A DNA Aptameric Ligand of Human Transferrin Receptor Generated by Cell-SELEX
Abstract
1. Introduction
2. Results and Discussion
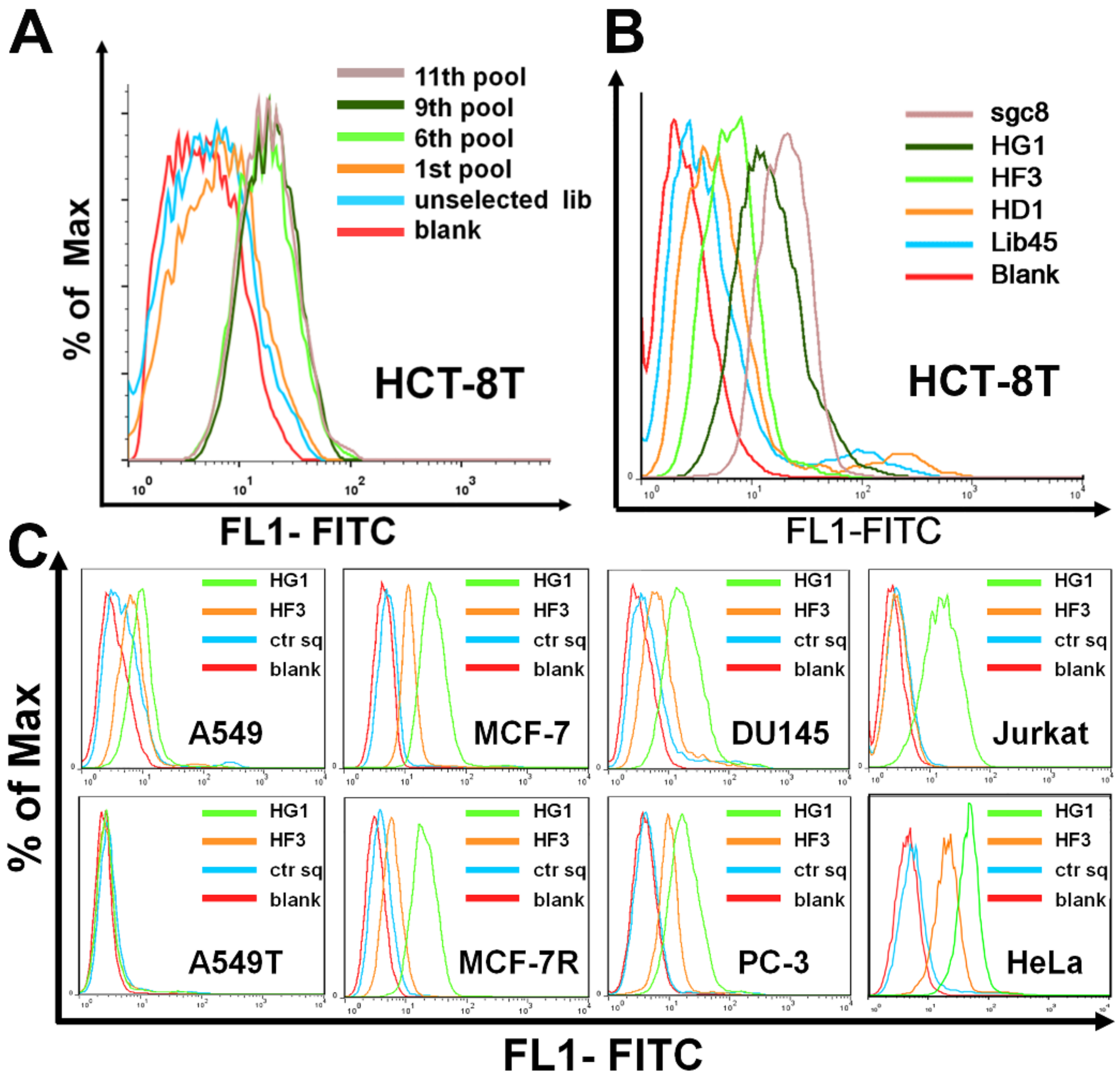
2.1. Selection of Aptamers against Malignant Tumor Cells
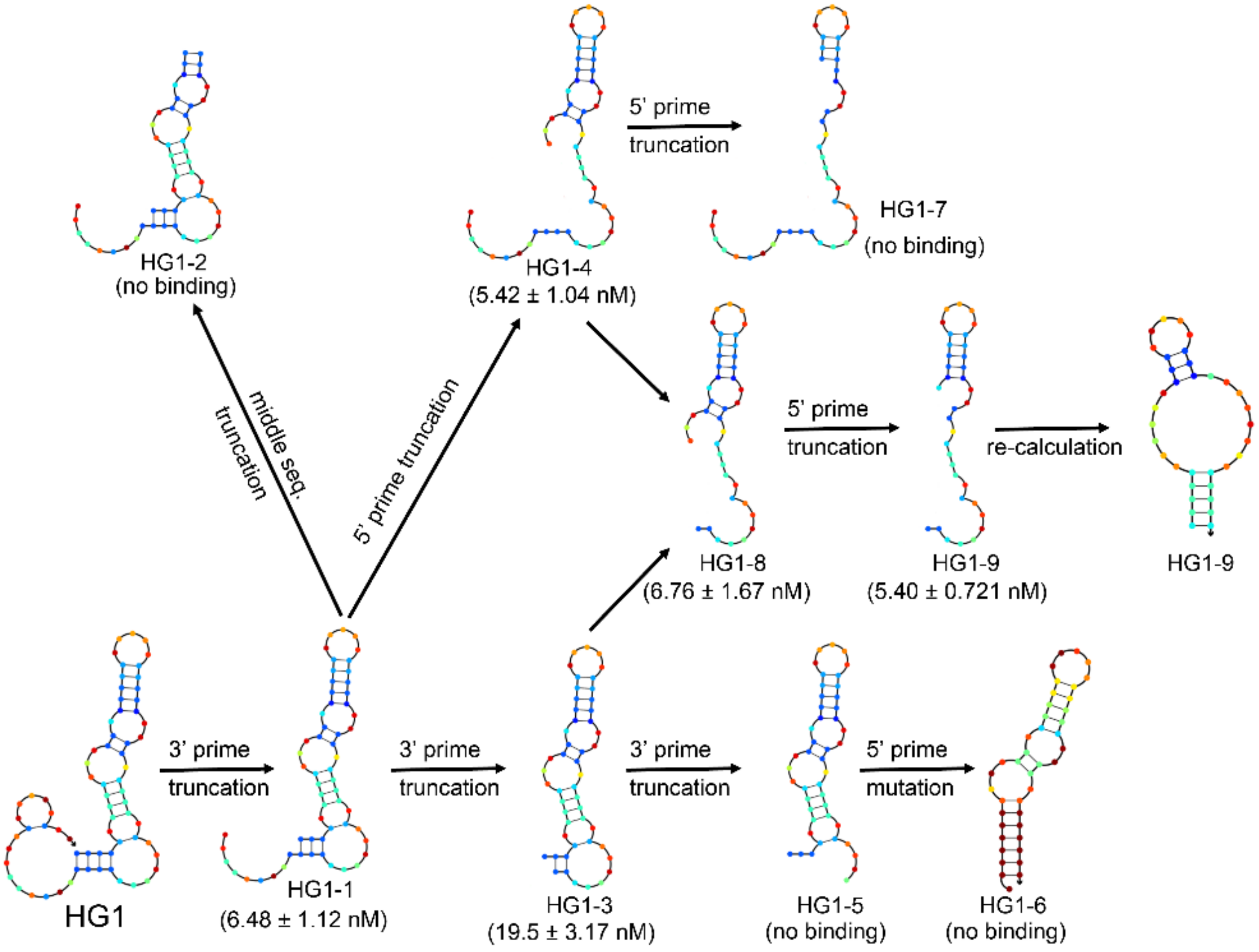
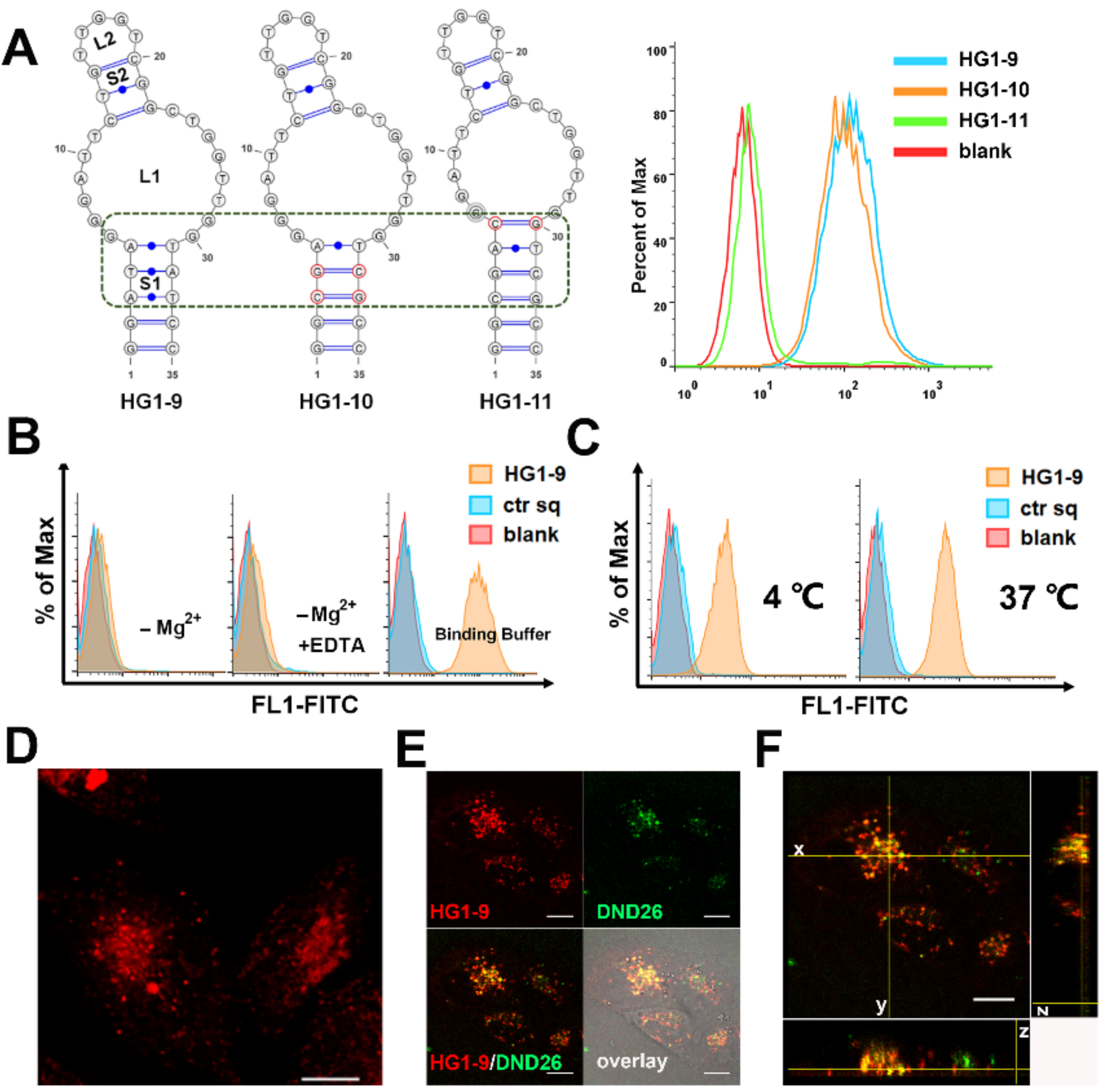
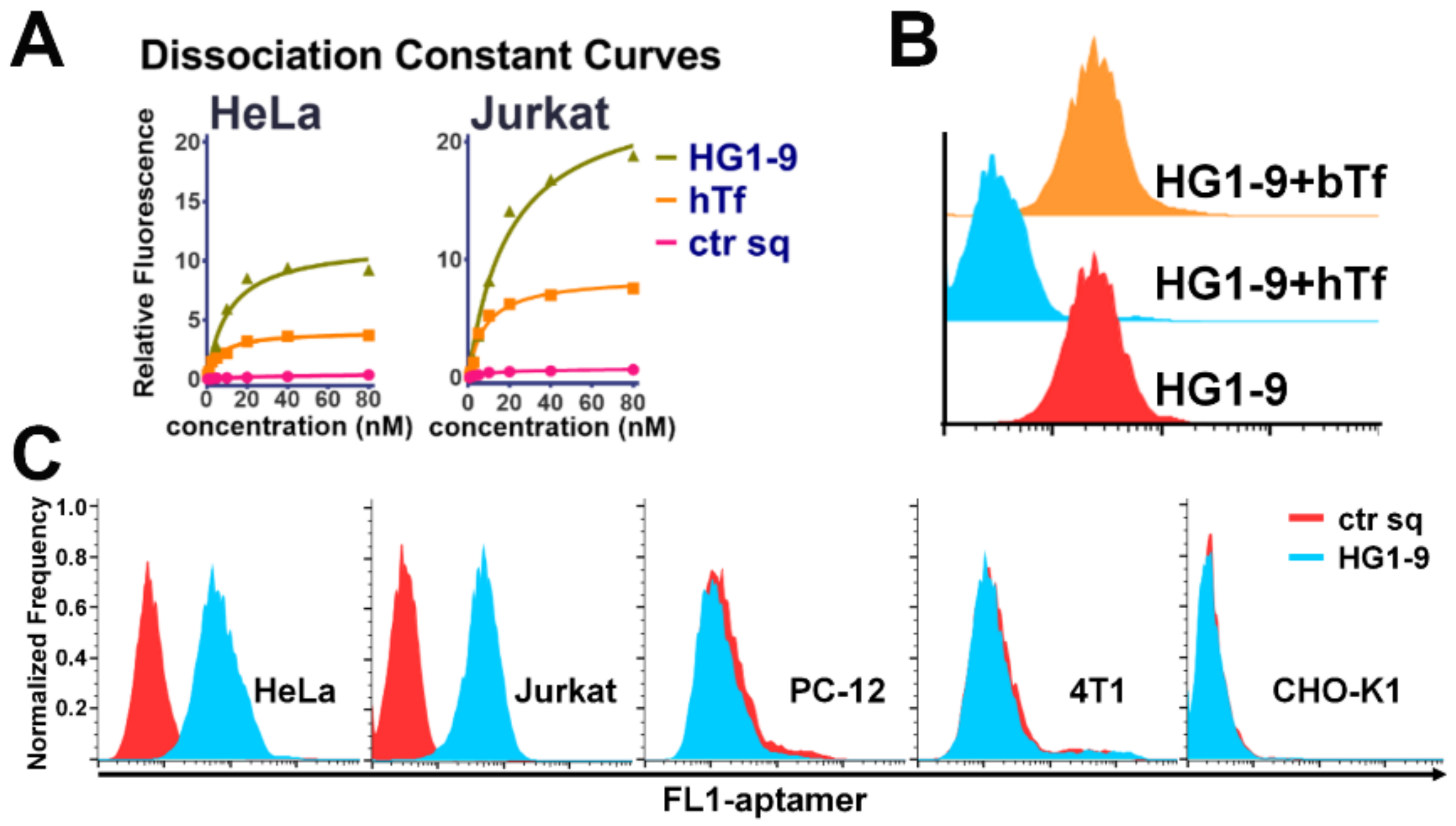
2.2. Characterization and Optimization of Selected Aptamers
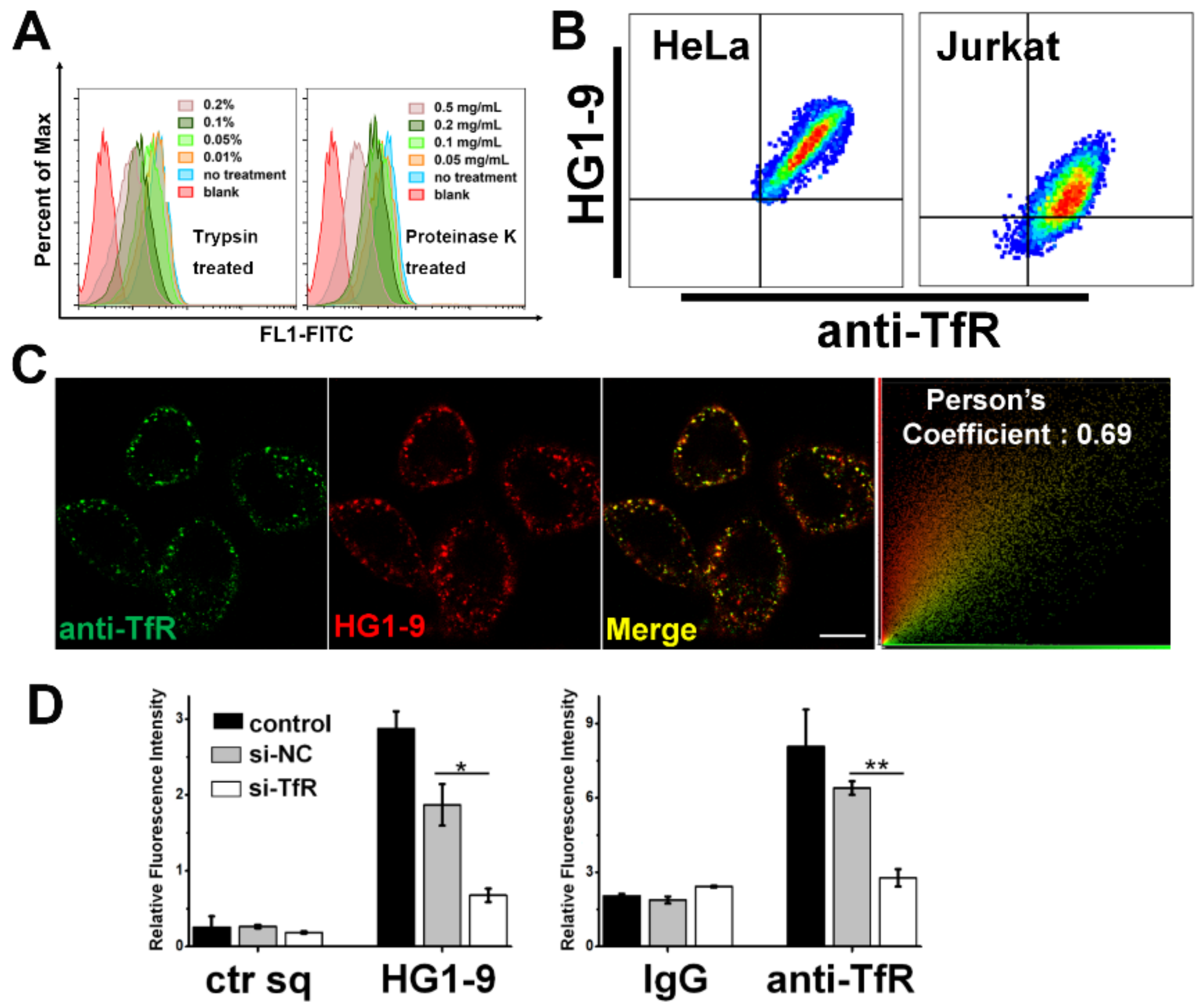
2.3. Identification of Target Protein of Aptamer HG1-9
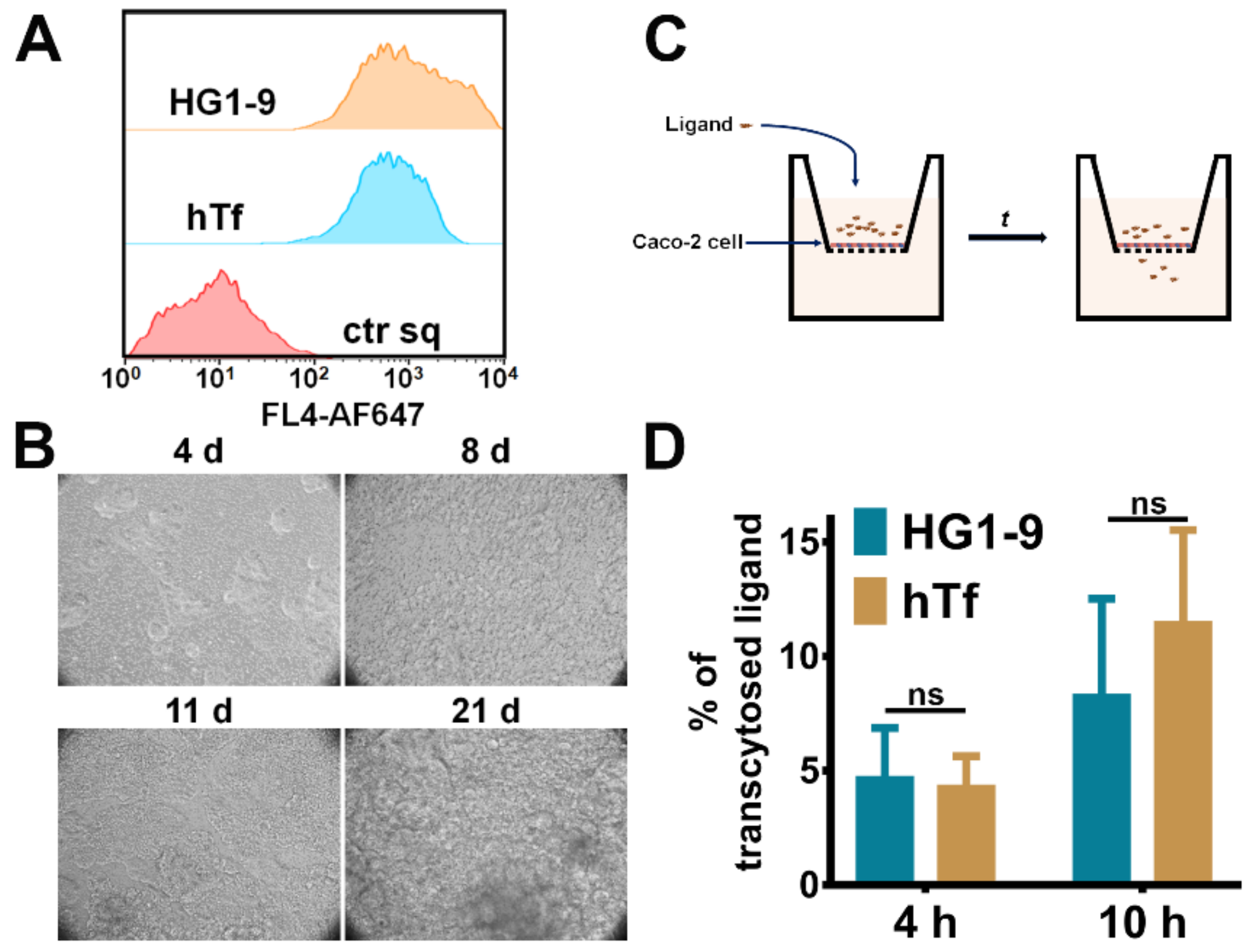
2.4. Comparison of Natural Ligand hTf and Aptamer HG1-9
2.5. The Transcytosis of Aptamer HG1-9 across Epithelium Barrier
3. Materials and Methods
3.1. Materials
3.2. Cell Lines and Cell Culture
3.3. Procedures of Cell-SELEX
3.4. Flow Cytometry Analysis
3.5. Confocal Microscopy Imaging
3.6. Proteinase Digestion
3.7. SILAC-Based Proteomic Assay for Target Protein Identification
3.8. siRNA Transfection
3.9. Investigation of the Transcytosis of hTf and HG1-9 by an Epithelium Model Established by Caco-2 Cells
3.10. Statistical Analysis
