Genetic Mapping of ms1s, a Recessive Gene for Male Sterility in Common Wheat
Abstract
:1. Introduction
2. Results
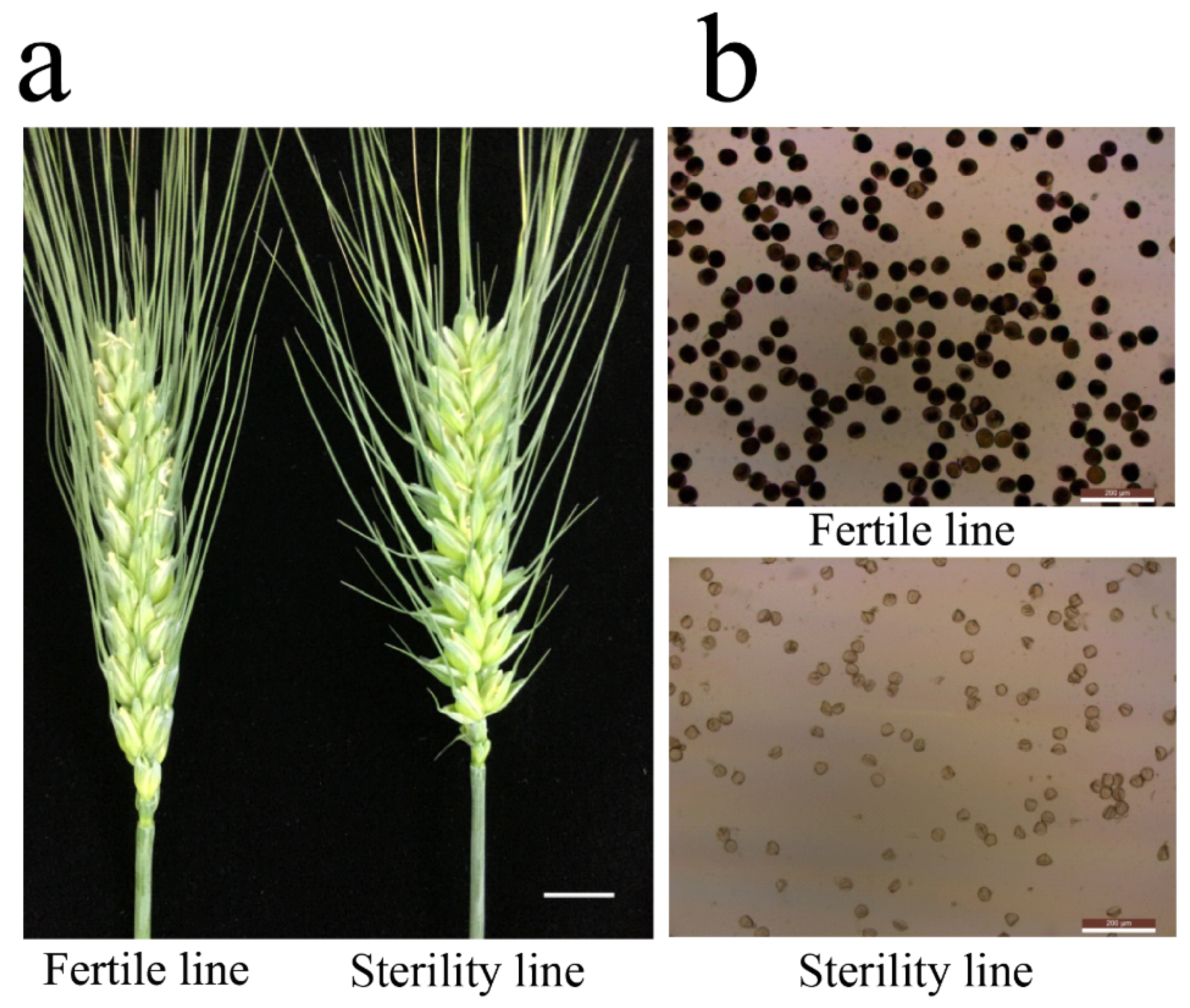
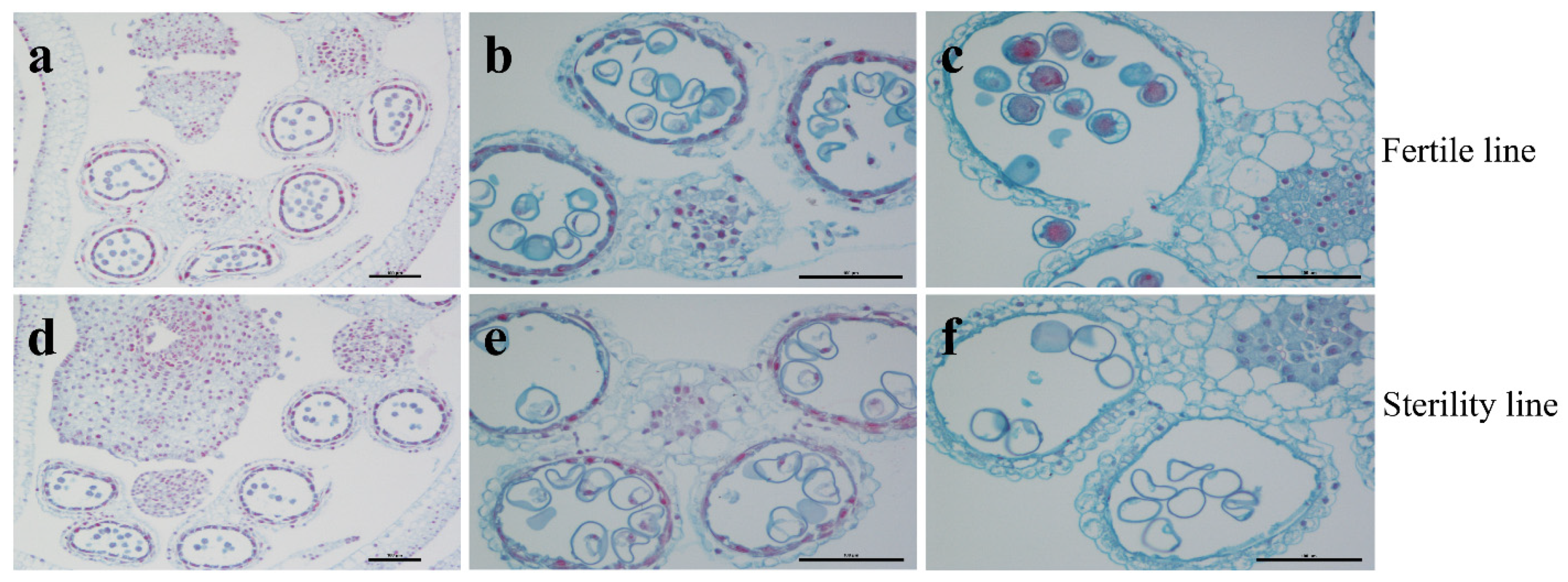
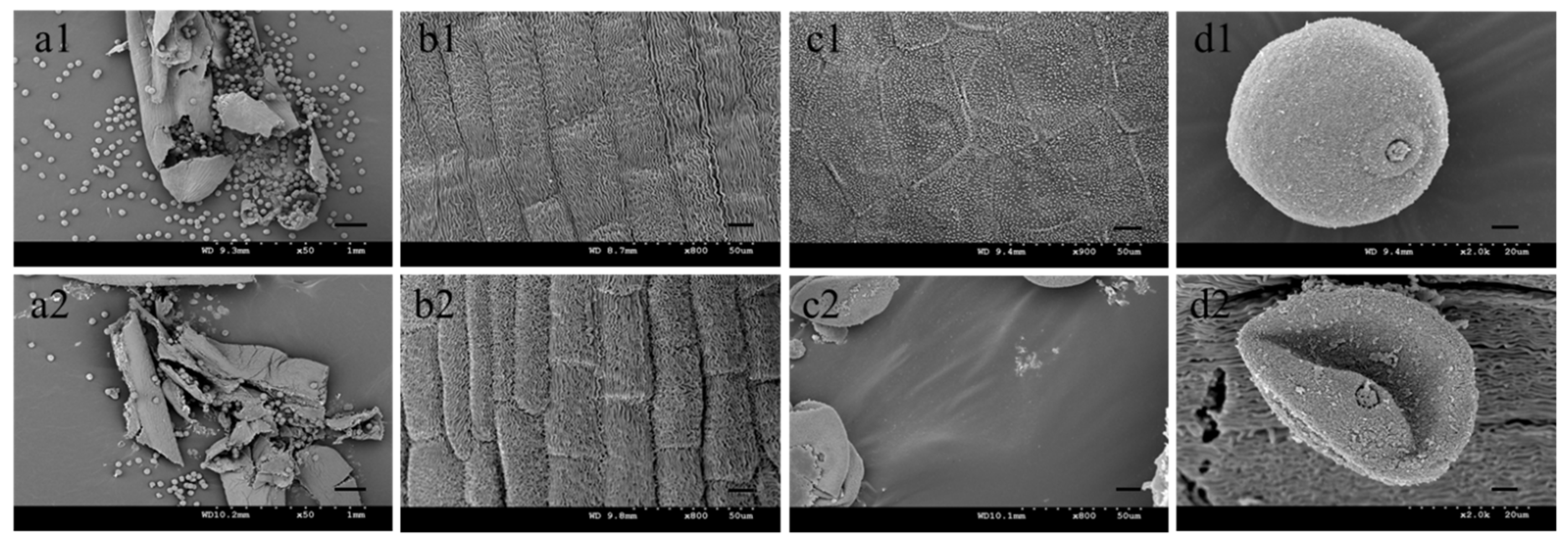
2.1. Sterile Plants in Line 15 Fan 03 Cannot Form Mature Pollen
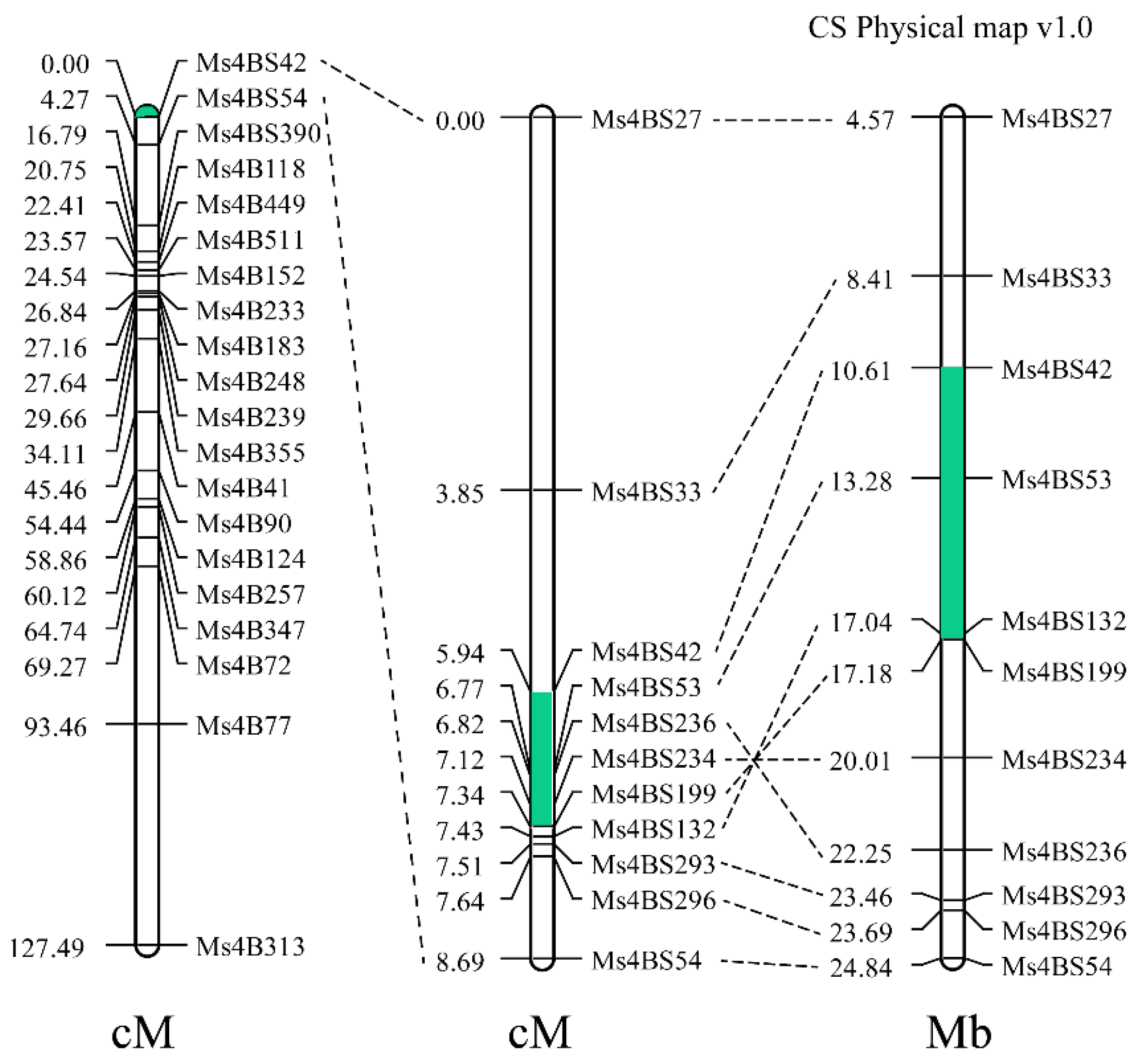
2.2. The Nuclear Male Sterility Gene Is Located on Chromosome 4BS
2.3. Sterility in Line 15 Fan 03 Is Caused by a Chromosome Deletion
2.4. Breeding Molecular Marker Development for ms1s
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Morphological Observations
4.3. Wheat660K SNP Chip Analysis
4.4. SSR Marker Development and Genetic Map Construction
4.5. Fluorescence In Situ Hybridization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, X.; Wang, H.; Tang, X.; Zhou, J.; Chen, H.; He, G.; Chen, L.; Xu, Z. Hybrid rice breeding welcomes a new era of molecular crop design. Sci. Sin. Vitae 2013, 43, 864–868. [Google Scholar]
- Chang, Z.; Chen, Z.; Wang, N.; Xie, G.; Lu, J.; Yan, W.; Zhou, J.; Tang, X.; Deng, X.W. Construction of a male sterility system for hybrid rice breeding and seed production using a nuclear male sterility gene. Proc. Natl. Acad. Sci. USA 2016, 113, 14145–14150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, J.A.; Ross, W.M. Cross breeding in wheat, Triticum aestivum L. II. hybrid seed set on a cytoplasmic male-sterile winter wheat composite subjected to cross-pollination. Crop. Sci. 1962, 2, 415–417. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.K.; Chatrath, R.; Mishra, B. Perspective of hybrid wheat research, A review. Indian J. Agric. Sci. 2010, 80, 1013–1027. [Google Scholar]
