Abstract
Proximal spinal muscular atrophy (SMA) is an autosomal recessive neurodegenerative disorder characterized by motor neuron loss and subsequent atrophy of skeletal muscle. SMA is caused by deficiency of the essential survival motor neuron (SMN) protein, canonically responsible for the assembly of the spliceosomal small nuclear ribonucleoproteins (snRNPs). Therapeutics aimed at increasing SMN protein levels are efficacious in treating SMA. However, it remains unknown how deficiency of SMN results in motor neuron loss, resulting in many reported cellular functions of SMN and pathways affected in SMA. Herein is a perspective detailing what genetics and biochemistry have told us about SMA and SMN, from identifying the SMA determinant region of the genome, to the development of therapeutics. Furthermore, we will discuss how genetics and biochemistry have been used to understand SMN function and how we can determine which of these are critical to SMA moving forward.
1. Introduction
Proximal spinal muscular atrophy (SMA) is an autosomal recessive neurodegenerative disorder that affects 1 in 10,000 individuals [1,2,3]. The disorder is caused by survival motor neuron (SMN) protein deficiency, resulting from loss of function mutation or deletion of the SMN1 gene, but retention of the companion SMN2 [4]. Until recently, SMA was the most common genetic cause of infant death [5], but in the last several years, three therapies have proven remarkably successful in challenging this claim [6,7,8]. Despite the efficacy of these therapeutics, we still do not know why deficiency of SMN results in SMA. Genetic and biochemical experiments have determined that SMN deficiency causes SMA. This perspective will detail how genetics can be used in the future to study the function of SMN and validate downstream targets that are altered by SMN deficiency.
2. Identifying SMN1 and SMN2 as the SMA Determinant Genes
SMA is an autosomal recessive neurodegenerative disorder characterized by the loss of spinal motor neurons and subsequent atrophy of muscle [9]. The disorder can be divided into five types, ranging in severity from Type 0, the most severe showing onset at birth, to Type 4, the mildest with onset in adulthood [9,10]. Early studies of patient pedigrees indicated different SMA types could occur in the same family, suggesting mutations in the same locus could give rise to all types of SMA [11,12,13,14,15,16]. The first steps in the molecular era were to map the causative gene using linkage analysis, which started by identification of genetic markers that segregated with the SMA phenotype [17,18,19,20]. The region was then narrowed with flanking markers until those markers were very close to the gene with no recombinants. It was interesting to find that the markers with no recombinants were in a region that was, at the very least, duplicated, indicating there were multiple copies of the gene of interest within that region. It later became clear this was due to duplication and insertion [4]. In 1995 it was determined that patients of differing SMA types all had mutations in a single gene, survival motor neuron 1 (SMN1) [4]. However, a nearly identical gene, SMN2, was always retained [4]. The SMA region is complex, and the multiple possible structures were not well represented in the genome assembly [4,21,22]. SMN1 and SMN2 were originally reported to exist in an inverted duplication; however, in our recent revisit of the region, we found a tandem duplication with an additional insertion (Figure 1) [4,22]. Our approach ensured assembly from one chromosome by requiring all single nucleotide polymorphisms (SNPs) aligned in a relatively large overlap.
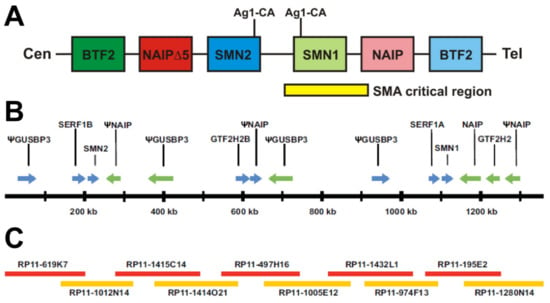
Figure 1.
Genomic organization of SMN1 and SMN2. (A) Original construction of the SMN region showing inverted duplication. The centromeric end of the chromosome is given on the left and telomeric end on the right. Ag1-CA markers are indicated above with lines to their approximate locations in SMN1 and SMN2, thus suggesting inverted duplication. Further genes in the region, BTF2 and NAIP, are also given, further suggesting inverted duplication. (B,C) Map of the SMA region that was assembled using overlapping clones that originate from the same chromosome. (B) Map of the region showing all genes and their orientation. SMN1 and SMN2 are in the same orientation and are approximately 848 kb away from each other. Lying between SMN1 and SMN2 are 2 NAIP pseudogenes (one containing NAIP exons 6–17 and the other contained NAIP exons 3 and 6–9) and 2 GUSBP3 pseudogenes, as well as SERF1A and GTF2H2B. Pseudogenes are indicated with a (Ψ). (C) Overlapping clones used to construct the region. The smallest overlap between clones was 32,833 bp while the average overlap was 71,850 bp. There was only a single mismatched base pair out of all the overlapping regions.
Both SMN1 and SMN2 were predicted to make SMN protein, however, SMN cDNAs derived from SMA patients showed the predominant transcript from SMN2 was truncated and lacked exon 7 (SMN∆7) [4,23,24]. A single nucleotide change from C to T was identified within SMN2 exon 7 affected the inclusion of exon 7 during pre-mRNA splicing [25,26]. This truncated SMN∆7 protein had decreased ability to oligomerize, resulting in degradation of the unstable product [27,28]. This was confirmed in SMA patient lymphoblasts where an SMN∆7 specific antibody could not detect the truncated protein [29]. Thus, in the case of SMA, where patients have a loss-of-function mutation or deletion in SMN1 but retention of SMN2, patients are reliant on the small amount of full-length SMN produced from SMN2 resulting in SMN protein deficiency [23,30,31]. The variable presentation of SMA in the clinic is then largely explained by duplications and additions of SMN2 copies. As such, more copies of SMN2 result in increased full-length SMN protein and a less severe phenotype [32]. In fact, our model of the SMA region as a straightforward duplication with additional insertion is compatible with the prediction that an unequal cross over results in loss of SMN1 with duplication of SMN2, thus explaining a mechanism for increased SMN2 copies in SMA patients [22]. The genetic understanding of SMN2 as a modifier of SMA, combined with the biochemical understanding that more copies of SMN2 results in increased full-length SMN protein, indicated a strong target for therapeutics to increase full-length SMN protein.
3. Genetics Informed SMA Therapeutics
As SMA is caused by SMN protein deficiency, the most obvious therapeutic approach was to increase SMN levels [30,31]. Since SMN deficiency is caused by inefficient inclusion of exon 7 in SMN2 transcripts, and that SMN2 is present in the entire patient population, there was strong rationale to screen for compounds that modulated SMN2 splicing. Indeed, it soon became clear that the C to T change in exon 7 removed an exon inclusion enhancer. In addition, several elements in both introns and exons that either enhanced exon 7 inclusion or inhibited exon 7 inclusion were identified including the intron splice silencer ISS-N1, which is the target of the antisense oligonucleotides (ASO) therapeutics [33,34,35,36]. Spinraza (Nusinersen) and Risdiplam (Evrysdi) were discovered and developed through screens for increasing full-length SMN production from SMN2 [34,37,38,39,40,41]. Both Spinraza and Risdiplam target intron 7; however, they enhance exon 7 inclusion by two different mechanisms. Spinraza is an ASO that targets ISS-N1 blocking inhibition of spliceosome recognition by hnRNPA1. Risdiplam is a small molecule drug that binds and stabilizes an unpaired adenine in the exon 7 5′ splice site which allosterically promotes binding of the U1-C zinc finger and U1-snRNP [42,43,44]. This acts by turning the weak 5′ splice site into a stronger one. Both therapies increase full-length SMN protein levels, survival, and motor function in SMA mice when delivered presymptomatically [38,39,40,44,45,46]. Clinical trials show these drugs are safe and efficacious [6,8,47,48]. In fact, presymptomatic two and three copy SMN2 SMA patients who received Spinraza achieve major motor milestones like sitting independently and walking with or without assistance [48]. Additionally, for those patients who receive treatment after onset of overt symptoms, both drugs have been shown to delay the progression of SMA [8,47]. The drugs are currently approved for treatment of SMA patients by both American and European medical authorities.
The presence of SMN2 in every SMA patient gives a unique target and an advantage in the development of gene therapy as well. The classic use of gene therapy delivers a cassette to a particular tissue(s) and expresses a gene product in trans to the genome. In SMA, this is performed by expression of the SMN cDNA from the ubiquitous chicken beta-actin (CBA) promoter, delivered by packaging it in adeno-associated virus serotype 9 (AAV9) [49,50,51]. The major advantage for a gene therapy approach in SMA is that patients already produce some SMN, thus reducing the possibility of immunological reaction to the introduced protein. AAV9-SMN gene therapy also increases survival and motor function in SMA mice when administered presymptomatically [50,52]. It is approved for treatment of SMA in the United States under the label Zolgensma (Onasemnogene abeparvovec). A single intravenous dose of Zolgensma resulted in similar achievement of major motor milestones consistent with Spinraza and Risdiplam [7]. Additionally, clinical trials with Zolgensma also indicate presymptomatic treatment yields the best results [7,53,54]. Trials are currently investigating an intrathecal delivery method [55].
Furthermore, SMN2 can be manipulated to act like SMN1 in what could potentially become a permanent fix to the level of SMN. There are many approaches that can be considered for manipulating SMN2, the most obvious of which is using an adenine base editor (ABEs) which can convert the T change back to a C, thus making SMN2 into a SMN1 gene [56,57,58]. Another possibility is the modification of negative regulators of SMN2 exon 7 inclusion such as ISS-N1, which when disrupted effectively turns SMN2 into SMN1, or at least SMN1-like [59]. This is an exciting prospect as it moves from a conventional gene therapy to the use of Cas or modifying base editors to make SMN2 function like SMN1 permanently. This will give the correct expression levels and not be in danger of loss of the expression cassette from the target tissue, or shutdown of expression by epigenetic mechanisms. The disadvantages for these approaches are the expression of the foreign enzymes, the off-target effects involved in the guide RNA-targeting mechanisms, in some cases the efficiency of the process, and the size constraints for packaging the cassettes in AAV. The future could entail a new breed of therapies that can truly fix the base defect. It is hoped that these could also be less expensive as technology moves forward and manufacturing of AAV gene therapy vectors advances.
4. How Do SMN Mutations Work
The vast majority of SMA cases result from a large deletion in SMN1; thus patients are entirely reliant on the full-length SMN produced from SMN2 [32,60]. However, 1% of SMA cases are caused by a loss of function missense mutation in SMN1 [32,60]. In every instance, missense mutations in SMN1 are always found in the presence of the SMN2 gene. Interestingly, some of these mutations are found in patients who present with milder symptoms than is expected from their SMN2 copy number; thus we refer to those variants as mild SMN missense mutations [61]. Conversely, there are missense mutations in SMN1 that do not modify the expected SMA phenotype as suggested by the SMN2 copy number [61] and these are referred to as severe mutations. Severe SMN missense mutations are predominantly found in the SMN Tudor domain—important for Sm-ring assembly (exon 3)—and the oligomerization domain (exon 6), both highly conserved regions of the SMN protein [61]. Thus, some severe mutations interfere with dimerization and disrupt SMN function. Missense mutations have been found in every SMN exon except exon 2B and exon 5 (Table 1). A pictorial representation of Table 1 is given in Figure 2.

Table 1.
Loss of function missense mutations in SMN1 identified in SMA patients.
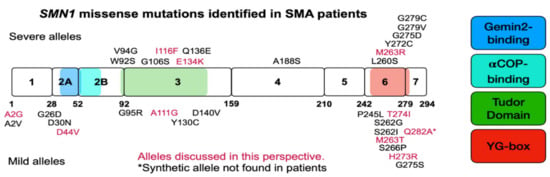
Figure 2.
Pictorial representation of SMN exons, domains, and mutations identified in SMA patients. SMN coding region depicted in block form with number designation for exons. Numbers underneath blocks designate the amino acids that end each exon, or are before the translation termination signal. Severe SMN missense mutations are given above blocks and mild mutations are given below blocks, in the approximate location they occur. The mutant alleles given in magenta are discussed within this manuscript. The colored boxes indicate domains of SMN in order from left to right: Gemin2-binding, alpha-COP-binding, Tudor Domain, and YG-box oligomerization domain. Please consult Table 1 for more detailed information about each missense mutation, the SMA type, SMN2 copy number, and publication.
As mild SMN missense mutations can alter SMN severity, it was suggested these alleles are partially functional. We addressed this question by first expressing an SMN missense allele in a cell line conditionally deleted for endogenous full-length Smn [77]. We generated an immortalized mouse embryonic fibroblast (iMEF) line that contains the Smn knockout exon 2A neomycin insertion allele (Smn−) and the floxed Smn exon 7 allele (SmnF7) [77,78,79]. When Cre-recombinase is expressed in these lines, Smn exon 7 is deleted (SmnD7), resulting in loss of functional Smn production in the cell and cell death [77,79]. Firstly, this indicates Smn is essential for cell survival and secondly, that SmnD7 does not make an Smn capable of essential function. We cloned the mild SMN missense mutant cDNAs A2G, D44V, A111G, T274I, and the severe SMNE134K missense mutant into mammalian expression constructs, transfected these into our Smn−/F7 iMEFs, and selected stable expressing lines [77]. When treated with Cre, removing the endogenous source of full-length wild-type Smn, these cells also die [77]. We found that none of the missense mutations on their own could rescue the loss of Smn protein and the cells died.
Next, we expressed mild SMN missense alleles transgenically to determine if overexpression could rescue embryonic lethality in Smn−/− mice [80,81,82,83]. Mice expressing the mild SMN missense mutants A2G, D44V, A111G, T274I, Q282A, or the severe SMNI116F missense mutant on an Smn− background were not viable [80,81,82]. These experiments indicated that missense mutations in SMN1 are incapable of the essential function of SMN in the absence of full-length SMN protein. It should be noted that missense mutations in C. elegans and Drosophila do not behave in the same manner as those in mice and humans [64,67,82,84,85,86]. For instance, the orthologous mutants for SMND44V can rescue the Smn null animals in worms and flies [85,86]. Additionally, expression of the orthologous severe SMNI116F mutant rescues Smn null in Drosophila [86]. We postulate that this incongruence between orthologous SMN missense mutant activity in these models may be due to a difference in the downstream targets of SMN function. Thus, it is important to test targets found in C. elegans and Drosophila in mammalian systems to ensure translatability.
