Altered Differentiation of Tendon-Derived Stem Cells in Diabetic Conditions Mediated by Macrophage Migration Inhibitory Factor
Abstract
:1. Introduction
2. Results
2.1. Confirmation of Streptozotocin(STZ)-Induced Diabetes(DM) Rat Model
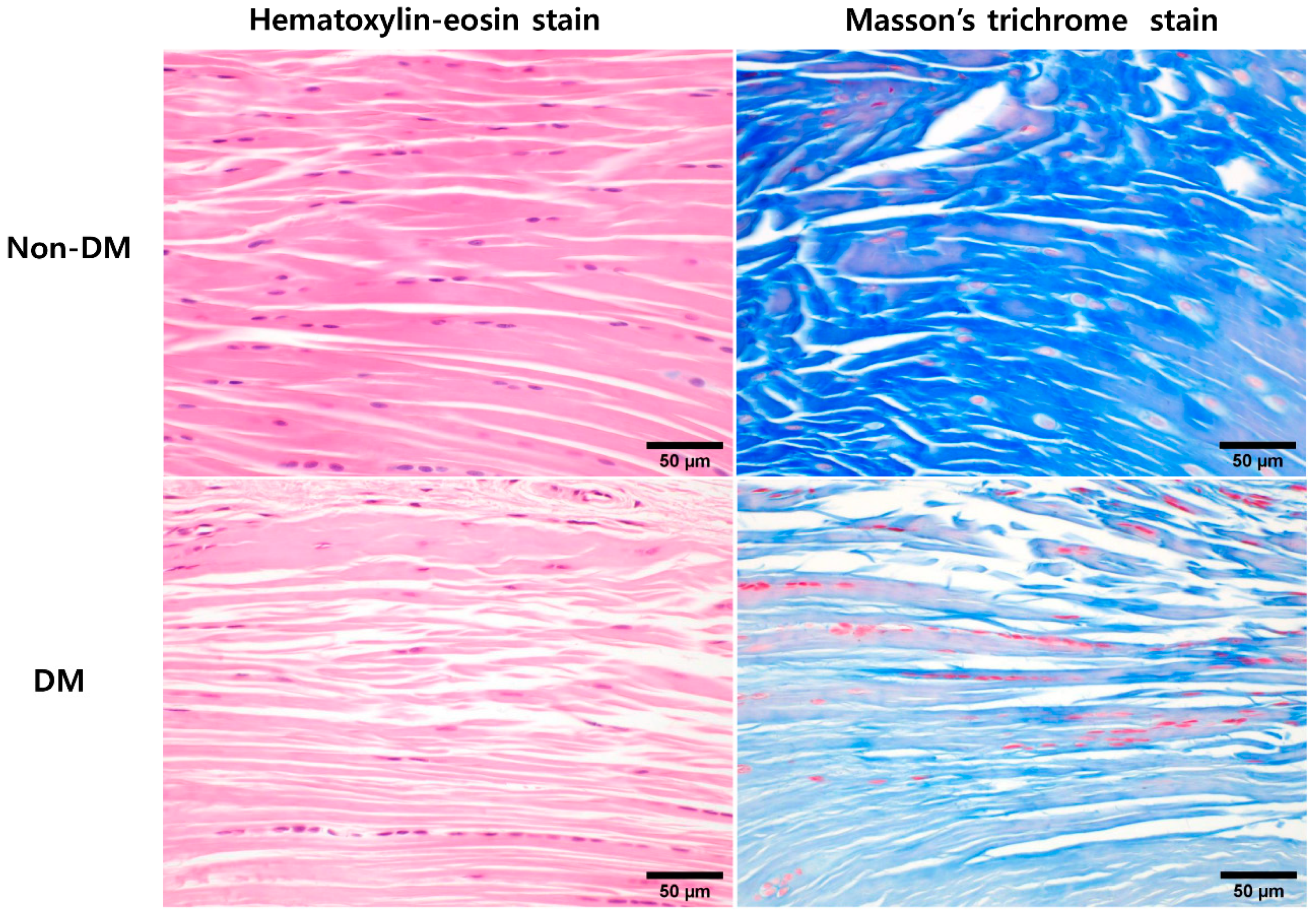
2.2. In Vivo Animal Experiments: Comparative Histological Analysis of DM and Non-DM Groups
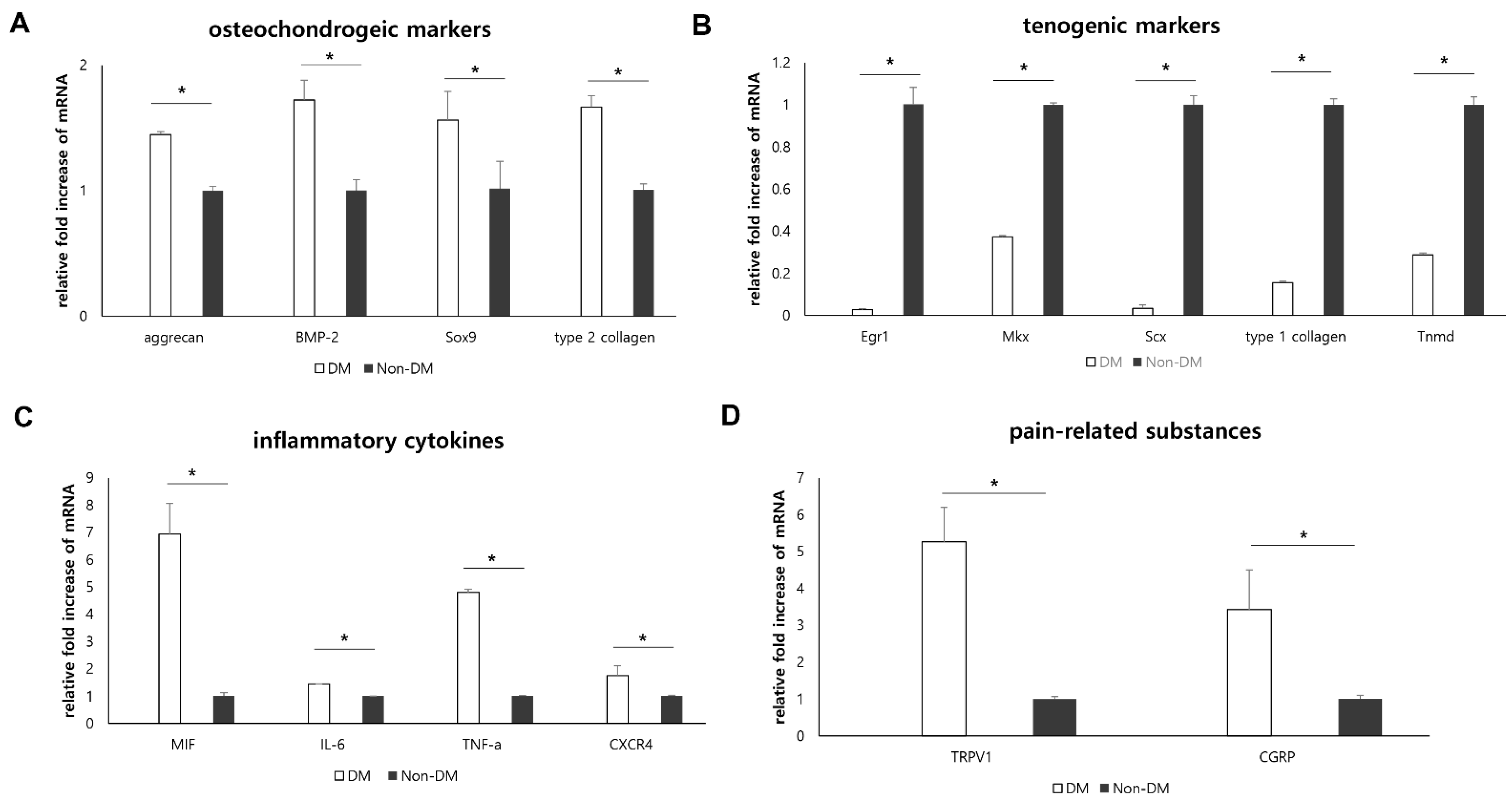
2.3. In Vivo Animal Experiments: Effect of Diabetic Condition on Supraspinatus Tendon
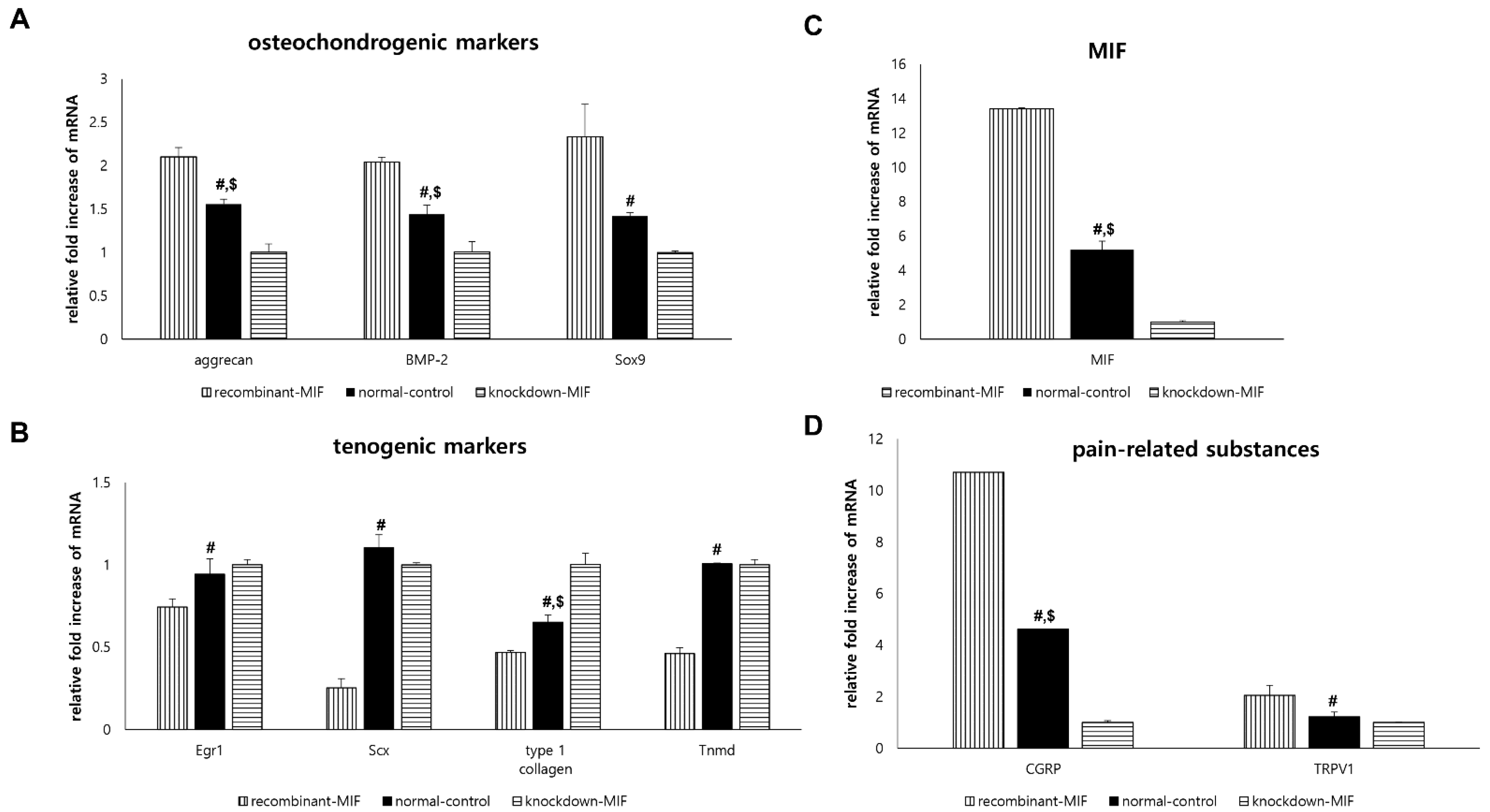
2.4. In Vitro Experiments: Effect of MIF on TdSCs under Non-Hyperglycemic Condition
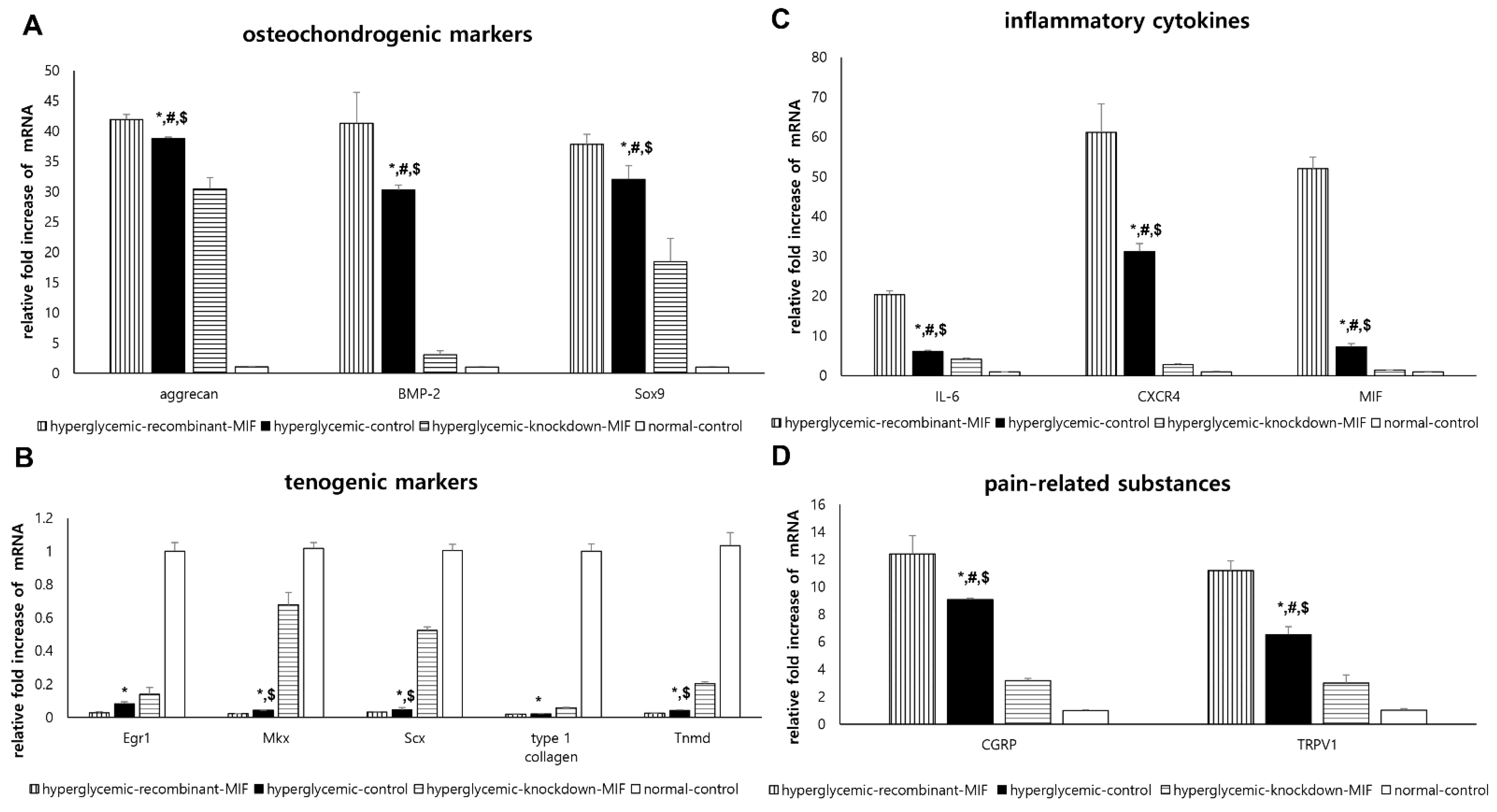
2.5. In Vitro Experiments: Effect of Hyperglycemic Condition and MIF on TdSCs
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. In Vivo Animal Experiments: Type 1 Diabetic Rat Model
4.3. In Vivo Animal Experiments: Tissue Sampling
4.4. In Vivo Animal Experiments: Supraspinatus Tendon Histology
4.5. In Vitro Experiments: TdSCs Culture
4.6. In Vitro TdSCs Experiments under Non-Hyperglycemic Normal Conditions: Recombinant MIF Treatment vs. Small Interference RNA Transfection
4.7. In Vitro TdSCs Experiments under Hyperglycemic Condition: Recombinant MIF Treatment vs. Small Interference RNA Transfection for TdSCs
4.8. In Vivo Animal Experiments & In Vitro TdSCs Experiments: RT-PCR
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sunwoo, J.Y.; Eliasberg, C.D.; Carballo, C.B.; Rodeo, S.A. The role of the macrophage in tendinopathy and tendon healing. J. Orthop. Res. 2020, 38, 1666–1675. [Google Scholar] [CrossRef]