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lawrence, C.M.; Ray, S.; Babyonyshev, M.; Galluser, R.; Borhani, D.W.; Harrison, S.C. Crystal structure of the ectodomain of human transferrin receptor. Science 1999, 286, 779–782. [Google Scholar] [CrossRef]
- Montemiglio, L.C.; Testi, C.; Ceci, P.; Falvo, E.; Pitea, M.; Savino, C.; Arcovito, A.; Peruzzi, G.; Baiocco, P.; Mancia, F.; et al. Cryo-EM structure of the human ferritin-transferrin receptor 1 complex. Nat. Commun. 2019, 10, 1121. [Google Scholar] [CrossRef]
- Daniels, T.R.; Delgado, T.; Rodriguez, J.A.; Helguera, G.; Penichet, M.L. The transferrin receptor part I: Biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin. Immunol. 2006, 121, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fang, C.J.; Ryan, J.C.; Niemi, E.C.; Lebron, J.A.; Bjorkman, P.J.; Arase, H.; Torti, F.M.; Torti, S.V.; Nakamura, M.C.; et al. Binding and uptake of H-ferritin are mediated by human transferrin receptor-1. Proc. Natl. Acad. Sci. USA 2010, 107, 3505–3510. [Google Scholar] [CrossRef] [PubMed]
