Uteroplacental Circulation in Normal Pregnancy and Preeclampsia: Functional Adaptation and Maladaptation
Abstract
1. Introduction
2. Uteroplacental Vascular (Mal)Adaptation in Normal Pregnancy and Preeclampsia
2.1. Myogenic Tone
2.2. Vasoreactivity
2.3. Shear Stress
2.4. Release of Endothelium-Derived Vasodilators
3. Mechanisms Underlying Uterine Vascular (Mal)Adaptation in Normal Pregnancy and Preeclampsia
3.1. The Estrogen-ER Signaling Pathway
3.2. Ca2+ Spark/STOC Coupling
3.3. HIFs, Oxidative Stress and Endoplasmic Reticulum Stress
3.4. Kinase Signaling
3.5. Angiogenic Balance
3.6. Inflammation
3.7. Autoimmunity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Monk, C.; Lugo-Candelas, C.; Trumpff, C. Prenatal Developmental Origins of Future Psychopathology: Mechanisms and Pathways. Annu. Rev. Clin. Psychol. 2019, 15, 317–344. [Google Scholar] [CrossRef]
- Osol, G.; Mandala, M. Maternal Uterine Vascular Remodeling During Pregnancy. Physiology 2009, 24, 58–71. [Google Scholar] [CrossRef]
- Hunter, S.; Robson, S.C. Adaptation of the maternal heart in pregnancy. Br. Heart J. 1992, 68, 540–543. [Google Scholar] [CrossRef]
- Mahendru, A.A.; Everett, T.R.; Wilkinson, I.B.; Lees, C.C.; McEniery, C.M. A longitudinal study of maternal cardiovascular function from preconception to the postpartum period. J. Hypertens. 2014, 32, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Sanghavi, M.; Rutherford, J.D. Cardiovascular Physiology of Pregnancy. Circulation 2014, 130, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Watson, A.L.; Hempstock, J.; Bao, Y.-P.; Skepper, J.N.; Burton, G.J. Onset of Maternal Arterial Blood Flow and Placental Oxidative Stress: A Possible Factor in Human Early Pregnancy Failure. Am. J. Pathol. 2000, 157, 2111–2122. [Google Scholar] [CrossRef]
- Saghian, R.; Bogle, G.; James, J.L.; Clark, A.R. Establishment of maternal blood supply to the placenta: Insights into plugging, unplugging and trophoblast behaviour from an agent-based model. Interface Focus 2019, 9, 20190019. [Google Scholar] [CrossRef]
- Lang, U.; Baker, R.; Braems, G.; Zygmunt, M.; Künzel, W.; Clark, K. Uterine blood flow—A determinant of fetal growth. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 110, S55–S61. [Google Scholar] [CrossRef]
- Huppertz, B. Placental origins of preeclampsia: Challenging the current hypothesis. Hypertension 2008, 51, 970–975. [Google Scholar] [CrossRef]
- Redman, C.W.; Sargent, I.L. Latest advances in understanding preeclampsia. Science 2005, 308, 1592–1594. [Google Scholar] [CrossRef]
- Gilbert, J.S.; Ryan, M.J.; Lamarca, B.B.; Sedeek, M.; Murphy, S.R.; Granger, J.P. Pathophysiology of hypertension during preeclampsia: Linking placental ischemia with endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H541–H550. [Google Scholar] [CrossRef]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S.; et al. Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.C.; Shennan, A.; Hyett, J.A.; Kapur, A.; Hadar, E.; Divakar, H.; McAuliffe, F.; Costa, F.D.S.; von Dadelszen, P.; McIntyre, H.D.; et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int. J. Gynecol. Obstet. 2019, 145, 1–33. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obs. Gynecol 2020, 135, e237–e260. [Google Scholar] [CrossRef]
- Tooher, J.; Thornton, C.; Makris, A.; Ogle, R.; Korda, A.; Hennessy, A. All Hypertensive Disorders of Pregnancy Increase the Risk of Future Cardiovascular Disease. Hypertension 2017, 70, 798–803. [Google Scholar] [CrossRef]
- Andraweera, P.H.; Gatford, K.L.; Care, A.S.; Bianco-Miotto, T.; Lassi, Z.S.; Dekker, G.A.; Arstall, M.; Roberts, C. Mechanisms linking exposure to preeclampsia in utero and the risk for cardiovascular disease. J. Dev. Orig. Health Dis. 2020, 11, 235–242. [Google Scholar] [CrossRef]
- Turbeville, H.R.; Sasser, J.M. Preeclampsia beyond Pregnancy: Long-Term Consequences for Mother and Child. Am. J. Physiol. Physiol. 2020, 318, F1315–F1326. [Google Scholar] [CrossRef]
- Melchiorre, K.; Thilaganathan, B.; Giorgione, V.; Ridder, A.; Memmo, A.; Khalil, A. Hypertensive Disorders of Pregnancy and Future Cardiovascular Health. Front. Cardiovasc. Med. 2020, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Degner, K.; Magness, R.R.; Shah, D.M. Establishment of the Human Uteroplacental Circulation: A Historical Perspective. Reprod. Sci. 2017, 24, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Enriquez, P.J.V.; McKerracher, L.J.; Elliot, M.G. Pre-Eclampsia and Maternal–Fetal Conflict. Evol. Med. Public Health 2018, 2018, 217–218. [Google Scholar] [CrossRef]
- Gatford, K.L.; Andraweera, P.H.; Roberts, C.T.; Care, A.S. Animal Models of Preeclampsia: Causes, Consequences, and Interventions. Hypertension 2020, 75, 1363–1381. [Google Scholar] [CrossRef]
- Campbell, S.; Diaz-Recasens, J.; Griffin, D.R.; Cohen-Overbeek, T.E.; Pearce, J.M.; Willson, K.; Teague, M.J. New doppler technique for assessing uteroplacental blood flow. Lancet 1983, 1, 675–677. [Google Scholar] [CrossRef]
- Konje, J.C.; Kaufmann, P.; Bell, S.C.; Taylor, D.J. A longitudinal study of quantitative uterine blood flow with the use of color power angiography in appropriate for gestational age pregnancies. Am. J. Obstet. Gynecol. 2001, 185, 608–613. [Google Scholar] [CrossRef]
- Palmer, S.K.; Zamudio, S.; Coffin, C.; Parker, S.; Stamm, E.; Moore, L. Quantitative estimation of human uterine artery blood flow and pelvic blood flow redistribution in pregnancy. Obstet. Gynecol. 1992, 80, 1000–1006. [Google Scholar]
- Flo, K.; Wilsgaard, T.; Acharya, G. A New Non-Invasive Method for Measuring Uterine Vascular Resistance and Its Relationship to Uterine Artery Doppler Indices: A Longitudinal Study. Ultrasound Obstet. Gynecol 2011, 37, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.R.; Morriss, F.H., Jr.; Makowski, E.L.; Meschia, G.; Battaglia, F.C. Circulatory changes in the reproductive tissues of ewes during pregnancy. Gynecol. Obstet. Investig. 1974, 5, 252–268. [Google Scholar] [CrossRef] [PubMed]
- Peeters, L.L.H.; Sparks, J.W.; Grutters, G.; Girard, J.; Battaglia, F.C. Uteroplacental Blood Flow during Pregnancy in Chronically Catheterized Guinea Pigs. Pediatr. Res. 1982, 16, 716–720. [Google Scholar] [CrossRef]
- Dowell, R.T.; Kauer, C.D. Maternal hemodynamics and uteroplacental blood flow throughout gestation in conscious rats. Methods Find. Exp. Clin. Pharmacol. 1997, 19, 613–625. [Google Scholar] [PubMed]
- Leiberman, J.R.; Meizner, I.; Mazor, M.; Insler, V. Blood supply to the uterus in preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 1988, 28, 23–27. [Google Scholar] [CrossRef]
- Lunell, N.O.; Nylund, L.E.; Lewander, R.; Sarby, B.; Thornström, S. Uteroplacental Blood Flow in Pre-Eclampsia Measurements with Indium-113M and a Computer-Linked Gamma Camera. Clin. Exp. Hypertens. Part B Hypertens. Pregnancy 1982, 1, 105–117. [Google Scholar] [CrossRef]
- Takata, M.; Nakatsuka, M.; Kudo, T. Differential Blood Flow in Uterine, Ophthalmic, and Brachial Arteries of Preeclamptic Women. Obstet. Gynecol. 2002, 100, 931–939. [Google Scholar] [CrossRef]
- Palmer, S.K.; Moore, L.G.; Young, D.A.; Cregger, B.; Berman, J.C.; Zamudio, S. Altered blood pressure course during normal pregnancy and increased preeclampsia at high altitude (3100 meters) in Colorado. Am. J. Obstet. Gynecol. 1999, 180, 1161–1168. [Google Scholar] [CrossRef]
- Zamudio, S. High-Altitude Hypoxia and Preeclampsia. Front. Biosci. 2007, 12, 2967–2977. [Google Scholar] [CrossRef] [PubMed]
- Zamudio, S.; Palmer, S.K.; Droma, T.; Stamm, E.; Coffin, C.; Moore, L.G. Effect of altitude on uterine artery blood flow during normal pregnancy. J. Appl. Physiol. 1995, 79, 7–14. [Google Scholar] [CrossRef]
- Tykocki, N.R.; Boerman, E.M.; Jackson, W.F. Smooth Muscle Ion Channels and Regulation of Vascular Tone in Resistance Arteries and Arterioles. Compr. Physiol. 2017, 7, 485–581. [Google Scholar] [CrossRef]
- Davis, M.J.; Hill, M. Signaling Mechanisms Underlying the Vascular Myogenic Response. Physiol. Rev. 1999, 79, 387–423. [Google Scholar] [CrossRef]
- Hill, M.A.; Yang, Y.; Ella, S.R.; Davis, M.J.; Braun, A.P. Large conductance, Ca2+ -activated K+ channels (BKCa) and arteriolar myogenic signaling. FEBS Lett. 2010, 584, 2033–2042. [Google Scholar] [CrossRef]
- Veerareddy, S.; Cooke, C.-L.M.; Baker, P.N.; Davidge, S.T. Vascular adaptations to pregnancy in mice: Effects on myogenic tone. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H2226–H2233. [Google Scholar] [CrossRef]
- Marshall, S.A.; Senadheera, S.N.; Jelinic, M.; O’Sullivan, K.; Parry, L.J.; Tare, M. Relaxin Deficiency Leads to Uterine Artery Dysfunction During Pregnancy in Mice. Front. Physiol. 2018, 9, 255. [Google Scholar] [CrossRef]
- Xiao, D.; Buchholz, J.N.; Zhang, L. Pregnancy attenuates uterine artery pressure-dependent vascular tone: Role of PKC/ERK pathway. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H2337–H2343. [Google Scholar] [CrossRef]
- Hu, X.-Q.; Xiao, D.; Zhu, R.; Huang, X.; Yang, S.; Wilson, S.; Zhang, L. Pregnancy Upregulates Large-Conductance Ca2+-Activated K+ Channel Activity and Attenuates Myogenic Tone in Uterine Arteries. Hypertension 2011, 58, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Osol, G.; Cipolla, M. Interaction of myogenic and adrenergic mechanisms in isolated, pressurized uterine radial arteries from late-pregnant and nonpregnant rats. Am. J. Obstet. Gynecol. 1993, 168, 697–705. [Google Scholar] [CrossRef]
- Eckman, D.M.; Gupta, R.; Rosenfeld, C.R.; Morgan, T.M.; Charles, S.M.; Mertz, H.; Moore, L.G. Pregnancy increases myometrial artery myogenic tone via NOS- or COX-independent mechanisms. Am. J. Physiol. Integr. Comp. Physiol. 2012, 303, R368–R375. [Google Scholar] [CrossRef] [PubMed]
- Kublickiene, K.R.; Lindblom, B.; Kruger, K.; Nisell, H. Preeclampsia: Evidence for impaired shear stress-mediated nitric oxide release in uterine circulation. Am. J. Obstet. Gynecol. 2000, 183, 160–166. [Google Scholar] [CrossRef]