After establishing that missense mutations in SMN1 are nonfunctional in mammals in the absence of full-length SMN, we asked, how are these mutations able to modify the SMA phenotype in patients? The functional mechanism likely involves oligomerization. SMN is an oligomeric protein and several experiments have indicated oligomerization is necessary for SMN function [27,28,77,82,83]. Furthermore, the predominant product of SMN2, the truncated SMN∆7 protein, inefficiently oligomerizes and is rapidly degraded [27,28]. Finally, to date all missense mutations that prevent SMN oligomerization are severe and all mild SMN missense mutants can oligomerize [27,87,88].
Previously, other oligomeric proteins have been shown to perform intragenic complementation, a situation in which two mutant proteins of a single gene form a heteromeric complex that has increased function when compared to a complex composed of each mutant alone [89]. This is evident with loss of function mutations in argininosuccinate lyase and calpain-3 [89,90,91,92]. We have demonstrated that intragenic complementation occurs with the SMN protein. Expression of all mild SMN mutants A2G, D44V, A111G, T274I, or Q282A in the presence of full-length SMN protein produced by SMN2 rescued weight, survival, and electrophysiology in Smn null mice [80,81,82]. This implies the mechanism for modification of the SMA phenotype in patients who have a mild SMN missense mutation lies in the ability of the missense mutant protein to form a functional SMN oligomer with the small amount of SMN produced from SMN2. This data strongly suggests the functional unit of SMN in the cell is the oligomer and not the monomer. Furthermore, we have confirmed in our iMEF cell system that expression of two SMN missense mutants also rescues cell survival in the absence of full-length wild-type SMN [77,83]. Notably, in iMEFs the expression level of SMN protein in some of the dually expressing SMN missense mutant iMEFs is less than has been tested for each SMN missense mutant expressed singly [77]. This means that rescue of iMEF survival is due to the presence of the SMN missense mutants and not merely due to a nominal increase in SMN protein abundance.
Recently a structure of the SMN oligomer has been determined in which SMN first dimerizes through the glycine zipper interface and then further oligomerizes in an antiparallel stacking formation to form tetrameric and octameric SMN oligomers [88]. Interestingly, SMN readily exists in tetrameric oligomers in pombe; however, gel filtration indicates human SMN adopts an octameric complex composed of a dimer of tetramers [88,93,94]. The authors clearly show severe SMN missense mutations that disrupt initial dimerization through the glycine zipper interface, abrogate higher order SMN oligomerization. The authors also show that mild SMN missense mutations, M263T and T264I that do not prevent SMN dimerization, disrupt the antiparallel stacking interaction, and shift SMN oligomer speciation away from the octameric and tetrameric forms and towards the dimer. This is interesting because the SMNM263T mutant which prefers the dimeric state was found in a patient who presented with Type 2 SMA, while having only one copy of SMN2 [73,88]. Furthermore, SMNT274I was found in a patient who presented with milder Type 3 SMA, while having only one copy of SMN2, and the SMNT274I oligomers preferentially adopt the dimeric and tetrameric states [72]. This indicates that first, the SMNM263T and SMNT274I mutants can interact with wild-type SMN produced from SMN2 and second, higher order oligomeric formation has an impact on the functional output of SMN. Our own experiments show that expression of the SMNT274I mutant does not rescue lethality of a Smn null when expressed in cells or mice, but rescue does occur when SMN from SMN2 or SMNA111G is present [77,82,83]. Thus, a homomeric mutant does not allow the formation of higher order oligomers but the addition of a wild-type domain does. Interestingly, the association of Gemin8 with SMNM263T and SMNT274I homomers is reduced, suggesting antiparallel stacking and higher order oligomer formation is important for proper SMN complex integrity and function [88]. It should be noted that while SMNT274I fully rescues SMA mice when SMN2 is present, this is not the case in humans as SMNT274I or SMNM263T, with one copy of SMN2, does result in SMA in humans. Thus, these oligomers have reduced function compared to wild-type SMN complexes in humans [72,82].
The combination of genetics and biochemistry has given insight into SMN and its function, but many questions remain regarding the SMN protein, its structure, function, and domains. For instance, we do not know the true stoichiometry of the SMN complex, nor have we determined how SMN and the Gemins are spatially oriented within the complex. Additionally, we determined the SMNA2G mutant does not complement the SMNT274I mutation whereas both SMND44V and SMNA111G do complement [77]. This suggests an interaction or shared function between the extreme N-terminus and C-terminus of SMN. Could the N- and C-terminus share a common interaction with Gemin8 or Gemin3 and stabilize the association of the fully assembled complex as a whole? We propose that a combination of genetics and biochemistry can continue to find answers to these questions.
5. Suppressor Screens Which Have Been Informative in Other Neurodegenerative Disorders
Here, we discuss two suppressors screens that have been informative in determining the critical function or targets for therapy in other neurodegenerative diseases. We focus on Spinocerebellar ataxia type 1 (SCA1) [95], where suppressors inform on the mechanism of the disease [96,97,98,99], and Rett Syndrome caused by MECP2 deficiency [100], in which N-ethyl-N-nitrosourea (ENU) random mutagenesis screens have yielded novel targets for therapy [101,102,103]. These examples were chosen because the SCA1 suppressor story can inform on experiments that show how SMN deficiency gives rise to SMA, whereas the Rett screen to suppress MECP2 deficiency can inform on possible additional targets for suppression of SMN deficiency.
SCA1 is caused by expansion of a CAG repeat in the ataxin 1 gene (ATXN1), yielding a large glutamine repeat in the protein, which causes cerebellar and brain stem degeneration and ataxia [95]. Toxicity from the expansion repeat was proposed to occur through RNA aggregation, RAN translation, and/or disruption of various protein interactions [99]. Reducing the levels of the ATXN1 partner protein, cognate partner capicua (CIC) reduced severity in mouse models of SCA1 [97]. Furthermore, mutating ATXN1 residues V591A and S602D, identified by structural studies followed by mutagenesis, resulted in abolishing the interaction with CIC in vitro and in vivo [98,99]. These mutations completely suppressed the SCA1 phenotype in mice, indicating that the toxicity caused by the expansion involves the critical partner CIC [99]. In essence, these studies eliminated the possibility that SCA1 toxicity resulted from RNA aggregation or RAN translation and demonstrate that toxicity is mediated by the aberrant interaction between mutant ATXN1 and CIC. CIC is a transcriptional repressor and alterations in the expression of some of its target genes, particularly ion channels, has been reported in SCA1 [104,105]. It remains unclear how these downstream targets contribute to the final phenotype of SCA1, however, those targets should be dependent on CIC repression. In a similar fashion, relevant downstream targets of SMN deficiency should directly link to SMN function. Moreover, like SCA1, similar screens in SMN partner proteins that interact with a mutant SMN can identify suppressors in SMA.
A second example is Rett Syndrome, which is caused by mutations that disrupt expression of the X chromosome encoded MECP2 gene. Male wild-type C57/6J mice were mutagenized with ENU and crossed to female mice which have a null allele of MECP2 (Mecp2tm1.1Bird/+). Male mice with the Mecp2 null allele were then selected and assessed for correction. These rescued mice formed the founders of this dominant modifier screen, were bred for three generations, and assessed for the corrective phenotype. Modifiers were initially located using linkage analysis with quantitative trait mapping, followed by exome capture and sequencing. The exome alteration, an early stop codon, was found to overlap the squalene epoxidase (SQLE) gene, which is a rate-limiting enzyme in cholesterol synthesis [101]. This indicated that Rett mice had an abnormality in cholesterol metabolism; thus the authors tested if statins could modify the phenotype [101]. The statins are now in clinical trials for Rett; however, it should be noted that the alteration in cholesterol metabolism was not consistent in different strains of mice [106]. Thus, in humans there is the possibility that lipid metabolism could vary due to the individual’s genetic background [106,107].
Since the initial identification of SQLE, additional ENU screens have been performed using both mendelian models for quantitative linkage mapping and association analysis with linear regression [102]. Exome sequencing over a larger region subsequently revealed several groups of genes that work together to ameliorate phenotypic severity [102]. For instance, Rett mice heterozygous for both the early termination SQLE allele and an early termination mutant Rbb8 allele have a markedly improved lifespan of 203–813 days. While mutagenesis screens are often performed in C. elegans, Drosophila, and zebrafish, they are much less common in mice. Since the Mecp2 gene is not found in invertebrates, these screens had to be performed in mice [101,106]. Screens such as this can also identify key elements and additional therapeutic targets in SMA. Unfortunately, thus far screens in C. elegans and Drosophila yielded suppressors that do not act in a similar fashion in SMA in mouse models. As in Rett Syndrome, association testing to identify multiple interacting loci is an excellent way to detect interacting loci as this technique considers the possibility of interacting mutants that may be informative in SMA. The SCA1 and Rett screens highlight how to identify suppressors or modifiers in neurodegenerative diseases with no clear target for effective therapy. In SMA we have a therapeutic target (SMN) thus our current quest is for additional therapeutic targets that act independently of SMN.
6. What Does SMN Do and What Does SMN Deficiency Affect
Reduction in SMN protein has been shown to affect many cellular and molecular pathways, including the biogenesis of the spliceosomal small nuclear ribonucleoproteins (snRNP) [108,109,110,111,112,113,114,115,116,117], U7 snRNP [118,119,120], telomerase [121,122,123], signal recognition particle (SRP) [124], translation regulation [125,126,127,128], and mRNA trafficking [129,130,131,132,133,134,135,136], of which there are many comprehensive reviews [137]. Unlike SMN, metabolic proteins like argininosuccinate lyase, glucocerebrosidase, iduronate-2-sulfatase, galactosylceramidase, and Wilson’s disease protein can be tested with biochemical assays that detect their enzymatic functions eliminating the need to rely on protein interactions to predict function. Multiple functions for SMN have been proposed due to protein interactions in immunoprecipitation experiments and protein alterations when SMN is deficient. The results of these experiments can be misleading if immunoprecipitations have low stringency or, in the case of alterations with SMN deficiency, if the effect is due to reduced SMN expression and not a downstream effector of SMN deficiency. For example, a measured effect of SMN deficiency could arise from altered splicing of a gene that then affects translation or mRNA trafficking. Proposed SMN functions can be broadly grouped into two classes: Sm-assembly dependent and Sm-assembly independent. We feel that identification of suppressors can help clarify the role SMN performs within these functions.
SMN is directly involved in the assembly of the heptameric Sm-ring onto the spliceosomal snRNAs and U7 snRNA, and likely involved in the premature Sm-assembled telomerase RNP prior to Lsm-ring assembly [108,109,110,112,117,118,119,120,122]. The most characterized of these assemblies is certainly snRNP biogenesis, in which SMN is associated with almost all steps from assembly of the Sm-ring onto snRNAs to delivery of the snRNP to the spliceosome [87,110,111,112,113,116,121,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157]. There are functional, biochemical assays showing SMN direct involvement in snRNP assembly, both in vivo and in vitro [82,87,110,153,158,159]. Additionally, similar assays show SMN direct involvement in Sm-ring assembly onto U7 snRNA in vivo [120]. In the case of telomerase, SMN was not shown to directly assemble the Sm-ring onto telomerase RNA. However the anti-Sm immunoprecipitations capture premature telomerase RNA in pombe [122]. Interestingly, this complex was shown to be transient, in which the Sm-ring is removed and replaced by an Lsm-ring, making a mature and active telomerase. As SMN is the only protein known to assemble Sm-rings, to date, SMN is invoked to perform this assembly. In addition to functional assays for spliceosomal and U7 snRNP assembly, a specific SMN complex can be isolated from cells that is responsible for this biochemical activity [87,110,160]. In the case of functions that are not Sm-assembly dependent, there are currently no biochemical assays for the proposed function that are clearly directed by SMN, nor is there clear characterization of the complex that performs this function; thus, it is difficult to determine what role they play in SMA.
The canonical SMN complex that functions in snRNP assembly consists of SMN, Gemin 2–8, and Unrip [111,113,116,150]. Immunoprecipitations using anti-SMN or various anti-Gemin antibodies always pull down this complex, clearly indicating it is the predominant SMN complex in cells [108,110,148,160,161,162,163,164,165,166,167]. Indeed, with a Flag tag on Gemin2, a complex can be relatively easily purified which performs snRNP assembly [87,110,160]. This snRNP assembly activity is tightly correlated to the amount of SMN present [158,159]. Gemin2, 3, and 8 directly bind SMN, with Gemin2 binding in the N-terminus within exon 2A and Gemin8 in the C-terminus [88,115,160,168,169,170]. It is currently unknown what region of SMN is responsible for binding Gemin3. Importantly, SMN missense mutations show defects in assembling this complex as well as in the ability to assemble snRNPs. For example, Gemin8 binding was recently shown to require higher order SMN oligomer formation, which is affected by the SMNT274I mild missense mutation [88]. Furthermore, Gemin8 binding is entirely abolished in SMNH273R mutant homomers [88]. The SMN missense mutation D44V lies within the SMN-Gemin2 interface as constructed by NMR [115]. Though it does not abolish interaction with Gemin2, the D44V mutation is suggested to destabilize the integrity of the SMN complex as SMND44V homomers fractionate at lower molecular weight complexes than those made by wild-type SMN in vitro [87]. There are also mutant SMN proteins which do not affect complex formation but affect SMN complex function. Particularly, SMNE134K can form stable SMN complexes, but showed decreased ability to assembly snRNPs as compared to wild-type SMN in vitro [87]. The bulk of these experiments have been carried out in in vitro systems due to the added complexity of interrogating these questions in vivo. However, we have shown that the heteromeric oligomers of complementing SMN missense mutants are functionally equivalent at snRNP assembly as homomeric oligomers consisting of wild-type SMN in Smn−/D7 iMEFs [77]. A caveat to this rule includes all SMNE13K heteromeric oligomers, which are less efficient at assembling snRNPs than wild-type SMN homomers [77]. This is interesting as it suggests SMNE134K loss of function is involved in snRNP assembly, parroting the in vitro experiments.