- Whitford, R.; Fleury, D.; Reif, J.C.; Garcia, M.; Okada, T.; Korzun, V.; Langridge, P. Hybrid breeding in wheat, technologies to improve hybrid wheat seed production. J. Exp. Bot. 2013, 64, 5411–5428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pugsley, A.T.; Oram, R.N. Genic male sterility in wheat. Aust. Plant Breed. Genet. Newsl. 1959, 14, 10–11. [Google Scholar]
- Liu, B.H.; Deng, J.Y. A dominant gene for male sterility in wheat. Plant Breed. 1986, 97, 204–209. [Google Scholar]
- Franckowiak, J.D.; Maan, S.S.; Williams, N.D. A proposal for hybrid wheat utilizing Aegilops squarrosa L. cytoplasm. Crop. Sci. 1976, 16, 725–728. [Google Scholar] [CrossRef]
- Maan, S.S.; Kianian, S.F. Third dominant male sterility gene in common wheat. Wheat Inf. Serv. 2001, 93, 27–31. [Google Scholar]
- Sasakuma, T.; Maan, S.S.; Williams, N.D. EMS-induced male-sterile mutants in euplasmic and alloplasmic common wheat. Crop. Sci. 1978, 18, 850–853. [Google Scholar] [CrossRef]
- Klindworth, D.L.; Williams, N.D.; Maan, S.S. Chromosomal location of genetic male sterility genes in four mutants of hexaploid wheat. Crop. Sci. 2002, 42, 1447–1450. [Google Scholar] [CrossRef] [Green Version]