- Reho, J.J.; Toot, J.D.; Peck, J.; Novak, J.; Yun, Y.H.; Ramirez, R.J. Increased Myogenic Reactivity of Uterine Arteries from Pregnant Rats with Reduced Uterine Perfusion Pressure. Pregnancy Hypertens. 2012, 2, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Xiao, D.; Huang, X.; Longo, L.D.; Zhang, L. Chronic hypoxia increases pressure-dependent myogenic tone of the uterine artery in pregnant sheep: Role of ERK/PKC pathway. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1840–H1849. [Google Scholar] [CrossRef]
- Hu, X.-Q.; Xiao, D.; Zhu, R.; Huang, X.; Yang, S.; Wilson, S.; Zhang, L. Chronic Hypoxia Suppresses Pregnancy-Induced Upregulation of Large-Conductance Ca2+-Activated K+ Channel Activity in Uterine Arteries. Hypertension 2012, 60, 214–222. [Google Scholar] [CrossRef]
- Vanhoutte, P.M. Endothelium and Control of Vascular Function. State of the Art lecture. Hypertension 1989, 13, 658–667. [Google Scholar] [CrossRef]
- Ni, Y.; Meyer, M.; Osol, G. Gestation increases nitric oxide–mediated vasodilation in rat uterine arteries. Am. J. Obstet. Gynecol. 1997, 176, 856–864. [Google Scholar] [CrossRef]
- Nelson, S.H.; Steinsland, O.S.; Suresh, M.S.; Lee, N.M. Pregnancy augments nitric oxide-dependent dilator response to acetylcholine in the human uterine artery. Hum. Reprod. 1998, 13, 1361–1367. [Google Scholar] [CrossRef]
- White, M.M.; McCullough, R.E.; Dyckes, R.; Robertson, A.D.; Moore, L.G. Chronic hypoxia, pregnancy, and endothelium-mediated relaxation in guinea pig uterine and thoracic arteries. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2069–H2075. [Google Scholar] [CrossRef]
- Thompson, L.P.; Weiner, C.P. Pregnancy enhances G protein activation and nitric oxide release from uterine arteries. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H2069–H2075. [Google Scholar] [CrossRef] [PubMed]
- Cooke, C.-L.M.; Davidge, S.T. Pregnancy-Induced Alterations of Vascular Function in Mouse Mesenteric and Uterine Arteries1. Biol. Reprod. 2003, 68, 1072–1077. [Google Scholar] [CrossRef]
- Gangula, P.R.; Thota, C.; Wimalawansa, S.J.; Bukoski, R.D.; Yallampalli, C. Mechanisms Involved in Calcitonin Gene-Related Peptide-Induced Relaxation in Pregnant Rat Uterine Artery. Biol. Reprod. 2003, 69, 1635–1641. [Google Scholar] [CrossRef]
- Ross, G.R.; Yallampalli, U.; Gangula, P.R.; Reed, L.; Sathishkumar, K.; Gao, H.; Chauhan, M.; Yallampalli, C. Adrenomedullin relaxes rat uterine artery: Mechanisms and influence of pregnancy and estradiol. Endocrinology 2010, 151, 4485–4493. [Google Scholar] [CrossRef] [PubMed]
- Weiner, C.P.; Thompson, L.P.; Liu, K.Z.; Herrig, J.E. Pregnancy reduces serotonin-induced contraction of guinea pig uterine and carotid arteries. Am. J. Physiol. Heart Circ. Physiol. 1992, 263, H1764–H1769. [Google Scholar] [CrossRef]
- Weiner, C.; Liu, K.Z.; Thompson, L.; Herrig, J.; Chestnut, D. Effect of pregnancy on endothelium and smooth muscle: Their role in reduced adrenergic sensitivity. Am. J. Physiol. Heart Circ. Physiol. 1991, 261, H1275–H1283. [Google Scholar] [CrossRef] [PubMed]
- Weiner, C.P.; Martinez, E.; Chestnut, D.H.; Ghodsi, A. Effect of pregnancy on uterine and carotid artery response to norepinephrine, epinephrine, and phenylephrine in vessels with documented functional endothelium. Am. J. Obstet. Gynecol. 1989, 161, 1605–1610. [Google Scholar] [CrossRef]
- Magness, R.R.; Rosenfeld, C.R. Systemic and uterine responses to α-adrenergic stimulation in pregnant and nonpregnant ewes. Am. J. Obstet. Gynecol. 1986, 155, 897–904. [Google Scholar] [CrossRef]
- Jovanovic, S.; Grbovic, L.; Jovanovic, A. Pregnancy is associated with altered response to neuropeptide Y in uterine artery. Mol. Hum. Reprod. 2000, 6, 352–360. [Google Scholar] [CrossRef][Green Version]
- McElvy, S.; Greenberg, S.G.; Mershon, J.L.; Yang, D.S.; Magill, C.; Clark, K.E. Mechanism of uterine vascular refractoriness to endothelin-1 in pregnant sheep. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H804–H812. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Clark, K.E. Effect of endothelin-1 on the uterine vasculature of the pregnant and estrogen-treated nonpregnant sheep. Am. J. Obstet. Gynecol. 1992, 167, 1642–1650. [Google Scholar] [CrossRef]
- McCarthy, A.L.; Woolfson, R.G.; Raju, S.K.; Poston, L. Abnormal endothelial cell function of resistance arteries from women with preeclampsia. Am. J. Obstet. Gynecol. 1993, 168, 1323–1330. [Google Scholar] [CrossRef]
- Knock, G.A.; Poston, L. Bradykinin-Mediated relaxation of isolated maternal resistance arteries in normal pregnancy and preeclampsia. Am. J. Obstet. Gynecol. 1996, 175, 1668–1674. [Google Scholar] [CrossRef]
- Ashworth, J.R.; Warren, A.Y.; Baker, P.; Johnson, I.R. Loss of endothelium-dependent relaxation in myometrial resistance arteries in pre-eclampsia. BJOG Int. J. Obstet. Gynaecol. 1997, 104, 1152–1158. [Google Scholar] [CrossRef]
- Ashworth, J.R.; Baker, P.N.; Warren, A.Y.; Phil, M.; Johnson, I.R. Mechanisms of Endothelium-Dependent Relaxation in Myometrial Resistance Vessels and Their Alteration in Preeclampsia. Hypertens. Pregnancy 1999, 18, 57–71. [Google Scholar] [CrossRef]
- Svedas, E.; Nisell, H.; Vanwijk, M.J.; Nikas, Y.; Kublickiene, K.R. Endothelial dysfunction in uterine circulation in preeclampsia: Can estrogens improve it? Am. J. Obstet. Gynecol. 2002, 187, 1608–1616. [Google Scholar] [CrossRef]
- Ong, S.S.; Moore, R.J.; Warren, A.Y.; Crocker, I.P.; Fulford, J.; Tyler, D.J.; Gowland, P.A.; Baker, P.N. Myometrial and placental artery reactivity alone cannot explain reduced placental perfusion in pre-eclampsia and intrauterine growth restriction. BJOG Int. J. Obstet. Gynaecol. 2003, 110, 909–915. [Google Scholar] [CrossRef]
- Wareing, M.; Myers, J.E.; O’Hara, M.; Kenny, L.C.; Warren, A.Y.; Taggart, M.J.; Skillern, L.; Machin, I.; Baker, P.N. Effects of a phosphodiesterase-5 (PDE5) inhibitor on endothelium-dependent relaxation of myometrial small arteries. Am. J. Obstet. Gynecol. 2004, 190, 1283–1290. [Google Scholar] [CrossRef]
- Luksha, L.; Luksha, N.; Kublickas, M.; Nisell, H.; Kublickiene, K. Diverse Mechanisms of Endothelium-Derived Hyperpolarizing Factor-Mediated Dilatation in Small Myometrial Arteries in Normal Human Pregnancy and Preeclampsia1. Biol. Reprod. 2010, 83, 728–735. [Google Scholar] [CrossRef]
- Moyes, A.J.; Gray, G.A.; Denison, F.C. Bradykinin B1receptor-Mediated Vasodilation is Impaired in Myometrial Arteries from Women with Pre-Eclampsia. Hypertens. Pregnancy 2013, 33, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Mazzuca, M.Q.; Li, W.; Reslan, O.M.; Yu, P.; Mata, K.M.; Khalil, R.A. Downregulation of Microvascular Endothelial Type B Endothelin Receptor Is a Central Vascular Mechanism in Hypertensive Pregnancy. Hypertension 2014, 64, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Aljunaidy, M.M.; Morton, J.S.; Cooke, C.-L.; Davidge, S.T. Maternal vascular responses to hypoxia in a rat model of intrauterine growth restriction. Am. J. Physiol. Integr. Comp. Physiol. 2016, 311, R1068–R1075. [Google Scholar] [CrossRef] [PubMed]
- Turan, S.; Aberdeen, G.W.; Thompson, L.P. Chronic hypoxia alters maternal uterine and fetal hemodynamics in the full-term pregnant guinea pig. Am. J. Physiol. Integr. Comp. Physiol. 2017, 313, R330–R339. [Google Scholar] [CrossRef]
- Anderson, C.M.; Lopez, F.; Zhang, H.-Y.; Pavlish, K.; Benoit, J.N. Reduced Uteroplacental Perfusion Alters Uterine Arcuate Artery Function in the Pregnant Sprague-Dawley Rat1. Biol. Reprod. 2005, 72, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Verlohren, S.; Niehoff, M.; Hering, L.; Geusens, N.; Herse, F.; Tintu, A.N.; Plagemann, A.; Lenoble, F.; Pijnenborg, R.; Muller, M.N.; et al. Uterine Vascular Function in a Transgenic Preeclampsia Rat Model. Hypertension 2008, 51, 547–553. [Google Scholar] [CrossRef]
- Pulgar, V.M.; Yamaleyeva, L.M.; Varagic, J.; McGee, C.M.; Bader, M.; Dechend, R.; Howlett, A.C.; Brosnihan, K.B. Increased angiotensin II contraction of the uterine artery at early gestation in a transgenic model of hypertensive pregnancy is reduced by inhibition of endocannabinoid hydrolysis. Hypertension 2014, 64, 619–625. [Google Scholar] [CrossRef]
- Ando, J.; Yamamoto, K. Flow detection and calcium signalling in vascular endothelial cells. Cardiovasc. Res. 2013, 99, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Kassab, G.S. Role of shear stress and stretch in vascular mechanobiology. J. R. Soc. Interface 2011, 8, 1379–1385. [Google Scholar] [CrossRef]
- Mateev, S.; Sillau, A.H.; Mouser, R.; McCullough, R.E.; White, M.M.; Young, D.A.; Moore, L.G. Chronic hypoxia opposes pregnancy-induced increase in uterine artery vasodilator response to flow. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H820–H829. [Google Scholar] [CrossRef]
- Sandoo, A.; Van Zanten, J.J.V.; Metsios, G.S.; Carroll, D.; Kitas, G. The Endothelium and Its Role in Regulating Vascular Tone. Open Cardiovasc. Med. J. 2010, 4, 302–312. [Google Scholar] [CrossRef]
- Murad, F. Shattuck Lecture. Nitric oxide and cyclic GMP in cell signaling and drug development. N. Engl. J. Med. 2006, 355, 2003–2011. [Google Scholar] [CrossRef]
- Narumiya, S.; Sugimoto, Y.; Ushikubi, F. Prostanoid Receptors: Structures, Properties, and Functions. Physiol. Rev. 1999, 79, 1193–1226. [Google Scholar] [CrossRef]
- Biringer, R.G. A Review of Prostanoid Receptors: Expression, Characterization, Regulation, and Mechanism of Action. J. Cell Commun. Signal. 2020, 15, 155–184. [Google Scholar] [CrossRef]
- Gheibi, S.; Jeddi, S.; Kashfi, K.; Ghasemi, A. Regulation of vascular tone homeostasis by NO and H2S: Implications in hypertension. Biochem. Pharmacol. 2018, 149, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Félétou, M. Endothelium-Dependent Hyperpolarization and Endothelial Dysfunction. J. Cardiovasc. Pharmacol. 2016, 67, 373–387. [Google Scholar] [CrossRef]