- Prutki, M.; Poljak-Blazi, M.; Jakopovic, M.; Tomas, D.; Stipancic, I.; Zarkovic, N. Altered iron metabolism, transferrin receptor 1 and ferritin in patients with colon cancer. Cancer Lett. 2006, 238, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Daniels, T.R.; Bernabeu, E.; Rodriguez, J.A.; Patel, S.; Kozman, M.; Chiappetta, D.A.; Holler, E.; Ljubimova, J.Y.; Helguera, G.; Penichet, M.L. The transferrin receptor and the targeted delivery of therapeutic agents against cancer. Biochim. Biophys. Acta 2012, 1820, 291–317. [Google Scholar] [CrossRef]
- Wiley, D.T.; Webster, P.; Gale, A.; Davis, M.E. Transcytosis and brain uptake of transferrin-containing nanoparticles by tuning avidity to transferrin receptor. Proc. Natl. Acad. Sci. USA 2013, 110, 8662–8667. [Google Scholar] [CrossRef]
- Tortorella, S.; Karagiannis, T.C. Transferrin receptor-mediated endocytosis: A useful target for cancer therapy. J. Membr. Biol. 2014, 247, 291–307. [Google Scholar] [CrossRef]
- Zhang, R.; Feng, G.; Zhang, C.J.; Cai, X.; Cheng, X.; Liu, B. Real-Time Specific Light-Up Sensing of Transferrin Receptor: Image-Guided Photodynamic Ablation of Cancer Cells through Controlled Cytomembrane Disintegration. Anal. Chem. 2016, 88, 4841–4848. [Google Scholar] [CrossRef]