SMN is a ubiquitously expressed protein that clearly performs an essential function in assembling spliceosomal snRNPs. Why is it that SMN deficiency causes motor neuron death and not the death of other cell types in SMA? One theory is SMN has specific functions pertaining to motor neurons and this logic has led to trafficking proteins and mRNAs down motor neuron axons for further processing at the axon terminal [129,130,131,132,133,135,171,172,173]. Importantly, these functions are said to occur in an Sm-independent fashion [124,131,174,175,176]. The signal recognition particle (SRP) is a ribonucleoprotein complex that recognizes a specific N-terminal sequence of newly synthesized peptides, particularly for transmembrane proteins and stalls their translation until they are correctly docked with the endoplasmic reticulum [177,178,179]. Immunoprecipitations of native SMN complexes were shown to pull down the 7S RNA of the SRP [124]. This binding was shown to specifically involve Gemin5 as 7S RNA could still associate in conditions that abolished SMN complex association as well as with recombinant purified Gemin5 [124]. Furthermore, and more importantly, anti-Sm immunoprecipitations with the Sm-specific Y12 antibody did not yield 7S RNA, suggesting SMN complex association with SRP is independent of Sm-ring assembly [124]. It is suggested that the SMN complex completes the final assembly of SRP biogenesis by assembling SRP54 onto the premature 7S RNP complex, however mechanisms furthering this theory have yet to be discovered [124]. Additionally, SMN missense mutations have not been analyzed in this association, which may be useful in uncovering the role of SMN in SRP biogenesis.
Working in a similar pathway to SRP, SMN has been shown to associate with the alpha-COP subunit of the COPI coatomer complex, important for trafficking proteins from the cis end of the Golgi back to the rough endoplasmic reticulum for further packaging, processing, and trafficking in membrane-coated vesicles [180]. An SMN and alpha-COP interaction was first reported to occur in a yeast two-hybrid screen, then confirmed in immunoprecipitations in neuronal-like cells in culture [180,181]. Alpha-COP was shown to specifically interact with lysine residues in SMN exon 2B in vitro [181,182]. When these lysines are mutated to alanines, the SMN–alpha-COP interaction is abolished [182]. Mutations in alpha-COP were also shown to remove the SMN interaction [182]. It was shown that overexpression of alpha-COP was capable of rescuing growth of axons in cell culture and zebrafish, but only increased survival of SMA mice from 11 to 18 days with no motor functions assessed [182,183,184]. This interaction is interesting, as our experiments have shown that expression of an Smn lacking exon 2B, which contains the lysine residues responsible for alpha-COP interaction, completely rescues survival and snRNP assembly in Smn−/D7 iMEFs [77]. These data suggest that the alpha-COP interaction is not essential to Smn function. As in the example for SCA1, where a mutant protein removed the ability to bind CIC resulted in removal of the toxic action of the glutamine expansion in ATXN1, we can ask if expression of an Smn which lacks the ability to bind alpha-COP modifies the motor outcomes and survival in SMA mice. It should be noted that an experiment regarding this question has been published, however expression from the mutated SMN transgene is quite low and this mutant has not been assayed for the ability to perform snRNP assembly [184]. Furthermore, if the Smn lacking exon 2B is crossed onto an Smn null, it can be determined whether the resulting mouse presents with an overt phenotype.
SMN has been reported in axons in RNPs that are not associated with Sm proteins [131,174] though time resolved quantitative proteomics have found SMN and SmB trafficking together in neuronal axons [185]. The composition of these RNPs has been largely uncharacterized. However one major interactor has been implicated: HuD (ELAV-like protein 4) [130,132,186]. HuD is a neuron-specific RNA-binding protein shown to increase the half-life of its AU-rich target mRNAs [132]. SMN–HuD interaction was first reported by expressing tagged SMN Tudor Domains in MN-1 cells, which pulled down HuD in an RNA-dependent manner [132]. HuD and SMN complex components were then shown to co-localize in the axons of neuronal cultures [132]. Overexpression of HuD resulted in the suppression of axonal growth defects in SMA zebrafish and neuronal cultures [132,186]. Interestingly, expression of the SMNE134K mutant in SMN deficient neuronal cultures and SMA zebrafish was shown to abolish HuD association [132,186]. This is a rather surprising feature of HuD–SMN binding as the SMNE134K appears to act in a dominant manner, in which the SMN heteromer should still be able to interact with HuD through the present wild-type SMN. This would suggest the suppression of the SMA phenotype observed in culture and zebrafish is working in an SMN-independent mechanism and is thus downstream of the critical function of SMN in regards to SMA. However, this can be addressed using suppressor screens, in which identifying a suppressor mutation in HuD that rescues SMNE134K–HuD interaction, or a specific mutation in SMN that disrupts HuD interaction, but not other SMN functions could clearly show SMN involvement in HuD-mediated mRNP trafficking.
Lastly, we discuss a possible role of SMN in regulation of translation. SMN has been shown to co-sediment with ribosomes, in what are called “SMN primed” ribosomes, and there is a decrease in fractional abundance of ribosomes in polysomes between control and SMA mice [127,128]. Particularly interesting is that there is a decrease in the relative abundance in ribosomal proteins in SMA animals as compared to wild-type [127]. However, SMN levels correlate with cell proliferation and the decrease in these proteins could be downstream of the critical function of SMN [187,188]. The SMN–ribosome interaction is further confounded by experiments in which recombinant SMN is incubated with an 80S ribosomal pellet [128]. Recombinant SMN is detected in relatively equivalent proportions in both unbound and bound fractions, regardless of whether SMN is incubated with the 80S ribosomal pellet, thus indicating a nonspecific association between SMN and ribosomes [128]. The SMN complex is stable under high salt conditions (500 mM NaCl) and sediments across 60S–80S fractions; therefore, an 80S ribosomal pellet likely contains the entire SMN complex and co-sedimentation is due to size, rather than protein binding events [160,175]. Lastly, an enrichment in mRNAs containing 5′UTRs with IRES motifs were shown to be preferentially affected by SMN deficiency [128]. Interestingly, Gemin5 is known to bind and negatively regulate translation of mRNAs with IRES motifs in their 5′UTRs, and also to directly interact with ribosomal subunits L3, L4, eIF3B, eIF4B, and eIF4E [176,189,190,191,192,193]. Thus, the direct role of SMN in translation is largely uncharacterized and speculative. However, we see immense value in these experiments, as the authors show intriguing changes in response to SMN deficiency with a clear mechanism to how those changes arise. We look forward to further characterization of these SMN–ribosome complexes and suggest that identification of SMN mutants that abolish ribosomal association while retaining SMN complex formation would be informative.
It is possible to identify suppressors of specific SMN missense mutations that inform which function of SMN is critical to SMA. In this regard, we have used the SMNE134K and performed a random mutagenesis screen, using our Smn−/F7 iMEFs to ask whether we can make a mutation that rescues SMNE134K loss of function. This experiment is pictorially represented in Figure 3. In this experiment, Smn−/F7;SMNE134K iMEFs are mutagenized with ENU, then full-length wild-type Smn is removed by transfection with a lentivirus delivering an improved Cre-recombinase (iCre), yielding Smn−/D7;SMNE134K. Thus, there is no functional Smn from the mouse locus and the cells are entirely reliant on the nonfunctional SMNE134K, which causes the cells to die [77]. However, if a mutation in a gene occurs that can restore the essential function lost by SMNE134K, the cells should live. Indeed, we have performed such a screen and we have identified several mutant suppressor lines which we have sequenced to locate the suppressor. Importantly, the rescue is dependent on having SMNE134K present. If suppression of Smn−/D7;SMNE134K lethality occurs in a specific function of SMN, i.e., Sm-assembly, the suppressor, and SMNE134K can then be introduced into SMA mice or Smn null mice to ask the question, what rescue occurs? Indeed, we know that suppressors in Sm proteins can be obtained. Do Smn null mice expressing SMNE134K, and the suppressor show complete rescue of motor neurons, all phenotypes, or do they still show phenotypic abnormality? If it is complete rescue, then Sm-assembly is the critical function of SMN. If partial rescue occurs, then Sm-assembly contributes to SMA, but other functions must also contribute. Lastly, if a true SMA phenotype results, then there is a new mouse model of SMA and Sm-assembly is not the critical pathway in SMA. Though we have used the SMNE134K missense mutant, this same suppressor screen can be extended to other SMN missense mutations as none rescue survival of the Smn−/D7 iMEFs. These experiments can inform on the specific functional interaction of other members of the SMN complex, other unique SMN complexes, and how they work in an unbiased manner.
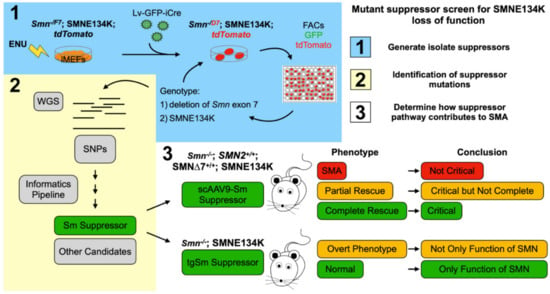
Figure 3.
Mutant suppressor screen for SMNE134K loss of function. (1) Pictorial representation of the random mutagenesis screen and isolation of SMNE134K mutant suppressor lines. Smn−/F7;SMNE134K iMEFs were mutagenized with N-ethyl-N-nitrosourea (ENU) then treated with a lentivirus delivering iCre-recombinase and Green Fluorescent Protein (GFP) to delete Smn exon 7 from the SmnF7 allele (now SmnD7). iCre activity is monitored by the removal of a floxed-stop codon upstream of tdTomato, activating tdTomato expression. Cells were sorted by FACs to enrich the tdTomato and GFP double positive population of cells and were seeded at low density. Surviving colonies were picked, expanded, and genotyped for retention of SMNE134K and deletion of Smn exon 7 using ddPCR. Cell populations which showed greater than 70% deletion of Smn exon 7 were kept and pushed to 100% deletion by additional lentivrial transfection of iCre and dilution cloning. (2) Whole genome sequencing (WGS) was performed on surviving Smn−/D7;SMNE134K mutant suppressor lines to identify variant SNPs not common to the unmutagenized Smn−/F7;SMNE134K cell lines. Resultant variant SNPs were prioritized in a candidate list and culminated in testing whether a mutation in an Sm protein was responsible for suppression of Smn−/D7;SMNE134K lethality. This Sm Suppressor indicates Sm-assembly is the essential function of SMN in iMEFs. (3) Outline of experiments that should determine whether rescue of Sm-assembly is critical to SMA. In ∆7 SMNE134K SMA mice, an AAV9 delivering the Sm suppressor can result in three outcomes: no modification of the SMA phenotype, a partial rescue, or complete rescue. Additionally, transgenic expression of the Sm suppressor in an Smn null, SMNE134K-expressing mouse can have two outcomes: development of an overt phenotype or normalcy. Each of these outcomes suggests a different conclusion, thus showing how suppressor screens can inform on the critical function of SMN regarding SMA.
7. Downstream Targets of SMN
The above section identifies the function of SMN that is critical for SMA. Once these are defined, it becomes possible to look for specific therapeutic targets or downstream targets of SMN deficiency. Currently we would say that targets are ill-defined and unclear in all cases. An example is the recently reported B-Raf which is implied to be critically impaired and result in motor neuron death [194]. The primary evidence given is that the neurotrophic pathway that supports motor neurons is reduced in presymptomatic mice. The first question that arises is how specific this is to SMA and whether this also occurs in SMA with respiratory distress (SMARD) or some other motor neuron disease control. This is required to know whether this neurotrophic pathway is specific to SMA or has the chance of being a secondary change. In addition, B-Raf null mice appear to have loss of both sensory neurons and motor neurons, begging the question as to whether the null really models the situation in SMA [195]. In this instance, identifying a hypomorphic allele would help clarify the situation as to whether B-Raf is required at higher levels in motor neurons. In addition, while sensory defects have been reported in SMA mice, it is not clear there is death of sensory neurons in mice or SMA patients. Lastly, the importance of the B-Raf pathway was confirmed using the C. elegans model of SMA and has yet to be replicated in mice or iPS cells [194]. We stress the importance of translating these experiments into mammalian models. Additionally, the authors state this will give access to a SMN-independent therapy strategy, but the reason for B-Raf reduction, if related to SMA, is deficiency of SMN. The report also states that the B-Raf change has not been directly related to SMN deficiency and could very well be downstream of the primary defect. This is problematic and we feel an approach that first defines the important pathway and then analyzes that pathway is essential to a logical path forward.
Given SMN role in spliceosomal snRNP biogenesis, it is plausible that a reduction in snRNP abundance could lead to inefficient or aberrant pre-mRNA splicing [196]. Many in the SMA community adhere to a stance that we have tested the gamut for splicing changes and since nothing has moved forward this cannot be the critical function of SMN regarding SMA. We feel the data on altered splicing is simply not adequate. Firstly, the most homogenous sample of mature motor neurons that has been collected for splicing analysis was performed on two SMA and two normal mice, with large variations in the levels of exon incorporation. Surely two disease, two controls, and no neurodegenerative disease control, with large variation between samples cannot be considered adequate. Additional studies have been performed in iPS motor neurons; however, these are not mature motor neurons, and it is not clear these cells are an accurate substitute [197,198]. However, before moving forward with sequencing experiments looking for splicing changes, the critical function of SMN needs to be defined and then the downstream target, like altered splicing, can be looked for. In addition, the downstream target should be significantly modified by SMN reduction and correcting this downstream target should restore a specific aspect of the SMA phenotype. In the context of an additive therapy to SMN restoration, the downstream targets should not be considered as an additive therapy of SMN without evidence that this is the case. Most will be SMN dependent and restored by SMN. Therefore, much work is needed in identifying downstream targets of SMN deficiency.