- Garland, C.J.; Dora, K.A. EDH: Endothelium-Dependent Hyperpolarization and Microvascular Signalling. Acta Physiol. 2016, 219, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.W. Eicosanoids in preeclampsia. Prostaglandins Leukot Essent Fat. Acids 2004, 70, 223–232. [Google Scholar] [CrossRef]
- Toda, N.; Toda, H.; Okamura, T. Regulation of myometrial circulation and uterine vascular tone by constitutive nitric oxide. Eur. J. Pharmacol. 2013, 714, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Holwerda, K.M.; Faas, M.M.; van Goor, H.; Lely, A.T. Gasotransmitters: A solution for the therapeutic dilemma in preeclampsia? Hypertension 2013, 62, 653–659. [Google Scholar] [CrossRef]
- Goulopoulou, S. Maternal Vascular Physiology in Preeclampsia. Hypertension 2017, 70, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Sutton, E.F.; Gemmel, M.; Powers, R.W. Nitric oxide signaling in pregnancy and preeclampsia. Nitric Oxide 2019, 95, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Osol, G.; Ko, N.L.; Mandalà, M. Altered Endothelial Nitric Oxide Signaling as a Paradigm for Maternal Vascular Maladaptation in Preeclampsia. Curr. Hypertens. Rep. 2017, 19, 82. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.H.; Steinsland, O.S.; Wang, Y.; Yallampalli, C.; Dong, Y.-L.; Sanchez, J.M. Increased Nitric Oxide Synthase Activity and Expression in the Human Uterine Artery During Pregnancy. Circ. Res. 2000, 87, 406–411. [Google Scholar] [CrossRef]
- Xiao, D.; Bird, I.M.; Magness, R.R.; Longo, L.D.; Zhang, L. Upregulation of eNOS in pregnant ovine uterine arteries by chronic hypoxia. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H812–H820. [Google Scholar] [CrossRef]
- Magness, R.R.; Sullivan, J.A.; Li, Y.; Phernetton, T.M.; Bird, I.M. Endothelial vasodilator production by uterine and systemic arteries. VI. Ovarian and pregnancy effects on eNOS and NO(x). Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H1692–H1698. [Google Scholar] [CrossRef]
- Magness, R.R.; Rosenfeld, C.R.; Hassan, A.; Shaul, P.W. Endothelial vasodilator production by uterine and systemic arteries. I. Effects of ANG II on PGI2 and NO in pregnancy. Am. J. Physiol. Heart Circ. Physiol. 1996, 270, H1914–H1923. [Google Scholar] [CrossRef]
- Xiao, D.; Liu, Y.; Pearce, W.; Zhang, L. Endothelial nitric oxide release in isolated perfused ovine uterine arteries: Effect of pregnancy. Eur. J. Pharmacol. 1999, 367, 223–230. [Google Scholar] [CrossRef]
- Amit, A.; Thaler, I.; Paz, Y.; Itskovitz-Eldor, J. The effect of a nitric oxide donor on Doppler flow velocity waveforms in the uterine artery during the first trimester of pregnancy. Ultrasound Obstet. Gynecol. 1998, 11, 94–98. [Google Scholar] [CrossRef]
- Miller, S.L.; Jenkin, G.; Walker, D.W. Effect of nitric oxide synthase inhibition on the uterine vasculature of the late-pregnant ewe. Am. J. Obstet. Gynecol. 1999, 180, 1138–1145. [Google Scholar] [CrossRef]
- Rosenfeld, C.R.; White, R.E.; Roy, T.; Cox, B.E. Calcium-Activated potassium channels and nitric oxide coregulate estrogen-induced vasodilation. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H319–H328. [Google Scholar] [CrossRef]
- Rosenfeld, C.R.; Roy, T. Prolonged uterine artery nitric oxide synthase inhibition modestly alters basal uteroplacental vasodilation in the last third of ovine pregnancy. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1196–H1203. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.R.; Cox, B.E.; Roy, T.; Magness, R.R. Nitric oxide contributes to estrogen-induced vasodilation of the ovine uterine circulation. J. Clin. Investig. 1996, 98, 2158–2166. [Google Scholar] [CrossRef]
- Wight, E.; Küng, C.F.; Moreau, P.; Takase, H.; Lüscher, T.F. Chronic Blockade of Nitric Oxide Synthase and Endothelin Receptors During Pregnancy in the Rat: Effect on Reactivity of the Uterine Artery In Vitro. J. Soc. Gynecol. Investig. 1998, 5, 288–295. [Google Scholar] [CrossRef]
- Kublickiene, K.R.; Nisell, H.; Poston, L.; Krüger, K.; Lindblom, B. Modulation of vascular tone by nitric oxide and endothelin 1 in myometrial resistance arteries from pregnant women at term. Am. J. Obstet. Gynecol. 2000, 182, 87–93. [Google Scholar] [CrossRef]
- Van Buren, G.A.; Yang, D.-S.; Clark, K.E. Estrogen-Induced uterine vasodilatation is antagonized by L-nitroarginine methyl ester, an inhibitor of nitric oxide synthesis. Am. J. Obstet. Gynecol. 1992, 167, 828–833. [Google Scholar] [CrossRef]
- Stanley, J.L.; Andersson, I.J.; Hirt, C.J.; Moore, L.; Dilworth, M.; Chade, A.R.; Sibley, C.P.; Davidge, S.T.; Baker, P. Effect of the Anti-Oxidant Tempol on Fetal Growth in a Mouse Model of Fetal Growth Restriction1. Biol. Reprod. 2012, 87, 1–8. [Google Scholar] [CrossRef]
- Kusinski, L.C.; Stanley, J.L.; Dilworth, M.R.; Hirt, C.J.; Andersson, I.J.; Renshall, L.J.; Baker, B.C.; Baker, P.N.; Sibley, C.P.; Wareing, M.; et al. eNOS Knockout Mouse as a Model of Fetal Growth Restriction with an Impaired Uterine Artery Function and Placental Transport Phenotype. Am. J. Physiol. Integr. Comp. Physiol. 2012, 303, R86–R93. [Google Scholar] [CrossRef]
- Kulandavelu, S.; Whiteley, K.J.; Qu, D.; Mu, J.; Bainbridge, S.A.; Adamson, S.L. Endothelial Nitric Oxide Synthase Deficiency Reduces Uterine Blood Flow, Spiral Artery Elongation, and Placental Oxygenation in Pregnant Mice. Hypertension 2012, 60, 231–238. [Google Scholar] [CrossRef]
- Rennie, M.Y.; Rahman, A.; Whiteley, K.J.; Sled, J.G.; Adamson, S.L. Site-Specific increases in utero- and fetoplacental arterial vascular resistance in eNOS-deficient mice due to impaired arterial enlargement. Biol. Reprod. 2015, 92, 48. [Google Scholar] [CrossRef]
- Shaamash, A.; Elsonosy, E.; Zakhari, M.; Radwan, S.; El-Dien, H. Placental nitric oxide synthase (NOS) activity and nitric oxide (NO) production in normal pregnancy, pre-eclampsia and eclampsia. Int. J. Gynecol. Obstet. 2001, 72, 127–133. [Google Scholar] [CrossRef]
- Smith-Jackson, K.; Hentschke, M.; Poli-De-Figueiredo, C.; da Costa, B.E.P.; Kurlak, L.; Pipkin, F.B.; Czajka, A.; Mistry, H. Placental expression of eNOS, iNOS and the major protein components of caveolae in women with pre-eclampsia. Placenta 2015, 36, 607–610. [Google Scholar] [CrossRef]
- Kim, Y.; Park, H.; Lee, H.; Ha, E.; Suh, S.; Oh, S.; Yoo, H.-S. Reduced l-arginine Level and Decreased Placental eNOS Activity in Preeclampsia. Placenta 2006, 27, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; He, F.; Kuang, L.; Tang, W.; Li, Y.; Chen, D. eNOS/iNOS And Endoplasmic Reticulum Stress-Induced Apoptosis in the Placentas of Patients with Preeclampsia. J. Hum. Hypertens. 2016, 31, 49–55. [Google Scholar] [CrossRef]
- Chen, J.; Gao, Q.; Jiang, L.; Feng, X.; Zhu, X.; Fan, X.; Mao, C.; Xu, Z. The NOX2-Derived reactive oxygen species damaged endothelial nitric oxide system via suppressed BKCa/SKCa in preeclampsia. Hypertens. Res. 2017, 40, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-F.; He, M.-Z.; Xie, Y.; Wu, Y.-Y.; Yang, M.-T.; Fan, Y.; Qiao, F.-Y.; Deng, D.-R. Involvement of dysregulated IKCa and SKCa channels in preeclampsia. Placenta 2017, 58, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, G.; Jahan, S.; Ain, Q.U.; Ullah, A.; Afsar, T.; Almajwal, A.; Alam, I.; Razak, S. Placental endothelial nitric oxide synthase expression and role of oxidative stress in susceptibility to preeclampsia in Pakistani women. Mol. Genet. Genom. Med. 2020, 8, e1019. [Google Scholar]
- Baker, P.; Davidge, S.T.; Roberts, J.M. Plasma from Women With Preeclampsia Increases Endothelial Cell Nitric Oxide Production. Hypertension 1995, 26, 244–248. [Google Scholar] [CrossRef]
- Davidge, S.T.; Baker, P.N.; Roberts, J.M. NOS Expression is Increased in Endothelial Cells Exposed to Plasma from Women with Preeclampsia. Am. J. Physiol. Heart Circ. Physiol. 1995, 269, H1106–H1112. [Google Scholar] [CrossRef]
- Kao, C.K.; Morton, J.S.; Quon, A.L.; Reyes, L.M.; Lopez-Jaramillo, P.; Davidge, S.T. Mechanism of vascular dysfunction due to circulating factors in women with pre-eclampsia. Clin. Sci. 2016, 130, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Tashie, W.; Fondjo, L.A.; Owiredu, W.K.B.A.; Ephraim, R.K.D.; Asare, L.; Adu-Gyamfi, E.A.; Seidu, L. Altered Bioavailability of Nitric Oxide and L-Arginine Is a Key Determinant of Endothelial Dysfunction in Preeclampsia. BioMed Res. Int. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Roggensack, A.M.; Zhang, Y.; Davidge, S.T. Evidence for Peroxynitrite Formation in the Vasculature of Women with Preeclampsia. Hypertension 1999, 33, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, B.M.; Cook, L.G.; Danchuk, S.; Puschett, J.B. Uncoupled Endothelial Nitric Oxide Synthase and Oxidative Stress in a Rat Model of Pregnancy-Induced Hypertension. Am. J. Hypertens. 2007, 20, 1297–1304. [Google Scholar] [CrossRef]
- Guerby, P.; Tasta, O.; Swiader, A.; Pont, F.; Bujold, E.; Parant, O.; Vayssiere, C.; Salvayre, R.; Negre-Salvayre, A. Role of oxidative stress in the dysfunction of the placental endothelial nitric oxide synthase in preeclampsia. Redox Biol. 2021, 40, 101861. [Google Scholar] [CrossRef] [PubMed]
- Noris, M.; Todeschini, M.; Cassis, P.; Pasta, F.; Cappellini, A.; Bonazzola, S.; Macconi, D.; Maucci, R.; Porrati, F.; Benigni, A.; et al. l-Arginine Depletion in Preeclampsia Orients Nitric Oxide Synthase Toward Oxidant Species. Hypertension 2004, 43, 614–622. [Google Scholar] [CrossRef]
- Sankaralingam, S.; Xu, H.; Davidge, S.T. Arginase contributes to endothelial cell oxidative stress in response to plasma from women with preeclampsia. Cardiovasc. Res. 2009, 85, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Lorca, R.A.; Lane, S.L.; Bales, E.S.; Nsier, H.; Yi, H.; Donnelly, M.A.; Euser, A.G.; Julian, C.G.; Moore, L.G. High Altitude Reduces NO-Dependent Myometrial Artery Vasodilator Response During Pregnancy. Hypertension 2019, 73, 1319–1326. [Google Scholar] [CrossRef]