- Pallotta, M.A.; Warner, P.; Kouidri, A.; Tucker, E.J.; Baes, M.; Suchecki, R.; Watson-Haigh, N.; Okada, T.; Garcia, M.; Sandhu, A.; et al. Wheat ms5 male-sterility is induced by recessive homoeologous A and D genome non-specific lipid transfer proteins. Plant J. 2019, 99, 673–685. [Google Scholar] [CrossRef]
- Xia, C.; Zhang, L.; Zou, C.; Gu, Y.; Duan, J.; Zhao, G.; Wu, J.; Liu, Y.; Fang, X.; Gao, L.; et al. A TRIM insertion in the promoter of Ms2 causes male sterility in wheat. Nat. Commun. 2017, 8, 15407. [Google Scholar] [CrossRef] [Green Version]
- Maan, S.S.; Carlson, K.M.; Williams, N.D.; Yang, T. Chromosomal arm location and gene-centromere distance of a dominant gene for male sterility in wheat. Crop. Sci. 1987, 27, 494–500. [Google Scholar] [CrossRef]
- Suneson, C.A. Use of Pugsley’s sterile wheat in cross breeding. Crop. Sci. 1962, 2, 534–535. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, S.; Feng, Y.; Ji, W.; Wang, G. A new male sterile mutant LZ in wheat (Triticum aestivum L.). Euphytica 2008, 159, 403–410. [Google Scholar] [CrossRef]
- Fossati, A.; Ingold, M. A male sterile mutant in Triticum aestivum. Wheat Inf. Serv. 1970, 30, 8–10. [Google Scholar]
- Driscoll, C.J. Registration of cornerstone male sterile wheat germplasm 1 (Reg. No.GP 74). Crop. Sci. 1977, 17, 190. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Chen, S.; Heng, Y.; Chen, Z.; Yang, J.; Zhou, K.; Pei, J.; He, H.; Deng, X.W.; et al. Poaceae-specific MS1 encodes a phospholipid-binding protein for male fertility in bread wheat. Proc. Natl. Acad. Sci. USA 