Lectin-Mediated Bacterial Modulation by the Intestinal Nematode Ascaris suum
Abstract
:1. Introduction
2. Results
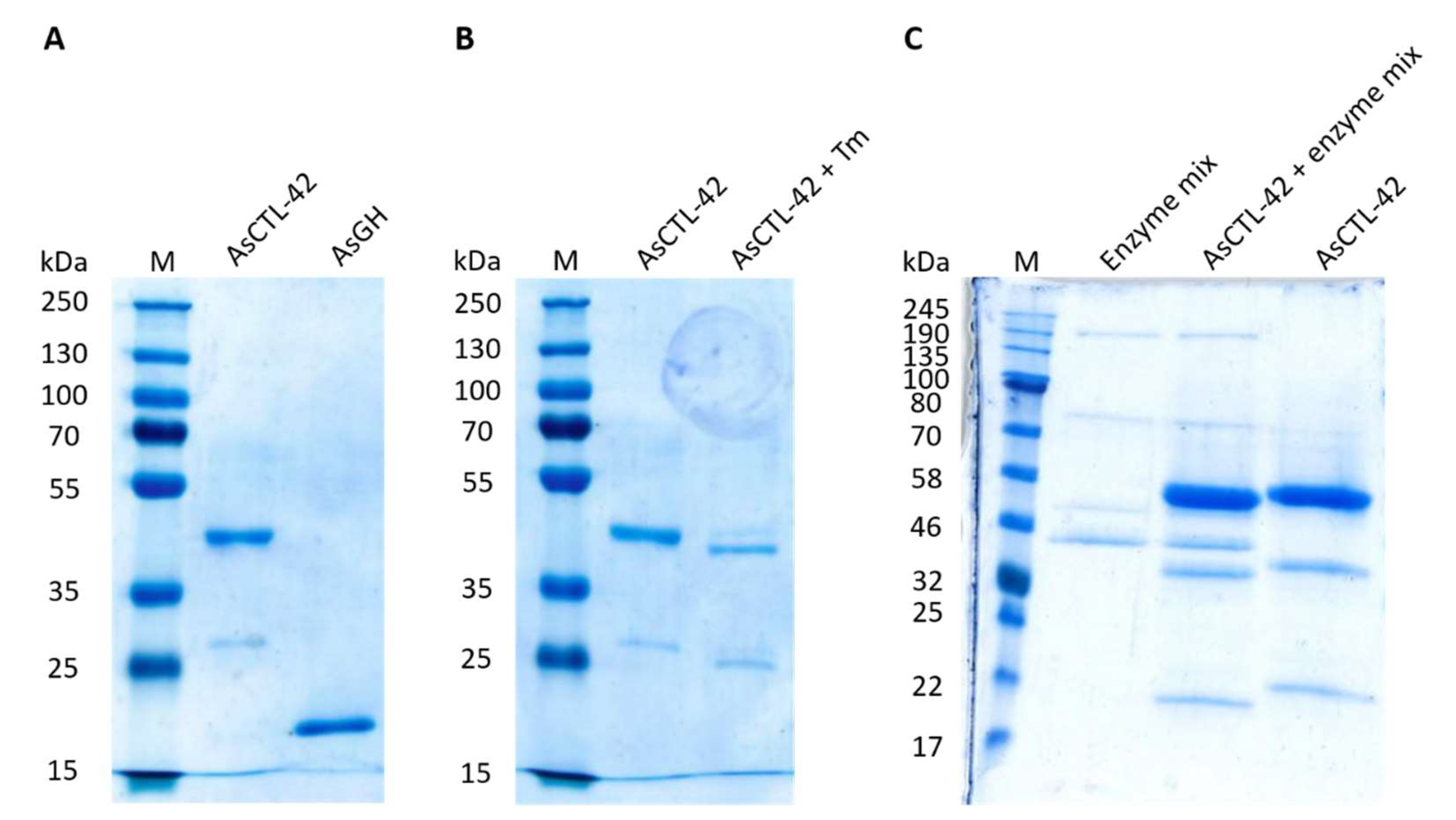
2.1. Eukaryotic Expression of AsCTL-42
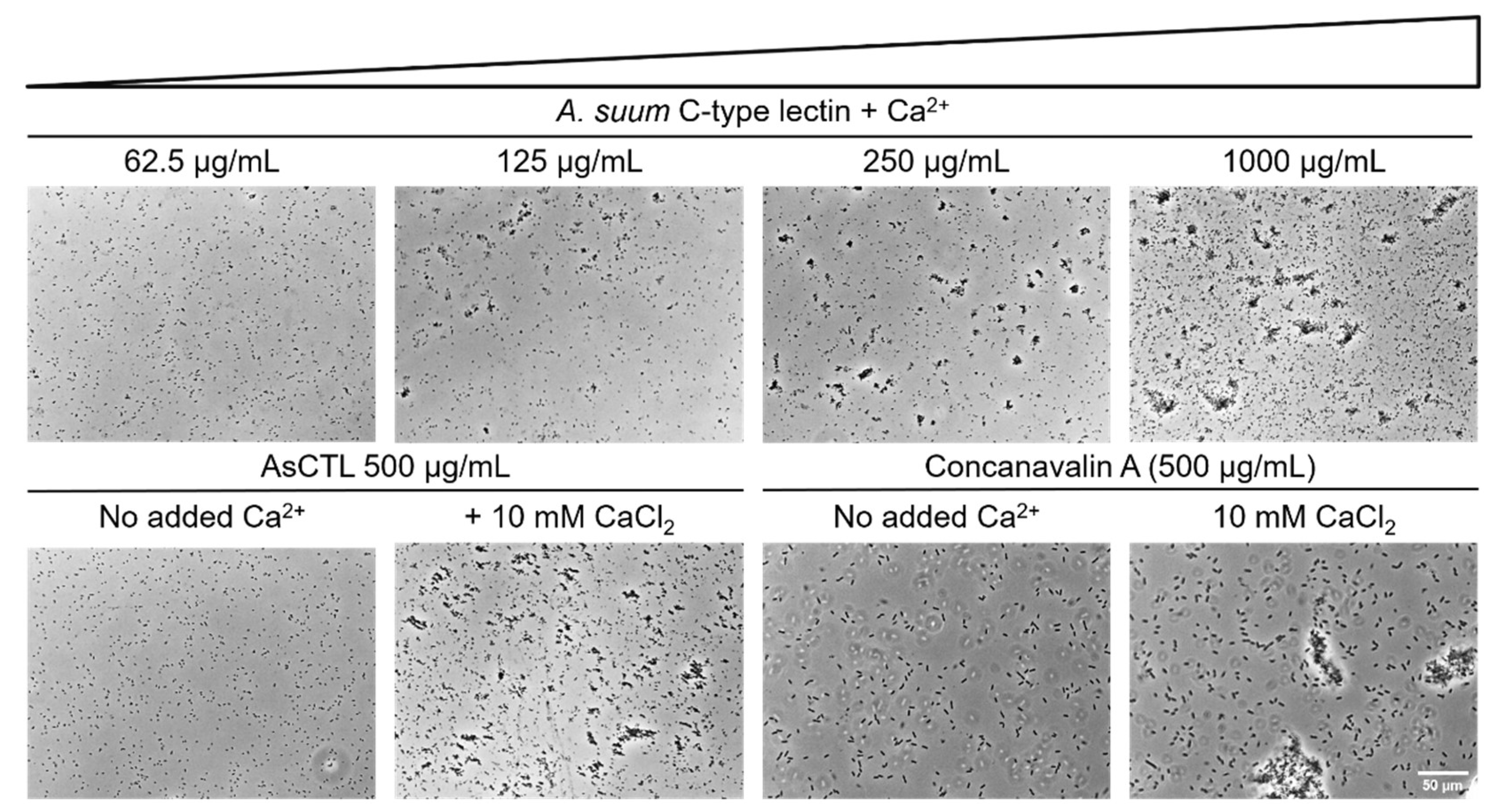
2.2. AsCTL-42 Agglutinates Salmonella
2.3. AsCTL-42 Does Not Inhibit Bacterial Growth
2.4. AsCTL-42 Does Not Bind to Bacterial Glycans
2.5. AsCTL-42 Decreases Invasion of Porcine Intestinal Epithelial Cells by Salmonella
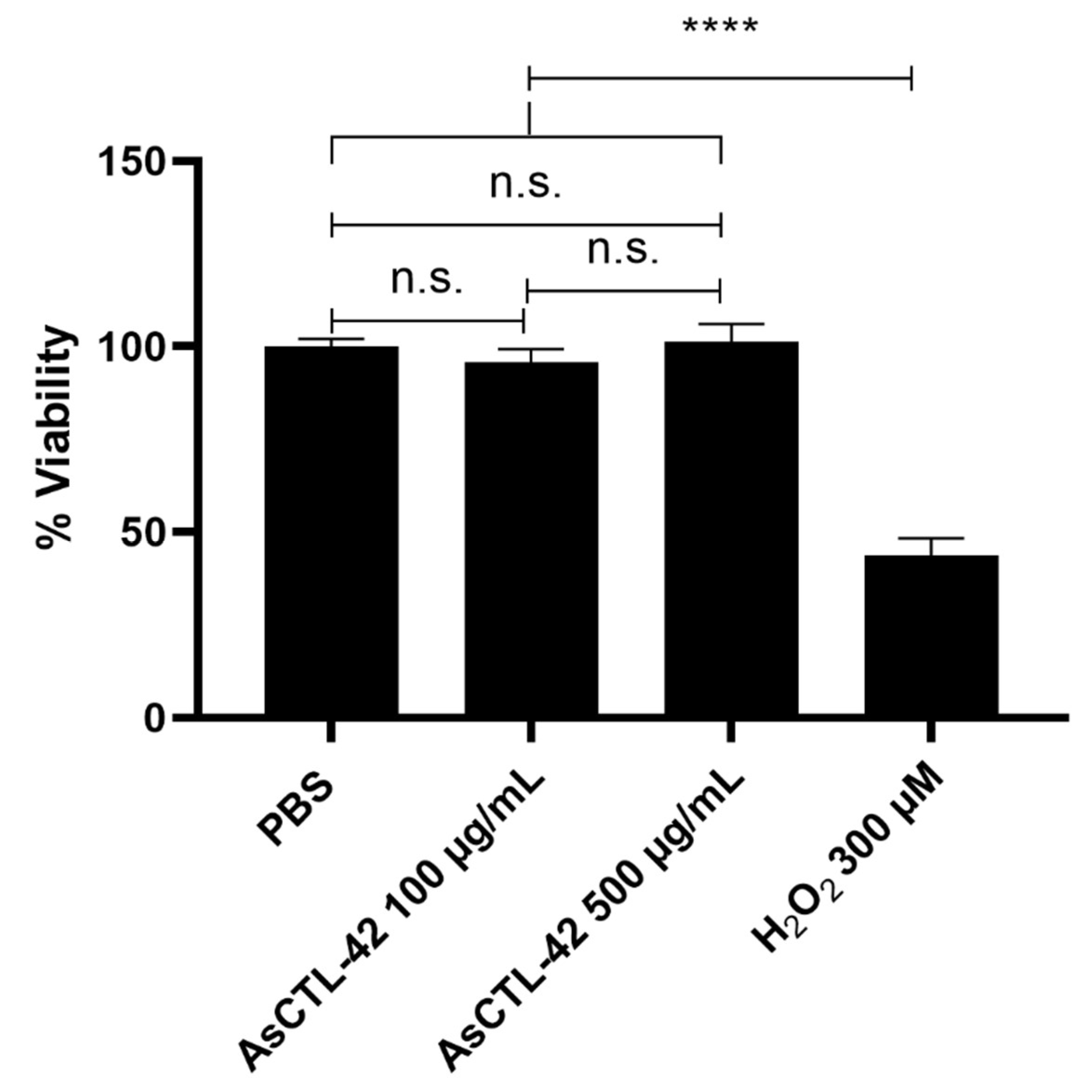
2.6. AsCTL-42 Does Not Interfere with Host Cell Viability
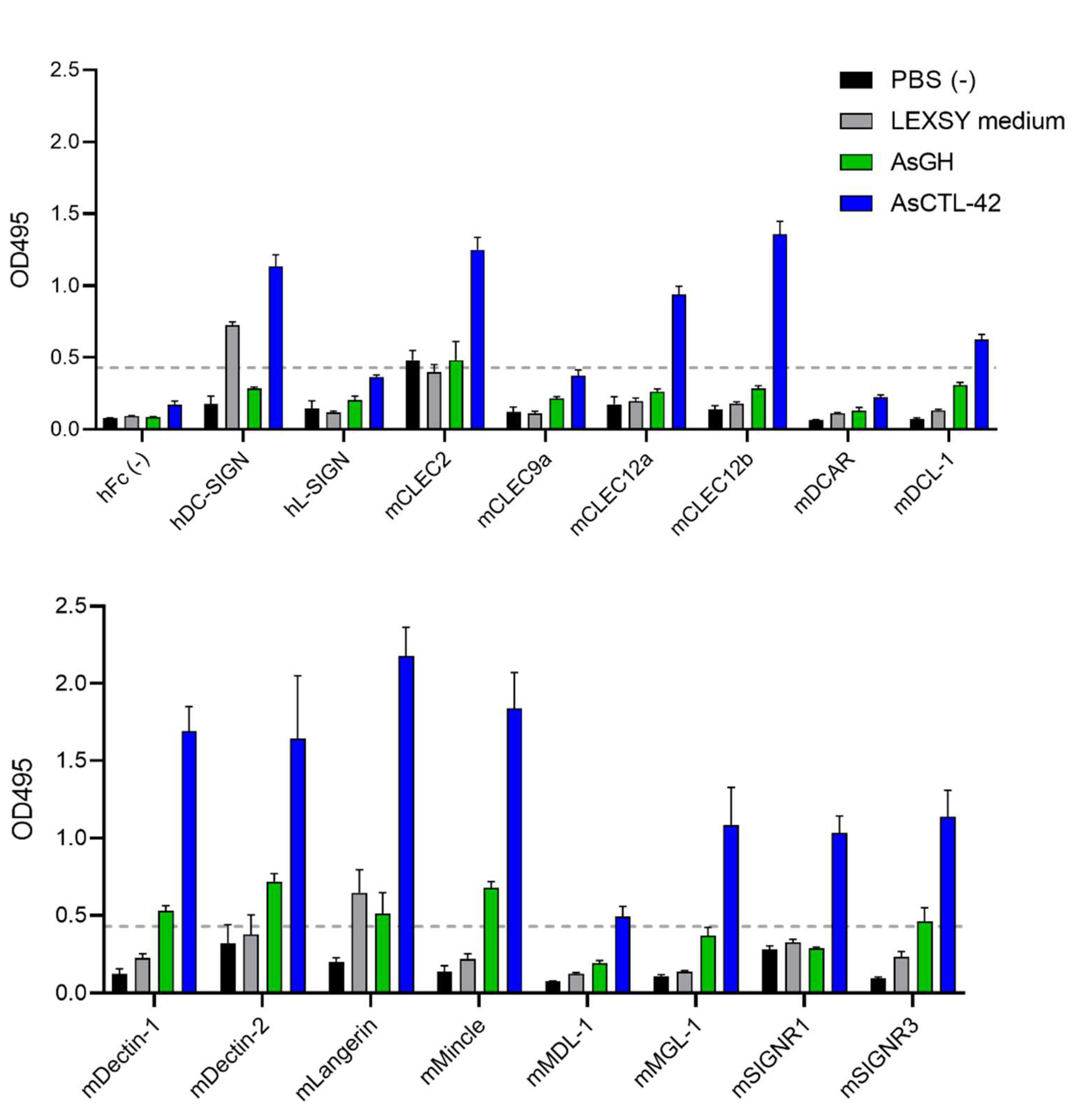
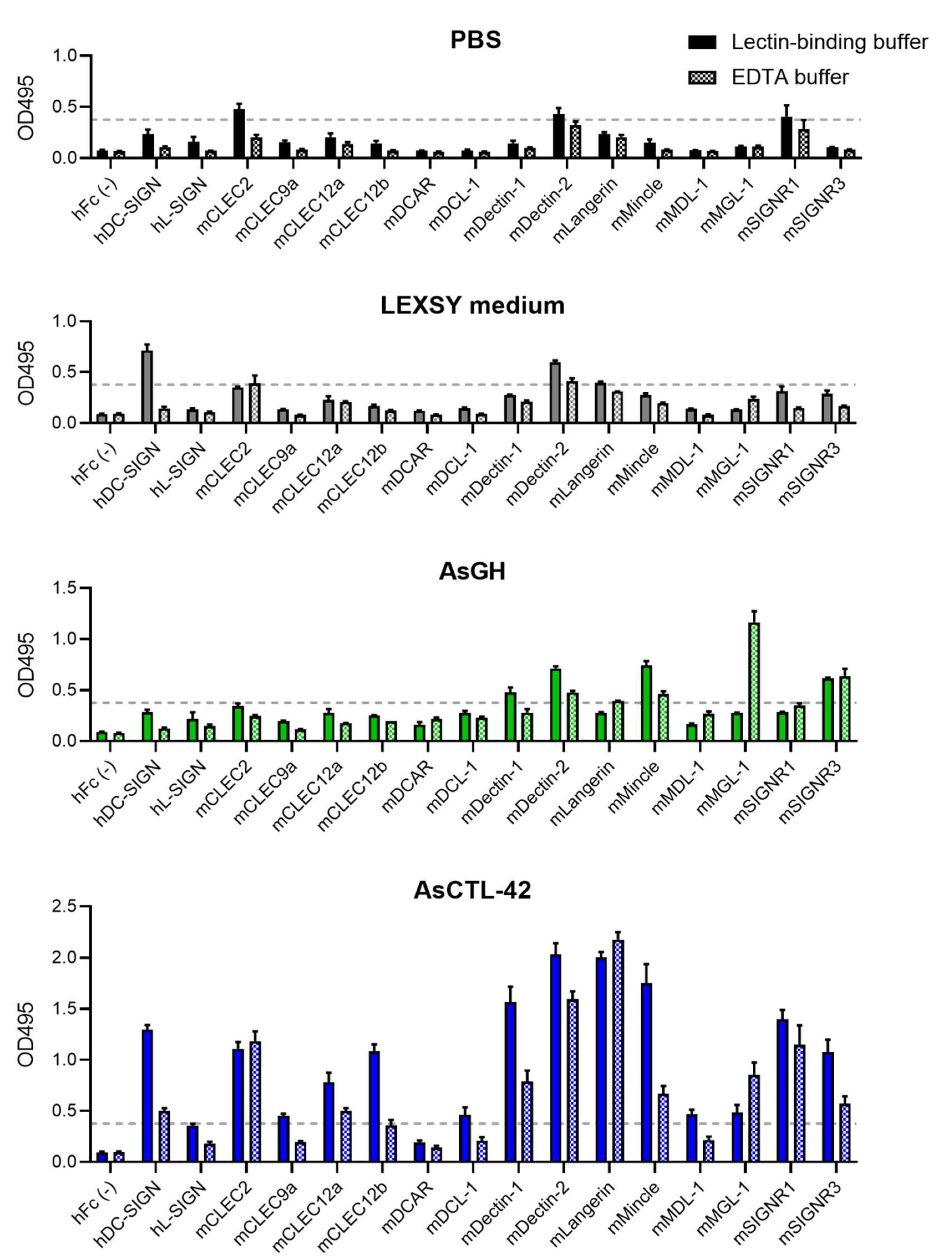
2.7. AsCTL-42 Binds Selected Mammalian C-Type Lectin Receptors
3. Discussion
4. Materials and Methods
4.1. Recombinant Expression of AsCTL-42 and Protein Analysis
4.2. Bacterial Strains
4.3. Radial Diffusion Assay
4.4. Cell Culture and Growth Conditions
4.5. Cell Viability Testing
4.6. Glycan Array
4.7. C-Type Lectin Receptor Screening
4.8. Agglutination Assay
4.9. Salmonella Invasion Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Fact Sheet: Soil-Transmitted Helminth Infections. Available online: http://www.who.int/mediacentre/factsheets/fs366/en/ (accessed on 27 March 2017).
- Katakam, K.K.; Thamsborg, S.M.; Dalsgaard, A.; Kyvsgaard, N.C.; Mejer, H. Environmental Contamination and Transmission of Ascaris Suum in Danish Organic Pig Farms. Parasites Vectors 2016, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Jardim-Botelho, A.; Raff, S.; Rodrigues, R.D.; Hoffman, H.J.; Diemert, D.J.; Corrêa-Oliveira, R.; Bethony, J.M.; Gazzinelli, M.F. Hookworm, Ascaris Lumbricoides Infection and Polyparasitism Associated with Poor Cognitive Performance in Brazilian Schoolchildren. Trop. Med. Int. Health 2008, 13, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Saathoff, E.; Olsen, A.; Kvalsvig, J.D.; Appleton, C.C. Patterns of Geohelminth Infection, Impact of Albendazole Treatment and Re-Infection after Treatment in Schoolchildren from Rural KwaZulu-Natal/South-Africa. BMC Infect. Dis. 2004, 4, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Yentur Doni, N.; Yildiz Zeyrek, F.; Simsek, Z.; Gurses, G.; Sahin, İ. Risk Factors and Relationship Between Intestinal Parasites and the Growth Retardation and Psychomotor Development Delays of Children in Şanlıurfa, Turkey. Türkiye Parazitolojii Derg. 2015, 39, 270–276. [Google Scholar] [CrossRef]
- Dold, C.; Holland, C.V. Ascaris and Ascariasis. Microbes Infect. 2011, 13, 632–637. [Google Scholar] [CrossRef]
- Holland, C.V.; Asaolu, S.O.; Crompton, D.W.; Stoddart, R.C.; Macdonald, R.; Torimiro, S.E. The Epidemiology of Ascaris Lumbricoides and Other Soil-Transmitted Helminths in Primary School Children from Ile-Ife, Nigeria. Parasitology 1989, 99 Pt 2, 275–285. [Google Scholar] [CrossRef]