- Shangguan, D.; Li, Y.; Tang, Z.; Cao, Z.C.; Chen, H.W.; Mallikaratchy, P.; Sefah, K.; Yang, C.J.; Tan, W. Aptamers evolved from live cells as effective molecular probes for cancer study. Proc. Natl. Acad. Sci. USA 2006, 103, 11838–11843. [Google Scholar] [CrossRef]
- Liu, J.; You, M.X.; Pu, Y.; Liu, H.X.; Ye, M.; Tan, W.H. Recent Developments in Protein and Cell-Targeted Aptamer Selection and Applications. Curr. Med. Chem. 2011, 18, 4117–4125. [Google Scholar] [CrossRef]
- Chen, C.H.B.; Dellamaggiore, K.R.; Ouellette, C.P.; Seclano, C.D.; Lizadjohry, M.; Chernis, G.A.; Gonzales, M.; Baltasar, F.E.; Fan, A.L.; Myerowitz, R.; et al. Aptamer-based endocytosis of a lysosomal enzyme. Proc. Natl. Acad. Sci. USA 2008, 105, 15908–15913. [Google Scholar] [CrossRef] [PubMed]
- Wilner, S.E.; Wengerter, B.; Maier, K.; Magalhaes, M.D.B.; Del Amo, D.S.; Pai, S.; Opazo, F.; Rizzoli, S.O.; Yan, A.; Levy, M. An RNA Alternative to Human Transferrin: A New Tool for Targeting Human Cells. Mol. Ther. Nucl. Acids 2012, 1, e21. [Google Scholar] [CrossRef] [PubMed]