8. Modifiers of the SMA Phenotype
In SMA the phenotype is largely determined by the copy number of SMN2, but there are exceptions that diverge from this rule. The first is discussed above, regarding mild missense mutations in SMN1. Second, there are variants that lie within the SMN2 gene that alter the incorporation of SMN exon 7 and result in the production of more full-length SMN from that particular SMN2 gene [22,199,200,201,202]. Lastly, there are exception cases that would appear to not involve either SMN1 or SMN2, and thus the variant must be located outside the SMA region. Since mild missense mutations in SMN1 are discussed above, we will focus on modifiers within SMN2 as well as modifiers outside the SMA locus in this section.
A strong example of modifiers in SMN2 is the c.859G > C variant located in exon 7 [200,201]. This variant increases the inclusion of exon 7 in the SMN2 transcripts and has never been reported in a Type 1 individual with two copies of SMN2. The c.859G>C variant is found in Type 2 or 3 patients [199,200,201]. The variant has also been reported in a Type 2 individual who has two copies of SMN2 and is heterozygous for the variant. Whereas in other cases which also have two copies of the variant is in a homozygous state, SMA Type 3b results [199]. SMN2 variants in intron 6 that alter the incorporation of exon 7 are also associated with a milder than expected phenotype [22,202]. Thus, at least one mechanism of modification is altering the amount of full-length SMN coming from SMN2. However, there are discordant siblings in which the SMN2 gene(s) are identical between the siblings [22,32,203,204,205,206,207]. In our experience, these discordant siblings are most common in three copy SMN2 cases and predominantly conform to a Type 2 for the severe sibling and a Type 3b, or milder, in the other sibling [22]. However, there are exceptions that occur in all SMA types [208] and thus a model that can be used for testing in the general population is defined as Type 1 with two copies of SMN2, Type 2 with three copies, and Type 3 with four copies which can be used to help identify these modifiers. We suggest that the modifiers that occur in discordant siblings are the same as in the general population such that with a sufficiently large population or when using an isolated population with a particular founder, association studies can be used to identify the critical areas where these modifiers lie.
The first modifier proposed for SMA that lies outside the SMA region was plastin 3 (PLS3). PLS3 was found to be more highly expressed in lymphoblasts isolated from mild or unaffected siblings in discordant families. PLS3 is located on the X chromosome, and thus was reported as a sex-dependent, partially penetrant modifier [207]. However, the partially penetrant phenotype without description of the PLS3 mutation responsible for this increase in expression is troubling. Furthermore, SMA families in which the more severe sibling has higher PLS3 expression than the mild sibling have been reported [209]. Consequently, some have suggested that modifiers only function in milder SMA cases, however, there are siblings with Type 1 and 2 SMA, as well as cases where the male sibling is less severely affected than their female sibling [12,208].
The overexpression of PLS3 has been studied in two severe SMA mouse models, Taiwanese SMA mice and ∆7SMA mice, with opposing results [210,211]. Transgenic overexpression in our hands in the ∆7SMA model did not alter the SMA phenotype including the electrophysiological properties of the NMJ [211]. A previous publication suggests modification by PLS3 only occurs in mild SMA, in which a low dose of ASO directed against ISS-N1 was administered to the Taiwanese SMA mice to elevate SMN levels and create a milder phenotype [207]. However, the results from these experiments are hard to interpret as the overexpression of PLS3 itself can alter the endocytosis pathways and alter uptake of the ASO [210,212,213,214]. Additionally, the PLS3 mutation, rs871773, that increases PLS3 expression in colon cancer does not associate with mild SMA exception cases [22,215]. Until there is more definitive data, we favor a more cautious approach that increased PLS3 expression is not responsible for the modification of the phenotype.
The second reported modifier of SMA, Neurocalcin Delta (NCALD), was found in a single family from a genome-wide linkage analysis. This analysis identified eight genomic regions that could possibly link with the milder SMA case, however none of these regions reached the required significance level to determine linkage [216]. Subsequent expression analysis revealed reduced expression of NCALD [216]. However, it is unclear if one of the other seven regions is also involved in impacting phenotypic severity. One mutation, a 17 base-pair deletion adjacent to a punitive super enhancer, was found 600 kilobases upstream of the NCALD gene, however this deletion on its own does not modify the SMA phenotype as it is clearly found in both mild and severe discordant siblings [22]. A second mutation, a CT insertion in intron 1 of the NCALD gene, [216] is predicted to be a strong cryptic splice site that might inhibit NCALD expression [22]. Again though, this variant also occurs in the severe sibling of a discordant pair and in a concordant sibling pair [22]. While it is possible both variants must occur on the same allele to modify the phenotype, it seems more likely that the predicted cryptic splice site causes reduced NCALD expression and the 17 bp deletion adjacent to the enhancer is merely a rare polymorphic variant. [216] As NCALD mutations occur in both mild and severe siblings more experiments are required to determine if NCALD really modifies the SMA phenotype in humans.
There are several other genes that have been reported to modify the SMA phenotype in mice, C. elegans, and Drosophila, but in no case has the candidate been shown to modify the phenotype in humans by an association analysis [217,218,219,220,221]. While expression analysis of potential modifying genes is important, establishing a direct genetic link to the phenotypic alteration is paramount in identifying true modifiers. Ideally, a large pool of well characterized genomic SMA patient samples should be used to test for candidate association or for analysis between concordant and discordant siblings. As a rule, the modifying variant should only associate with the mild SMA case and not in the severe or concordant siblings. These variants can be further tested in a large panel of patient samples (not siblings) that are divided according to their SMN2 copy. The model of SMN2 copy number that we have used states that two copies of SMN2 predicts Type 1 SMA, three copies of SMN2 predicts Type 2 SMA, and four copies of SMN2 predicts Type 3 SMA. Any sample not conforming to this copy number model was considered to be discordant. One can then perform an association analysis to test if variant show a statistically significant association with a milder than expected phenotype. This would indicate that the variant is a true phenotypic modifier [22].
Alternatively, studying a population derived from a limited number of founder individuals, such as the Mennonite, Amish, and Hutterite SMA patients, can be a highly effective way to identify phenotypic modifiers. For example, a common haplotype marking a SMN1 deletion with two copies of SMN2 on one chromosome occurs in the Hutterites. Some individuals homozygous for this haplotype have a deletion of SMN1 with four copies of SMN2 and display no obvious SMA phenotype while other have typical Type 3 SMA [222]. Comparison of these two groups of individuals with whole genome sequencing will likely reveal an additional chromosomal region containing modifier genes.
9. Conclusions
The first era of genetic discovery in SMA identified the causative SMN1 gene and the therapeutic SMN2 target that led to three incredibly effective FDA approved drugs in a remarkably short period of time. The future of genetic studies, in conjunction with biochemistry, will demonstrate how SMN missense mutations can disrupt the function of SMN through complementation. Studying the SMNA2G and SMNT274I mutations that do not complement will likely identify new interaction between the N- and C-terminus of SMN. Finally, the use of partially functional SMN mutants will identify suppressors of specific SMN missense mutations in novel protein binding partners. Identification of true modifiers of the SMA phenotype will provide additional targets for therapy to improve outcomes of symptomatic SMA patients. Together genetic and biochemical studies have the potential unravel the basic biology of the disease and give us a clearer understanding of the function of SMN that is critical to the development of SMA.
Author Contributions
A.J.B.III and A.H.M.B. wrote this manuscript. V.L.M. edited and restructured the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The work of the authors was funded by NICHD R01HD060586 to A.H.M.B., CureSMA, MDA, and the Marshall Heritage Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to acknowledge Kaitlyn Kray for critiquing word usage and concepts.
Conflicts of Interest
A.H.M.B. consults for Novartis.
References
- Pearn, J. Incidence, prevalence, and gene frequency studies of chronic childhood spinal muscular atrophy. J. Med. Genet. 1978, 15, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Pearn, J.H. The gene frequency of acute Werdnig-Hoffmann disease (SMA type 1). A total population survey in North-East England. J. Med. Genet. 1973, 10, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Sugarman, E.A.; Nagan, N.; Zhu, H.; Akmaev, V.R.; Zhou, Z.; Rohlfs, E.M.; Flynn, K.; Hendrickson, B.C.; Scholl, T.; Sirko-Osadsa, D.A.; et al. Pan-ethnic carrier screening and prenatal diagnosis for spinal muscular atrophy: Clinical laboratory analysis of >72,400 specimens. Eur. J. Hum. Genet. 2012, 20, 27–32. [Google Scholar] [CrossRef]
- Lefebvre, S.; Burglen, L.; Reboullet, S.; Clermont, O.; Burlet, P.; Viollet, L.; Benichou, B.; Cruaud, C.; Millasseau, P.; Zeviani, M.; et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995, 80, 155–165. [Google Scholar] [CrossRef]
- Roberts, D.F.; Chavez, J.; Court, S.D. The genetic component in child mortality. Arch. Dis. Child. 1970, 45, 33–38. [Google Scholar] [CrossRef]
- Finkel, R.S.; Mercuri, E.; Darras, B.T.; Connolly, A.M.; Kuntz, N.L.; Kirschner, J.; Chiriboga, C.A.; Saito, K.; Servais, L.; Tizzano, E.; et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1723–1732. [Google Scholar] [CrossRef]
- Mendell, J.R.; Al-Zaidy, S.; Shell, R.; Arnold, W.D.; Rodino-Klapac, L.R.; Prior, T.W.; Lowes, L.; Alfano, L.; Berry, K.; Church, K.; et al. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1713–1722. [Google Scholar] [CrossRef]
- Baranello, G.; Darras, B.T.; Day, J.W.; Deconinck, N.; Klein, A.; Masson, R.; Mercuri, E.; Rose, K.; El-Khairi, M.; Gerber, M.; et al. Risdiplam in Type 1 Spinal Muscular Atrophy. N. Engl. J. Med. 2021, 384, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Arnold, W.D.; Kassar, D.; Kissel, J.T. Spinal muscular atrophy: Diagnosis and management in a new therapeutic era. Muscle Nerve 2015, 51, 157–167. [Google Scholar] [CrossRef]
- Arnold, W.D.; Burghes, A.H. Spinal muscular atrophy: Development and implementation of potential treatments. Ann. Neurol. 2013, 74, 348–362. [Google Scholar] [CrossRef]
- Becker, P.E. Atrophia musculorum spinalis pseudomyopathica: Heriditare neurogenic proximale Amytrophie von Kugelberg und Welander. Z. Menschl. Verbungsforsch. Konst. 1964, 37, 193–220. [Google Scholar]
- Burghes, A.H.; Ingraham, S.E.; Kote-Jarai, Z.; Rosenfeld, S.; Herta, N.; Nadkarni, N.; DiDonato, C.J.; Carpten, J.; Hurko, O.; Florence, J.; et al. Linkage mapping of the spinal muscular atrophy gene. Hum. Genet. 1994, 93, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Dubowitz, V. Infantile Muscular Atrophy. A Prospective Study with Particular Reference to a Slowly Progressive Variety. Brain A. J. Neurol. 1964, 87, 707–718. [Google Scholar] [CrossRef]
- Muller, B.; Melki, J.; Burlet, P.; Clerget-Darpoux, F. Proximal spinal muscular atrophy (SMA) types II and III in the same sibship are not caused by different alleles at the SMA locus on 5q. Am. J. Hum. Genet. 1992, 50, 892–895. [Google Scholar]
- Schmid, P.C. Study of the clinical aspects of infantile spinal progressive muscular atrophy of the Werdnig-Hoffman type. Z Kinderheilkd 1958, 81, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Zellweger, H.; Schneider, H.J.; Schuldt, D.R.; Mergner, W. Heritable spinal muscular atrophies. Helv. Paediatr. Acta 1969, 24, 92–105. [Google Scholar]
- Brzustowicz, L.M.; Lehner, T.; Castilla, L.H.; Penchaszadeh, G.K.; Wilhelmsen, K.C.; Daniels, R.; Davies, K.E.; Leppert, M.; Ziter, F.; Wood, D.; et al. Genetic mapping of chronic childhood-onset spinal muscular atrophy to chromosome 5q11.2-13.3. Nature 1990, 344, 540–541. [Google Scholar] [CrossRef] [PubMed]
- Gilliam, T.C.; Brzustowicz, L.M.; Castilla, L.H.; Lehner, T.; Penchaszadeh, G.K.; Daniels, R.J.; Byth, B.C.; Knowles, J.; Hislop, J.E.; Shapira, Y.; et al. Genetic homogeneity between acute and chronic forms of spinal muscular atrophy. Nature 1990, 345, 823–825. [Google Scholar] [CrossRef]
- Melki, J.; Abdelhak, S.; Sheth, P.; Bachelot, M.F.; Burlet, P.; Marcadet, A.; Aicardi, J.; Barois, A.; Carriere, J.P.; Fardeau, M.; et al. Gene for chronic proximal spinal muscular atrophies maps to chromosome 5q. Nature 1990, 344, 767–768. [Google Scholar] [CrossRef]