- Murrell, K.D.; Eriksen, L.; Nansen, P.; Slotved, H.C.; Rasmussen, T. Ascaris Suum: A Revision of Its Early Migratory Path and Implications for Human Ascariasis. J. Parasitol. 1997, 83, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Roepstorff, A.; Eriksen, L.; Slotved, H.C.; Nansen, P. Experimental Ascaris Suum Infection in the Pig: Worm Population Kinetics Following Single Inoculations with Three Doses of Infective Eggs. Parasitology 1997, 115, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Meurens, F.; Summerfield, A.; Nauwynck, H.; Saif, L.; Gerdts, V. The Pig: A Model for Human Infectious Diseases. Trends Microbiol. 2012, 20, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Heinritz, S.N.; Mosenthin, R.; Weiss, E. Use of Pigs as a Potential Model for Research into Dietary Modulation of the Human Gut Microbiota. Nutr. Res. Rev. 2013, 26, 191–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crompton, D.W. Ascaris and Ascariasis. Adv. Parasitol. 2001, 48, 285–375. [Google Scholar] [CrossRef]
- Bonardi, S. Salmonella in the Pork Production Chain and Its Impact on Human Health in the European Union. Epidemiol. Infect. 2017, 145, 1513–1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, A.R.; Krych, L.; Ahmad, H.F.; Nejsum, P.; Skovgaard, K.; Nielsen, D.S.; Thamsborg, S.M. A Polyphenol-Enriched Diet and Ascaris Suum Infection Modulate Mucosal Immune Responses and Gut Microbiota Composition in Pigs. PLoS ONE 2017, 12, e0186546. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, F.; Urban, J.F.; Paerewijck, O.; Geldhof, P.; Li, R.W. Ascaris Suum Infection Was Associated with a Worm-Independent Reduction in Microbial Diversity and Altered Metabolic Potential in the Porcine Gut Microbiome. Int. J. Parasitol. 2019, 49, 247–256. [Google Scholar] [CrossRef]
- Midha, A.; Ebner, F.; Schlosser-Brandenburg, J.; Rausch, S.; Hartmann, S. Trilateral Relationship: Ascaris, Microbiota, and Host Cells. Trends Parasitol. 2021, 37, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Van Steendam, K.; Dhaenens, M.; Vlaminck, J.; Deforce, D.; Jex, A.R.; Gasser, R.B.; Geldhof, P. Proteomic Analysis of the Excretory-Secretory Products from Larval Stages of Ascaris Suum Reveals High Abundance of Glycosyl Hydrolases. PLoS Negl. Trop. Dis. 2013, 7, e2467. [Google Scholar] [CrossRef] [Green Version]
- Midha, A.; Janek, K.; Niewienda, A.; Henklein, P.; Guenther, S.; Serra, D.O.; Schlosser, J.; Hengge, R.; Hartmann, S. The Intestinal Roundworm Ascaris Suum Releases Antimicrobial Factors Which Interfere With Bacterial Growth and Biofilm Formation. Front. Cell Infect. Microbiol. 2018, 8, 271. [Google Scholar] [CrossRef] [Green Version]