2017, 114, 12614–12619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, B.S.; Anand, S.C. Genetic male sterility for hybrid seed production in wheat. Crop. Sci. 1970, 10, 385–386. [Google Scholar] [CrossRef]
- Driscoll, C.J. XYZ system of producing hybrid wheat. Crop. Sci. 1972, 12, 516–517. [Google Scholar] [CrossRef]
- Driscoll, C.J. Modified XYZ system of producing hybrid wheat. Crop. Sci. 1985, 25, 1115–1116. [Google Scholar] [CrossRef]
- Huang, S.S.; Xue, C.P.; Xu, J.; Li, W.L. A preliminary report of selections and studies on male sterile-maintainable line of blue-grained wheat. Acta Bot. Boreali-Occident. Sin. 1988, 8, 162–166. [Google Scholar]
- Huang, S.S.; Li, W.L.; Xu, J.; Xue, C.P. The development of a blue marked nucleus male sterile line and its maintainer in bread wheat. Acta Agron. Sin. 1991, 17, 81–87. [Google Scholar]
- Zhou, K.; Wang, S.; Feng, Y.; Liu, Z.; Wang, G. The 4E-ms system of producing hybrid wheat. Crop. Sci. 2006, 46, 250–255. [Google Scholar] [CrossRef]
- Tucker, E.J.; Baumann, U.; Kouidri, A.; Suchecki, R.; Baes, M.; Garcia, M.; Okada, T.; Dong, C.; Wu, Y.; Sandhu, A.; et al. Molecular identification of the wheat male fertility gene Ms1 and its prospects for hybrid breeding. Nat. Commun. 2017, 8, 869. [Google Scholar] [CrossRef]
- Athwal, D.S.; Phul, P.S.; Minocha, J.L. Genetic male sterility in wheat. Euphytica 1967, 16, 354–360. [Google Scholar] [CrossRef]
