and Atlantic salmon. PLoS ONE 2019, 14, e0212123. [Google Scholar] [CrossRef]
- Le, T.N.M.; Teh, C.; Shyh-Chang, N.; Xie, H.; Zhou, B.; Korzh, V.; Lodish, H.F.; Lim, B. MicroRNA-125b is a novel negative regulator of p53. Genes Dev. 2009, 23, 862–876. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.U.; Kaimal, V.; Chen, J.; Jegga, A.G. Dissecting microregulation of a master regulatory network. BMC Genom. 2008, 9, 88. [Google Scholar] [CrossRef]
- Kamstra, J.H.; Hurem, S.; Martin, L.M.; Lindeman, L.C.; Legler, J.; Oughton, D.; Salbu, B.; Brede, D.A.; Lyche, J.L.; Aleström, P. Ionizing radiation induces transgenerational effects of DNA methylation in zebrafish. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Martín, L.; Kamstra, J.H.; Hurem, S.; Lindeman, L.C.; Brede, D.A.; Aanes, H.; Babiak, I.; Arenal, A.; Oughton, D.; Salbu, B.; et al. Altered non-coding RNA expression profile in F1 progeny 1 year after parental irradiation is linked to adverse effects in zebrafish. Sci. Rep. 2021, 11, 1–17. [Google Scholar] [CrossRef]
- Dimri, M.; Joshi, J.; Chakrabarti, R.; Sehgal, N.; Sureshbabu, A.; Prem Kumar, I. Todralazine Protects Zebrafish from Lethal Effects of Ionizing Radiation: Role of Hematopoietic Cell Expansion. Zebrafish 2015, 12, 33–47. [Google Scholar] [CrossRef]
- Daroczi, B.; Kari, G.; McAleer, M.F.; Wolf, J.C.; Rodeck, U.; Dicker, A.P. In Vivo Radioprotection by the Fullerene Nanoparticle DF-1 as Assessed in a Zebrafish Model. Clin Cancer Res. 2006, 12, 7086–7091. [Google Scholar] [CrossRef] [PubMed]
- Dimri, M.; Joshi, J.; Shrivastava, N.; Ghosh, S.; Chakraborti, R.; Indracanti, P.K. Prilocaine hydrochloride protects zebrafish from lethal effects of ionizing radiation: Role of hematopoietic cell expansion. Tokai J. Exp. Clin. Med. 2015, 40, 8–15. [Google Scholar]
- Lee, W.W.; Kim, E.A.; Um, J.H.; Kang, N.; Han, E.J.; Oh, J.Y.; Park, S.Y.; Jeon, Y.J.; Ahn, G. Radio-Protective Effects of Octopus ocellatus Meat Consisted of a Plentiful Taurine Against Damages Caused by Gamma Ray Irradiation. Adv. Exp. Med. Biol. 2017, 975 Pt 2, 955–971. [Google Scholar]
- Szabó, E.R.; Plangár, I.; Tőkés, T.; Mán, I.; Polanek, R.; Kovács, R.; Fekete, G.; Szabó, Z.; Csenki, Z.; Baska, F.; et al. l-Alpha Glycerylphosphorylcholine as a Potential Radioprotective Agent in Zebrafish Embryo Model. Zebrafish 2016, 13, 481–488. [Google Scholar] [CrossRef]
- Gan, L.; Guo, M.; Si, J.; Zhang, J.; Liu, Z.; Zhao, J.; Wang, F.; Yan, J.; Li, H.; Zhang, H. Protective effects of phenformin on zebrafish embryonic neurodevelopmental toxicity induced by X-ray radiation. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4202–4210. [Google Scholar] [CrossRef] [PubMed]