- Melki, J.; Sheth, P.; Abdelhak, S.; Burlet, P.; Bachelot, M.F.; Lathrop, M.G.; Frezal, J.; Munnich, A. Mapping of acute (type I) spinal muscular atrophy to chromosome 5q12-q14. The French Spinal Muscular Atrophy Investigators. Lancet 1990, 336, 271–273. [Google Scholar] [CrossRef]
- Burghes, A. When is a deletion not a deletion? When it is converted. Am. J. Hum. Genet. 1997, 61, 9. [Google Scholar] [CrossRef]
- Ruhno, C.; McGovern, V.L.; Avenarius, M.R.; Snyder, P.J.; Prior, T.W.; Nery, F.C.; Muhtaseb, A.; Roggenbuck, J.S.; Kissel, J.T.; Sansone, V.A.; et al. Complete sequencing of the SMN2 gene in SMA patients detects SMN gene deletion junctions and variants in SMN2 that modify the SMA phenotype. Hum. Genet. 2019, 138, 241–256. [Google Scholar] [CrossRef]
- Gennarelli, M.; Lucarelli, M.; Capon, F.; Pizzuti, A.; Merlini, L.; Angelini, C.; Novelli, G.; Dallapiccola, B. Survival motor neuron gene transcript analysis in muscles from spinal muscular atrophy patients. Biochem. Biophys. Res. Commun. 1995, 213, 342–348. [Google Scholar] [CrossRef]
- Parsons, D.W.; McAndrew, P.E.; Monani, U.R.; Mendell, J.R.; Burghes, A.H.; Prior, T.W. An 11 base pair duplication in exon 6 of the SMN gene produces a type I spinal muscular atrophy (SMA) phenotype: Further evidence for SMN as the primary SMA-determining gene. Hum. Mol. Genet. 1996, 5, 1727–1732. [Google Scholar] [CrossRef] [PubMed]
- Lorson, C.L.; Hahnen, E.; Androphy, E.J.; Wirth, B. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc. Natl. Acad. Sci. USA 1999, 96, 6307–6311. [Google Scholar] [CrossRef] [PubMed]
- Monani, U.R.; Lorson, C.L.; Parsons, D.W.; Prior, T.W.; Androphy, E.J.; Burghes, A.H.; McPherson, J.D. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum. Mol. Genet. 1999, 8, 1177–1183. [Google Scholar] [CrossRef]
- Lorson, C.L.; Strasswimmer, J.; Yao, J.M.; Baleja, J.D.; Hahnen, E.; Wirth, B.; Le, T.; Burghes, A.H.; Androphy, E.J. SMN oligomerization defect correlates with spinal muscular atrophy severity. Nat. Genet. 1998, 19, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Burnett, B.G.; Munoz, E.; Tandon, A.; Kwon, D.Y.; Sumner, C.J.; Fischbeck, K.H. Regulation of SMN protein stability. Mol. Cell Biol. 2009, 29, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Vitte, J.; Fassier, C.; Tiziano, F.D.; Dalard, C.; Soave, S.; Roblot, N.; Brahe, C.; Saugier-Veber, P.; Bonnefont, J.P.; Melki, J. Refined characterization of the expression and stability of the SMN gene products. Am. J. Pathol. 2007, 171, 1269–1280. [Google Scholar] [CrossRef]
- Coovert, D.D.; Le, T.T.; McAndrew, P.E.; Strasswimmer, J.; Crawford, T.O.; Mendell, J.R.; Coulson, S.E.; Androphy, E.J.; Prior, T.W.; Burghes, A.H. The survival motor neuron protein in spinal muscular atrophy. Hum. Mol. Genet. 1997, 6, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, S.; Burlet, P.; Liu, Q.; Bertrandy, S.; Clermont, O.; Munnich, A.; Dreyfuss, G.; Melki, J. Correlation between severity and SMN protein level in spinal muscular atrophy. Nat. Genet. 1997, 16, 265–269. [Google Scholar] [CrossRef]
- McAndrew, P.; Parsons, D.; Simard, L.; Rochette, C.; Ray, P.; Mendell, J.; Prior, T.; Burghes, A. Identification of proximal spinal muscular atrophy carriers and patients by analysis of SMNT and SMNC gene copy number. Am. J. Hum. Genet. 1997, 60, 1411–1422. [Google Scholar] [CrossRef]
- Cartegni, L.; Krainer, A.R. Disruption of an SF2/ASF-dependent exonic splicing enhancer in SMN2 causes spinal muscular atrophy in the absence of SMN1. Nat. Genet. 2002, 30, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Vickers, T.A.; Baker, B.F.; Bennett, C.F.; Krainer, A.R. Enhancement of SMN2 exon 7 inclusion by antisense oligonucleotides targeting the exon. PLoS Biol. 2007, 5, e73. [Google Scholar] [CrossRef]
- Singh, N.K.; Singh, N.N.; Androphy, E.J.; Singh, R.N. Splicing of a critical exon of human Survival Motor Neuron is regulated by a unique silencer element located in the last intron. Mol. Cell Biol. 2006, 26, 1333–1346. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.N. Evolving concepts on human SMN pre-mRNA splicing. RNA Biol. 2007, 4, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Vickers, T.A.; Okunola, H.L.; Bennett, C.F.; Krainer, A.R. Antisense masking of an hnRNP A1/A2 intronic splicing silencer corrects SMN2 splicing in transgenic mice. Am. J. Hum. Genet. 2008, 82, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Sahashi, K.; Rigo, F.; Hung, G.; Horev, G.; Bennett, C.F.; Krainer, A.R. Peripheral SMN restoration is essential for long-term rescue of a severe spinal muscular atrophy mouse model. Nature 2011, 478, 123–126. [Google Scholar] [CrossRef]
- Passini, M.A.; Bu, J.; Richards, A.M.; Kinnecom, C.; Sardi, S.P.; Stanek, L.M.; Hua, Y.; Rigo, F.; Matson, J.; Hung, G.; et al. Antisense oligonucleotides delivered to the mouse CNS ameliorate symptoms of severe spinal muscular atrophy. Transl. Sci. Transl. Med. 2011, 3, 72. [Google Scholar] [CrossRef]
- Porensky, P.N.; Mitrpant, C.; McGovern, V.L.; Bevan, A.K.; Foust, K.D.; Kaspar, B.K.; Wilton, S.D.; Burghes, A.H. A single administration of morpholino antisense oligomer rescues spinal muscular atrophy in mouse. Hum. Mol. Genet. 2012, 21, 1625–1638. [Google Scholar] [CrossRef]
- Naryshkin, N.A.; Weetall, M.; Dakka, A.; Narasimhan, J.; Zhao, X.; Feng, Z.; Ling, K.K.; Karp, G.M.; Qi, H.; Woll, M.G.; et al. Motor neuron disease. SMN2 splicing modifiers improve motor function and longevity in mice with spinal muscular atrophy. Science 2014, 345, 688–693. [Google Scholar] [CrossRef]
- Campagne, S.; Boigner, S.; Rudisser, S.; Moursy, A.; Gillioz, L.; Knorlein, A.; Hall, J.; Ratni, H.; Clery, A.; Allain, F.H. Structural basis of a small molecule targeting RNA for a specific splicing correction. Nat. Chem. Biol. 2019, 15, 1191–1198. [Google Scholar] [CrossRef]
- Palacino, J.; Swalley, S.E.; Song, C.; Cheung, A.K.; Shu, L.; Zhang, X.; Van Hoosear, M.; Shin, Y.; Chin, D.N.; Keller, C.G.; et al. SMN2 splice modulators enhance U1-pre-mRNA association and rescue SMA mice. Nat. Chem. Biol. 2015, 11, 511–517. [Google Scholar] [CrossRef]
- Sivaramakrishnan, M.; McCarthy, K.D.; Campagne, S.; Huber, S.; Meier, S.; Augustin, A.; Heckel, T.; Meistermann, H.; Hug, M.N.; Birrer, P.; et al. Binding to SMN2 pre-mRNA-protein complex elicits specificity for small molecule splicing modifiers. Nat. Commun. 2017, 8, 1476. [Google Scholar] [CrossRef]
- Arnold, W.D.; Porensky, P.N.; McGovern, V.L.; Iyer, C.C.; Duque, S.; Li, X.; Meyer, K.; Schmelzer, L.; Kaspar, B.K.; Kolb, S.J.; et al. Electrophysiological Biomarkers in Spinal Muscular Atrophy: Preclinical Proof of Concept. Ann. Clin. Transl. Neurol. 2014, 1, 34–44. [Google Scholar] [CrossRef]
- Ratni, H.; Karp, G.M.; Weetall, M.; Naryshkin, N.A.; Paushkin, S.V.; Chen, K.S.; McCarthy, K.D.; Qi, H.; Turpoff, A.; Woll, M.G.; et al. Specific Correction of Alternative Survival Motor Neuron 2 Splicing by Small Molecules: Discovery of a Potential Novel Medicine To Treat Spinal Muscular Atrophy. J. Med. Chem. 2016, 59, 6086–6100. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Darras, B.T.; Chiriboga, C.A.; Day, J.W.; Campbell, C.; Connolly, A.M.; Iannaccone, S.T.; Kirschner, J.; Kuntz, N.L.; Saito, K.; et al. Nusinersen versus Sham Control in Later-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2018, 378, 625–635. [Google Scholar] [CrossRef] [PubMed]
- De Vivo, D.C.; Bertini, E.; Swoboda, K.J.; Hwu, W.L.; Crawford, T.O.; Finkel, R.S.; Kirschner, J.; Kuntz, N.L.; Parsons, J.A.; Ryan, M.M.; et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: Interim efficacy and safety results from the Phase 2 NURTURE study. Neuromuscul. Disord. 2019, 29, 842–856. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, E.; Marais, T.; Chatauret, N.; Benkhelifa-Ziyyat, S.; Duque, S.; Ravassard, P.; Carcenac, R.; Astord, S.; Pereira de Moura, A.; Voit, T.; et al. Intravenous scAAV9 delivery of a codon-optimized SMN1 sequence rescues SMA mice. Hum. Mol. Genet. 2011, 20, 681–693. [Google Scholar] [CrossRef]
- Foust, K.D.; Wang, X.; McGovern, V.L.; Braun, L.; Bevan, A.K.; Haidet, A.M.; Le, T.T.; Morales, P.R.; Rich, M.M.; Burghes, A.H. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat. Biotechnol. 2010, 28, 271. [Google Scholar] [CrossRef] [PubMed]
- Valori, C.F.; Ning, K.; Wyles, M.; Mead, R.J.; Grierson, A.J.; Shaw, P.J.; Azzouz, M. Systemic delivery of scAAV9 expressing SMN prolongs survival in a model of spinal muscular atrophy. Transl. Sci. Transl. Med. 2010, 2, 35. [Google Scholar] [CrossRef]
- Meyer, K.; Ferraiuolo, L.; Schmelzer, L.; Braun, L.; McGovern, V.; Likhite, S.; Michels, O.; Govoni, A.; Fitzgerald, J.; Morales, P.; et al. Improving single injection CSF delivery of AAV9-mediated gene therapy for SMA: A dose-response study in mice and nonhuman primates. Mol. Ther. 2015, 23, 477–487. [Google Scholar] [CrossRef]
- Strauss, K.; Muntoni, F.; Farrar, M.; Saito, K.; Mendell, J.; Servais, L.; McMillan, H.; Swoboda, K.; Kwon, J.; Zaidman, C.; et al. Onasemnogene abeparvovec gene therapy in presymptomatic spinal muscular atrophy (SMA): SPR1NT study update in children with 2 copies of SMN2. In Proceedings of the Muscular Dystrophy Association Conference, Virtual Broadcast, Porto, Portugal, 15–18 March 2020; p. 67. Available online: https://mdaconference.org/node/1158 (accessed on 27 July 2021).
- Strauss, K.; Muntoni, F.; Farrar, M.; Saito, K.; Mendell, J.; Servais, L.; McMillan, H.; Swoboda, K.; Kwon, J.; Zaidman, C.; et al. Onasemnogene abeparvovec gene therapy in presymptomatic spinal muscular atrophy (SMA): SPR1NT study update in children with 3 copies of SMN2. In Proceedings of the Muscular Dystrophy Association Conference, Virtual Broadcast, Porto, Portugal, 5–18 March 2021; p. 68. Available online: https://mdaconference.org/node/1159 (accessed on 27 July 2021).
- Novartis Gene Therapies. Study of Intrathecal Administration of Onasemnogene Abeparvovec-Xioi for Spinanl Muscular Atrophy (STRONG). National Institutes of Health, U.S. National Library of Medicine. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT03381729 (accessed on 27 July 2021).
- Arbab, M.; Shen, M.W.; Mok, B.; Wilson, C.; Matuszek, Z.; Cassa, C.A.; Liu, D.R. Determinants of Base Editing Outcomes from Target Library Analysis and Machine Learning. Cell 2020, 182, 463–480. [Google Scholar] [CrossRef]
- Gaudelli, N.M.; Komor, A.C.; Rees, H.A.; Packer, M.S.; Badran, A.H.; Bryson, D.I.; Liu, D.R. Programmable base editing of A*T to G*C in genomic DNA without DNA cleavage. Nature 2017, 551, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.W.; Arbab, M.; Hsu, J.Y.; Worstell, D.; Culbertson, S.J.; Krabbe, O.; Cassa, C.A.; Liu, D.R.; Gifford, D.K.; Sherwood, R.I. Predictable and precise template-free CRISPR editing of pathogenic variants. Nature 2018, 563, 646–651. [Google Scholar] [CrossRef]
- Li, J.J.L.X.; Tang, C.; Lu, Y.Q.; Hu, X.; Zuo, E.; Li, H.; Ying, W.; Sun, Y.; Lai, L.L.; Hai-Zhu, C.; et al. Disruption of splicing-regulatory elements using CRISPR/Cas9 rescues spinal muscular atrophy in human iPSCs and mice. Natl. Sci. Rev. 2020, 7, 92–101. [Google Scholar] [CrossRef]
- Wirth, B.; Herz, M.; Wetter, A.; Moskau, S.; Hahnen, E.; Rudnik-Schoneborn, S.; Wienker, T.; Zerres, K. Quantitative analysis of survival motor neuron copies: Identification of subtle SMN1 mutations in patients with spinal muscular atrophy, genotype-phenotype correlation, and implications for genetic counseling. Am. J. Hum. Genet. 1999, 64, 1340–1356. [Google Scholar] [CrossRef] [PubMed]
- Burghes, A.H.; Beattie, C.E. Spinal muscular atrophy: Why do low levels of survival motor neuron protein make motor neurons sick? Nat. Rev. Neurosci. 2009, 10, 597. [Google Scholar] [CrossRef]
- Parsons, D.W.; McAndrew, P.E.; Iannaccone, S.T.; Mendell, J.R.; Burghes, A.H.; Prior, T.W. Intragenic telSMN mutations: Frequency, distribution, evidence of a founder effect, and modification of the spinal muscular atrophy phenotype by cenSMN copy number. Am. J. Hum. Genet. 1998, 63, 1712–1723. [Google Scholar] [CrossRef] [PubMed]
- Vinette, K.M.; Kirwin, S.M.; Gonzalez, I.L.; Funanage, V.L. A Review of SMN1 mutations in a Molecular Diagnostics Laboratory. In Proceedings of the 12th Annual International Spinal Muscular Atrophy Research Group Meeting, Boston, MA, USA, 9 June 2008. [Google Scholar]
- Sun, Y.; Grimmler, M.; Schwarzer, V.; Schoenen, F.; Fischer, U.; Wirth, B. Molecular and functional analysis of intragenic SMN1 mutations in patients with spinal muscular atrophy. Hum. Mutat 2005, 25, 64–71. [Google Scholar] [CrossRef]
- Kotani, T.; Sutomo, R.; Sasongko, T.H.; Sadewa, A.H.; Gunadi, M.T.; Fujii, E.; Endo, S.; Lee, M.J.; Ayaki, H.; Harada, Y.; et al. A novel mutation at the N-terminal of SMN Tudor domain inhibits its interaction with target proteins. J. Neurol. 2007, 254, 624–630. [Google Scholar] [CrossRef]
- Clermont, O.; Burlet, P.; Benit, P.; Chanterau, D.; Saugier-Veber, P.; Munnich, A.; Cusin, V. Molecular analysis of SMA patients without homozygous SMN1 deletions using a new strategy for identification of SMN1 subtle mutations. Hum. Mutat. 2004, 24, 417–427. [Google Scholar] [CrossRef]
- Cusco, I.; Barcelo, M.J.; del Rio, E.; Baiget, M.; Tizzano, E.F. Detection of novel mutations in the SMN Tudor domain in type I SMA patients. Neurology 2004, 63, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Mihal, D.C.; Bridgeman, S.J.; Pryatt, R.E.; Prior, T.W. Sequence analysis of small SMNq mutations in SMA compound heterozygotes. In Proceedings of the Annual Meeting of The American Society of Human Genetics, New Orleans, Louisiana, 9–13 October 2006; Available online: www.ashg.org/genetics/ashg06s/index.shtml (accessed on 27 July 2021).