- Bauters, L.; Naalden, D.; Gheysen, G. The Distribution of Lectins across the Phylum Nematoda: A Genome-Wide Search. Int. J. Mol. Sci. 2017, 18, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rausch, S.; Midha, A.; Kuhring, M.; Affinass, N.; Radonic, A.; Kühl, A.A.; Bleich, A.; Renard, B.Y.; Hartmann, S. Parasitic Nematodes Exert Antimicrobial Activity and Benefit From Microbiota-Driven Support for Host Immune Regulation. Front. Immunol. 2018, 9, 2282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaishnava, S.; Yamamoto, M.; Severson, K.M.; Ruhn, K.A.; Yu, X.; Koren, O.; Ley, R.; Wakeland, E.K.; Hooper, L.V. The Antibacterial Lectin RegIIIgamma Promotes the Spatial Segregation of Microbiota and Host in the Intestine. Science 2011, 334, 255–258. [Google Scholar] [CrossRef] [Green Version]
- Mallo, G.V.; Kurz, C.L.; Couillault, C.; Pujol, N.; Granjeaud, S.; Kohara, Y.; Ewbank, J.J. Inducible Antibacterial Defense System in C. Elegans. Curr. Biol. 2002, 12, 1209–1214. [Google Scholar] [CrossRef] [Green Version]
- O’Rourke, D.; Baban, D.; Demidova, M.; Mott, R.; Hodgkin, J. Genomic Clusters, Putative Pathogen Recognition Molecules, and Antimicrobial Genes Are Induced by Infection of C. Elegans with M. Nematophilum. Genome Res. 2006, 16, 1005–1016. [Google Scholar] [CrossRef] [Green Version]
- Miltsch, S.M.; Seeberger, P.H.; Lepenies, B. The C-Type Lectin-like Domain Containing Proteins Clec-39 and Clec-49 Are Crucial for Caenorhabditis Elegans Immunity against Serratia Marcescens Infection. Dev. Comp. Immunol. 2014, 45, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Xiao, X.; Liu, Y.; Zhang, R.; Liu, J.; Liu, Q.; Wang, P.; Cheng, G. Mosquito C-Type Lectins Maintain Gut Microbiome Homeostasis. Nat. Microbiol. 2016, 1, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hewitson, J.P.; Grainger, J.R.; Maizels, R.M. Helminth Immunoregulation: The Role of Parasite Secreted Proteins in Modulating Host Immunity. Mol. Biochem. Parasitol. 2009, 167, 1–11. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Haney, E.F.; Gill, E.E. The Immunology of Host Defence Peptides: Beyond Antimicrobial Activity. Nat. Rev. Immunol. 2016, 16, 321–334. [Google Scholar] [CrossRef]
- Breitling, R.; Klingner, S.; Callewaert, N.; Pietrucha, R.; Geyer, A.; Ehrlich, G.; Hartung, R.; Müller, A.; Contreras, R.; Beverley, S.M.; et al. Non-Pathogenic Trypanosomatid Protozoa as a Platform for Protein Research and Production. Protein Expr. Purif. 2002, 25, 209–218. [Google Scholar] [CrossRef]