- Zhoua, R.; Songe, J.; Sia, J.; Zhanga, H.; Liue, B.; Gana, L.; Zhoua, X.; Wanga, Y.; Yana, J.; Zhang, Q. Effects of Ru(CO)3Cl-glycinate on the developmental toxicities induced by X-ray and carbon-ion irradiation in zebrafish embryos. Mutat. Res. 2016, 793–794, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Rachakonda, G.; Morre, D.J.; Morre, D.M.; Crooks, P.A.; Sonar, V.N.; Roti Roti, J.L.; Rogers, B.E.; Greco, S.; Ye, F.; et al. Indolylquinuclidinols inhibit ENOX activity and endothelial cell morphogenesis while enhancing radiation-mediated control of tumor vasculature. FASEB J. 2009, 23, 2986–2995. [Google Scholar] [CrossRef] [PubMed]
- Bladen, C.L.; Kozlowski, D.J.; Dynan, W.S. Effects of Low-Dose Ionizing Radiation and Menadione, an Inducer of Oxidative Stress, Alone and in Combination in a Vertebrate Embryo Model. Radiat. Res. 2012, 178, 499–503. [Google Scholar] [CrossRef]
- Lally, B.E.; Geiger, G.A.; Kridel, S.; Arcury-Quandt, A.E.; Robbins, M.E.; Kock, N.D.; Wheeler, K.; Peddi, P.; Georgakilas, A.; Kao, G.D.; et al. Identification and Biological Evaluation of a Novel and Potent Small Molecule Radiation Sensitizer via an Unbiased Screen of a Chemical Library. Cancer Res. 2007, 67, 8791–8799. [Google Scholar] [CrossRef]
- Bladen, C.L.; Navarre, S.; Dynan, W.S.; Kozlowski, D.J. Expression of the Ku70 subunit (XRCC6) and protection from low dose ionizing radiation during zebrafish embryogenesis. Neurosci. Lett. 2007, 422, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Sorrells, S.; Carbonneau, S.; Harrington, E.; Chen, A.T.; Hast, B.; Milash, B.; Pyati, U.; Major, M.B.; Zhou, Y.; Zon, L.I.; et al. Ccdc94 Protects Cells from Ionizing Radiation by Inhibiting the Expression of p53. PLoS Genet. 2012, 8, e1002922. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.H.; Shah, R.B.; Li, Y.; Arora, A.; Ung, P.M.-U.; Raman, R.; Gorbatenko, A.; Kozono, S.; Zhou, X.Z.; Brechin, V.; et al. An IRAK1–PIN1 signalling axis drives intrinsic tumour resistance to radiation therapy. Nat. Cell Biol. 2019, 21, 203–213. [Google Scholar] [CrossRef]
- Kong, E.Y.; Yeung, W.K.; Chan, T.K.Y.; Cheng, S.H.; Yu, K.N. Exogenous Nitric Oxide Suppresses In Vivo X-ray-Induced Targeted and Non-Targeted Effects in Zebrafish Embryos. Int. J. Mol. Sci. 2016, 17, 1321. [Google Scholar] [CrossRef] [PubMed]
- Duffy, K.T.; Wickstrom, E. Zebrafish tp53 knockdown extends the survival of irradiated zebrafish embryos more effectively than the p53 inhibitor pifithrin. Cancer Biol. Ther. 2007, 6, 675–678. [Google Scholar] [CrossRef][Green Version]
- Daroczi, B.; Kari, G.; Ren, Q.; Dicker, A.P.; Rodeck, U. NF-κB inhibitors alleviate and the proteasome inhibitor PS-341 exacerbates radiation toxicity in zebrafish embryos. Mol. Cancer Ther. 2009, 8, 2625–2634. [Google Scholar] [CrossRef]
- Jette, C.A.; Flanagan, A.M.; Ryan, J.; Pyati, U.J.; Carbonneau, S.; Stewart, R.A.; Langenau, D.M.; Look, A.T.; Letai, A. BIM and other BCL-2 family proteins exhibit cross-species conservation of function between zebrafish and mammals. Cell Death Differ. 2008, 15, 1063–1072. [Google Scholar] [CrossRef]