- Sun, L.; Yang, W.; Li, Y.; Shan, Q.; Ye, X.; Wang, D.; Yu, K.; Lu, W.; Xin, P.; Pei, Z.; et al. A wheat dominant dwarfing line with Rht12 which reduces stem cell length and affects GA synthesis, is a 5AL terminal deletion line. Plant J. 2019, 97, 887–900. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, J.; Che, Y.; Liu, W.; Lu, Y.; Yang, X.; Li, X.; Jia, J.; Liu, X.; Li, L. Construction of Agropyron Gaertn genetic linkage maps using a wheat 660K SNP array reveals a homoeologous relationship with the wheat genome. Plant Biotechnol. J. 2018, 16, 818–827. [Google Scholar] [CrossRef] [Green Version]
- Stein, N.; Herren, G.; Keller, B. A new DNA extraction method for high-throughput marker analysis in a large-genome species such as Triticum aestivum. Plant Breed. 2001, 120, 354–356. [Google Scholar] [CrossRef]
- da Maia, L.C.; Palmieri, D.A.; de Souza, V.Q.; Kopp, M.M.; de Carvalho, F.I.; Costa de Oliveira, A. SSR locator, tool for simple sequence repeat discovery integrated with primer design and PCR simulation. Int. J. Plant Genom. 2008, 2008, 412696. [Google Scholar] [CrossRef] [Green Version]
- Stam, P. Construction of integrated genetic linkage maps by means of a new computer package, Join Map. Plant J. 1993, 3, 739–744. [Google Scholar] [CrossRef]
- Voorrips, R.E. MapChart, software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, F.; Liu, B.; Fedak, G.; Liu, Z. Genomic constitution and variation in five partial amphiploids of wheat-Thinopyrum intermedium as revealed by GISH, multicolor GISH and seed storage protein analysis. Theor. Appl. Genet. 2004, 109, 1070–1076. [Google Scholar] [CrossRef] [PubMed]