- Jin, S.-P.; Chung, J.H. Inhibition of N-Glycosylation by Tunicamycin Attenuates Cell–Cell Adhesion via Impaired Desmosome Formation in Normal Human Epidermal Keratinocytes. Biosci. Rep. 2018, 38, BSR20171641. [Google Scholar] [CrossRef] [Green Version]
- Hammerum, A.M. Enterococci of Animal Origin and Their Significance for Public Health. Clin. Microbiol. Infect. 2012, 18, 619–625. [Google Scholar] [CrossRef]
- Khanna, T.; Friendship, R.; Dewey, C.; Weese, J.S. Methicillin Resistant Staphylococcus Aureus Colonization in Pigs and Pig Farmers. Vet. Microbiol. 2008, 128, 298–303. [Google Scholar] [CrossRef]
- Luppi, A. Swine Enteric Colibacillosis: Diagnosis, Therapy and Antimicrobial Resistance. Porc. Health Manag. 2017, 3, 1–18. [Google Scholar] [CrossRef]
- Casanova-Higes, A.; Marín-Alcalá, C.M.; Andrés-Barranco, S.; Cebollada-Solanas, A.; Alvarez, J.; Mainar-Jaime, R.C. Weaned Piglets: Another Factor to Be Considered for the Control of Salmonella Infection in Breeding Pig Farms. Vet. Res. 2019, 50, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Geissner, A.; Reinhardt, A.; Rademacher, C.; Johannssen, T.; Monteiro, J.; Lepenies, B.; Thépaut, M.; Fieschi, F.; Mrázková, J.; Wimmerova, M.; et al. Microbe-Focused Glycan Array Screening Platform. Proc. Natl. Acad. Sci. USA 2019, 116, 1958–1967. [Google Scholar] [CrossRef] [Green Version]
- Cummings, R.D.; Darvill, A.G.; Etzler, M.E.; Hahn, M.G. Glycan-Recognizing Probes as Tools. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2015. [Google Scholar]
- Singh, V.; Finke-Isami, J.; Hopper-Chidlaw, A.C.; Schwerk, P.; Thompson, A.; Tedin, K. Salmonella Co-Opts Host Cell Chaperone-Mediated Autophagy for Intracellular Growth *. J. Biol. Chem. 2017, 292, 1847–1864. [Google Scholar] [CrossRef] [Green Version]
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blázquez-Castro, A. Tetrazolium Salts and Formazan Products in Cell Biology: Viability Assessment, Fluorescence Imaging, and Labeling Perspectives. Acta Histochem. 2018, 120, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.; Wescott, R.B. Infectivity, Fecundity, and Survival of Nematospiroides Dubius in Gnotobiotic Mice. Exp. Parasitol. 1972, 32, 327–334. [Google Scholar] [CrossRef]
- Wescott, R.B.; Todd, A.C. A Comparison of the Development of Nippostrongylus Brasiliensis in Germ-Free and Conventional Mice. J. Parasitol. 1964, 50, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Fricke, W.F.; Song, Y.; Wang, A.