Effect of 9,12-Octadecadiynoic Acid on Neurobehavioral Development in Caenorhabditis elegans
Abstract
1. Introduction
2. Results
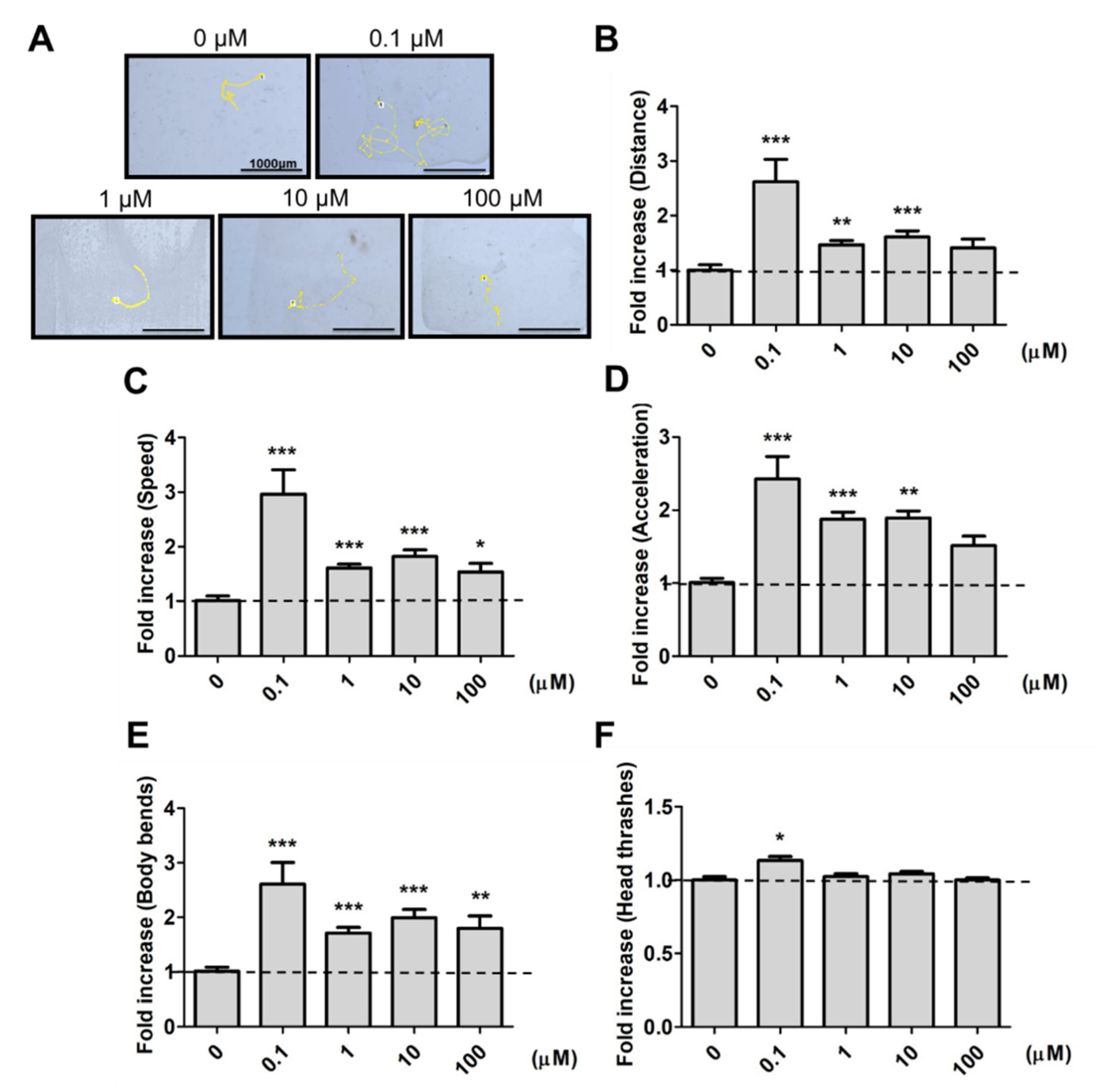
2.1. Effects of 9,12-Octadecadiynoic Acid Supplementation on Neurobehavioral Indicators in Worms
2.2. Effects of 9,12-Octadecadiynoic Acid Supplementation on Aggregative Behavior in Worms
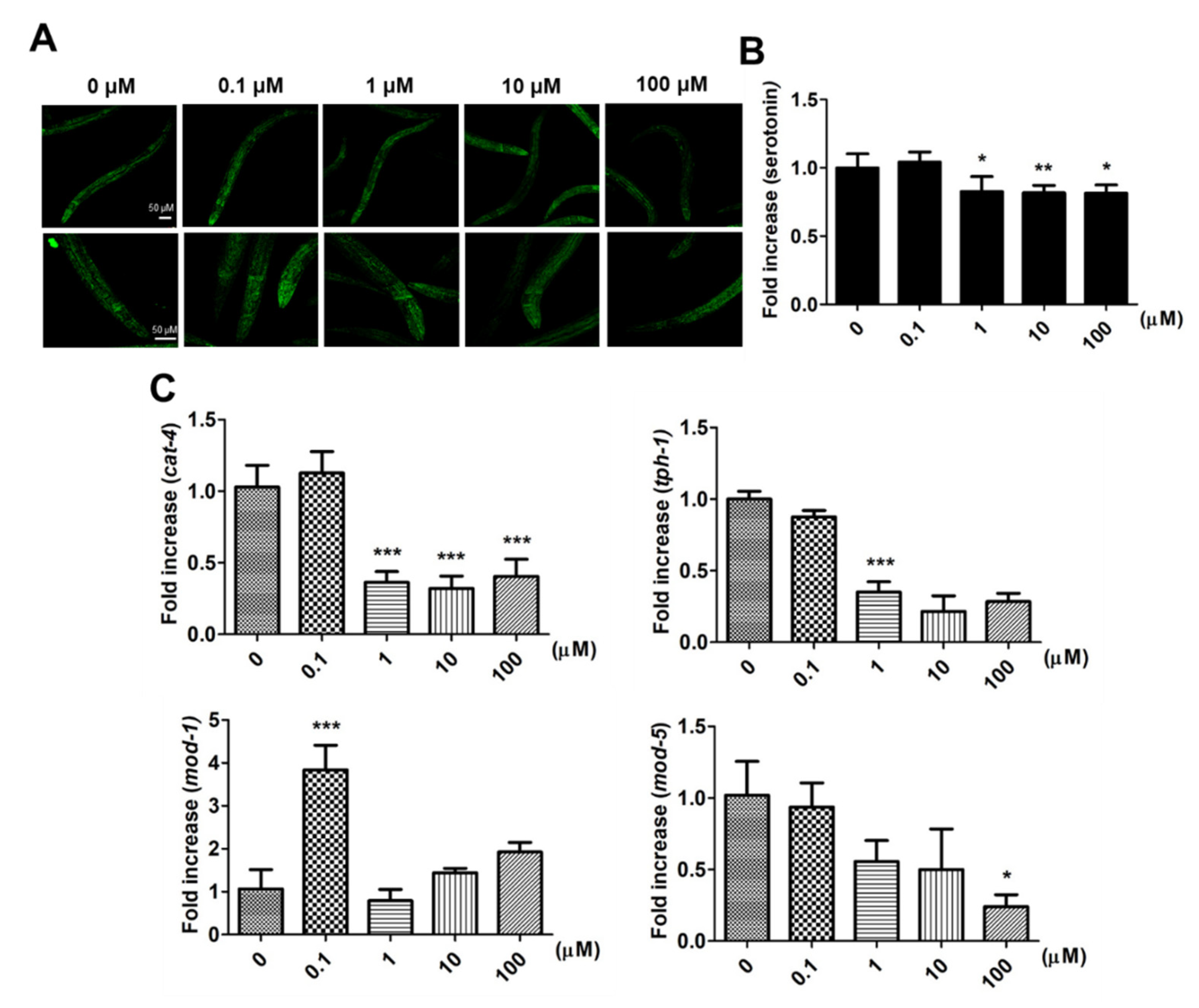
2.3. Supplementary 9,12-Octadecadiynoic Acid in Worms Influences Serotonin Synthesis and Serotonin-Related Gene Expression
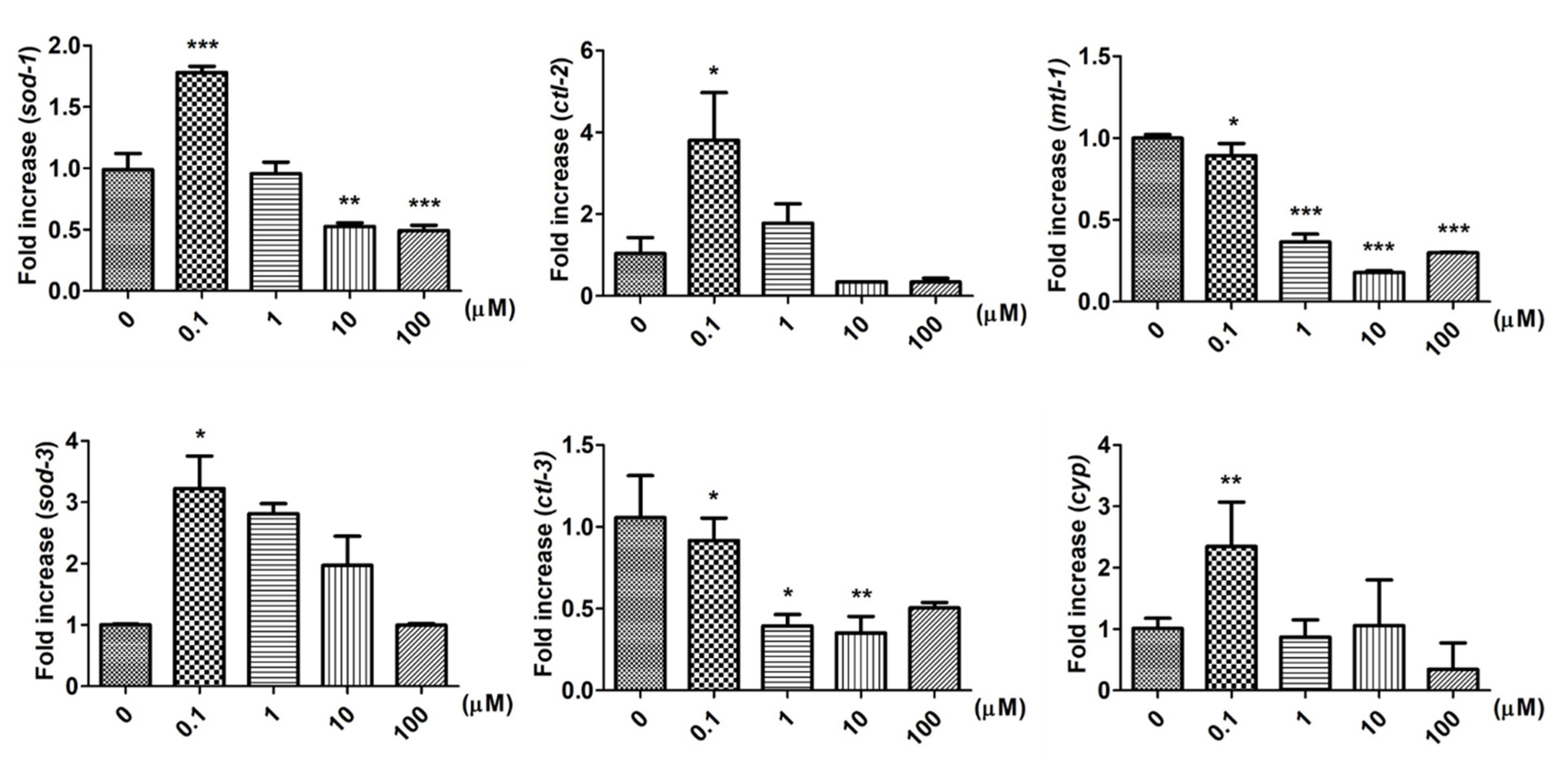
2.4. Supplementary 9,12-Octadecadiynoic Acid in Worms Influences Stress-Related Gene Expression
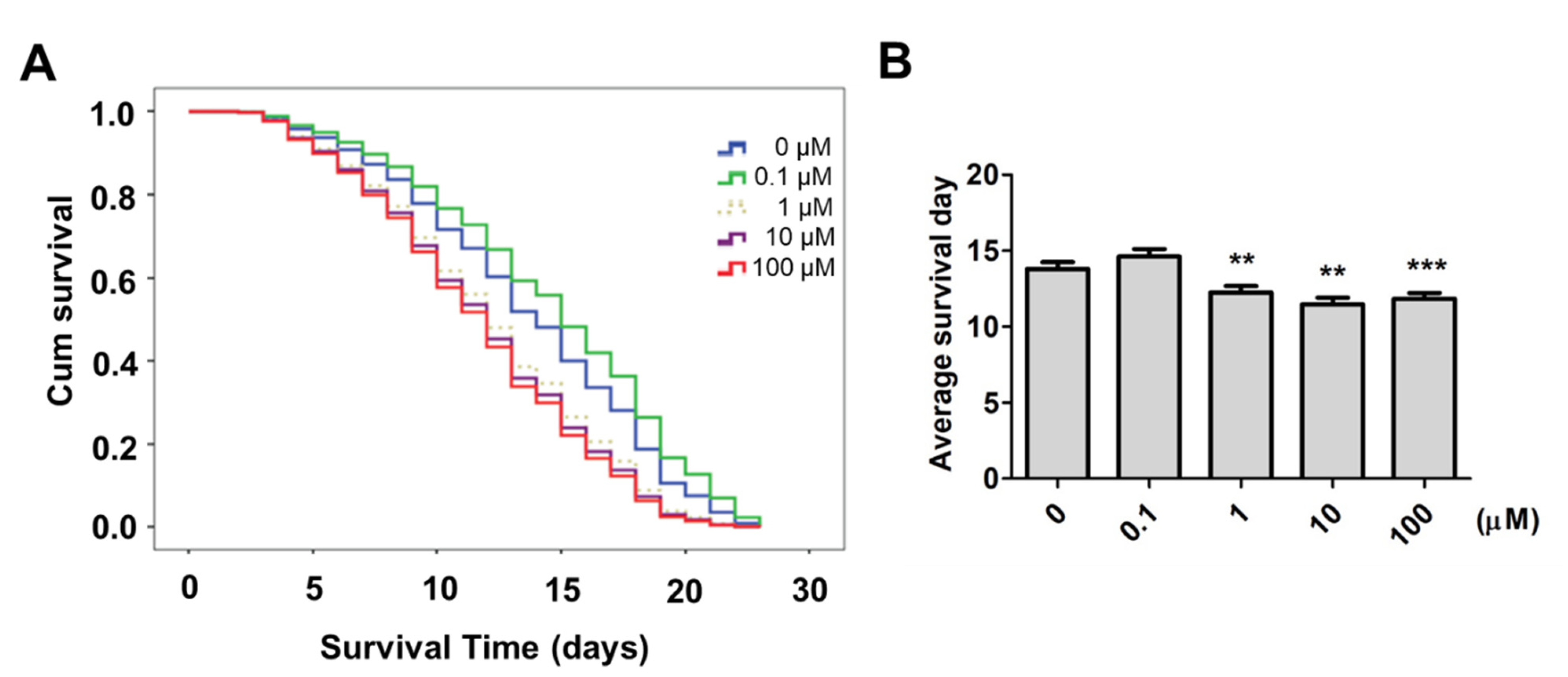