- Xu, L.; Xu, K.; Wu, Z.; Chen, Z.; He, Y.; Ma, C.; Moqbel, S.A.A.; Ran, J.; Zhang, C.; Wu, L.; et al. Pioglitazone attenuates advanced glycation end products-induced apoptosis and calcification by modulating autophagy in tendon-derived stem cells. J. Cell. Mol. Med. 2020, 24, 2240–2251. [Google Scholar] [CrossRef]
- Jomaa, G.; Kwan, C.K.; Fu, S.C.; Ling, S.K.; Chan, K.M.; Yung, P.S.; Rolf, C. A systematic review of inflammatory cells and markers in human tendinopathy. BMC Musculoskelet. Disord. 2020, 21, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.Y.; Hua, Y.H. Achilles Tendinopathy: Current Concepts about the Basic Science and Clinical Treatments. BioMed Res. Int. 2016, 2016, 6492597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kader, D.; Saxena, A.; Movin, T.; Maffulli, N. Achilles tendinopathy: Some aspects of basic science and clinical management. Br. J. Sports Med. 2002, 36, 239–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirchgesner, T.; Larbi, A.; Omoumi, P.; Malghem, J.; Zamali, N.; Manelfe, J.; Lecouvet, F.; Vande Berg, B.; Djebbar, S.; Dallaudière, B. Drug-induced tendinopathy: From physiology to clinical applications. Jt. Bone Spine 2014, 81, 485–492. [Google Scholar] [CrossRef]
- Buck, F.M.; Grehn, H.; Hilbe, M.; Pfirrmann, C.W.; Manzanell, S.; Hodler, J. Magnetic resonance histologic correlation in rotator cuff tendons. J. Magn. Reson. Imaging 2010, 32, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Leong, H.T.; Fu, S.C.; He, X.; Oh, J.H.; Yamamoto, N.; Hang, S. Risk factors for rotator cuff tendinopathy: A systematic review and meta-analysis. J. Rehabil. Med. 2019, 51, 627–637. [Google Scholar] [CrossRef] [Green Version]