-J.; Smith, A.; Grinchuk, V.; Mongodin, E.; Pei, C.; Ma, B.; Lu, N.; Urban, J.F.; et al. Type 2 Immunity-Dependent Reduction of Segmented Filamentous Bacteria in Mice Infected with the Helminthic Parasite Nippostrongylus Brasiliensis. Microbiome 2015, 3, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, L.A.; Smith, K.A.; Filbey, K.J.; Harcus, Y.; Hewitson, J.P.; Redpath, S.A.; Valdez, Y.; Yebra, M.J.; Finlay, B.B.; Maizels, R.M. Commensal-Pathogen Interactions in the Intestinal Tract: Lactobacilli Promote Infection with, and Are Promoted by, Helminth Parasites. Gut Microbes 2014, 5, 522–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebner, F.; Hepworth, M.R.; Rausch, S.; Janek, K.; Niewienda, A.; Kühl, A.; Henklein, P.; Lucius, R.; Hamelmann, E.; Hartmann, S. Therapeutic Potential of Larval Excretory/Secretory Proteins of the Pig Whipworm Trichuris Suis in Allergic Disease. Allergy 2014, 69, 1489–1497. [Google Scholar] [CrossRef]
- Kuramoto, T.; Uzuyama, H.; Hatakeyama, T.; Tamura, T.; Nakashima, T.; Yamaguchi, K.; Oda, T. Cytotoxicity of a GalNAc-Specific C-Type Lectin CEL-I toward Various Cell Lines. J. Biochem. 2005, 137, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Coelho, L.C.B.B.; dos Santos Silva, P.M.; de Oliveira, W.F.; de Moura, M.C.; Pontual, E.V.; Gomes, F.S.; Paiva, P.M.G.; Napoleão, T.H.; dos Santos Correia, M.T. Lectins as Antimicrobial Agents. J. Appl. Microbiol. 2018, 125, 1238–1252. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, D.E.; Gibson, M.I. Latent Ice Recrystallization Inhibition Activity in Nonantifreeze Proteins: Ca2+-Activated Plant Lectins and Cation-Activated Antimicrobial Peptides. Biomacromolecules 2015, 16, 3411–3416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Snyder, S.; Feng, P.; Azadi, P.; Zhang, S.; Bulgheresi, S.; Sanderson, K.E.; He, J.; Klena, J.; Chen, T. Role of N-Acetylglucosamine within Core Lipopolysaccharide of Several Species of Gram-Negative Bacteria in Targeting the DC-SIGN (CD209). J. Immunol. 2006, 177, 4002–4011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindenwald, D.L.; Lepenies, B. C-Type Lectins in Veterinary Species: Recent Advancements and Applications. Int. J. Mol. Sci. 2020, 21, 5122. [Google Scholar] [CrossRef] [PubMed]
- van Die, I.; van Vliet, S.J.; Nyame, A.K.; Cummings, R.D.; Bank, C.M.C.; Appelmelk, B.; Geijtenbeek, T.B.H.; van Kooyk, Y. The Dendritic Cell–Specific C-Type Lectin DC-SIGN Is a Receptor for Schistosoma Mansoni Egg Antigens and Recognizes the Glycan Antigen Lewis x. Glycobiology 2003, 13, 471–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guasconi, L.; Serradell, M.C.; Garro, A.P.; Iacobelli, L.; Masih, D.T. C-Type Lectins on Macrophages Participate in the Immunomodulatory Response to Fasciola Hepatica Products. Immunology 2011, 133, 386–396. [Google Scholar] [CrossRef]