| Gene ID | Forward Primer | Reverse Primer | Position (Mb) |
|---|---|---|---|
| TraesCS4B01G015000 | GCCTATCTTTGAACCGGCGA | AATTAGGCGAGCCCAAGAGC | 10.610 |
| TraesCS4B01G015200LC | CCCGGGCTTATGAAGGCTTG | ATGGAAGGAGGAGGCATTGA | 10.712 |
| TraesCS4B01G015400LC | GTATGCGACGACTCCCGAAG | AGTACGCCGACTTCCCCTAT | 10.789 |
| TraesCS4B01G015200 | CGTCGGACGATACGGACACA | TGTCACCCTCCATCCTACGTAC | 11.433 |
| TraesCS4B01G016200 | CGCCGGTAGGTAGTAATCCG | TCCTGAGCCTGACCATCTCG | 11.904 |
| TraesCS4B01G017400 | CCCTGTGCGGAATCTTCCAA | CCAGAGTCACAAACCGCTCA | 12.526 |
| TraesCS4B01G017500 | ACATTCACTTGACAGCGACCT | CCACGGTACTCGCATGCTC | 13.053 |
| TraesCS4B01G017900 (Ms1) | ACGCAGAAACGAACCTCGAA | GCTAGAAGTGCTCCGGTTTG | 13.126 |
| TraesCS4B01G018300 | TACTTGTCGGCCAAACGGA | CGTCCGTCAAGAACACACCA | 13.297 |
| TraesCS4B01G019100 | GTGGAGCAAGTGGACTCACC | CCAGGCAAGTCGTCGATTCA | 13.977 |
| TraesCS4B01G020300 | GAAAACCCTAGACGCTCCCC | GCACCAACAAAACCGTCAGA | 14.389 |
| TraesCS4B01G021100LC | ACTTTTGCGCCAGGTATCCA | GCCTCTCGCGAAAGAAATGC | 14.539 |
| TraesCS4B01G021300 | GGCATGTGCCTAGTGGGAAT | CAGCAGCACAAGGTTGGTTC | 15.409 |
| TraesCS4B01G021600 | GAGGTCATTTACCAGTGCCAGT | TTCCACCGGTACTCTTCAGC | 15.596 |
| TraesCS4B01G022600 | GGGCTACCGGCAACGTAATA | AAGCGCACCCTCACAAGAAA | 16.680 |
| TraesCS4B01G023300 | CGCTGGTTGGAGTTTACCCT | TGTAAGGTCTTAGCGCCCAC | 17.017 |