- Ranger, T.A.; Wong, A.M.; Cook, J.L.; Gaida, J.E. Is there an association between tendinopathy and diabetes mellitus? A systematic review with meta-analysis. Br. J. Sports Med. 2016, 50, 982–989. [Google Scholar] [CrossRef]
- Roh, Y.H.; Oh, M.; Noh, J.H.; Gong, H.S.; Baek, G.H. Effect of Metabolic Syndrome on the Functional Outcome of Corticosteroid Injection for Lateral Epicondylitis: Retrospective Matched Case-Control Study. Sci. Rep. 2017, 7, 10845. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.J.; McDougall, C.; Brown, I.D.; Jaberoo, M.C.; Stearns, A.; Ashraf, R.; Fisher, M.; Kelly, I.G. Prevalence of symptoms and signs of shoulder problems in people with diabetes mellitus. J. Shoulder Elb. Surg. 2007, 16, 748–751. [Google Scholar] [CrossRef]
- Afolabi, B.I.; Idowu, B.M.; Onigbinde, S.O. Achilles tendon degeneration on ultrasound in type 2 diabetic patients. J. Ultrason. 2021, 20, e291–e299. [Google Scholar] [CrossRef]
- Ackerman, J.E.; Best, K.T.; Muscat, S.N.; Loiselle, A.E. Metabolic Regulation of Tendon Inflammation and Healing Following Injury. Curr. Rheumatol. Rep. 2021, 23, 15. [Google Scholar] [CrossRef]
- Chbinou, N.; Frenette, J. Insulin-dependent diabetes impairs the inflammatory response and delays angiogenesis following Achilles tendon injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R952–R957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Partridge, L.; Rajbhandari, S. Achilles Tendon in Diabetes. Curr. Diabetes Rev. 2017, 13, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Studentsova, V.; Mora, K.M.; Glasner, M.F.; Buckley, M.R.; Loiselle, A.E. Obesity/Type II Diabetes Promotes Function-limiting Changes in Murine Tendons that are not reversed by Restoring Normal Metabolic Function. Sci. Rep. 2018, 8, 9218. [Google Scholar] [CrossRef] [Green Version]