- Maier, K.E.; Jangra, R.K.; Shieh, K.R.; Cureton, D.K.; Xiao, H.; Snapp, E.L.; Whelan, S.P.; Chandran, K.; Levy, M. A New Transferrin Receptor Aptamer Inhibits New World Hemorrhagic Fever Mammarenavirus Entry. Mol. Ther.-Nucl. Acids 2016, 5, e321. [Google Scholar] [CrossRef]
- Wu, X.; Liu, H.; Han, D.; Peng, B.; Zhang, H.; Zhang, L.; Li, J.; Liu, J.; Cui, C.; Fang, S.; et al. Elucidation and structural modeling of CD71 as a molecular target for cell-specific aptamer binding. J. Am. Chem. Soc. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhao, Z.; Bai, H.; Fu, T.; Yang, C.; Hu, X.; Liu, Q.; Champanhac, C.; Teng, I.T.; Ye, M.; et al. DNA Aptamer Selected against Pancreatic Ductal Adenocarcinoma for in vivo Imaging and Clinical Tissue Recognition. Theranostics 2015, 5, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Li, W.M.; Bing, T.; Wei, J.Y.; Chen, Z.Z.; Shangguan, D.H.; Fang, J. Cell-SELEX-based selection of aptamers that recognize distinct targets on metastatic colorectal cancer cells. Biomaterials 2014, 35, 6998–7007. [Google Scholar] [CrossRef] [PubMed]
- Tolle, F.; Wilke, J.; Wengel, J.; Mayer, G. By-Product Formation in Repetitive PCR Amplification of DNA Libraries during SELEX. PLoS ONE 2014, 9, e114693. [Google Scholar] [CrossRef]