- Leles, D.; Gardner, S.L.; Reinhard, K.; Iñiguez, A.; Araujo, A. Are Ascaris Lumbricoides and Ascaris Suum a Single Species? Parasites Vectors 2012, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, N.; French, T.; Rausch, S.; Kühl, A.; Hemminger, K.; Dunay, I.R.; Steinfelder, S.; Hartmann, S. Toxoplasma Co-Infection Prevents Th2 Differentiation and Leads to a Helminth-Specific Th1 Response. Front. Cell Infect. Microbiol. 2017, 7, 341. [Google Scholar] [CrossRef] [Green Version]
- van der Wolf, P.J.; Wolbers, W.B.; Elbers, A.R.; van der Heijden, H.M.; Koppen, J.M.; Hunneman, W.A.; van Schie, F.W.; Tielen, M.J. Herd Level Husbandry Factors Associated with the Serological Salmonella Prevalence in Finishing Pig Herds in The Netherlands. Vet. Microbiol. 2001, 78, 205–219. [Google Scholar] [CrossRef]
- Reynolds, L.A.; Redpath, S.A.; Yurist-Doutsch, S.; Gill, N.; Brown, E.M.; van der Heijden, J.; Brosschot, T.P.; Han, J.; Marshall, N.C.; Woodward, S.E.; et al. Enteric Helminths Promote Salmonella Co-Infection by Altering the Intestinal Metabolome. J. Infect. Dis. 2017, 215, 1245–1254. [Google Scholar] [CrossRef]
- Tielens, A.G.M.; van Grinsven, K.W.A.; Henze, K.; van Hellemond, J.J.; Martin, W. Acetate Formation in the Energy Metabolism of Parasitic Helminths and Protists. Int. J. Parasitol. 2010, 40, 387–397. [Google Scholar] [CrossRef]
- Lawhon, S.D.; Maurer, R.; Suyemoto, M.; Altier, C. Intestinal Short-Chain Fatty Acids Alter Salmonella Typhimurium Invasion Gene Expression and Virulence through BarA/SirA. Mol. Microbiol. 2002, 46, 1451–1464. [Google Scholar] [CrossRef]
- Jacobson, A.; Lam, L.; Rajendram, M.; Tamburini, F.; Honeycutt, J.; Pham, T.; Treuren, W.V.; Pruss, K.; Stabler, S.R.; Lugo, K.; et al. A Gut Commensal-Produced Metabolite Mediates Colonization Resistance to Salmonella Infection. Cell Host Microbe 2018, 24, 296–307.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamas, A.; Regal, P.; Vázquez, B.; Cepeda, A.; Franco, C.M. Short Chain Fatty Acids Commonly Produced by Gut Microbiota Influence Salmonella Enterica Motility, Biofilm Formation, and Gene Expression. Antibiotics 2019, 8, 265. [Google Scholar] [CrossRef] [Green Version]
- José, M.V.; Ruiz, A.; Bobadilla, J.R. Prevalence of Infection, Mean Worm Burden and Degree of Worm Aggregation as Determinants of Prevalence of Disease Due to Intestinal Helminths. Arch. Med. Res. 1997, 28, 121–127. [Google Scholar]
- Newey, S.; Shaw, D.J.; Kirby, A.; Montieth, P.; Hudson, P.J.; Thirgood, S.J. Prevalence, Intensity and Aggregation of Intestinal Parasites in Mountain Hares and Their Potential Impact on Population Dynamics. Int. J. Parasitol. 2005, 35, 367–373. [Google Scholar] [CrossRef]