- Nichols, A.E.C.; Oh, I.; Loiselle, A.E. Effects of Type II Diabetes Mellitus on Tendon Homeostasis and Healing. J. Orthop. Res. 2020, 38, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.S.; Moon, S.C.; Jeon, J.W.; Rhee, Y.G. The influence of diabetes mellitus on clinical and structural outcomes after arthroscopic rotator cuff repair. Am. J. Sports Med. 2015, 43, 991–997. [Google Scholar] [CrossRef]
- Mall, N.A.; Tanaka, M.J.; Choi, L.S.; Paletta, G.A., Jr. Factors affecting rotator cuff healing. J. Bone Jt. Surg. Am. 2014, 96, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Snedeker, J.G.; Gautieri, A. The role of collagen crosslinks in ageing and diabetes—The good, the bad, and the ugly. Muscles Ligaments Tendons J. 2014, 4, 303–308. [Google Scholar] [CrossRef]
- Kwan, C.K.; Fu, S.C.; Yung, P.S. A high glucose level stimulate inflammation and weaken pro-resolving response in tendon cells—A possible factor contributing to tendinopathy in diabetic patients. Asia Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 2020, 19, 1–6. [Google Scholar] [CrossRef]
- Lui, P.P.; Chan, K.M. Tendon-derived stem cells (TDSCs): From basic science to potential roles in tendon pathology and tissue engineering applications. Stem Cell Rev. Rep. 2011, 7, 883–897. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Li, Y.J.; Dai, G.C.; Lin, Y.C.; Li, G.; Wang, C.; Chen, H.; Rui, Y.F. Impaired function of tendon-derived stem cells in experimental diabetes mellitus rat tendons: Implications for cellular mechanism of diabetic tendon