- Zadeh, J.N.; Steenberg, C.D.; Bois, J.S.; Wolfe, B.R.; Pierce, M.B.; Khan, A.R.; Dirks, R.M.; Pierce, N.A. NUPACK: Analysis and design of nucleic acid systems. J. Comput. Chem. 2011, 32, 170–173. [Google Scholar] [CrossRef]
- Sendra, G.H.; Hoerth, C.H.; Wunder, C.; Lorenz, H. 2D map projections for visualization and quantitative analysis of 3D fluorescence micrographs. Sci. Rep. 2015, 5, 12457. [Google Scholar] [CrossRef]
- Bing, T.; Zhang, N.; Shangguan, D.H. Cell-SELEX, an Effective Way to the Discovery of Biomarkers and Unexpected Molecular Events. Adv. Biosyst. 2019, 3, 1900193. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Bing, T.; Zhang, N.; Wang, L.; Wang, J.; Liu, X.; Shangguan, D. A Nucleus-Targeting DNA Aptamer for Dead Cell Indication. ACS Sens. 2019, 4, 1612–1618. [Google Scholar] [CrossRef]
- Bing, T.; Shangguan, D.; Wang, Y.S. Facile Discovery of Cell-Surface Protein Targets of Cancer Cell Aptamers. Mol. Cell Proteom. 2015, 14, 2692–2700. [Google Scholar] [CrossRef]
- Reed, N.A.; Raliya, R.; Tang, R.; Xu, B.; Mixdorf, M.; Achilefu, S.; Biswas, P. Electrospray Functionalization of Titanium Dioxide Nanoparticles with Transferrin for Cerenkov Radiation Induced Cancer Therapy. ACS Appl. Bio Mater. 2019, 2, 1141–1147. [Google Scholar] [CrossRef]