- Prior, T.W.; (Pathology Department Case Western Reserve University, Cleveland, OH, USA). Personal Communication, 2021.
- Zapletalova, E.; Hedvicakova, P.; Kozak, L.; Vondracek, P.; Gaillyova, R.; Marikova, T.; Kalina, Z.; Juttnerova, V.; Fajkus, J.; Fajkusova, L. Analysis of point mutations in the SMN1 gene in SMA patients bearing a single SMN1 copy. Neuromuscul. Disord. 2007, 17, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Rochette, C.F.; Surh, L.C.; Ray, P.N.; McAndrew, P.E.; Prior, T.W.; Burghes, A.H.; Vanasse, M.; Simard, L.R. Molecular diagnosis of non-deletion SMA patients using quantitative PCR of SMN exon 7. Neurogenetics 1997, 1, 141–147. [Google Scholar] [CrossRef]
- Hahnen, E.; Schonling, J.; Rudnik-Schoneborn, S.; Raschke, H.; Zerres, K.; Wirth, B. Missense mutations in exon 6 of the survival motor neuron gene in patients with spinal muscular atrophy (SMA). Hum. Mol. Genet. 1997, 6, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Alias, L.; Bernal, S.; Fuentes-Prior, P.; Barcelo, M.J.; Also, E.; Martinez-Hernandez, R.; Rodriguez-Alvarez, F.J.; Martin, Y.; Aller, E.; Grau, E.; et al. Mutation update of spinal muscular atrophy in Spain: Molecular characterization of 745 unrelated patients and identification of four novel mutations in the SMN1 gene. Hum. Genet. 2009, 125, 29–39. [Google Scholar] [CrossRef]
- Wang, C.H.; Papendick, B.D.; Bruinsma, P.; Day, J.K. Identification of a novel missense mutation of the SMN(T) gene in two siblings with spinal muscular atrophy. Neurogenetics 1998, 1, 273–276. [Google Scholar]
- Talbot, K.; Ponting, C.P.; Theodosiou, A.M.; Rodrigues, N.R.; Surtees, R.; Mountford, R.; Davies, K.E. Missense mutation clustering in the survival motor neuron gene: A role for a conserved tyrosine and glycine rich region of the protein in RNA metabolism? Hum. Mol. Genet. 1997, 6, 497–500. [Google Scholar] [CrossRef]
- Carrel, T.L.; McWhorter, M.L.; Workman, E.; Zhang, H.; Wolstencroft, E.C.; Lorson, C.; Bassell, G.J.; Burghes, A.H.; Beattie, C.E. Survival motor neuron function in motor axons is independent of functions required for small nuclear ribonucleoprotein biogenesis. J. Neurosci. 2006, 26, 11014–11022. [Google Scholar] [CrossRef]
- Blatnik, A.J.; McGovern, V.L.; Le, T.T.; Iyer, C.C.; Kaspar, B.K.; Burghes, A.H.M. Conditional deletion of SMN in cell culture identifies functional SMN alleles. Hum. Mol. Genet. 2020, 29, 3477–3492. [Google Scholar] [CrossRef]
- Schrank, B.; Gotz, R.; Gunnersen, J.M.; Ure, J.M.; Toyka, K.V.; Smith, A.G.; Sendtner, M. Inactivation of the survival motor neuron gene, a candidate gene for human spinal muscular atrophy, leads to massive cell death in early mouse embryos. Proc. Natl. Acad. Sci. USA 1997, 94, 9920–9925. [Google Scholar] [CrossRef]
- Frugier, T.; Tiziano, F.D.; Cifuentes-Diaz, C.; Miniou, P.; Roblot, N.; Dierich, A.; Le Meur, M.; Melki, J. Nuclear targeting defect of SMN lacking the C-terminus in a mouse model of spinal muscular atrophy. Hum. Mol. Genet. 2000, 9, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Monani, U.R.; Pastore, M.T.; Gavrilina, T.O.; Jablonka, S.; Le, T.T.; Andreassi, C.; DiCocco, J.M.; Lorson, C.; Androphy, E.J.; Sendtner, M.; et al. A transgene carrying an A2G missense mutation in the SMN gene modulates phenotypic severity in mice with severe (type I) spinal muscular atrophy. J. Cell Biol. 2003, 160, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Workman, E.; Saieva, L.; Carrel, T.L.; Crawford, T.O.; Liu, D.; Lutz, C.; Beattie, C.E.; Pellizzoni, L.; Burghes, A.H. A SMN missense mutation complements SMN2 restoring snRNPs and rescuing SMA mice. Hum. Mol. Genet. 2009, 18, 2215–2229. [Google Scholar] [CrossRef] [PubMed]
- Iyer, C.C.; Corlett, K.M.; Massoni-Laporte, A.; Duque, S.I.; Madabusi, N.; Tisdale, S.; McGovern, V.L.; Le, T.T.; Zaworski, P.G.; Arnold, W.D.; et al. Mild SMN missense alleles are only functional in the presence of SMN2 in mammals. Hum. Mol. Genet. 2018, 27, 3404–3416. [Google Scholar] [CrossRef] [PubMed]
- McGovern, V.L.; Kray, K.M.; Arnold, W.D.; Duque, S.I.; Iyer, C.C.; Massoni-Laporte, A.; Workman, E.; Patel, A.; Battle, D.J.; Burghes, A.H.M. Intragenic complementation of amino and carboxy terminal SMN missense mutations can rescue Smn null mice. Hum. Mol. Genet. 2020, 29, 3493–3503. [Google Scholar] [CrossRef]
- Miguel-Aliaga, I.; Chan, Y.B.; Davies, K.E.; van den Heuvel, M. Disruption of SMN function by ectopic expression of the human SMN gene in Drosophila. FEBS Lett. 2000, 486, 99–102. [Google Scholar] [CrossRef]
- Sleigh, J.N.; Buckingham, S.D.; Esmaeili, B.; Viswanathan, M.; Cuppen, E.; Westlund, B.M.; Sattelle, D.B. A novel Caenorhabditis elegans allele, smn-1(cb131), mimicking a mild form of spinal muscular atrophy, provides a convenient drug screening platform highlighting new and pre-approved compounds. Hum. Mol. Genet. 2011, 20, 245–260. [Google Scholar] [CrossRef]
- Praveen, K.; Wen, Y.; Gray, K.M.; Noto, J.J.; Patlolla, A.R.; Van Duyne, G.D.; Matera, A.G. SMA-causing missense mutations in survival motor neuron (Smn) display a wide range of phenotypes when modeled in Drosophila. PLoS Genet. 2014, 10, e1004489. [Google Scholar] [CrossRef]
- Neuenkirchen, N.; Englbrecht, C.; Ohmer, J.; Ziegenhals, T.; Chari, A.; Fischer, U. Reconstitution of the human U snRNP assembly machinery reveals stepwise Sm protein organization. EMBO J. 2015, 34, 1925–1941. [Google Scholar] [CrossRef]
- Veepaschit, J.; Viswanathan, A.; Bordonne, R.; Grimm, C.; Fischer, U. Identification and structural analysis of the Schizosaccharomyces pombe SMN complex. Nucleic Acids Res. 2021, 49, 7207–7223. [Google Scholar] [CrossRef]
- Turner, M.A.; Simpson, A.; McInnes, R.R.; Howell, P.L. Human argininosuccinate lyase: A structural basis for intragenic complementation. Proc. Natl. Acad. Sci. USA 1997, 94, 9063–9068. [Google Scholar] [CrossRef]
- Yu, B.; Howell, P.L. Intragenic complementation and the structure and function of argininosuccinate lyase. Cell Mol. Life Sci 2000, 57, 1637–1651. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Thompson, G.D.; Yip, P.; Howell, P.L.; Davidson, A.R. Mechanisms for intragenic complementation at the human argininosuccinate lyase locus. Biochemistry 2001, 40, 15581–15590. [Google Scholar] [CrossRef]
- Ono, Y.; Shindo, M.; Doi, N.; Kitamura, F.; Gregorio, C.C.; Sorimachi, H. The N- and C-terminal autolytic fragments of CAPN3/p94/calpain-3 restore proteolytic activity by intermolecular complementation. Proc. Natl. Acad. Sci. USA 2014, 111, E5527–E5536. [Google Scholar] [CrossRef]
- Martin, R.; Gupta, K.; Ninan, N.S.; Perry, K.; Van Duyne, G.D. The survival motor neuron protein forms soluble glycine zipper oligomers. Structure 2012, 20, 1929–1939. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Martin, R.; Sharp, R.; Sarachan, K.L.; Ninan, N.S.; Van Duyne, G.D. Oligomeric Properties of Survival Motor Neuron.Gemin2 Complexes. J. Biol. Chem. 2015, 290, 20185–20199. [Google Scholar] [CrossRef]
- Orr, H.T.; Zoghbi, H.Y. SCA1 molecular genetics: A history of a 13 year collaboration against glutamines. Hum. Mol. Genet. 2001, 10, 2307–2311. [Google Scholar] [CrossRef] [PubMed]
- Duvick, L.; Barnes, J.; Ebner, B.; Agrawal, S.; Andresen, M.; Lim, J.; Giesler, G.J.; Zoghbi, H.Y.; Orr, H.T. SCA1-like disease in mice expressing wild-type ataxin-1 with a serine to aspartic acid replacement at residue 776. Neuron 2010, 67, 929–935. [Google Scholar] [CrossRef]
- Fryer, J.D.; Yu, P.; Kang, H.; Mandel-Brehm, C.; Carter, A.N.; Crespo-Barreto, J.; Gao, Y.; Flora, A.; Shaw, C.; Orr, H.T.; et al. Exercise and genetic rescue of SCA1 via the transcriptional repressor Capicua. Science 2011, 334, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Lu, H.C.; Zoghbi, H.Y.; Song, J.J. Structural basis of protein complex formation and reconfiguration by polyglutamine disease protein Ataxin-1 and Capicua. Genes Dev. 2013, 27, 590–595. [Google Scholar] [CrossRef]
- Rousseaux, M.W.C.; Tschumperlin, T.; Lu, H.C.; Lackey, E.P.; Bondar, V.V.; Wan, Y.W.; Tan, Q.; Adamski, C.J.; Friedrich, J.; Twaroski, K.; et al. ATXN1-CIC Complex Is the Primary Driver of Cerebellar Pathology in Spinocerebellar Ataxia Type 1 through a Gain-of-Function Mechanism. Neuron 2018, 97, 1235–1243.e5. [Google Scholar] [CrossRef]
- Liyanage, V.R.; Rastegar, M. Rett syndrome and MeCP2. Neuromolecular. Med. 2014, 16, 231–264. [Google Scholar] [CrossRef]
- Buchovecky, C.M.; Turley, S.D.; Brown, H.M.; Kyle, S.M.; McDonald, J.G.; Liu, B.; Pieper, A.A.; Huang, W.; Katz, D.M.; Russell, D.W.; et al. A suppressor screen in Mecp2 mutant mice implicates cholesterol metabolism in Rett syndrome. Nat. Genet. 2013, 45, 1013–1020. [Google Scholar] [CrossRef]
- Enikanolaiye, A.; Ruston, J.; Zeng, R.; Taylor, C.; Schrock, M.; Buchovecky, C.M.; Shendure, J.; Acar, E.; Justice, M.J. Suppressor mutations in Mecp2-null mice implicate the DNA damage response in Rett syndrome pathology. Genome Res. 2020, 30, 540–552. [Google Scholar] [CrossRef]
- Nagy, G.; Ackerman, S.L. Cholesterol metabolism and Rett syndrome pathogenesis. Nat. Genet. 2013, 45, 965–967. [Google Scholar] [CrossRef]
- Bushart, D.D.; Shakkottai, V.G. Ion channel dysfunction in cerebellar ataxia. Neurosci. Lett. 2019, 688, 41–48. [Google Scholar] [CrossRef]
- Chopra, R.; Bushart, D.D.; Cooper, J.P.; Yellajoshyula, D.; Morrison, L.M.; Huang, H.; Handler, H.P.; Man, L.J.; Dansithong, W.; Scoles, D.R.; et al. Altered Capicua expression drives regional Purkinje neuron vulnerability through ion channel gene dysregulation in spinocerebellar ataxia type 1. Hum. Mol. Genet. 2020, 29, 3249–3265. [Google Scholar] [CrossRef]
- Kyle, S.M.; Vashi, N.; Justice, M.J. Rett syndrome: A neurological disorder with metabolic components. Open Biol. 2018, 8, 216. [Google Scholar] [CrossRef]
- Justice, M.J.; Buchovecky, C.M.; Kyle, S.M.; Djukic, A. A role for metabolism in Rett syndrome pathogenesis: New clinical findings and potential treatment targets. Rare Dis. 2013, 1, e27265. [Google Scholar] [CrossRef]
- Fischer, U.; Liu, Q.; Dreyfuss, G. The SMN-SIP1 complex has an essential role in spliceosomal snRNP biogenesis. Cell 1997, 90, 1023–1029. [Google Scholar] [CrossRef]
- Pellizzoni, L.; Kataoka, N.; Charroux, B.; Dreyfuss, G. A novel function for SMN, the spinal muscular atrophy disease gene product, in pre-mRNA splicing. Cell 1998, 95, 615–624. [Google Scholar] [CrossRef]
- Pellizzoni, L.; Yong, J.; Dreyfuss, G. Essential role for the SMN complex in the specificity of snRNP assembly. Science 2002, 298, 1775–1779. [Google Scholar] [CrossRef]
- Gubitz, A.K.; Feng, W.; Dreyfuss, G. The SMN complex. Exp. Cell Res. 2004, 296, 51–56. [Google Scholar] [CrossRef]
- Battle, D.J.; Lau, C.K.; Wan, L.; Deng, H.; Lotti, F.; Dreyfuss, G. The Gemin5 protein of the SMN complex identifies snRNAs. Mol. Cell 2006, 23, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Kolb, S.J.; Battle, D.J.; Dreyfuss, G. Molecular functions of the SMN complex. J. Child. Neurol. 2007, 22, 990–994. [Google Scholar] [CrossRef]