- Polhemus, D.J.; Lefer, D.J. Emergence of Hydrogen Sulfide as an Endogenous Gaseous Signaling Molecule in Cardiovascular Disease. Circ. Res. 2014, 114, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Sheibani, L.; Lechuga, T.J.; Zhang, H.; Hameed, A.; Wing, D.A.; Kumar, S.; Rosenfeld, C.R.; Chen, D.-B. Augmented H2S production via cystathionine-beta-synthase upregulation plays a role in pregnancy-associated uterine vasodilation. Biol. Reprod. 2017, 96, 664–672. [Google Scholar] [CrossRef]
- Lechuga, T.J.; Qi, Q.R.; Magness, R.R.; Chen, D.B. Ovine uterine artery hydrogen sulfide biosynthesis in vivo: Effects of ovarian cycle and pregnancydagger. Biol. Reprod. 2019, 100, 1630–1636. [Google Scholar] [CrossRef] [PubMed]
- Bok, R.; Guerra, D.D.; Lorca, R.A.; Wennersten, S.A.; Harris, P.S.; Rauniyar, A.K.; Stabler, S.P.; MacLean, K.N.; Roede, J.R.; Brown, L.D.; et al. Cystathionine gamma-lyase promotes estrogen-stimulated uterine artery blood flow via glutathione homeostasis. Redox Biol. 2021, 40, 101827. [Google Scholar] [CrossRef]
- Cindrova-Davies, T.; Herrera, E.A.; Niu, Y.; Kingdom, J.; Giussani, D.A.; Burton, G.J. Reduced cystathionine gamma-lyase and increased miR-21 expression are associated with increased vascular resistance in growth-restricted pregnancies: Hydrogen sulfide as a placental vasodilator. Am. J. Pathol. 2013, 182, 1448–1458. [Google Scholar] [CrossRef]
- Holwerda, K.; Bos, E.; Rajakumar, A.; Ris-Stalpers, C.; van Pampus, M.; Timmer, A.; Erwich, J.; Faas, M.; van Goor, H.; Lely, A. Hydrogen sulfide producing enzymes in pregnancy and preeclampsia. Placenta 2012, 33, 518–521. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ahmad, S.; Cai, M.; Rennie, J.; Fujisawa, T.; Crispi, F.; Baily, J.; Miller, M.R.; Cudmore, M.; Hadoke, P.W.; et al. Dysregulation of hydrogen sulfide producing enzyme cystathionine gamma-lyase contributes to maternal hypertension and placental abnormalities in preeclampsia. Circulation 2013, 127, 2514–2522. [Google Scholar] [CrossRef] [PubMed]
- Saif, J.; Ahmad, S.; Rezai, H.; Litvinova, K.; Sparatore, A.; Alzahrani, F.A.; Wang, K.; Ahmed, A. Hydrogen sulfide releasing molecule MZe786 inhibits soluble Flt-1 and prevents preeclampsia in a refined RUPP mouse model. Redox Biol. 2021, 38, 101814. [Google Scholar] [CrossRef]
- Janowiak, M.A.; Magness, R.R.; Habermehl, D.A.; Bird, I.M. Pregnancy Increases Ovine Uterine Artery Endothelial Cyclooxygenase-1 Expression. Endocrinology 1998, 139, 765–771. [Google Scholar] [CrossRef]
- Di, T.; Sullivan, J.A.; Rupnow, H.L.; Magness, R.R.; Bird, I.M. Pregnancy Induces Expression of cPLA2 in Ovine Uterine Artery but Not Systemic Artery Endothelium. J. Soc. Gynecol. Investig. 1999, 6, 301–306. [Google Scholar] [CrossRef]
- Magness, R.R.; Shideman, C.R.; Habermehl, D.A.; Sullivan, J.A.; Bird, I.M. Endothelial vasodilator production by uterine and systemic arteries. V. Effects of ovariectomy, the ovarian cycle, and pregnancy on prostacyclin synthase expression. Prostaglandins Other Lipid Mediat. 2000, 60, 103–118. [Google Scholar] [CrossRef]
- Magness, R.R.; Rosenfeld, C.R.; Faucher, D.J.; Mitchell, M. Uterine prostaglandin production in ovine pregnancy: Effects of angiotensin II and indomethacin. Am. J. Physiol. Heart Circ. Physiol. 1992, 263, H188–H197. [Google Scholar] [CrossRef] [PubMed]
- Gerber, J.G.; Payne, N.A.; Murphy, R.C.; Nies, A.S. Prostacyclin produced by the pregnant uterus in the dog may act as a circulating vasodepressor substance. J. Clin. Investig. 1981, 67, 632–636. [Google Scholar] [CrossRef]
- Clark, K.E.; Harrington, D.J. Effect of the prostacyclin synthetase inhibitor tranylcypromine on uterine blood flow in pregnancy. Prostaglandins 1982, 23, 227–236. [Google Scholar] [CrossRef]
- Khan, I.; Al-Yatama, M.; Nandakumaran, M. Expression of the Na -H exchanger isoform-1 and cyclooxygenases in human placentas: Their implications in preeclampsia. IUBMB Life 1999, 47, 715–722. [Google Scholar] [CrossRef]
- Börekçi, B.; Aksoy, H.; Toker, A.; Özkan, A. Placental tissue cyclo-oxygenase 1 and 2 in pre-eclamptic and normal pregnancy. Int. J. Gynecol. Obstet. 2006, 95, 127–131. [Google Scholar] [CrossRef]
- Hu, S.; Li, J.; Tong, M.; Li, Q.; Chen, Y.; Lu, H.; Wang, Y.; Min, L. MicroRNA1443p may participate in the pathogenesis of preeclampsia by targeting Cox2. Mol Med Rep 2019, 19, 4655–4662. [Google Scholar] [PubMed]
- Fitzgerald, D.J.; Entman, S.S.; Mulloy, K.; Fitzgerald, G.A. Decreased prostacyclin biosynthesis preceding the clinical manifestation of pregnancy-induced hypertension. Circulation 1987, 75, 956–963. [Google Scholar] [CrossRef]
- Walsh, S.W. Preeclampsia: An imbalance in placental prostacyclin and thromboxane Production. Am. J. Obstet. Gynecol. 1985, 152, 335–340. [Google Scholar] [CrossRef]
- Remuzzi, G.; Marchesi, D.; Zoja, C.; Muratore, D.; Mecca, G.; Misiani, R.; Rossi, E.; Barbato, M.; Capetta, P.; Donati, M.B.; et al. Reduced umbilical and placental vascular prostacyclin in severe pre-eclampsia. Prostaglandins 1980, 20, 105–110. [Google Scholar] [CrossRef]
- Suzuki, Y.; Hattori, T.; Kajikuri, J.; Yamamoto, T.; Suzumori, K.; Itoh, T. Reduced function of endothelial prostacyclin in human omental resistance arteries in pre-eclampsia. J. Physiol. 2002, 545, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Gokina, N.I.; Kuzina, O.Y.; Vance, A.M. Augmented EDHF signaling in rat uteroplacental vasculature during late pregnancy. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1642–H1652. [Google Scholar] [CrossRef]
- Mazzuca, M.Q.; Tare, M.; Parkington, H.C.; Dragomir, N.M.; Parry, L.J.; Wlodek, M.E. Uteroplacental insufficiency programmes vascular dysfunction in non-pregnant rats: Compensatory adaptations in pregnancy. J. Physiol. 2012, 590, 3375–3388. [Google Scholar] [CrossRef]
- Feletou, M.; Vanhoutte, P.M. Endothelium-Derived hyperpolarizing factor: Where are we now? Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Kenny, L.; Baker, P.; Kendall, D.A.; Randall, M.D.; Dunn, W.R. The role of gap junctions in mediating endothelium-dependent responses to bradykinin in myometrial small arteries isolated from pregnant women. Br. J. Pharmacol. 2002, 136, 1085–1088. [Google Scholar] [CrossRef]
- Luksha, L.; Nisell, H.; Luksha, N.; Kublickas, M.; Hultenby, K.; Kublickiene, K. Endothelium-derived hyperpolarizing factor in preeclampsia: Heterogeneous contribution, mechanisms, and morphological prerequisites. Am. J. Physiol. Integr. Comp. Physiol. 2008, 294, R510–R519. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Hu, X.Q.; Xiao, D.; Yang, S.; Wilson, S.M.; Longo, L.D.; Zhang, L. Chronic hypoxia inhibits pregnancy-induced upregulation of SKCa channel expression and function in uterine arteries. Hypertension 2013, 62, 367–374. [Google Scholar] [CrossRef]
- Choi, S.; Kim, J.A.; Na, H.-Y.; Park, S.; Han, K.-H.; Kim, Y.J.; Suh, S.H. NADPH Oxidase 2-Derived Superoxide Downregulates Endothelial KCa3.1 in Preeclampsia. Free Radic. Biol. Med. 2013, 57, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Abbassi-Ghanavati, M.; Greer, L.G.; Cunningham, F.G. Pregnancy and Laboratory Studies: A Reference Table for Clinicians. Obstet. Gynecol. 2010, 115, 868–869. [Google Scholar] [CrossRef]
- Chang, K.; Lubo, Z. Review article: Steroid hormones and uterine vascular adaptation to pregnancy. Reprod. Sci. 2008, 15, 336–348. [Google Scholar] [CrossRef]
- Pastore, M.B.; Jobe, S.O.; Ramadoss, J.; Magness, R.R. Estrogen receptor-alpha and estrogen receptor-beta in the uterine vascular endothelium during pregnancy: Functional implications for regulating uterine blood flow. Semin. Reprod. Med. 2012, 30, 46–61. [Google Scholar]
- Hu, X.-Q.; Song, R.; Zhang, L. Effect of Oxidative Stress on the Estrogen-NOS-NO-KCa Channel Pathway in Uteroplacental Dysfunction: Its Implication in Pregnancy Complications. Oxidative Med. Cell. Longev. 2019, 2019, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Mandalà, M. Influence of Estrogens on Uterine Vascular Adaptation in Normal and Preeclamptic Pregnancies. Int. J. Mol. Sci. 2020, 21, 2592. [Google Scholar] [CrossRef]
- Bai, J.; Qi, Q.-R.; Li, Y.; Day, R.; Makhoul, J.; Magness, R.R.; Chen, D.-B. Estrogen Receptors and Estrogen-Induced Uterine Vasodilation in Pregnancy. Int. J. Mol. Sci. 2020, 21, 4349. [Google Scholar] [CrossRef] [PubMed]
- Magness, R.R.; Rosenfeld, C.R. Local and systemic estradiol-17 beta: Effects on uterine and systemic vasodilation. Am. J. Physiol. Content 1989, 256, E536–E542. [Google Scholar] [CrossRef]
- Magness, R.R.; Parker, C.R.; Rosenfeld, C.R. Systemic and uterine responses to chronic infusion of estradiol-17 beta. Am. J. Physiol. Metab. 1993, 265, E690–E698. [Google Scholar] [CrossRef] [PubMed]
- Magness, R.R.; Phernetton, T.M.; Gibson, T.C.; Chen, D.B. Uterine blood flow responses to ICI 182 780 in ovariectomized oestradiol-17beta-treated, intact follicular and pregnant sheep. J. Physiol. 2005, 565, 71–83. [Google Scholar] [CrossRef]
- Corcoran, J.J.; Nicholson, C.; Sweeney, M.; Charnock, J.C.; Robson, S.C.; Westwood, M.; Taggart, M.J. Human uterine and placental arteries exhibit tissue-specific acute responses to 17beta-estradiol and estrogen-receptor-specific agonists. Mol. Hum. Reprod. 2014, 20, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Tropea, T.; De Francesco, E.M.; Rigiracciolo, D.C.; Maggiolini, M.; Wareing, M.; Osol, G.; Mandalà, M. Pregnancy Augments G Protein Estrogen Receptor (GPER) Induced Vasodilation in Rat Uterine Arteries via the Nitric Oxide—cGMP Signaling Pathway. PLoS ONE 2015, 10, e0141997. [Google Scholar] [CrossRef]
- Scott, P.-A.; Tremblay, A.; Brochu, M.; St-Louis, J. Vasorelaxant action of 17β-estradiol in rat uterine arteries: Role of nitric oxide synthases and estrogen receptors. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3713–H3719. [Google Scholar] [CrossRef]
- Berkane, N.; Liere, P.; Oudinet, J.-P.; Hertig, A.; Lefèvre, G.; Pluchino, N.; Schumacher, M.; Chabbert-Buffet, N. From Pregnancy to Preeclampsia: A Key Role for Estrogens. Endocr. Rev. 2017, 38, 123–144. [Google Scholar] [CrossRef] [PubMed]