| Primer | Sequence (5′-3′) | Product Size (bp) |
|---|---|---|
| ms1sCAPS1F | AAGAGCCCATATGCACCTTG | 361 |
| ms1sCAPS1R | GGTACCAGGGGGTTGAAGAT | |
| ms1sCAPS2F | AAGAGCCCATATGCACCTTG | 347 |
| ms1sCAPS2R | GAAGATAACTGGGCGTGGAA | |
| ms1sCAPS3F | AAGAGCCCATATGCACCTTG | 395 |
| ms1sCAPS3R | TGCGAAAGTGTTGTGCTACC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Li, Y.; Sun, L.; Shoaib, M.; Sun, J.; Wang, D.; Li, X.; Liu, D.; Zhan, K.; Zhang, A. Genetic Mapping of ms1s, a Recessive Gene for Male Sterility in Common Wheat. Int. J. Mol. Sci. 2021, 22, 8541. https://doi.org/10.3390/ijms22168541
Yang W, Li Y, Sun L, Shoaib M, Sun J, Wang D, Li X, Liu D, Zhan K, Zhang A. Genetic Mapping of ms1s, a Recessive Gene for Male Sterility in Common Wheat. International Journal of Molecular Sciences. 2021; 22(16):8541. https://doi.org/10.3390/ijms22168541
Chicago/Turabian StyleYang, Wenlong, Yafei Li, Linhe Sun, Muhammad Shoaib, Jiazhu Sun, Dongzhi Wang, Xin Li, Dongcheng Liu, Kehui Zhan, and Aimin Zhang. 2021. "Genetic Mapping of ms1s, a Recessive Gene for Male Sterility in Common Wheat" International Journal of Molecular Sciences 22, no. 16: 8541. https://doi.org/10.3390/ijms22168541
APA StyleYang, W., Li, Y., Sun, L., Shoaib, M., Sun, J., Wang, D., Li, X., Liu, D., Zhan, K., & Zhang, A. (2021). Genetic Mapping of ms1s, a Recessive Gene for Male Sterility in Common Wheat. International Journal of Molecular Sciences, 22(16), 8541. https://doi.org/10.3390/ijms22168541