- Wan, L.; Ottinger, E.; Cho, S.; Dreyfuss, G. Inactivation of the SMN complex by oxidative stress. Mol. Cell 2008, 31, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; So, B.R.; Li, P.; Yong, J.; Glisovic, T.; Wan, L.; Dreyfuss, G. Structure of a key intermediate of the SMN complex reveals Gemin2’s crucial function in snRNP assembly. Cell 2011, 146, 384–395. [Google Scholar] [CrossRef]
- Li, D.K.; Tisdale, S.; Lotti, F.; Pellizzoni, L. SMN control of RNP assembly: From post-transcriptional gene regulation to motor neuron disease. Semin. Cell Dev. Biol. 2014, 32, 22–29. [Google Scholar] [CrossRef] [PubMed]
- So, B.R.; Wan, L.; Zhang, Z.; Li, P.; Babiash, E.; Duan, J.; Younis, I.; Dreyfuss, G. A U1 snRNP-specific assembly pathway reveals the SMN complex as a versatile hub for RNP exchange. Nat. Struct Mol. Biol. 2016, 23, 225–230. [Google Scholar] [CrossRef]
- Pillai, R.S.; Will, C.L.; Luhrmann, R.; Schumperli, D.; Muller, B. Purified U7 snRNPs lack the Sm proteins D1 and D2 but contain Lsm10, a new 14 kDa Sm D1-like protein. EMBO J. 2001, 20, 5470–5479. [Google Scholar] [CrossRef] [PubMed]
- Pillai, R.S.; Grimmler, M.; Meister, G.; Will, C.L.; Lührmann, R.; Fischer, U.; Schümperli, D. Unique Sm core structure of U7 snRNPs: Assembly by a specialized SMN complex and the role of a new component, Lsm11, in histone RNA processing. Genes Dev. 2003, 17, 2321–2333. [Google Scholar] [CrossRef]
- Tisdale, S.; Lotti, F.; Saieva, L.; Van Meerbeke, J.P.; Crawford, T.O.; Sumner, C.J.; Mentis, G.Z.; Pellizzoni, L. SMN is essential for the biogenesis of U7 small nuclear ribonucleoprotein and 3’-end formation of histone mRNAs. Cell Rep. 2013, 5, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, S.; Henriksson, S.; Weibrecht, I.; Smith, S.; Soderberg, O.; Stromblad, S.; Wiman, K.G.; Farnebo, M. WRAP53 is essential for Cajal body formation and for targeting the survival of motor neuron complex to Cajal bodies. PLoS Biol. 2010, 8, e1000521. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Kannan, R.; Blanchette, M.; Baumann, P. Telomerase RNA biogenesis involves sequential binding by Sm and Lsm complexes. Nature 2012, 484, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Poole, A.R.; Hebert, M.D. SMN and coilin negatively regulate dyskerin association with telomerase RNA. Biol. Open 2016, 5, 726–735. [Google Scholar] [CrossRef]
- Piazzon, N.; Schlotter, F.; Lefebvre, S.; Dodre, M.; Mereau, A.; Soret, J.; Besse, A.; Barkats, M.; Bordonne, R.; Branlant, C.; et al. Implication of the SMN complex in the biogenesis and steady state level of the signal recognition particle. Nucleic Acids Res. 2013, 41, 1255–1272. [Google Scholar] [CrossRef]
- Sanchez, G.; Dury, A.Y.; Murray, L.M.; Biondi, O.; Tadesse, H.; El Fatimy, R.; Kothary, R.; Charbonnier, F.; Khandjian, E.W.; Cote, J. A novel function for the survival motoneuron protein as a translational regulator. Hum. Mol. Genet. 2013, 22, 668–684. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, G.; Bondy-Chorney, E.; Laframboise, J.; Paris, G.; Didillon, A.; Jasmin, B.J.; Cote, J. A novel role for CARM1 in promoting nonsense-mediated mRNA decay: Potential implications for spinal muscular atrophy. Nucleic Acids Res. 2016, 44, 2661–2676. [Google Scholar] [CrossRef]
- Bernabo, P.; Tebaldi, T.; Groen, E.J.N.; Lane, F.M.; Perenthaler, E.; Mattedi, F.; Newbery, H.J.; Zhou, H.; Zuccotti, P.; Potrich, V.; et al. In Vivo Translatome Profiling in Spinal Muscular Atrophy Reveals a Role for SMN Protein in Ribosome Biology. Cell Rep. 2017, 21, 953–965. [Google Scholar] [CrossRef] [PubMed]
- Lauria, F.; Bernabo, P.; Tebaldi, T.; Groen, E.J.N.; Perenthaler, E.; Maniscalco, F.; Rossi, A.; Donzel, D.; Clamer, M.; Marchioretto, M.; et al. SMN-primed ribosomes modulate the translation of transcripts related to spinal muscular atrophy. Nat. Cell Biol. 2020, 22, 1239–1251. [Google Scholar] [CrossRef]
- Tadesse, H.; Deschenes-Furry, J.; Boisvenue, S.; Cote, J. KH-type splicing regulatory protein interacts with survival motor neuron protein and is misregulated in spinal muscular atrophy. Hum. Mol. Genet. 2008, 17, 506–524. [Google Scholar] [CrossRef] [PubMed]
- Akten, B.; Kye, M.J.; Hao, L.T.; Wertz, M.H.; Singh, S.; Nie, D.; Huang, J.; Merianda, T.T.; Twiss, J.L.; Beattie, C.E.; et al. Interaction of survival of motor neuron (SMN) and HuD proteins with mRNA cpg15 rescues motor neuron axonal deficits. Proc. Natl. Acad. Sci. USA 2011, 108, 10337–10342. [Google Scholar] [CrossRef]
- Fallini, C.; Zhang, H.; Su, Y.; Silani, V.; Singer, R.H.; Rossoll, W.; Bassell, G.J. The survival of motor neuron (SMN) protein interacts with the mRNA-binding protein HuD and regulates localization of poly(A) mRNA in primary motor neuron axons. J. Neurosci. 2011, 31, 3914–3925. [Google Scholar] [CrossRef]
- Hubers, L.; Valderrama-Carvajal, H.; Laframboise, J.; Timbers, J.; Sanchez, G.; Cote, J. HuD interacts with survival motor neuron protein and can rescue spinal muscular atrophy-like neuronal defects. Hum. Mol. Genet. 2011, 20, 553–579. [Google Scholar] [CrossRef]
- Rage, F.; Boulisfane, N.; Rihan, K.; Neel, H.; Gostan, T.; Bertrand, E.; Bordonne, R.; Soret, J. Genome-wide identification of mRNAs associated with the protein SMN whose depletion decreases their axonal localization. RNA 2013, 19, 1755–1766. [Google Scholar] [CrossRef] [PubMed]
- Dombert, B.; Sivadasan, R.; Simon, C.M.; Jablonka, S.; Sendtner, M. Presynaptic localization of Smn and hnRNP R in axon terminals of embryonic and postnatal mouse motoneurons. PLoS ONE 2014, 9, e110846. [Google Scholar] [CrossRef]
- Fallini, C.; Rouanet, J.P.; Donlin-Asp, P.G.; Guo, P.; Zhang, H.; Singer, R.H.; Rossoll, W.; Bassell, G.J. Dynamics of survival of motor neuron (SMN) protein interaction with the mRNA-binding protein IMP1 facilitates its trafficking into motor neuron axons. Dev. Neurobiol. 2014, 74, 319–332. [Google Scholar] [CrossRef]
- Fallini, C.; Donlin-Asp, P.G.; Rouanet, J.P.; Bassell, G.J.; Rossoll, W. Deficiency of the Survival of Motor Neuron Protein Impairs mRNA Localization and Local Translation in the Growth Cone of Motor Neurons. J. Neurosci. 2016, 36, 3811–3820. [Google Scholar] [CrossRef]
- Singh, R.N.; Howell, M.D.; Ottesen, E.W.; Singh, N.N. Diverse role of survival motor neuron protein. Biochim. Biophys. Acta. Gene Regul. Mech. 2017, 1860, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Mattaj, I.W. Cap trimethylation of U snRNA is cytoplasmic and dependent on U snRNP protein binding. Cell 1986, 46, 905–911. [Google Scholar] [CrossRef]
- Fischer, U.; Luhrmann, R. An essential signaling role for the m3G cap in the transport of U1 snRNP to the nucleus. Science 1990, 249, 786–790. [Google Scholar] [CrossRef]
- Hamm, J.; Darzynkiewicz, E.; Tahara, S.M.; Mattaj, I.W. The trimethylguanosine cap structure of U1 snRNA is a component of a bipartite nuclear targeting signal. Cell 1990, 62, 569–577. [Google Scholar] [CrossRef]
- Fischer, U.; Sumpter, V.; Sekine, M.; Satoh, T.; Luhrmann, R. Nucleo-cytoplasmic transport of U snRNPs: Definition of a nuclear location signal in the Sm core domain that binds a transport receptor independently of the m3G cap. EMBO J. 1993, 12, 573–583. [Google Scholar] [CrossRef]
- Palacios, I.; Hetzer, M.; Adam, S.A.; Mattaj, I.W. Nuclear import of U snRNPs requires importin beta. EMBO J. 1997, 16, 6783–6792. [Google Scholar] [CrossRef]
- Huber, J.; Cronshagen, U.; Kadokura, M.; Marshallsay, C.; Wada, T.; Sekine, M.; Luhrmann, R. Snurportin1, an m3G-cap-specific nuclear import receptor with a novel domain structure. EMBO J. 1998, 17, 4114–4126. [Google Scholar] [CrossRef]
- Kambach, C.; Walke, S.; Young, R.; Avis, J.M.; de la Fortelle, E.; Raker, V.A.; Luhrmann, R.; Li, J.; Nagai, K. Crystal structures of two Sm protein complexes and their implications for the assembly of the spliceosomal snRNPs. Cell 1999, 96, 375–387. [Google Scholar] [CrossRef]
- Meister, G.; Buhler, D.; Pillai, R.; Lottspeich, F.; Fischer, U. A multiprotein complex mediates the ATP-dependent assembly of spliceosomal U snRNPs. Nat. Cell Biol. 2001, 3, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Meister, G.; Eggert, C.; Buhler, D.; Brahms, H.; Kambach, C.; Fischer, U. Methylation of Sm proteins by a complex containing PRMT5 and the putative U snRNP assembly factor pICln. Curr. Biol. 2001, 11, 1990–1994. [Google Scholar] [CrossRef]
- Bachand, F.; Boisvert, F.M.; Cote, J.; Richard, S.; Autexier, C. The product of the survival of motor neuron (SMN) gene is a human telomerase-associated protein. Mol. Biol. Cell 2002, 13, 3192–3202. [Google Scholar] [CrossRef] [PubMed]
- Massenet, S.; Pellizzoni, L.; Paushkin, S.; Mattaj, I.W.; Dreyfuss, G. The SMN complex is associated with snRNPs throughout their cytoplasmic assembly pathway. Mol. Cell. Biol. 2002, 22, 6533–6541. [Google Scholar] [CrossRef]
- Narayanan, U.; Ospina, J.K.; Frey, M.R.; Hebert, M.D.; Matera, A.G. SMN, the spinal muscular atrophy protein, forms a pre-import snRNP complex with snurportin1 and importin beta. Hum. Mol. Genet. 2002, 11, 1785–1795. [Google Scholar] [CrossRef]
- Paushkin, S.; Gubitz, A.K.; Massenet, S.; Dreyfuss, G. The SMN complex, an assemblyosome of ribonucleoproteins. Curr. Opin. Cell Biol. 2002, 14, 305–312. [Google Scholar] [CrossRef]
- Mouaikel, J.; Narayanan, U.; Verheggen, C.; Matera, A.G.; Bertrand, E.; Tazi, J.; Bordonne, R. Interaction between the small-nuclear-RNA cap hypermethylase and the spinal muscular atrophy protein, survival of motor neuron. EMBO Rep. 2003, 4, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, U.; Achsel, T.; Luhrmann, R.; Matera, A.G. Coupled in vitro import of U snRNPs and SMN, the spinal muscular atrophy protein. Mol. Cell 2004, 16, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Chari, A.; Golas, M.M.; Klingenhager, M.; Neuenkirchen, N.; Sander, B.; Englbrecht, C.; Sickmann, A.; Stark, H.; Fischer, U. An assembly chaperone collaborates with the SMN complex to generate spliceosomal SnRNPs. Cell 2008, 135, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.; Kasim, M.; Bachorik, J.L.; Wan, L.; Dreyfuss, G. Gemin5 delivers snRNA precursors to the SMN complex for snRNP biogenesis. Mol. Cell 2010, 38, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.; Englbrecht, C.; Chari, A. Biogenesis of spliceosomal small nuclear ribonucleoproteins. Wiley Interdiscip. Rev. RNA 2011, 2, 718–731. [Google Scholar] [CrossRef]
- Makarov, E.M.; Owen, N.; Bottrill, A.; Makarova, O.V. Functional mammalian spliceosomal complex E contains SMN complex proteins in addition to U1 and U2 snRNPs. Nucleic Acids Res. 2012, 40, 2639–2652. [Google Scholar] [CrossRef]
- Borg, R.M.; Fenech Salerno, B.; Vassallo, N.; Bordonne, R.; Cauchi, R.J. Disruption of snRNP biogenesis factors Tgs1 and pICln induces phenotypes that mirror aspects of SMN-Gemins complex perturbation in Drosophila, providing new insights into spinal muscular atrophy. Neurobiol. Dis. 2016, 94, 245–258. [Google Scholar] [CrossRef]