2.5. Effects of 9,12-Octadecadiynoic Acid Supplementation on Lifespan in Worms
3. Discussion
4. Material and Methods
4.1. Lipid Analysis and Neurodevelopmental Test
4.2. C. elegans Culture and 9,12-Octadecadiynoic Acid Feeding Protocol
4.3. Evaluation of Locomotive Behaviors
4.4. Foraging Behavior
4.5. Evaluation of Aggregation
4.6. Immunohistochemistry Staining
4.7. Gene Expression Assay
4.8. Lifespan Assay
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delplanque, B.; Gibson, R.; Koletzko, B.; Lapillonne, A.; Strandvik, B. Lipid Quality in Infant Nutrition: Current Knowledge and Future Opportunities. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, E.L.; Michaelsen, K.F.; Sanders, S.A.; Reinisch, J.M. The association between duration of breastfeeding and adult intelligence. JAMA 2002, 287, 2365–2371. [Google Scholar] [CrossRef] [PubMed]
- Thomas Oakland, P.L.H. Adaptive Behavior Assessment System-II; Elsevier Inc.: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Lee, I.H.; Procko, C.; Lu, Y.; Shaham, S. Stress-Induced Neural Plasticity Mediated by Glial GPCR REMO-1 Promotes C. elegans Adaptive Behavior. Cell Rep. 2021, 34, 108607. [Google Scholar] [CrossRef] [PubMed]
- Wolf, C.; Linden, D.E. Biological pathways to adaptability--interactions between genome, epigenome, nervous system and environment for adaptive behavior. Genes Brain Behav. 2012, 11, 3–28. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Quilez, S.; Homer, K.; Hendricks, M. Environmental Programming of Adult Foraging Behavior in C. elegans. Curr. Biol. 2019, 29, 2867–2879.e4. [Google Scholar] [CrossRef]
- Srinivasan, J.; von Reuss, S.H.; Bose, N.; Zaslaver, A.; Mahanti, P.; Ho, M.C.; O’Doherty, O.G.; Edison, A.S.; Sternberg, P.W.; Schroeder, F.C. A modular library of small molecule signals regulates social behaviors in Caenorhabditis elegans. PLoS Biol. 2012, 10, e1001237. [Google Scholar] [CrossRef]
- Artyukhin, A.B.; Yim, J.J.; Cheong Cheong, M.; Avery, L. Starvation-induced collective behavior in C. elegans. Sci. Rep. 2015, 5, 10647. [Google Scholar] [CrossRef] [PubMed]
- Bargmann, C.I.; Chemosensation in C. elegans. In WormBook; WormBook. 2006; pp. 1–29. Available online: http://www.wormbook.org (accessed on 15 July 2021).