- Hertig, A.; Liere, P.; Chabbert-Buffet, N.; Fort, J.; Pianos, A.; Eychenne, B.; Cambourg, A.; Schumacher, M.; Berkane, N.; Lefevre, G.; et al. Steroid profiling in preeclamptic women: Evidence for aromatase deficiency. Am. J. Obstet. Gynecol. 2010, 203, 477.e1–477.e9. [Google Scholar] [CrossRef]
- Jobe, S.O.; Tyler, C.T.; Magness, R.R. Aberrant Synthesis, Metabolism, and Plasma Accumulation of Circulating Estrogens and Estrogen Metabolites in Preeclampsia Implications for Vascular Dysfunction. Hypertension 2013, 61, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Hu, Z.; Zeng, K.; Yin, Y.; Zhao, M.; Chen, M.; Chen, Q. The reduction in circulating levels of estrogen and progesterone in women with preeclampsia. Pregnancy Hypertens. 2017, 11, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Sobrevilla, L.A.; Romero, I.; Kruger, F.; Whittembury, J. Low estrogen excretion during pregnancy at high altitude. Am. J. Obstet. Gynecol. 1968, 102, 828–833. [Google Scholar] [CrossRef]
- Zamudio, S.; Leslie, K.; White, M.; Hagerman, D.D.; Moore, L.G. Low Serum Estradiol and High Serum Progesterone Concentrations Characterize Hypertensive Pregnancies at High Altitude. J. Soc. Gynecol. Investig. 1994, 1, 197–205. [Google Scholar] [CrossRef]
- Berkane, N.; Liere, P.; Lefevre, G.; Alfaidy, N.; Nahed, R.A.; Vincent, J.; Oudinet, J.-P.; Pianos, A.; Cambourg, A.; Rozenberg, P.; et al. Abnormal steroidogenesis and aromatase activity in preeclampsia. Placenta 2018, 69, 40–49. [Google Scholar] [CrossRef]
- Pérez-Sepúlveda, A.; Monteiro, L.J.; Dobierzewska, A.; España-Perrot, P.P.; Venegas-Araneda, P.; Guzmán-Rojas, A.M.; González, M.I.; Palominos-Rivera, M.; Irarrazabal, C.E.; Figueroa-Diesel, H.; et al. Placental Aromatase Is Deficient in Placental Ischemia and Preeclampsia. PLoS ONE 2015, 10, e0139682. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Mendelson, C. USF1 and USF2 Mediate Inhibition of Human Trophoblast Differentiation and CYP19 Gene Expression by Mash-2 and Hypoxia. Mol. Cell. Biol. 2003, 23, 6117–6128. [Google Scholar] [CrossRef]
- Murphy, E. Estrogen Signaling and Cardiovascular Disease. Circ. Res. 2011, 109, 687–696. [Google Scholar] [CrossRef]
- Byers, M.J.; Zangl, A.; Phernetton, T.M.; Lopez, G.; Chen, D.B.; Magness, R.R. Endothelial vasodilator production by ovine uterine and systemic arteries: Ovarian steroid and pregnancy control of ERalpha and ERbeta levels. J. Physiol. 2005, 565, 85–99. [Google Scholar] [CrossRef]
- Chang, K.; Xiao, D.; Huang, X.; Xue, Z.; Yang, S.; Longo, L.D.; Zhang, L. Chronic Hypoxia Inhibits Sex Steroid Hormone-Mediated Attenuation of Ovine Uterine Arterial Myogenic Tone in Pregnancy. Hypertension 2010, 56, 750–757. [Google Scholar] [CrossRef][Green Version]
- Chen, M.; Xiao, D.; Hu, X.Q.; Dasgupta, C.; Yang, S.; Zhang, L. Hypoxia Represses ER-alpha Expression and Inhibits Estrogen-Induced Regulation of Ca2+-Activated K+ Channel Activity and Myogenic Tone in Ovine Uterine Arteries: Causal Role of DNA Methylation. Hypertension 2015, 66, 44–51. [Google Scholar] [CrossRef]
- Lambertini, E.; Penolazzi, L.; Giordano, S.; Del Senno, L.; Piva, R. Expression of the human oestrogen receptor-alpha gene is regulated by promoter F in MG-63 osteoblastic cells. Biochem. J. 2003, 372, 831–839. [Google Scholar] [CrossRef]
- Dasgupta, C.; Chen, M.; Zhang, H.; Yang, S.; Zhang, L. Chronic Hypoxia During Gestation Causes Epigenetic Repression of the Estrogen Receptor-α Gene in Ovine Uterine Arteries via Heightened Promoter Methylation. Hypertension 2012, 60, 697–704. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hu, X.Q.; Dasgupta, C.; Chen, M.; Xiao, D.; Huang, X.; Han, L.; Yang, S.; Xu, Z.; Zhang, L. Pregnancy Reprograms Large-Conductance Ca(2+)-Activated K(+) Channel in Uterine Arteries: Roles of Ten-Eleven Translocation Methylcytosine Dioxygenase 1-Mediated Active Demethylation. Hypertension 2017, 69, 1181–1191. [Google Scholar] [CrossRef]
- Park, M.; Park, K.; Lee, J.; Shin, Y.Y.; An, S.; Kang, S.S.; Cho, W.; An, B.; Kim, S.C. The expression and activation of sex steroid receptors in the preeclamptic placenta. Int. J. Mol. Med. 2018, 41, 2943–2951. [Google Scholar] [CrossRef] [PubMed]
- Lan, K.-C.; Lai, Y.-J.; Cheng, H.-H.; Tsai, N.-C.; Su, Y.-T.; Tsai, C.-C.; Hsu, T.-Y. Levels of sex steroid hormones and their receptors in women with preeclampsia. Reprod. Biol. Endocrinol. 2020, 18, 1–7. [Google Scholar] [CrossRef]
- Hu, X.-Q.; Chen, M.; Dasgupta, C.; Xiao, D.; Huang, X.; Yang, S.; Zhang, L. Chronic hypoxia upregulates DNA methyltransferase and represses large conductance Ca2+-activated K+ channel function in ovine uterine arteries. Biol. Reprod. 2017, 96, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-Q.; Dasgupta, C.; Xiao, D.; Huang, X.; Yang, S.; Zhang, L. MicroRNA-210 Targets Ten-Eleven Translocation Methylcytosine Dioxygenase 1 and Suppresses Pregnancy-Mediated Adaptation of Large Conductance Ca2+-Activated K+ Channel Expression and Function in Ovine Uterine Arteries. Hypertension 2017, 70, 601–612. [Google Scholar] [CrossRef]
- Hu, X.-Q.; Dasgupta, C.; Xiao, J.; Yang, S.; Zhang, L. Long-term high altitude hypoxia during gestation suppresses large conductance Ca2+-activated K+ channel function in uterine arteries: A causal role for microRNA-210. J. Physiol. 2018, 596, 5891–5906. [Google Scholar] [CrossRef]
- Feng, X.; Zhou, L.; Mao, X.; Tong, C.; Chen, X.; Zhao, D.; Baker, P.N.; Xia, Y.; Zhang, H. Association of a Reduction of G-protein Coupled Receptor 30 Expression and the Pathogenesis of Preeclampsia. Mol. Med. Rep. 2017, 16, 5997–6003. [Google Scholar] [CrossRef]
- Pastore, M.B.; Talwar, S.; Conley, M.R.; Magness, R.R. Identification of Differential ER-Alpha Versus ER-Beta Mediated Activation of eNOS in Ovine Uterine Artery Endothelial Cells1. Biol. Reprod. 2016, 94, 139. [Google Scholar] [CrossRef] [PubMed]
- Salhab, W.A.; Shaul, P.W.; Cox, B.E.; Rosenfeld, C.R. Regulation of types I and III NOS in ovine uterine arteries by daily and acute estrogen exposure. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2134–H2142. [Google Scholar] [CrossRef]
- Rupnow, H.L.; Phernetton, T.M.; Shaw, C.E.; Modrick, M.L.; Bird, I.M.; Magness, R.R. Endothelial vasodilator production by uterine and systemic arteries. VII. Estrogen and progesterone effects on eNOS. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H1699–H1705. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.R.; Chen, C.; Roy, T.; Liu, X.T. Estrogen Selectively Up-Regulates eNOS and nNOS in Reproductive Arteries by Transcriptional Mechanisms. J. Soc. Gynecol. Investig. 2003, 10, 205–215. [Google Scholar] [CrossRef]
- Hayashi, T.; Yamada, K.; Esaki, T.; Kuzuya, M.; Satake, S.; Ishikawa, T.; Hidaka, H.; Iguchi, A. Estrogen Increases Endothelial Nitric Oxide by a Receptor Mediated System. Biochem. Biophys. Res. Commun. 1995, 214, 847–855. [Google Scholar] [CrossRef]
- Lechuga, T.J.; Zhang, H.H.; Sheibani, L.; Karim, M.; Jia, J.; Magness, R.R.; Rosenfeld, C.R.; Chen, D.B. Estrogen Replacement Therapy in Ovariectomized Nonpregnant Ewes Stimulates Uterine Artery Hydrogen Sulfide Biosynthesis by Selectively Up-Regulating Cystathionine beta-Synthase Expression. Endocrinology 2015, 156, 2288–2298. [Google Scholar] [CrossRef]
- Lechuga, T.J.; Qi, Q.R.; Kim, T.; Magness, R.R.; Chen, D.B. E2beta stimulates ovine uterine artery endothelial cell H2S production in vitro by estrogen receptor-dependent upregulation of cystathionine beta-synthase and cystathionine gamma-lyase expressiondagger. Biol. Reprod. 2019, 100, 514–522. [Google Scholar] [CrossRef]
- Lechuga, T.J.; Bilg, A.K.; Patel, B.A.; Nguyen, N.A.; Qi, Q.R.; Chen, D.B. Estradiol-17beta stimulates H2 S biosynthesis by ER-dependent CBS and CSE transcription in uterine artery smooth muscle cells in vitro. J. Cell Physiol. 2019, 234, 9264–9273. [Google Scholar] [CrossRef]
- Sobrino, A.; Oviedo, P.J.; Novella, S.; Laguna-Fernandez, A.; Bueno, C.; Garcia-Perez, M.A.; Tarin, J.J.; Cano, A.; Hermenegildo, C. Estradiol selectively stimulates endothelial prostacyclin production through estrogen receptor-α. J. Mol. Endocrinol. 2010, 44, 237–246. [Google Scholar] [CrossRef]
- Jobe, S.O.; Ramadoss, J.; Wargin, A.J.; Magness, R.R. Estradiol-17beta and its cytochrome P450- and catechol-O-methyltransferase-derived metabolites selectively stimulate production of prostacyclin in uterine artery endothelial cells: Role of estrogen receptor-alpha versus estrogen receptor-beta. Hypertension 2013, 61, 509–518. [Google Scholar] [CrossRef]
- Burger, N.Z.; Kuzina, O.Y.; Osol, G.; Gokina, N.I. Estrogen replacement enhances EDHF-mediated vasodilation of mesenteric and uterine resistance arteries: Role of endothelial cell Ca2+. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E503–E512. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.S.; Gopalakrishnan, K.; Kumar, S. Pregnancy upregulates angiotensin type 2 receptor expression and increases blood flow in uterine arteries of rats. Biol. Reprod. 2018, 99, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.S.; Te Riele, G.M.; Qi, Q.R.; Lechuga, T.J.; Gopalakrishnan, K.; Chen, D.B.; Kumar, S. Estrogen Receptor-beta Mediates Estradiol-Induced Pregnancy-Specific Uterine Artery Endothelial Cell Angiotensin Type-2 Receptor Expression. Hypertension 2019, 74, 967–974. [Google Scholar] [CrossRef]
- Pluger, S.; Faulhaber, J.; Furstenau, M.; Lohn, M.; Waldschutz, R.; Gollasch, M.; Haller, H.; Luft, F.C.; Ehmke, H.; Pongs, O. Mice with disrupted BK channel beta1 subunit gene feature abnormal Ca(2+) spark/STOC coupling and elevated blood pressure. Circ. Res. 2000, 87, E53–E60. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.T.; Cheng, H.; Rubart, M.; Santana, L.F.; Bonev, A.D.; Knot, H.J.; Lederer, W.J. Relaxation of Arterial Smooth Muscle by Calcium Sparks. Science 1995, 270, 633–637. [Google Scholar] [CrossRef]