- Walker, M.; Hall, A.; Basáñez, M.-G. Chapter 7—Ascaris lumbricoides: New Epidemiological Insights and Mathematical Approaches. In Ascaris: The Neglected Parasite; Holland, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 155–201. ISBN 978-0-12-396978-1. [Google Scholar]
- Loukas, A.; Maizels, R.M. Helminth C-Type Lectins and Host-Parasite Interactions. Parasitol. Today 2000, 16, 333–339. [Google Scholar] [CrossRef]
- Harcus, Y.; Nicoll, G.; Murray, J.; Filbey, K.; Gomez-Escobar, N.; Maizels, R.M. C-Type Lectins from the Nematode Parasites Heligmosomoides Polygyrus and Nippostrongylus Brasiliensis. Parasitol. Int. 2009, 58, 461–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caraballo, L.; Zakzuk, J.; Acevedo, N. Helminth-Derived Cystatins: The Immunomodulatory Properties of an Ascaris Lumbricoides Cystatin. Parasitology 2021, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.W.; Donnelly, S.; Hutchinson, A.T.; To, J.; Taylor, N.L.; Norton, R.S.; Perugini, M.A.; Dalton, J.P. A Family of Helminth Molecules That Modulate Innate Cell Responses via Molecular Mimicry of Host Antimicrobial Peptides. PLoS Pathog. 2011, 7, e1002042. [Google Scholar] [CrossRef] [Green Version]
- Smyth, D.J.; Harcus, Y.; White, M.P.J.; Gregory, W.F.; Nahler, J.; Stephens, I.; Toke-Bjolgerud, E.; Hewitson, J.P.; Ivens, A.; McSorley, H.J.; et al. TGF-β Mimic Proteins Form an Extended Gene Family in the Murine Parasite Heligmosomoides Polygyrus. Int. J. Parasitol. 2018, 48, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, G.; Mueller, M.; Hartmann, S.; Steinfelder, S. Differential Immunomodulation in Human Monocytes versus Macrophages by Filarial Cystatin. PLoS ONE 2017, 12, e0188138. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.-H.; Shang, L.; Xue, L.-X.; Ding, W.; Chen, S.; Ma, R.-F.; Huang, J.-F.; Xiong, K. The Effect and Underlying Mechanism of Timosaponin B-II on RGC-5 Necroptosis Induced by Hydrogen Peroxide. BMC Complement. Altern. Med. 2014, 14, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maglinao, M.; Eriksson, M.; Schlegel, M.K.; Zimmermann, S.; Johannssen, T.; Götze, S.; Seeberger, P.H.; Lepenies, B. A Platform to Screen for C-Type Lectin Receptor-Binding Carbohydrates and Their Potential for Cell-Specific Targeting and Immune Modulation. J. Control. Release 2014, 175, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Mayer, S.; Moeller, R.; Monteiro, J.T.; Ellrott, K.; Josenhans, C.; Lepenies, B. C-Type Lectin Receptor (CLR)–Fc Fusion Proteins As Tools to Screen for Novel CLR/Bacteria Interactions: An Exemplary Study on Preselected Campylobacter Jejuni Isolates. Front. Immunol. 2018, 9, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prado Acosta, M.; Goyette-Desjardins, G.; Scheffel, J.; Dudeck, A.; Ruland, J.; Lepenies, B. S-Layer From Lactobacillus Brevis Modulates Antigen-Presenting Cell Functions via the Mincle-Syk-Card9 Axis. Front. Immunol. 2021, 12, 511. [Google Scholar] [CrossRef] [PubMed]