- Lee, K.S.; Iwanir, S.; Kopito, R.B.; Scholz, M.; Calarco, J.A.; Biron, D.; Levine, E. Serotonin-dependent kinetics of feeding bursts underlie a graded response to food availability in C. elegans. Nat. Commun. 2017, 8, 14221. [Google Scholar] [CrossRef]
- Cao, X.; Wang, X.; Chen, H.; Li, H.; Tariq, M.; Wang, C.; Zhou, Y.; Liu, Y. Neurotoxicity of nonylphenol exposure on Caenorhabditis elegans induced by reactive oxidative species and disturbance synthesis of serotonin. Environ. Pollut. 2019, 244, 947–957. [Google Scholar] [CrossRef]
- Ranganathan, R.; Sawin, E.R.; Trent, C.; Horvitz, H.R. Mutations in the Caenorhabditis elegans serotonin reuptake transporter MOD-5 reveal serotonin-dependent and -independent activities of fluoxetine. J. Neurosci. 2001, 21, 5871–5884. [Google Scholar] [CrossRef]
- Ranganathan, R.; Cannon, S.C.; Horvitz, H.R. MOD-1 is a serotonin-gated chloride channel that modulates locomotory behaviour in C. elegans. Nature 2000, 408, 470–475. [Google Scholar] [CrossRef]
- Bijlsma, R.; Loeschcke, V. Environmental stress, adaptation and evolution: An overview. J. Evol. Biol. 2005, 18, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Nojima, Y.; Sakamoto, T.; Iwabuchi, K.; Nakazato, T.; Bono, H.; Toyoda, A.; Fujiyama, A.; Kanost, M.R.; Tabunoki, H. Comparative analysis of seven types of superoxide dismutases for their ability to respond to oxidative stress in Bombyx mori. Sci. Rep. 2019, 9, 2170. [Google Scholar] [CrossRef]
- Dampc, J.; Kula-Maximenko, M.; Molon, M.; Durak, R. Enzymatic Defense Response of Apple Aphid Aphis pomi to Increased Temperature. Insects 2020, 11, 436. [Google Scholar] [CrossRef]
- Russell, R.J.; Scott, C.; Jackson, C.J.; Pandey, R.; Pandey, G.; Taylor, M.C.; Coppin, C.W.; Liu, J.W.; Oakeshott, J.G. The evolution of new enzyme function: Lessons from xenobiotic metabolizing bacteria versus insecticide-resistant insects. Evol. Appl. 2011, 4, 225–248. [Google Scholar] [CrossRef] [PubMed]
- Thangavelu, S.R.; Tripathi, P.P.; Arya, U.; Mishra, H.K.; Subramaniam, J.R. ALS associated mutant SOD1 impairs the motor neurons and astrocytes and wild type astrocyte secreted-factors reverse the impaired motor neurons. Ann. Neurosci. 2011, 18, 48–55. [Google Scholar]
- Yakunin, E.; Kisos, H.; Kulik, W.; Grigoletto, J.; Wanders, R.J.; Sharon, R. The regulation of catalase activity by PPAR gamma is affected by alpha-synuclein. Ann. Clin. Transl. Neurol. 2014, 1, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Anbalagan, C.; Lafayette, I.; Antoniou-Kourounioti, M.; Haque, M.; King, J.; Johnsen, B.; Baillie, D.; Gutierrez, C.; Martin, J.A.; de Pomerai, D. Transgenic nematodes as biosensors for metal stress in soil pore water samples. Ecotoxicology 2012, 21, 439–455. [Google Scholar] [CrossRef][Green Version]
- Herzog, J.I.; Schmahl, C. Adverse Childhood Experiences and the Consequences on Neurobiological, Psychosocial, and Somatic Conditions Across the Lifespan. Front. Psychiatry 2018, 9, 420. [Google Scholar] [CrossRef]