- Gnosa, S.; Capodanno, A.; Murthy, R.V.; Dahl, L.; Jensen, E.; Sun, X.F. AEG-1 Knockdown in Colon Cancer Cell Lines Inhibits Radiation-Enhanced Migration and Invasion in Vitro and in a Novel In Vivo Zebrafish Model. Oncotarget. 2016, 7, 81634–81644. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.M.; Weinstein, B.M. Studying vascular development in the zebrafish. Trends Cardiovasc. Med. 2000, 10, 352–360. [Google Scholar] [CrossRef]
- Sztal, T.E.; Ruparelia, A.A.; Williams, C.; Bryson-Richardson, R.J. Using Touch-evoked Response and Locomotion Assays to Assess Muscle Performance and Function in Zebrafish. J. Vis. Exp. 2016, e54431. [Google Scholar] [CrossRef] [PubMed]
- Tucker, B.; Lardelli, M. A Rapid Apoptosis Assay Measuring Relative Acridine Orange Fluorescence in Zebrafish Embryos. Zebrafish 2007, 4, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Parng, C.; Seng, W.L.; Semino, C.; McGrath, P. Zebrafish: A Preclinical Model for Drug Screening. ASSAY Drug Dev. Technol. 2002, 1 Pt 1, 41–48. [Google Scholar] [CrossRef]
- Kumar, M.P.; Shyama, S.; Kashif, S.; Dubey, S.; Avelyno, D.; Sonaye, B.; Samit, B.K.; Chaubey, R. Effects of gamma radiation on the early developmental stages of Zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2017, 142, 95–101. [Google Scholar] [CrossRef]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Si, J.; Zhang, H.; Wang, Z.; Li, J.; Zhou, X.; Gan, L.; Liu, Y. The effects of X-ray radiation on the eye development of zebrafish. Hum. Exp. Toxicol. 2014, 33, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Azzam, E.I.; Jay-Gerin, J.-P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef]
- Kryston, T.B.; Georgiev, A.B.; Pissis, P.; Georgakilas, A.G. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat. Res. Mol. Mech. Mutagen. 2011, 711, 193–201. [Google Scholar] [CrossRef]
- Calvaruso, M.; Pucci, G.; Musso, R.; Bravatà, V.; Cammarata, F.P.; Russo, G.; Forte, G.I.; Minafra, L. Nutraceutical Compounds as Sensitizers for Cancer Treatment in Radiation Therapy. Int J. Mol. Sci. 2019, 20, 5267. [Google Scholar] [CrossRef] [PubMed]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Plangar, I.; Szabo, E.R.; Tokes, T.; Man, I.; Brinyiczki, K.; Fekete, G.; Németh, I.; Ghyczy, M.; Boros, M.; Hideghéty, K. Radio-neuroprotective effect of L-alpha glycerylphosphorylcholine (GPC) in an experimental rat model. J. Neurooncol 2014, 119, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Ghyczy, M.; Torday, C.; Kaszaki, J.; Szabó, A.; Czóbel, M.; Boros, M. Hypoxia-induced generation of methane in mitochondria and eukaryotic cells: An alternative approach to methanogenesis. Cell Physiol. Biochem. 2008, 21, 251–258. [Google Scholar] [CrossRef]
- Tokés, T.; Eros, G.; Bebes, A.; Hartmann, P.; Várszegi, S.; Varga, G.; Kaszaki, J.; Gulya, K.; Ghyczy, M.; Boros, M. Protective effects of a phosphatidylcholine-enriched diet in lipopolysaccharide-induced experimental neuroinflammation in the rat. Shock 2011, 36, 458–465. [Google Scholar] [CrossRef]
- Lisby, M.; Rothstein, R. DNA repair: Keeping it together. Curr. Biol. 2004, 14, R994–R996. [Google Scholar] [CrossRef][Green Version]