| E. faecium DSM20477 | S. aureus IMT29828 | E. coli IMT19224 | S. Typhimurium 4/74 | |
|---|---|---|---|---|
| AsCTL-42 (1 mg/mL) | - | - | - | - |
| Pexiganan (1.25 µg/mL) | 5.0 | 12.0 | 11.0 | 11.0 |
| PBS | - | - | - | - |
| Human (h) or Murine (m) Protein | Human or Murine Gene | Corresponding Porcine Protein | Corresponding Porcine Gene |
|---|---|---|---|
| hDC-SIGN | CD209/CLEC4L | CD209 | CD209 |
| hL-SIGN | CD209L/CLEC4M | CD209 | CD209 |
| mCLEC2 | Clec1b | CLEC1b | CLEC1B |
| mCLEC9a | Clec9a | CLEC9a | CLEC9A |
| mCLEC12a | Clec12a | CLEC12a | CLEC12A |
| mCLEC12b | Clec12b | CLEC12b | CLEC12B |
| mDCAR | Clec4b1 | CLEC4A | CLEC4A |
| mDCL-1 | Clec2i | CLEC2D | CLEC2D |
| mDectin-1 | Clec7a | CLEC7A | CLEC7A |
| mDectin-2 | Clec6a | No corresponding protein | No corresponding gene |
| mLangerin | Cd207 | CLEC4K | CD207 |
| mMincle | Clec4e | CLEC4E | CLEC4E |
| mMDL-1 | Clec5a | CLEC5A | CLEC5A |
| mMGL-1 | Clec10a | Asialoglycoprotein receptor 1 | ASGR1 |
| mSIGNR1 | Cd209b | CD209 | CD209 |
| mSIGNR3 | Cd209d | CD209 | CD209 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Midha, A.; Goyette-Desjardins, G.; Goerdeler, F.; Moscovitz, O.; Seeberger, P.H.; Tedin, K.; Bertzbach, L.D.; Lepenies, B.; Hartmann, S. Lectin-Mediated Bacterial Modulation by the Intestinal Nematode Ascaris suum. Int. J. Mol. Sci. 2021, 22, 8739. https://doi.org/10.3390/ijms22168739
Midha A, Goyette-Desjardins G, Goerdeler F, Moscovitz O, Seeberger PH, Tedin K, Bertzbach LD, Lepenies B, Hartmann S. Lectin-Mediated Bacterial Modulation by the Intestinal Nematode Ascaris suum. International Journal of Molecular Sciences. 2021; 22(16):8739. https://doi.org/10.3390/ijms22168739
Chicago/Turabian StyleMidha, Ankur, Guillaume Goyette-Desjardins, Felix Goerdeler, Oren Moscovitz, Peter H. Seeberger, Karsten Tedin, Luca D. Bertzbach, Bernd Lepenies, and Susanne Hartmann. 2021. "Lectin-Mediated Bacterial Modulation by the Intestinal Nematode Ascaris suum" International Journal of Molecular Sciences 22, no. 16: 8739. https://doi.org/10.3390/ijms22168739
APA StyleMidha, A., Goyette-Desjardins, G., Goerdeler, F., Moscovitz, O., Seeberger, P. H., Tedin, K., Bertzbach, L. D., Lepenies, B., & Hartmann, S. (2021). Lectin-Mediated Bacterial Modulation by the Intestinal Nematode Ascaris suum. International Journal of Molecular Sciences, 22(16), 8739. https://doi.org/10.3390/ijms22168739