- Jaggar, J.H.; Wellman, G.C.; Heppner, T.J.; Porter, V.; Perez, G.; Gollasch, M.; Kleppisch, T.; Rubart, M.; Stevenson, A.S.; Lederer, W.J.; et al. Ca2+ channels, ryanodine receptors and Ca2+-activated K+ channels: A functional unit for regulating arterial tone. Acta Physiol. Scand. 1998, 164, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-Q.; Song, R.; Romero, M.; Dasgupta, C.; Huang, X.; Holguin, M.A.; Williams, V.; Xiao, D.; Wilson, S.M.; Zhang, L. Pregnancy Increases Ca2+ Sparks/Spontaneous Transient Outward Currents and Reduces Uterine Arterial Myogenic Tone. Hypertension 2019, 73, 691–702. [Google Scholar] [CrossRef]
- Song, R.; Hu, X.-Q.; Romero, M.; Holguin, M.A.; Kagabo, W.; Xiao, D.; Wilson, S.M.; Zhang, L. Ryanodine receptor subtypes regulate Ca2+ sparks/spontaneous transient outward currents and myogenic tone of uterine arteries in pregnancy. Cardiovasc. Res. 2020, 117, 792–804. [Google Scholar] [CrossRef]
- Nagar, D.; Liu, X.T.; Rosenfeld, C.R. Estrogen regulates β-subunit expression in Ca(2+)-activated K(+) channels in arteries from reproductive tissues. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1417–H1427. [Google Scholar] [CrossRef]
- Rosenfeld, C.R.; Liu, X.T.; DeSpain, K. Pregnancy modifies the large conductance Ca2+-activated K+ channel and cGMP-dependent signaling pathway in uterine vascular smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1878–H1887. [Google Scholar] [CrossRef][Green Version]
- Rosenfeld, C.R.; Cornfield, D.N.; Roy, T. Ca(2+)-activated K(+) channels modulate basal and E(2)beta-induced rises in uterine blood flow in ovine pregnancy. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H422–H431. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.R.; Roy, T.; DesPain, K.; Cox, B.E. Large-Conductance Ca2+-Dependent K+ Channels Regulate Basal Uteroplacental Blood Flow in Ovine Pregnancy. J. Soc. Gynecol. Investig. 2005, 12, 402–408. [Google Scholar] [CrossRef]
- Lorca, R.A.; Wakle-Prabagaran, M.; Freeman, W.E.; Pillai, M.K.; England, S.K. The large-conductance voltage- and Ca(2+)-activated K(+) channel and its gamma1-subunit modulate mouse uterine artery function during pregnancy. J. Physiol. 2018, 596, 1019–1033. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.R.; Word, R.A.; DesPain, K.; Liu, X.-T. Large Conductance Ca2+—Activated K+ Channels Contribute to Vascular Function in Nonpregnant Human Uterine Arteries. Reprod. Sci. 2008, 15, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bai, J.; Yang, Y.-H.; Hoshi, N.; Chen, D.-B. Hydrogen Sulfide Relaxes Human Uterine Artery via Activating Smooth Muscle BKCa Channels. Antioxidants 2020, 9, 1127. [Google Scholar] [CrossRef]
- Valverde, M.A.; Rojas, P.; Amigo, J.; Cosmelli, D.; Orio, P.; Bahamonde, M.I.; Mann, G.E.; Vergara, C.; Latorre, R. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the beta subunit. Science 1999, 285, 1929–1931. [Google Scholar] [CrossRef] [PubMed]
- De Wet, H.; Allen, M.; Holmes, C.; Stobbart, M.; Lippiat, J.D.; Callaghan, R. Modulation of the BK channel by estrogens: Examination at single channel level. Mol. Membr. Biol. 2006, 23, 420–429. [Google Scholar] [CrossRef]
- Rosenfeld, C.R.; Hynan, L.S.; Liu, X.-T.; Roy, T. Large Conductance Ca2+-Activated K+ Channels Modulate Uterine α1-Adrenergic Sensitivity in Ovine Pregnancy. Reprod. Sci. 2013, 21, 456–464. [Google Scholar] [CrossRef][Green Version]
- Dimitropoulou, C.; White, R.E.; Fuchs, L.; Zhang, H.; Catravas, J.D.; Carrier, G.O. Angiotensin II Relaxes Microvessels Via the AT 2 Receptor and Ca2+ -Activated K+ (BK Ca) Channels. Hypertension 2001, 37, 301–307. [Google Scholar] [CrossRef]
- Xiao, D.; Zhu, R.; Zhang, L. Gestational Hypoxia Up-regulates Protein Kinase C and Inhibits Calcium-Activated Potassium Channels in Ovine Uterine Arteries. Int. J. Med. Sci. 2014, 11, 886–892. [Google Scholar] [CrossRef]
- He, M.; Li, F.; Yang, M.; Fanfan, L.; Beejadhursing, R.; Xie, Y.; Mengzhou, H.; Deng, D. Impairment of BKca channels in human placental chorionic plate arteries is potentially relevant to the development of preeclampsia. Hypertens. Res. 2017, 41, 126–134. [Google Scholar] [CrossRef]
- Chen, M.; Dasgupta, C.; Xiong, F.; Zhang, L. Epigenetic Upregulation of Large-Conductance Ca2+ -Activated K+ Channel Expression in Uterine Vascular Adaptation to Pregnancy. Hypertension 2014, 64, 610–618. [Google Scholar] [CrossRef][Green Version]
- Hu, X.-Q.; Song, R.; Romero, M.; Dasgupta, C.; Min, J.; Hatcher, D.; Xiao, D.; Blood, A.; Wilson, S.M.; Zhang, L. Gestational Hypoxia Inhibits Pregnancy-Induced Upregulation of Ca2+ Sparks and Spontaneous Transient Outward Currents in Uterine Arteries Via Heightened Endoplasmic Reticulum/Oxidative Stress. Hypertension 2020, 76, 930–942. [Google Scholar] [CrossRef]
- Soleymanlou, N.; Jurisica, I.; Nevo, O.; Ietta, F.; Zhang, X.; Zamudio, S.; Post, M.; Caniggia, I. Molecular Evidence of Placental Hypoxia in Preeclampsia. J. Clin. Endocrinol. Metab. 2005, 90, 4299–4308. [Google Scholar] [CrossRef]
- Rajakumar, A.; Brandon, H.M.; Daftary, A.; Ness, R.; Conrad, K.P. Evidence for the functional activity of hypoxia-inducible transcription factors overexpressed in preeclamptic placentae. Placenta 2004, 25, 763–769. [Google Scholar] [CrossRef]
- Xiao, D.; Hu, X.-Q.; Huang, X.; Zhou, J.; Wilson, S.; Yang, S.; Zhang, L. Chronic Hypoxia during Gestation Enhances Uterine Arterial Myogenic Tone via Heightened Oxidative Stress. PLoS ONE 2013, 8, e73731. [Google Scholar] [CrossRef]
- Watson, C.J.; Collier, P.; Tea, I.; Neary, R.; Watson, J.A.; Robinson, C.; Phelan, D.; Ledwidge, M.; McDonald, K.; McCann, A.; et al. Hypoxia-Induced epigenetic modifications are associated with cardiac tissue fibrosis and the development of a myofibroblast-like phenotype. Hum. Mol. Genet. 2013, 23, 2176–2188. [Google Scholar] [CrossRef]
- Enquobahrie, D.A.; Abetew, D.F.; Sorensen, T.K.; Willoughby, D.; Chidambaram, K.; Williams, M.A. Placental microRNA expression in pregnancies complicated by preeclampsia. Am. J. Obstet. Gynecol. 2011, 204, 178.e12–178.e21. [Google Scholar] [CrossRef] [PubMed]
- Muralimanoharan, S.; Maloyan, A.; Mele, J.; Guo, C.; Myatt, L. MIR-210 Modulates Mitochondrial Respiration in Placenta with Preeclampsia. Placenta 2012, 33, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Yung, H.W.; Cox, M.; van Patot, M.T.; Burton, G.J. Evidence of endoplasmic reticulum stress and protein synthesis inhibition in the placenta of non-native women at high altitude. FASEB J. 2012, 26, 1970–1981. [Google Scholar] [CrossRef]
- Zhu, R.; Huang, X.; Hu, X.-Q.; Xiao, D.; Zhang, L. Gestational Hypoxia Increases Reactive Oxygen Species and Inhibits Steroid Hormone–Mediated Upregulation of Ca2+ -Activated K+ Channel Function in Uterine Arteries. Hypertension 2014, 64, 415–422. [Google Scholar] [CrossRef]
- Hu, X.-Q.; Huang, X.; Xiao, D.; Zhang, L. Direct effect of chronic hypoxia in suppressing large conductance Ca2+-activated K+ channel activity in ovine uterine arteries via increasing oxidative stress. J. Physiol. 2015, 594, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Mattheson, H.; Veerbeek, J.H.W.; Charnock-Jones, D.S.; Burton, G.J.; Yung, H.W. Morphological and molecular changes in the murine placenta exposed to normobaric hypoxia throughout pregnancy. J. Physiol. 2015, 594, 1371–1388. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-Q.; Dasgupta, C.; Song, R.; Romero, M.; Wilson, S.M.; Zhang, L. MicroRNA-210 Mediates Hypoxia-Induced Repression of Spontaneous Transient Outward Currents in Sheep Uterine Arteries during Gestation. Hypertension 2021, 77, 1412–1427. [Google Scholar] [CrossRef]
- Sun, W.-T.; Wang, X.-C.; Mak, S.-K.; He, G.-W.; Liu, X.-C.; Underwood, M.J.; Yang, Q. Activation of PERK branch of ER stress mediates homocysteine-induced BKCa channel dysfunction in coronary artery via FoxO3a-dependent regulation of atrogin-1. Oncotarget 2017, 8, 51462–51477. [Google Scholar] [CrossRef] [PubMed]
- Munzel, T.; Feil, R.; Mulsch, A.; Lohmann, S.M.; Hofmann, F.; Walter, U. Physiology and pathophysiology of vascular signaling controlled by guanosine 3′,5′-cyclic monophosphate-dependent protein kinase. Circulation 2003, 108, 2172–2183. [Google Scholar] [CrossRef]
- Ringvold, H.; Khalil, R. Protein Kinase C as Regulator of Vascular Smooth Muscle Function and Potential Target in Vascular Disorders. Adv. Pharmacol. 2017, 78, 203–301. [Google Scholar] [CrossRef] [PubMed]
- Feil, R.; Lohmann, S.M.; de Jonge, H.; Walter, U.; Hofmann, F. Cyclic GMP-Dependent protein kinases and the cardiovascular system: Insights from genetically modified mice. Circ. Res. 2003, 93, 907–916. [Google Scholar] [CrossRef]
- Porter, V.A.; Bonev, A.D.; Knot, H.J.; Heppner, T.J.; Stevenson, A.S.; Kleppisch, T.; Lederer, W.J.; Nelson, M.T. Frequency modulation of Ca2+ sparks is involved in regulation of arterial diameter by cyclic nucleotides. Am. J. Physiol. Content 1998, 274, C1346–C1355. [Google Scholar] [CrossRef] [PubMed]
- Fukao, M.; Mason, H.S.; Britton, F.; Kenyon, J.; Horowitz, B.; Keef, K.D. Cyclic GMP-Dependent Protein Kinase Activates Cloned BKCa Channels Expressed in Mammalian Cells by Direct Phosphorylation at Serine 1072. J. Biol. Chem. 1999, 274, 10927–10935. [Google Scholar] [CrossRef]
- Hu, X.-Q.; Zhang, L. Function and regulation of large conductance Ca2+-activated K+ channel in vascular smooth muscle cells. Drug Discov. Today 2012, 17, 974–987. [Google Scholar] [CrossRef] [PubMed]
- Khavandi, K.; Baylie, R.L.; Sugden, S.A.; Ahmed, M.; Csato, V.; Eaton, P.; Hill-Eubanks, D.C.; Bonev, A.D.; Nelson, M.T.; Greenstein, A.S. Pressure-Induced oxidative activation of PKG enables vasoregulation by Ca2+ sparks and BK channels. Sci. Signal. 2016, 9, ra100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiao, D.; Hu, X. Effect of cGMP on Pharmacomechanical Coupling in the Uterine Artery of Near-Term Pregnant Sheep. J. Pharmacol. Exp. Ther. 2008, 327, 425–431. [Google Scholar] [CrossRef]