- Mayle, K.M.; Le, A.M.; Kamei, D.T. The intracellular trafficking pathway of transferrin. Biochim. Biophys. Acta 2012, 1820, 264–281. [Google Scholar] [CrossRef]
- Mazzucchelli, S.; Truffi, M.; Baccarini, F.; Beretta, M.; Sorrentino, L.; Bellini, M.; Rizzuto, M.A.; Ottria, R.; Ravelli, A.; Ciuffreda, P.; et al. H-Ferritin-nanocaged olaparib: A promising choice for both BRCA-mutated and sporadic triple negative breast cancer. Sci. Rep. 2017, 7, 7505. [Google Scholar] [CrossRef]
- Kelly, R.B.; Bonzelius, F.; Cleves, A.; Clift-O’Grady, L.; Grote, E.; Herman, G. Biogenesis of synaptic vesicles. J. Cell Sci. Suppl. 1993, 17, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.L.; Bonzelius, F.; Scully, R.M.; Kelly, R.B.; Herman, G.A. GLUT4 and transferrin receptor are differentially sorted along the endocytic pathway in CHO cells. J. Cell Biol. 1998, 140, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.J.; Norouziyan, F.; Shen, W.C. Accumulation of transferrin in Caco-2 cells: A possible mechanism of intestinal transferrin absorption. J. Control. Release 2007, 122, 393–398. [Google Scholar] [CrossRef] [PubMed][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Bing, T.; Shen, L.; Feng, L.; Liu, X.; Shangguan, D. A DNA Aptameric Ligand of Human Transferrin Receptor Generated by Cell-SELEX. Int. J. Mol. Sci. 2021, 22, 8923. https://doi.org/10.3390/ijms22168923
Zhang N, Bing T, Shen L, Feng L, Liu X, Shangguan D. A DNA Aptameric Ligand of Human Transferrin Receptor Generated by Cell-SELEX. International Journal of Molecular Sciences. 2021; 22(16):8923. https://doi.org/10.3390/ijms22168923
Chicago/Turabian StyleZhang, Nan, Tao Bing, Luyao Shen, Le Feng, Xiangjun Liu, and Dihua Shangguan. 2021. "A DNA Aptameric Ligand of Human Transferrin Receptor Generated by Cell-SELEX" International Journal of Molecular Sciences 22, no. 16: 8923. https://doi.org/10.3390/ijms22168923
APA StyleZhang, N., Bing, T., Shen, L., Feng, L., Liu, X., & Shangguan, D. (2021). A DNA Aptameric Ligand of Human Transferrin Receptor Generated by Cell-SELEX. International Journal of Molecular Sciences, 22(16), 8923. https://doi.org/10.3390/ijms22168923