- Syro, L.V.; Rotondo, F.; Camargo, M.; Ortíz, C.A.S.; Serna, C.A.; Kovacs, K. Temozolomide and Pituitary Tumors: Current Understanding, Unresolved Issues, and Future Directions. Front. Endocrinol. 2018, 9, 318. [Google Scholar] [CrossRef]
- Khoronenkova, S.V.; Dianov, G.L. ATM prevents DSB formation by coordinating SSB repair and cell cycle progression. Proc. Natl. Acad. Sci. USA 2015, 112, 3997–4002. [Google Scholar] [CrossRef]
- Biau, J.; Chautard, E.; Verrelle, P.; Dutreix, M. Altering DNA Repair to Improve Radiation Therapy: Specific and Multiple Pathway Targeting. Front. Oncol. 2019, 9, 1009. [Google Scholar] [CrossRef]
- Knyazhanskaya, E.; Anisenko, A.; Shadrina, O.; Kalinina, A.; Zatsepin, T.; Zalevsky, A.; Mazurov, D.; Gottikh, M. NHEJ pathway is involved in post-integrational DNA repair due to Ku70 binding to HIV-1 integrase. Retrovirology 2019, 16, 30. [Google Scholar] [CrossRef]
- Clancy, S. DNA Damage & Repair: Mechanisms for Maintaining DNA Integrity. Nat. Educ. 2008, 1, 103. [Google Scholar]
- Liang, G.; Salem, C.E.; Yu, M.C.; Nguyen, H.D.; Gonzales, F.A.; Nguyen, T.D.T.; Nichols, P.W.; Jones, P.A. DNA Methylation Differences Associated with Tumor Tissues Identified by Genome Scanning Analysis. Genomics 1998, 53, 260–268. [Google Scholar] [CrossRef]
- Richardson, C.; Jasin, M. Coupled homologous and nonhomologous repair of a double-strand break preserves genomic integrity in mammalian cells. Mol. Cell. Biol. 2000, 20, 9068–9075. [Google Scholar] [CrossRef]
- Suzuki, M.; Shigematsu, H.; Shames, D.S.; Sunaga, N.; Takahashi, T.; Shivapurkar, N.; Iizasa, T.; Frenkel, E.P.; Minna, J.D.; Fujisawa, T.; et al. DNA methylation-associated inactivation of TGFβ-related genes, DRM/Gremlin, RUNX3, and HPP1 in human cancers. Br. J. Cancer 2013, 109, 3132. [Google Scholar] [CrossRef] [PubMed]
- Perkins, N.D.; Gilmore, T.D. Good cop, bad cop: The different faces of NF-kappaB. Cell Death Differ. 2006, 13, 759–772. [Google Scholar] [CrossRef]
- Zavrski, I.; Kleeberg, L.; Kaiser, M.; Fleissner, C.; Heider, U.; Sterz, J.; Jakob, C.; Sezer, O. Proteasome as an Emerging Therapeutic Target in Cancer. Curr. Pharm. Des. 2007, 13, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Rallis, K.S.; Yau, T.H.L.; Sideris, M. Chemoradiotherapy in Cancer Treatment: Rationale and Clinical Applications. Anticancer Res. 2021, 41, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pajonk, F.; Vlashi, E.; McBride, W.H. Radiation Resistance of Cancer Stem Cells: The 4 R’s of Radiobiology Revisited. Stem Cells 2010, 28, 639–648. [Google Scholar] [CrossRef]
| Reagent | Role | Concentration | Affected Pathways | Radiation Dose | References |
|---|---|---|---|---|---|
| Flavopiridol + HypNA-pPNA | Radiosensitizer | 0–500 nM + 5 mM | Cell cycle, Apoptosis | 10–40 Gy ɣ rays | [11] |
| Temozolomide | Radiosensitizer | 100 µM | DNA damage | 10 Gy ɣ rays | [26] |
| Todralazine | Radioprotector | 5 µM | Oxidative stress | 20 Gy ɣ rays | [34] |
| Metoprolol | Radioprotector | 5 µM | Oxidative stress | 20 Gy ɣ rays | [34] |