disorder. Stem Cell Res. Ther. 2019, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Rui, Y.F.; Lui, P.P.; Wong, Y.M.; Tan, Q.; Chan, K.M. Altered fate of tendon-derived stem cells isolated from a failed tendon-healing animal model of tendinopathy. Stem Cells Dev. 2013, 22, 1076–1085. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.Q.; Jin, W.D.; Li, Y.S. Roles of macrophage migration inhibitory factor in polymyositis: Inflammation and regeneration. J. Int. Med. Res. 2018, 46, 732–738. [Google Scholar] [CrossRef]
- Abu El-Asrar, A.M.; Ahmad, A.; Siddiquei, M.M.; De Zutter, A.; Allegaert, E.; Gikandi, P.W.; De Hertogh, G.; Van Damme, J.; Opdenakker, G.; Struyf, S. The Proinflammatory and Proangiogenic Macrophage Migration Inhibitory Factor Is a Potential Regulator in Proliferative Diabetic Retinopathy. Front. Immunol. 2019, 10, 2752. [Google Scholar] [CrossRef] [Green Version]
- Khalilpour, J.; Roshan-Milani, S.; Gharalari, F.H.; Fard, A.A. Macrophage migration inhibitory factor antagonist (p425) ameliorates kidney histopathological and functional changes in diabetic rats. J. Bras. Nefrol. 2019, 41, 315–322. [Google Scholar] [CrossRef]
- Noh, S.U.; Lee, W.Y.; Kim, W.S.; Lee, Y.T.; Yoon, K.J. Expression of macrophage migration inhibitory factor in footpad skin lesions with diabetic neuropathy. Mol. Pain 2018, 14, 1744806918775482. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Wang, X.; Deng, X.; Zhang, Y.; Gao, W. Correlation between Plasma Macrophage Migration Inhibitory Factor Levels and Long-Term Prognosis in Patients with Acute Myocardial Infarction Complicated with Diabetes. Mediat. Inflamm. 2019, 2019, 8276180. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Rui, Y.F.; Li, G.; Wang, C. Alterations of tendons in diabetes mellitus: What are the current findings? Int. Orthop. 2015, 39, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Bernhagen, J.; Krohn, R.; Lue, H.; Gregory, J.L.; Zernecke, A.; Koenen, R.R.; Dewor, M.; Georgiev, I.; Schober, A.; Leng, L.; et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat. Med. 2007, 13, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Korf, H.; Breser, L.; Van Hoeck, J.; Godoy, J.; Cook, D.P.; Stijlemans, B.; De Smidt, E.; Moyson, C.; Monteiro Carvalho Mori Cunha, J.P.; Rivero, V.; et al. MIF inhibition interferes with the inflammatory and T cell-stimulatory capacity of NOD macrophages and delays autoimmune diabetes onset. PLoS ONE 2017, 12, e0187455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Q.L.; Wang, X.Z.; Xie, W.; Chen, X.W.; Zhu, Y.L.; Li, X.G. Macrophage migration inhibitory factor may contribute to hypertrophy of lumbar ligamentum flavum in type 2 diabetes mellitus. Chin. Med. J. 2020, 133, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Batista, F.; Nery, C.; Pinzur, M.; Monteiro, A.C.; de Souza, E.F.; Felippe, F.H.; Alcântara, M.C.; Campos, R.S. Achilles tendinopathy in diabetes mellitus. Foot Ankle Int. 2008, 29, 498–501. [Google Scholar] [CrossRef]
- Kameyama, M.; Chen, K.R.; Mukai, K.; Shimada, A.; Atsumi, Y.; Yanagimoto, S. Histopathological characteristics of stenosing flexor tenosynovitis in diabetic patients and possible associations with diabetes-related variables. J. Hand Surg. Am. 2013, 38, 1331–1339. [Google Scholar] [CrossRef]