- Chen, J.; Ren, W.; Lin, L.; Zeng, S.; Huang, L.; Tang, J.; Bi, S.; Pan, J.; Chen, D.; Du, L. Abnormal cGMP-Dependent Protein Kinase I-Mediated Decidualization in Preeclampsia. Hypertens. Res. 2021, 44, 318–324. [Google Scholar] [CrossRef]
- Singh, D.K.; Sarkar, J.; Raghavan, A.; Reddy, S.P.; Raj, J.U. Hypoxia Modulates the Expression of Leucine Zipper-Positive MYPT1 and its Interaction with Protein Kinase G and Rho Kinases in Pulmonary Arterial Smooth Muscle Cells. Pulm. Circ. 2011, 1, 487–498. [Google Scholar] [CrossRef]
- Thorpe, R.B.; Hubbell, M.C.; Silpanisong, J.; Williams, J.M.; Pearce, W.J. Chronic hypoxia attenuates the vasodilator efficacy of protein kinase G in fetal and adult ovine cerebral arteries. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H207–H219. [Google Scholar] [CrossRef]
- Takenaka, T.; Forster, H.; Epstein, M. Protein kinase C and calcium channel activation as determinants of renal vasoconstriction by angiotensin II and endothelin. Circ. Res. 1993, 73, 743–750. [Google Scholar] [CrossRef]
- Fallgren, B.; Bergstrand, H.; Edvinsson, L. Calcium influx and protein kinase C activation involved in uterine vasoconstriction in guinea pigs. Eur. J. Pharmacol. 1989, 170, 61–67. [Google Scholar] [CrossRef]
- Bonev, A.D.; Jaggar, J.H.; Rubart, M.; Nelson, M.T. Activators of protein kinase C decrease Ca2+ spark frequency in smooth muscle cells from cerebral arteries. Am. J. Physiol. Content 1997, 273, C2090–C2095. [Google Scholar] [CrossRef] [PubMed]
- Magness, R.R.; Rosenfeld, C.R.; Carr, B.R. Protein kinase C in uterine and systemic arteries during ovarian cycle and pregnancy. Am. J. Physiol. Metab. 1991, 260, E464–E470. [Google Scholar] [CrossRef] [PubMed]
- Kanashiro, C.A.; Cockrell, K.L.; Alexander, B.T.; Granger, J.P.; Khalil, R.A. Pregnancy-associated reduction in vascular protein kinase C activity rebounds during inhibition of NO synthesis. Am. J. Physiol. Integr. Comp. Physiol. 2000, 278, R295–R303. [Google Scholar] [CrossRef]
- Xiao, D.; Huang, X.; Yang, S.; Zhang, L. Direct chronic effect of steroid hormones in attenuating uterine arterial myogenic tone: Role of protein kinase c/extracellular signal-regulated kinase 1/2. Hypertension 2009, 54, 352–358. [Google Scholar] [CrossRef]
- Goulopoulou, S.; Hannan, J.; Matsumoto, T.; Webb, R.C. Pregnancy reduces RhoA/Rho kinase and protein kinase C signaling pathways downstream of thromboxane receptor activation in the rat uterine artery. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H2477–H2488. [Google Scholar] [CrossRef]
- Xiao, D.; Huang, X.; Longo, L.D.; Zhang, L. PKC Regulates α1-Adrenoceptor-Mediated Contractions and Baseline Ca2+ Sensitivity in the Uterine Arteries of Nonpregnant and Pregnant Sheep Acclimatized to High Altitude Hypoxia. High Alt. Med. Biol. 2010, 11, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Haller, H.; Hempel, A.; Homuth, V.; Mandelkow, A.; Busjahn, A.; Maasch, C.; Drab, M.; Lindschau, C.; Jupner, A.; Vetter, K.; et al. Endothelial-Cell permeability and protein kinase C in pre-eclampsia. Lancet 1998, 351, 945–949. [Google Scholar] [CrossRef]
- Xiao, D.; Huang, X.; Zhang, L. Chronic Hypoxia Differentially Up-Regulates Protein Kinase C-Mediated Ovine Uterine Arterial Contraction via Actin Polymerization Signaling in Pregnancy1. Biol. Reprod. 2012, 87, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; May, V.; Braas, K.; Osol, G. Pregnancy augments uteroplacental vascular endothelial growth factor gene expression and vasodilator effects. Am. J. Physiol. Content 1997, 273, H938–H944. [Google Scholar] [CrossRef] [PubMed]
- Hoeben, A.; Landuyt, B.; Highley, M.S.; Wildiers, H.; Van Oosterom, A.T.; De Bruijn, E.A. Vascular Endothelial Growth Factor and Angiogenesis. Pharmacol. Rev. 2004, 56, 549–580. [Google Scholar] [CrossRef]
- Ribatti, D. The discovery of the placental growth factor and its role in angiogenesis: A historical review. Angiogenesis 2008, 11, 215–221. [Google Scholar] [CrossRef]
- Itoh, S.; Brawley, L.; Wheeler, T.; Anthony, F.W.; Poston, L.; Hanson, M.A. Vasodilation to Vascular Endothelial Growth Factor in the Uterine Artery of the Pregnant Rat Is Blunted by Low Dietary Protein Intake. Pediatr. Res. 2002, 51, 485–491. [Google Scholar] [CrossRef]
- Osol, G.; Celia, G.; Gokina, N.I.; Barron, C.; Chien, E.; Mandala, M.; Luksha, L.; Kublickiene, K. Placental growth factor is a potent vasodilator of rat and human resistance arteries. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1381–H1387. [Google Scholar] [CrossRef] [PubMed]
- David, A.L.; Torondel, B.; Zachary, I.; Wigley, V.; Abi-Nader, K.; Mehta, V.; Buckley, S.M.; Cook, T.; Boyd, M.; Rodeck, C.H.; et al. Local delivery of VEGF adenovirus to the uterine artery increases vasorelaxation and uterine blood flow in the pregnant sheep. Gene Ther. 2008, 15, 1344–1350. [Google Scholar] [CrossRef]
- Mehta, V.; Abi-Nader, K.N.; Peebles, D.M.; Benjamin, E.; Wigley, V.; Torondel, B.; Filippi, E.; Shaw, S.W.; Boyd, M.; Martin, J.; et al. Long-term increase in uterine blood flow is achieved by local overexpression of VEGF-A165 in the uterine arteries of pregnant sheep. Gene Ther. 2011, 19, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.; Abi-Nader, K.N.; Shangaris, P.; Shaw, S.W.; Filippi, E.; Benjamin, E.; Boyd, M.; Peebles, D.M.; Martin, J.; Zachary, I.; et al. Local over-expression of VEGF-DDeltaNDeltaC in the uterine arteries of pregnant sheep results in long-term changes in uterine artery contractility and angiogenesis. PLoS ONE 2014, 9, e100021. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.D.; Zsengeller, Z.; Khankin, E.; Lo, A.S.; Rajakumar, A.; Dupont, J.J.; McCurley, A.; Moss, M.E.; Zhang, D.; Clark, C.D.; et al. Soluble fms-like tyrosine kinase 1 promotes angiotensin II sensitivity in preeclampsia. J. Clin. Investig. 2016, 126, 2561–2574. [Google Scholar] [CrossRef] [PubMed]
- Storment, J.M.; Meyer, M.; Osol, G. Estrogen augments the vasodilatory effects of vascular endothelial growth factor in the uterine circulation of the rat. Am. J. Obstet. Gynecol. 2000, 183, 449–453. [Google Scholar] [CrossRef]
- Grummer, M.A.; Sullivan, J.A.; Magness, R.R.; Bird, I.M. Vascular endothelial growth factor acts through novel, pregnancy-enhanced receptor signalling pathways to stimulate endothelial nitric oxide synthase activity in uterine artery endothelial cells. Biochem. J. 2008, 417, 501–511. [Google Scholar] [CrossRef]
- Zhang, H.-H.; Chen, J.C.; Sheibani, L.; Lechuga, T.J.; Chen, N.-B. Pregnancy Augments VEGF-Stimulated In Vitro Angiogenesis and Vasodilator (NO and H2S) Production in Human Uterine Artery Endothelial Cells. J. Clin. Endocrinol. Metab. 2017, 102, 2382–2393. [Google Scholar] [CrossRef]
- Boeldt, D.S.; Grummer, M.A.; Magness, R.R.; Bird, I.M. Altered VEGF-stimulated Ca2+ signaling in part underlies pregnancy-adapted eNOS activity in UAEC. J. Endocrinol. 2014, 223, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.; Betancourt, A.; Belfort, M.A.; Shamshirsaz, A.A.; Fox, K.A.; Yallampalli, C. Placental growth factor blunts uterine artery responses to angiotensin II. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.J.; Maynard, S.E.; Qian, C.; Lim, K.-H.; England, L.J.; Yu, K.F.; Schisterman, E.; Thadhani, R.; Sachs, B.P.; Epstein, F.H.; et al. Circulating Angiogenic Factors and the Risk of Preeclampsia. N. Engl. J. Med. 2004, 350, 672–683. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmed, A. Elevated Placental Soluble Vascular Endothelial Growth Factor Receptor-1 Inhibits Angiogenesis in Preeclampsia. Circ. Res. 2004, 95, 884–891. [Google Scholar] [CrossRef]
- Shibata, E.; Rajakumar, A.; Powers, R.W.; Larkin, R.W.; Gilmour, C.; Bodnar, L.M.; Crombleholme, W.R.; Ness, R.B.; Roberts, J.M.; Hubel, C.A. Soluble fms-Like Tyrosine Kinase 1 Is Increased in Preeclampsia but Not in Normotensive Pregnancies with Small-for-Gestational-Age Neonates: Relationship to Circulating Placental Growth Factor. J. Clin. Endocrinol. Metab. 2005, 90, 4895–4903. [Google Scholar] [CrossRef] [PubMed]
- Sahay, A.S.; Patil, V.V.; Sundrani, D.P.; Joshi, A.A.; Wagh, G.N.; Gupte, S.A.; Joshi, S.R. A longitudinal study of circulating angiogenic and antiangiogenic factors and AT1-AA levels in preeclampsia. Hypertens. Res. 2014, 37, 753–758. [Google Scholar] [CrossRef]
- Palmer, K.; Kaitu’U-Lino, T.J.; Hastie, R.; Hannan, N.J.; Ye, L.; Binder, N.; Cannon, P.; Tuohey, L.; Johns, T.; Shub, A.; et al. Placental-Specific sFLT-1 e15a Protein Is Increased in Preeclampsia, Antagonizes Vascular Endothelial Growth Factor Signaling, and Has Antiangiogenic Activity. Hypertension 2015, 66, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Munaut, C.; Lorquet, S.; Pequeux, C.; Blacher, S.; Berndt, S.; Frankenne, F.; Foidart, J.-M. Hypoxia is responsible for soluble vascular endothelial growth factor receptor-1 (VEGFR-1) but not for soluble endoglin induction in villous trophoblast. Hum. Reprod. 2008, 23, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Nevo, O.; Soleymanlou, N.; Wu, Y.; Xu, J.; Kingdom, J.; Many, A.; Zamudio, S.; Caniggia, I. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am. J. Physiol. Integr. Comp. Physiol. 2006, 291, R1085–R1093. [Google Scholar] [CrossRef]
- Sasagawa, T.; Nagamatsu, T.; Morita, K.; Mimura, N.; Iriyama, T.; Fujii, T.; Shibuya, M. HIF-2α, but not HIF-1α, mediates hypoxia-induced up-regulation of Flt-1 gene expression in placental trophoblasts. Sci. Rep. 2018, 8, 17375. [Google Scholar] [CrossRef]
- Kendall, R.L.; Thomas, K.A. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc. Natl. Acad. Sci. USA 1993, 90, 10705–10709. [Google Scholar] [CrossRef]
- Maynard, S.E.; Min, J.-Y.; Merchan, J.; Lim, K.-H.; Li, J.; Mondal, S.; Libermann, T.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef]
- Baker, P.; Krasnow, J.; Roberts, J.M.; Yeo, K.-T. Elevated serum levels of vascular endothelial growth factor in patients with preeclampsia. Obstet. Gynecol. 1995, 86, 815–821. [Google Scholar] [CrossRef]