| Fullerene derivative DF-1 | Radioprotector | 100 µM | Oxidative stress | 20–40 Gy ɣ rays | [35] |
| Prilocaine hydrochloride | Radioprotector | 10–40 µM | Oxidative stress | 20 Gy ɣ rays | [36] |
| Octopus ocellatus meet (OMA) | Radioprotector | 62.50–250 µg/mL | Oxidative stress | 30 Gy ɣ rays | [37] |
| L-alpha-Glycerylphosphorylcholine (GPC) | Radioprotector | 194–1944 µM | Oxidative stress, Fibrosis | 5–20 Gy ɣ rays | [38] |
| Phenformin Hydrochloride | Radioprotector | 25 µM | Oxidative stress, Apoptosis | 4 Gy X rays | [39] |
| Ru(CO)3Cl-glycinate (CORM-3) | Radioprotector | 10 µM | Oxidative stress, Apoptosis | 4 Gy X rays | [40] |
| (Z)-(+/−)-2-(1-benzylindol-3-ylmethylene)-1-azabicyclo [2.2.2]octan-3-ol (VJ115) | Radiosensitizer | 50 µM | Oxidative stress, Apoptosis | 5–20 Gy ɣ rays | [41] |
| 2-methyl-1,4-naphthoquinone (Menadione) | Radiosensitizer | 0–10 µM | DNA damage | 0.15–1.5 Gy ɣ rays | [42] |
| 4′-bromo-3′-nitropropiophenone (NS-123) | Radiosensitizer | 30 µM | DNA damage | 10 Gy ɣ rays | [43] |
| Ku70 MOs | Radiosensitizer | 4–5 ng | DNA damage | 50 cGy ɣ rays | [44] |
| Radiosensitizing mutation 7 (rs7) | Radiosensitizer | - * | Apoptosis, DNA damage | 8–15 Gy X rays | [45] |
| Oxofendazole | Radiosensitizer | 20 µg/mL | Cell cycle, Apoptosis, DNA damage | 15 Gy ɣ rays | [46] |
| GȌ6976 | Radiosensitizer | 20 µg/mL | Cell cycle, Apoptosis, DNA damage | 15 Gy ɣ rays | [46] |
| S-nitroso-N-acetylpenicillamine (SNAP) | Radioprotector | 20–100 µM | Apoptosis | 75 mGy X rays | [47] |
| HypNA-pPNA 16-mer | Radioprotector | 0.5 pmol | Cell cycle, Apoptosis | 0–40 Gy X rays | [48] |
| pifthrin-a (PFTa) | Radioprotector | 1 µM | Cell cycle, Apoptosis | 0–40 Gy X rays | [48] |
| PS-341 | Radiosensitizer | 1 µM | Apoptosis | 0–20 Gy X rays | [49] |
| mcherry-BAD mRNA | Radiosensitizer | 100 ng/µL | Apoptosis | 15 Gy ɣ rays | [50] |
| AEG-1 knockdown cells | Radiosensitizer | - * | Migration, invasion | 0–10 Gy X rays | [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pucci, G.; Forte, G.I.; Cavalieri, V. Evaluation of Epigenetic and Radiomodifying Effects during Radiotherapy Treatments in Zebrafish. Int. J. Mol. Sci. 2021, 22, 9053. https://doi.org/10.3390/ijms22169053
Pucci G, Forte GI, Cavalieri V. Evaluation of Epigenetic and Radiomodifying Effects during Radiotherapy Treatments in Zebrafish. International Journal of Molecular Sciences. 2021; 22(16):9053. https://doi.org/10.3390/ijms22169053
Chicago/Turabian StylePucci, Gaia, Giusi Irma Forte, and Vincenzo Cavalieri. 2021. "Evaluation of Epigenetic and Radiomodifying Effects during Radiotherapy Treatments in Zebrafish" International Journal of Molecular Sciences 22, no. 16: 9053. https://doi.org/10.3390/ijms22169053
APA StylePucci, G., Forte, G. I., & Cavalieri, V. (2021). Evaluation of Epigenetic and Radiomodifying Effects during Radiotherapy Treatments in Zebrafish. International Journal of Molecular Sciences, 22(16), 9053. https://doi.org/10.3390/ijms22169053