- Renner, P.; Roger, T.; Calandra, T. Macrophage migration inhibitory factor: Gene polymorphisms and susceptibility to inflammatory diseases. Clin. Infect. Dis. 2005, 41 (Suppl. 7), S513–S519. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.L.; Morand, E.F. The role of macrophage migration inhibitory factor in the inflammatory immune response and rheumatoid arthritis. Wien. Med. Wochenschr. 2006, 156, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.K.; Cox, G.M.; Tian, J.B.; Zha, A.M.; Wei, P.; Kigerl, K.A.; Reddy, M.K.; Dagia, N.M.; Sielecki, T.; Zhu, M.X.; et al. Macrophage migration inhibitory factor (MIF) is essential for inflammatory and neuropathic pain and enhances pain in response to stress. Exp. Neurol. 2012, 236, 351–362. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Song, D.H.; Kim, S.J. Characteristics of tendon derived stem cells according to different factors to induce the tendinopathy. J. Cell. Physiol. 2018, 233, 6196–6206. [Google Scholar] [CrossRef]
| Forward | Reverse | |
|---|---|---|
| Aggrecan | 5′-AAGTGCTATGCTGGCTGGTT-3′ | 5′-GGTCTGGTTGGGGTAGAGGT-3′ |
| BMP-2 | 5′-TAGTGACTTTTGGCCACGACG-3′ | 5′-GCTTCCGCTGTTTGTGTTTG-3′ |
| Sox9 | 5′-CTGCGACCTCAGAAGGAAAG-3′ | 5′-CGCTGGTATTCAGGGAGGTA-3′ |
| Erg1 | 5′-TTATCCCAGCCAAACTACCC-3′ | 5′-CAGAGGAAGACGATGAAGCA-3′ |
| type 2 collagen | 5′-CTCAAGTCGCTGAACAACCA-3′ | 5′-GTCTCCGCTCTTCCACTCTG-3′ |
| Mkx | 5′-CTCCGGTTTCGATTGAGAAG-3′ | 5′-AGTTGTTCACGGCTCTTCGT-3′ |
| Scx | 5′-AACACGGCCTTCACTGCGCTG-3′ | 5′-CAGTAGCACGTTGCCCAGGTG-3′ |
| Tnmd | 5′-GTGGTCCCACAAGTGAAGGT-3′ | 5′-GTCTTCCTCGCTTGCTTGTC-3′ |
| type 1 collagen | 5′-TGGAGACAGGTCAGACCTG-3′ | 5′-TATTCGATGACTGTCTTGCC-3′ |
| CGRP | 5′-CTGTCGAGTTTCCACAGGTTCC-3′ | 5′-TTGCGCAGAGGTACGGTTCC-3′ |