- Bosio, P.M.; Wheeler, T.; Anthony, F.; Conroy, R.; O’Herlihy, C.; McKenna, P. Maternal plasma vascular endothelial growth factor concentrations in normal and hypertensive pregnancies and their relationship to peripheral vascular resistance. Am. J. Obstet. Gynecol. 2001, 184, 146–152. [Google Scholar] [CrossRef]
- McKeeman, G.C.; Ardill, J.E.; Caldwell, C.M.; Hunter, A.J.; McClure, N. Soluble vascular endothelial growth factor receptor-1 (sFlt-1) is increased throughout gestation in patients who have preeclampsia develop. Am. J. Obstet. Gynecol. 2004, 191, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Aranguren, L.; Espinosa-González, C.T.; González-Ortiz, L.M.; Sanabria-Barrera, S.M.; Riaño-Medina, C.E.; Nuñez, A.F.; Ahmed, A.; Vasquez-Vivar, J.; López, M. Soluble Fms-Like Tyrosine Kinase-1 Alters Cellular Metabolism and Mitochondrial Bioenergetics in Preeclampsia. Front. Physiol. 2018, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.R.; LaMarca, B.B.D.; Cockrell, K.; Granger, J.P. Role of Endothelin in Mediating Soluble fms-Like Tyrosine Kinase 1–Induced Hypertension in Pregnant Rats. Hypertension 2010, 55, 394–398. [Google Scholar] [CrossRef]
- Wang, Y.; Walsh, S.W. TNF alpha concentrations and mRNA expression are increased in preeclamptic placentas. J. Reprod. Immunol. 1996, 32, 157–169. [Google Scholar] [CrossRef]
- Rinehart, B.K.; Terrone, D.A.; Lagoo-Deenadayalan, S.; Barber, W.H.; Hale, E.A.; Martin, J.N., Jr.; Bennett, W.A. Expression of the placental cytokines tumor necrosis factor alpha, interleukin 1beta, and interleukin 10 is increased in preeclampsia. Am. J. Obstet. Gynecol. 1999, 181, 915–920. [Google Scholar] [CrossRef]
- Benyo, D.F.; Smárason, A.; Redman, C.W.G.; Sims, C.; Conrad, K.P. Expression of Inflammatory Cytokines in Placentas from Women with Preeclampsia1. J. Clin. Endocrinol. Metab. 2001, 86, 2505–2512. [Google Scholar] [CrossRef] [PubMed]
- Conrad, K.P.; Miles, T.M.; Benyo, D.F. Circulating Levels of Immunoreactive Cytokines in Women with Preeclampsia. Am. J. Reprod. Immunol. 1998, 40, 102–111. [Google Scholar] [CrossRef]
- Peraçoli, J.C.; Rudge, M.V.C.; Peracoli, M.T. Tumor Necrosis Factor-alpha in Gestation and Puerperium of Women with Gestational Hypertension and Pre-eclampsia. Am. J. Reprod. Immunol. 2007, 57, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Satyam, A.; Sharma, J.B. Leptin, IL-10 and Inflammatory Markers (TNF-? IL-6 and IL-8) in Pre-Eclamptic, Normotensive Pregnant and Healthy Non-Pregnant Women. Am. J. Reprod. Immunol. 2007, 58, 21–30. [Google Scholar] [CrossRef]
- Lau, S.Y.; Guild, S.-J.; Barrett, C.; Chen, Q.; McCowan, L.; Jordan, V.; Chamley, L. Tumor Necrosis Factor-Alpha, Interleukin-6, and Interleukin-10 Levels are Altered in Preeclampsia: A Systematic Review and Meta-Analysis. Am. J. Reprod. Immunol. 2013, 70, 412–427. [Google Scholar] [CrossRef]
- Benyo, D.F.; Miles, T.M.; Conrad, K.P. Hypoxia Stimulates Cytokine Production by Villous Explants from the Human Placenta1. J. Clin. Endocrinol. Metab. 1997, 82, 1582–1588. [Google Scholar] [CrossRef]
- Hung, T.H.; Charnock-Jones, D.S.; Skepper, J.N.; Burton, G.J. Secretion of tumor necrosis factor-alpha from human placental tissues induced by hypoxia-reoxygenation causes endothelial cell activation in vitro: A potential mediator of the inflammatory response in preeclampsia. Am. J. Pathol. 2004, 164, 1049–1061. [Google Scholar] [CrossRef]
- Corda, S.; LaPlace, C.; Vicaut, E.; Duranteau, J. Rapid Reactive Oxygen Species Production by Mitochondria in Endothelial Cells Exposed to Tumor Necrosis Factor- α Is Mediated by Ceramide. Am. J. Respir. Cell Mol. Biol. 2001, 24, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.F.; Mohamed, F.; Monge, J.C.; Stewart, D.J. Downregulation of eNOS mRNA expression by TNFalpha: Identification and functional characterization of RNA-protein interactions in the 3’UTR. Cardiovasc Res. 2003, 59, 160–168. [Google Scholar] [CrossRef]
- Yan, G.; You, B.; Chen, S.-P.; Liao, J.K.; Sun, J. Tumor Necrosis Factor-α Downregulates Endothelial Nitric Oxide Synthase mRNA Stability via Translation Elongation Factor 1-α 1. Circ. Res. 2008, 103, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.-X.; Boeldt, D.S.; Gifford, S.M.; Sullivan, J.A.; Grummer, M.A.; Magness, R.R.; Bird, I.M. Pregnancy Enhances Sustained Ca2+ Bursts and Endothelial Nitric Oxide Synthase Activation in Ovine Uterine Artery Endothelial Cells Through Increased Connexin 43 Function1. Biol. Reprod. 2010, 82, 66–75. [Google Scholar] [CrossRef][Green Version]
- Ampey, A.C.; Boeldt, D.S.; Clemente, L.; Grummer, M.A.; Yi, F.; Magness, R.R.; Bird, I.M. TNF-alpha inhibits pregnancy-adapted Ca2+ signaling in uterine artery endothelial cells. Mol. Cell. Endocrinol. 2019, 488, 14–24. [Google Scholar] [CrossRef]
- Lamarca, B.B.D.; Cockrell, K.; Sullivan, E.; Bennett, W.; Granger, J.P. Role of Endothelin in Mediating Tumor Necrosis Factor-Induced Hypertension in Pregnant Rats. Hypertension 2005, 46, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, N.S.; Thomson, S.E.; Heffernan, S.J.; Lim, S.; Thompson, J.; Ogle, R.; McKenzie, P.; Kirwan, P.J.; Makris, A.; Hennessy, A. Tumor necrosis factor α induces a model of preeclampsia in pregnant baboons (Papio hamadryas). Cytokine 2011, 56, 192–199. [Google Scholar] [CrossRef]
- Badran, M.; Abuyassin, B.; Ayas, N.; Laher, I. Intermittent hypoxia impairs uterine artery function in pregnant mice. J. Physiol. 2019, 597, 2639–2650. [Google Scholar] [CrossRef] [PubMed]
- Small, H.Y.; Nosalski, R.; Morgan, H.; Beattie, E.; Guzik, T.J.; Graham, D.; Delles, C. Role of Tumor Necrosis Factor-α and Natural Killer Cells in Uterine Artery Function and Pregnancy Outcome in the Stroke-Prone Spontaneously Hypertensive Rat. Hypertension 2016, 68, 1298–1307. [Google Scholar] [CrossRef]
- Travis, O.K.; Tardo, G.A.; Giachelli, C.; Siddiq, S.; Nguyen, H.T.; Crosby, M.T.; Johnson, T.; Brown, A.K.; Williams, J.M.; Cornelius, D.C. Tumor Necrosis Factor-Alpha Blockade Improves Uterine Artery Resistance, Maternal Blood Pressure, and Fetal Growth in Placental Ischemic Rats. Pregnancy Hypertens. 2021, 25, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, F.; Chang, H.; Zhang, S.; Yang, L.; Wang, X.; Cheng, X.; Zhang, M.; Ma, X.L.; Liu, H. Autoantibody against AT1 receptor from preeclamptic patients induces vasoconstriction through angiotensin receptor activation. J. Hypertens. 2008, 26, 1629–1635. [Google Scholar] [CrossRef]
- Wallukat, G.; Homuth, V.; Fischer, T.; Lindschau, C.; Horstkamp, B.; Jüpner, A.; Baur, E.; Nissen, E.; Vetter, K.; Neichel, D.; et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J. Clin. Investig. 1999, 103, 945–952. [Google Scholar] [CrossRef]
- Wallukat, G.; Neichel, D.; Nissen, E.; Homuth, V.; Luft, F. Agonistic autoantibodies directed against the angiotensin II AT1 receptor in patients with preeclampsia. Can. J. Physiol. Pharmacol. 2003, 81, 79–83. [Google Scholar] [CrossRef]
- Walther, T.; Wallukat, G.; Jank, A.; Bartel, S.; Schultheiss, H.-P.; Faber, R.; Stepan, H. Angiotensin II Type 1 Receptor Agonistic Antibodies Reflect Fundamental Alterations in the Uteroplacental Vasculature. Hypertension 2005, 46, 1275–1279. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Faulkner, J.L.; Amaral, L.M.; Cornelius, D.; Cunningham, M.W.; Ibrahim, T.; Heep, A.; Campbell, N.; Usry, N.; Wallace, K.; Herse, F.; et al. Vitamin D supplementation reduces some AT1-AA-induced downstream targets implicated in preeclampsia including hypertension. Am. J. Physiol. Integr. Comp. Physiol. 2017, 312, R125–R131. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.W.; Castillo, J.; Ibrahim, T.; Cornelius, D.C.; Campbell, N.; Amaral, L.; Vaka, V.R.; Usry, N.; Williams, J.M.; LaMarca, B. AT1-AA (Angiotensin II Type 1 Receptor Agonistic Autoantibody) Blockade Prevents Preeclamptic Symptoms in Placental Ischemic Rats. Hypertension 2018, 71, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Deer, E.; Vaka, V.R.; McMaster, K.M.; Wallace, K.; Cornelius, D.C.; Amaral, L.M.; Cunningham, M.W.; LaMarca, B. Vascular endothelial mitochondrial oxidative stress in response to preeclampsia: A role for angiotension II type 1 autoantibodies. Am. J. Obstet. Gynecol. MFM 2021, 3, 100275. [Google Scholar] [CrossRef] [PubMed]
- Vaka, V.R.; Cunningham, M.W.; Deer, E.; Franks, M.; Ibrahim, T.; Amaral, L.M.; Usry, N.; Cornelius, D.C.; Dechend, R.; Wallukat, G.; et al. Blockade of endogenous angiotensin II type I receptor agonistic autoantibody activity improves mitochondrial reactive oxygen species and hypertension in a rat model of preeclampsia. Am. J. Physiol. Integr. Comp. Physiol. 2020, 318, R256–R262. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, S.; Ren, J.; Yan, L.; Bai, L.; Wang, L.; Bian, J.; Yin, X.; Liu, H. The inhibitory effect of BKCa channels induced by autoantibodies against angiotensin II type 1 receptor is independent of AT1R. Acta Biochim. Biophys. Sin. 2018, 50, 560–566. [Google Scholar] [CrossRef] [PubMed]
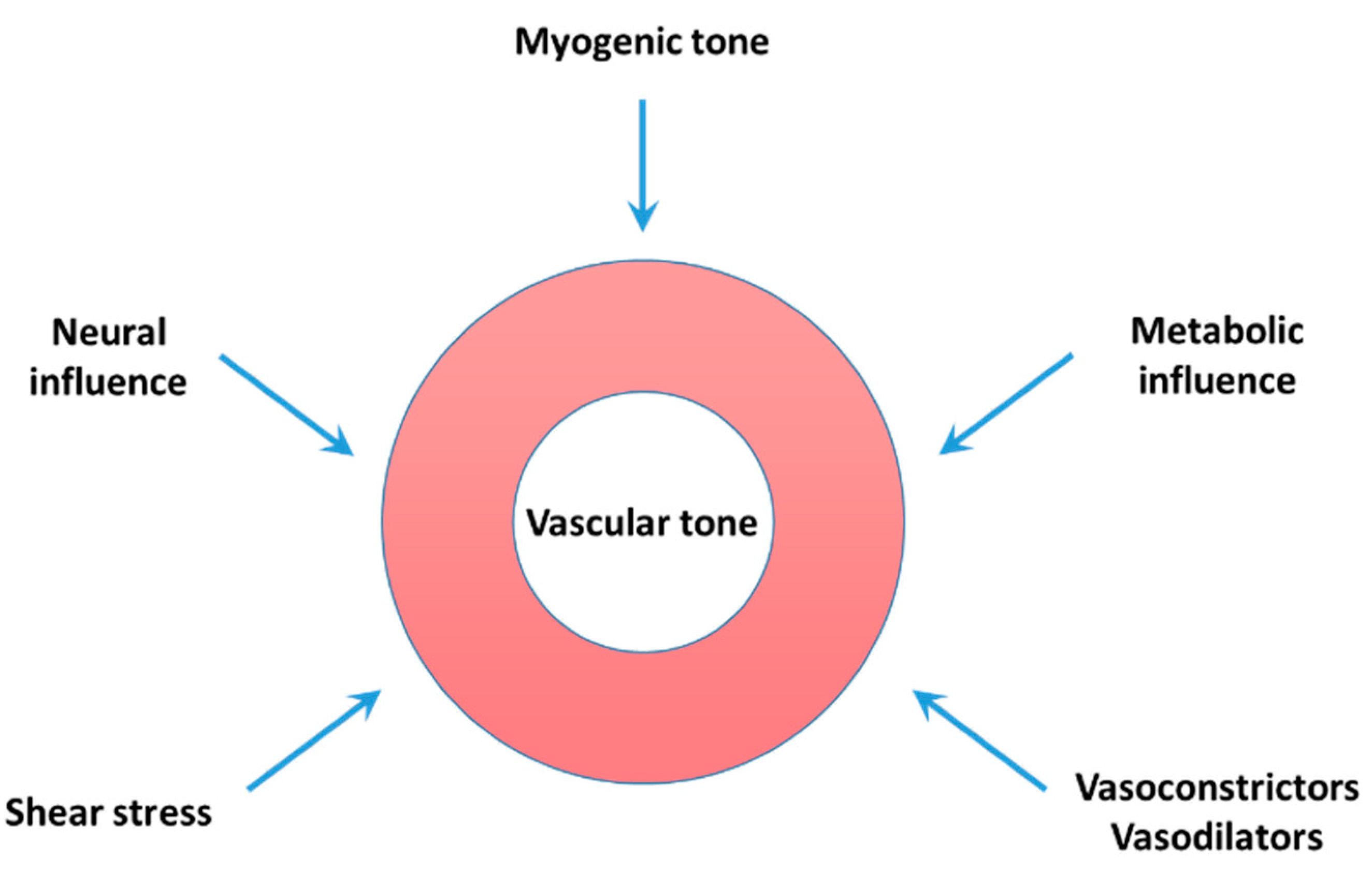
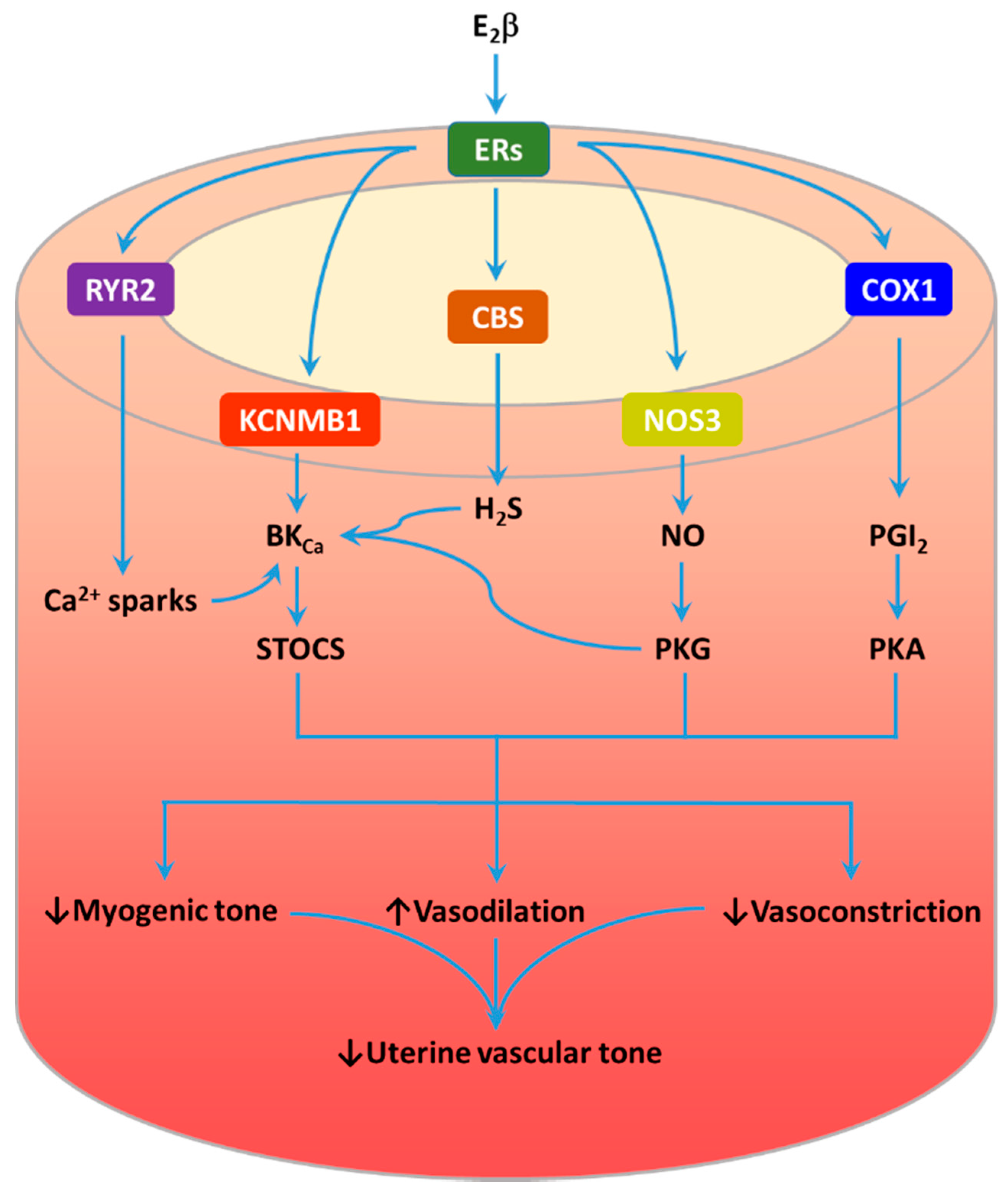
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Zhang, L. Uteroplacental Circulation in Normal Pregnancy and Preeclampsia: Functional Adaptation and Maladaptation. Int. J. Mol. Sci. 2021, 22, 8622. https://doi.org/10.3390/ijms22168622
Hu X, Zhang L. Uteroplacental Circulation in Normal Pregnancy and Preeclampsia: Functional Adaptation and Maladaptation. International Journal of Molecular Sciences. 2021; 22(16):8622. https://doi.org/10.3390/ijms22168622
Chicago/Turabian StyleHu, Xiangqun, and Lubo Zhang. 2021. "Uteroplacental Circulation in Normal Pregnancy and Preeclampsia: Functional Adaptation and Maladaptation" International Journal of Molecular Sciences 22, no. 16: 8622. https://doi.org/10.3390/ijms22168622
APA StyleHu, X., & Zhang, L. (2021). Uteroplacental Circulation in Normal Pregnancy and Preeclampsia: Functional Adaptation and Maladaptation. International Journal of Molecular Sciences, 22(16), 8622. https://doi.org/10.3390/ijms22168622