- Lin, K.; Hsin, H.; Libina, N.; Kenyon, C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat. Genet. 2001, 28, 139–145. [Google Scholar] [CrossRef]
- Libina, N.; Berman, J.R.; Kenyon, C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell 2003, 115, 489–502. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Del Rosario, C.; Slevin, M.; Molloy, E.J.; Quigley, J.; Nixon, E. How to use the Bayley Scales of Infant and Toddler Development. Arch. Dis. Child. Educ. Pr. 2021, 106, 108–112. [Google Scholar] [CrossRef]
- Nagy, B.E.; Kenyhercz, F. Adaptive Behavioral, Social-Emotional, and Neurodevelopmental Outcomes at 2 Years of Age in Hungarian Preterm Infants Based on Bayley III. Dev. Neurorehabil. 2021, 24, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Raabe, R.C.; Mathies, L.D.; Davies, A.G.; Bettinger, J.C. The omega-3 fatty acid eicosapentaenoic acid is required for normal alcohol response behaviors in C. elegans. PLoS ONE 2014, 9, e105999. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.S.; Kuo, W.J.; Lee, C.L.; Chu, I.H.; Chen, C.S. Exercise in an electrotactic flow chamber ameliorates age-related degeneration in Caenorhabditis elegans. Sci. Rep. 2016, 6, 28064. [Google Scholar] [CrossRef]


| Description | Compounds Description (rt_m/z) | Formula | Category | β estimate (p-Value) |
|---|---|---|---|---|
| Bullatacinone | 1.05_621.4340 | C37H66O7 | Fatty acid | 0.247 (0.013) |
| 9,12-octadecadiynoic acid | 1.55_275.2061 | C18H28O2 | Fatty acid | 0.285 (0.005) |
| 16:1(5Z) | 2.02_253.2221 | C16H30O2 | Fatty acid | −0.203 (0.037) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.-C.; Chao, H.-R.; Wu, C.-Y.; Lai, Y.-R.; Chen, C.-H.; Yoshioka, T.; Hsu, W.-L.; Tsai, M.-H. Effect of 9,12-Octadecadiynoic Acid on Neurobehavioral Development in Caenorhabditis elegans. Int. J. Mol. Sci. 2021, 22, 8917. https://doi.org/10.3390/ijms22168917
Chen T-C, Chao H-R, Wu C-Y, Lai Y-R, Chen C-H, Yoshioka T, Hsu W-L, Tsai M-H. Effect of 9,12-Octadecadiynoic Acid on Neurobehavioral Development in Caenorhabditis elegans. International Journal of Molecular Sciences. 2021; 22(16):8917. https://doi.org/10.3390/ijms22168917
Chicago/Turabian StyleChen, Tun-Chieh, How-Ran Chao, Ching-Ying Wu, Yun-Ru Lai, Chu-Huang Chen, Tohru Yoshioka, Wen-Li Hsu, and Ming-Hsien Tsai. 2021. "Effect of 9,12-Octadecadiynoic Acid on Neurobehavioral Development in Caenorhabditis elegans" International Journal of Molecular Sciences 22, no. 16: 8917. https://doi.org/10.3390/ijms22168917
APA StyleChen, T.-C., Chao, H.-R., Wu, C.-Y., Lai, Y.-R., Chen, C.-H., Yoshioka, T., Hsu, W.-L., & Tsai, M.-H. (2021). Effect of 9,12-Octadecadiynoic Acid on Neurobehavioral Development in Caenorhabditis elegans. International Journal of Molecular Sciences, 22(16), 8917. https://doi.org/10.3390/ijms22168917