| TRPV1 | 5′- CCACAGCGGTGGTGACGC -3′ | 5′- GGAGCTGTCAGGTGGCCG -3′ |
| CXCR4 | 5′-ATCATCTCCAAGCTGTCACACTCC-3′ | 5′-GTGATGGAGATCCACTTGTGCAC-3′ |
| MIF | 5′-CCCAGAACCGCAACTACAGCAA-3′ | 5′-CGTTGGCTGCGTTCATGTCGTAAT-3′ |
| TNF-α | 5′-CCCACGTCGTAGCAAACCACCA-3′ | 5′-CCATTGGCCAGGAGGGCGTTG-3′ |
| IL-6 | 5′-CCTGGAGTTTGTGAAGAACAACT-3′ | 5′-GGAAGTTGGGGTAGGAAGGA-3′ |
| GAPDH | 5′-AGTCTACTGGCGTCTTCA-3′ | 5′-TTGTCATATTTCTCGTGGT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-H.; Noh, S.-U.; Chae, S.-W.; Kim, S.-J.; Lee, Y.-T. Altered Differentiation of Tendon-Derived Stem Cells in Diabetic Conditions Mediated by Macrophage Migration Inhibitory Factor. Int. J. Mol. Sci. 2021, 22, 8983. https://doi.org/10.3390/ijms22168983
Kim D-H, Noh S-U, Chae S-W, Kim S-J, Lee Y-T. Altered Differentiation of Tendon-Derived Stem Cells in Diabetic Conditions Mediated by Macrophage Migration Inhibitory Factor. International Journal of Molecular Sciences. 2021; 22(16):8983. https://doi.org/10.3390/ijms22168983
Chicago/Turabian StyleKim, Du-Hwan, Sun-Up Noh, Seoung-Wan Chae, Sang-Jun Kim, and Yong-Taek Lee. 2021. "Altered Differentiation of Tendon-Derived Stem Cells in Diabetic Conditions Mediated by Macrophage Migration Inhibitory Factor" International Journal of Molecular Sciences 22, no. 16: 8983. https://doi.org/10.3390/ijms22168983
APA StyleKim, D.-H., Noh, S.-U., Chae, S.-W., Kim, S.-J., & Lee, Y.-T. (2021). Altered Differentiation of Tendon-Derived Stem Cells in Diabetic Conditions Mediated by Macrophage Migration Inhibitory Factor. International Journal of Molecular Sciences, 22(16), 8983. https://doi.org/10.3390/ijms22168983