- Wan, L.; Battle, D.J.; Yong, J.; Gubitz, A.K.; Kolb, S.J.; Wang, J.; Dreyfuss, G. The survival of motor neurons protein determines the capacity for snRNP assembly: Biochemical deficiency in spinal muscular atrophy. Mol. Cell Biol. 2005, 25, 5543–5551. [Google Scholar] [CrossRef] [PubMed]
- Gabanella, F.; Butchbach, M.E.; Saieva, L.; Carissimi, C.; Burghes, A.H.; Pellizzoni, L. Ribonucleoprotein assembly defects correlate with spinal muscular atrophy severity and preferentially affect a subset of spliceosomal snRNPs. PLoS ONE 2007, 2, e921. [Google Scholar] [CrossRef]
- Otter, S.; Grimmler, M.; Neuenkirchen, N.; Chari, A.; Sickmann, A.; Fischer, U. A comprehensive interaction map of the human survival of motor neuron (SMN) complex. J. Biol. Chem. 2007, 282, 5825–5833. [Google Scholar] [CrossRef]
- Charroux, B.; Pellizzoni, L.; Perkinson, R.A.; Shevchenko, A.; Mann, M.; Dreyfuss, G. Gemin3: A novel DEAD box protein that interacts with SMN, the spinal muscular atrophy gene product, and is a component of gems. J. Cell Biol. 1999, 147, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Charroux, B.; Pellizzoni, L.; Perkinson, R.A.; Yong, J.; Shevchenko, A.; Mann, M.; Dreyfuss, G. Gemin4. A novel component of the SMN complex that is found in both gems and nucleoli. J. Cell Biol. 2000, 148, 1177–1186. [Google Scholar] [CrossRef]
- Baccon, J.; Pellizzoni, L.; Rappsilber, J.; Mann, M.; Dreyfuss, G. Identification and Characterization of Gemin7, a Novel Component of the Survival of Motor Neuron Complex. J. Biol. Chem. 2002, 277, 31957–31962. [Google Scholar] [CrossRef]
- Pellizzoni, L.; Baccon, J.; Rappsilber, J.; Mann, M.; Dreyfuss, G. Purification of native survival of motor neurons complexes and identification of Gemin6 as a novel component. J. Biol. Chem. 2002, 277, 7540–7545. [Google Scholar] [CrossRef]
- Grimmler, M.; Otter, S.; Peter, C.; Muller, F.; Chari, A.; Fischer, U. Unrip, a factor implicated in cap-independent translation, associates with the cytosolic SMN complex and influences its intracellular localization. Hum. Mol. Genet. 2005, 14, 3099–3111. [Google Scholar] [CrossRef] [PubMed]
- Carissimi, C.; Saieva, L.; Baccon, J.; Chiarella, P.; Maiolica, A.; Sawyer, A.; Rappsilber, J.; Pellizzoni, L. Gemin8 is a novel component of the survival motor neuron complex and functions in small nuclear ribonucleoprotein assembly. J. Biol. Chem. 2006, 281, 8126–8134. [Google Scholar] [CrossRef]
- Carissimi, C.; Saieva, L.; Gabanella, F.; Pellizzoni, L. Gemin8 is required for the architecture and function of the survival motor neuron complex. J. Biol. Chem. 2006, 281, 37009–37016. [Google Scholar] [CrossRef]
- Liu, Q.; Fischer, U.; Wang, F.; Dreyfuss, G. The spinal muscular atrophy disease gene product, SMN, and its associated protein SIP1 are in a complex with spliceosomal snRNP proteins. Cell 1997, 90, 1013–1021. [Google Scholar] [CrossRef]
- Lorson, C.L.; Androphy, E.J. The domain encoded by exon 2 of the survival motor neuron protein mediates nucleic acid binding. Hum. Mol. Genet. 1998, 7, 1269–1275. [Google Scholar] [CrossRef][Green Version]
- Ogawa, C.; Usui, K.; Aoki, M.; Ito, F.; Itoh, M.; Kai, C.; Kanamori-Katayama, M.; Hayashizaki, Y.; Suzuki, H. Gemin2 plays an important role in stabilizing the survival of motor neuron complex. J. Biol. Chem. 2007, 282, 11122–11134. [Google Scholar] [CrossRef]
- Jablonka, S.; Rossoll, W.; Schrank, B.; Sendtner, M. The role of SMN in spinal muscular atrophy. J. Neurol. 2000, 247 (Suppl. S1), I37–142. [Google Scholar] [CrossRef] [PubMed]
- Rossoll, W.; Jablonka, S.; Andreassi, C.; Kroning, A.K.; Karle, K.; Monani, U.R.; Sendtner, M. Smn, the spinal muscular atrophy-determining gene product, modulates axon growth and localization of beta-actin mRNA in growth cones of motoneurons. J. Cell Biol. 2003, 163, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Piazzon, N.; Rage, F.; Schlotter, F.; Moine, H.; Branlant, C.; Massenet, S. In vitro and in cellulo evidences for association of the survival of motor neuron complex with the fragile X mental retardation protein. J. Biol. Chem. 2008, 283, 5598–5610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xing, L.; Rossoll, W.; Wichterle, H.; Singer, R.H.; Bassell, G.J. Multiprotein complexes of the survival of motor neuron protein SMN with Gemins traffic to neuronal processes and growth cones of motor neurons. J. Neurosci. 2006, 26, 8622–8632. [Google Scholar] [CrossRef]
- Battle, D.J.; Kasim, M.; Wang, J.; Dreyfuss, G. SMN-independent subunits of the SMN complex. Identification of a small nuclear ribonucleoprotein assembly intermediate. J. Biol. Chem. 2007, 282, 27953–27959. [Google Scholar] [CrossRef]
- Martinez-Salas, E.; Embarc-Buh, A.; Francisco-Velilla, R. Emerging Roles of Gemin5: From snRNPs Assembly to Translation Control. Int. J. Mol. Sci 2020, 21, 3868. [Google Scholar] [CrossRef]
- Keenan, R.J.; Freymann, D.M.; Stroud, R.M.; Walter, P. The signal recognition particle. Annu. Rev. Biochem. 2001, 70, 755–775. [Google Scholar] [CrossRef]
- Cross, B.C.; Sinning, I.; Luirink, J.; High, S. Delivering proteins for export from the cytosol. Nat. Rev. Mol. Cell Biol. 2009, 10, 255–264. [Google Scholar] [CrossRef]
- Saraogi, I.; Shan, S.O. Molecular mechanism of co-translational protein targeting by the signal recognition particle. Traffic 2011, 12, 535–542. [Google Scholar] [CrossRef]
- Peter, C.J.; Evans, M.; Thayanithy, V.; Taniguchi-Ishigaki, N.; Bach, I.; Kolpak, A.; Bassell, G.J.; Rossoll, W.; Lorson, C.L.; Bao, Z.Z.; et al. The COPI vesicle complex binds and moves with survival motor neuron within axons. Hum. Mol. Genet. 2011, 20, 1701–1711. [Google Scholar] [CrossRef]
- Fan, L.; Simard, L.R. Survival motor neuron (SMN) protein: Role in neurite outgrowth and neuromuscular maturation during neuronal differentiation and development. Hum. Mol. Genet. 2002, 11, 1605–1614. [Google Scholar] [CrossRef]
- Custer, S.K.; Todd, A.G.; Singh, N.N.; Androphy, E.J. Dilysine motifs in exon 2b of SMN protein mediate binding to the COPI vesicle protein alpha-COP and neurite outgrowth in a cell culture model of spinal muscular atrophy. Hum. Mol. Genet. 2013, 22, 4043–4052. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Custer, S.K.; Gilson, T.; Hao, L.T.; Beattie, C.E.; Androphy, E.J. alpha-COP binding to the survival motor neuron protein SMN is required for neuronal process outgrowth. Hum. Mol. Genet. 2015, 24, 7295–7307. [Google Scholar] [CrossRef]
- Custer, S.K.; Astroski, J.W.; Li, H.X.; Androphy, E.J. Interaction between alpha-COP and SMN ameliorates disease phenotype in a mouse model of spinal muscular atrophy. Biochem. Biophys. Res. Commun. 2019, 514, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Prescott, A.R.; Bales, A.; James, J.; Trinkle-Mulcahy, L.; Sleeman, J.E. Time-resolved quantitative proteomics implicates the core snRNP protein SmB together with SMN in neural trafficking. J. Cell Sci. 2014, 127, 812–827. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.T.; Duy, P.Q.; An, M.; Talbot, J.; Iyer, C.C.; Wolman, M.; Beattie, C.E. HuD and the Survival Motor Neuron Protein Interact in Motoneurons and Are Essential for Motoneuron Development, Function, and mRNA Regulation. J. Neurosci. 2017, 37, 11559–11571. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dreyfuss, G. A cell system with targeted disruption of the SMN gene: Functional conservation of the SMN protein and dependence of Gemin2 on SMN. J. Biol. Chem. 2001, 276, 9599–9605. [Google Scholar] [CrossRef] [PubMed]
- Li, D.K.; Tisdale, S.; Espinoza-Derout, J.; Saieva, L.; Lotti, F.; Pellizzoni, L. A cell system for phenotypic screening of modifiers of SMN2 gene expression and function. PLoS ONE 2013, 8, e71965. [Google Scholar] [CrossRef]
- Pacheco, A.; Lopez de Quinto, S.; Ramajo, J.; Fernandez, N.; Martinez-Salas, E. A novel role for Gemin5 in mRNA translation. Nucleic Acids Res. 2009, 37, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Workman, E.; Kalda, C.; Patel, A.; Battle, D.J. Gemin5 Binds to the Survival Motor Neuron mRNA to Regulate SMN Expression. J. Biol. Chem. 2015, 290, 15662–15669. [Google Scholar] [CrossRef] [PubMed]
- Francisco-Velilla, R.; Fernandez-Chamorro, J.; Ramajo, J.; Martinez-Salas, E. The RNA-binding protein Gemin5 binds directly to the ribosome and regulates global translation. Nucleic Acids Res. 2016, 44, 8335–8351. [Google Scholar] [CrossRef] [PubMed]
- Simsek, D.; Tiu, G.C.; Flynn, R.A.; Byeon, G.W.; Leppek, K.; Xu, A.F.; Chang, H.Y.; Barna, M. The Mammalian Ribo-interactome Reveals Ribosome Functional Diversity and Heterogeneity. Cell 2017, 169, 1051–1065.e18. [Google Scholar] [CrossRef] [PubMed]
- Francisco-Velilla, R.; Fernandez-Chamorro, J.; Dotu, I.; Martinez-Salas, E. The landscape of the non-canonical RNA-binding site of Gemin5 unveils a feedback loop counteracting the negative effect on translation. Nucleic Acids Res. 2018, 46, 7339–7353. [Google Scholar] [CrossRef]
- Hensel, N.; Cieri, F.; Santonicola, P.; Tapken, I.; Schuning, T.; Taiana, M.; Pagliari, E.; Joseph, A.; Fischer, S.; Heidrich, N.; et al. Impairment of the neurotrophic signaling hub B-Raf contributes to motoneuron degeneration in spinal muscular atrophy. Proc. Natl. Acad. Sci. USA 2021, 118, 518. [Google Scholar] [CrossRef] [PubMed]
- Wiese, S.; Pei, G.; Karch, C.; Troppmair, J.; Holtmann, B.; Rapp, U.R.; Sendtner, M. Specific function of B-Raf in mediating survival of embryonic motoneurons and sensory neurons. Nat. Neurosci. 2001, 4, 137–142. [Google Scholar] [CrossRef]
- Zhang, Z.; Pinto, A.M.; Wan, L.; Wang, W.; Berg, M.G.; Oliva, I.; Singh, L.N.; Dengler, C.; Wei, Z.; Dreyfuss, G. Dysregulation of synaptogenesis genes antecedes motor neuron pathology in spinal muscular atrophy. Proc. Natl. Acad. Sci. USA 2013, 110, 19348–19353. [Google Scholar] [CrossRef]
- Nichterwitz, S.; Nijssen, J.; Storvall, H.; Schweingruber, C.; Comley, L.H.; Allodi, I.; Lee, M.V.; Deng, Q.; Sandberg, R.; Hedlund, E. LCM-seq reveals unique transcriptional adaptation mechanisms of resistant neurons and identifies protective pathways in spinal muscular atrophy. Genome Res. 2020, 30, 1083–1096. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, F.; Nizzardo, M.; Vashisht, S.; Molteni, E.; Melzi, V.; Taiana, M.; Salani, S.; Santonicola, P.; Di Schiavi, E.; Bucchia, M.; et al. Key role of SMN/SYNCRIP and RNA-Motif 7 in spinal muscular atrophy: RNA-Seq and motif analysis of human motor neurons. Brain 2019, 142, 276–294. [Google Scholar] [CrossRef] [PubMed]
- Bernal, S.; Alias, L.; Barcelo, M.J.; Also-Rallo, E.; Martinez-Hernandez, R.; Gamez, J.; Guillen-Navarro, E.; Rosell, J.; Hernando, I.; Rodriguez-Alvarez, F.J.; et al. The c.859G>C variant in the SMN2 gene is associated with types II and III SMA and originates from a common ancestor. J. Med. Genet. 2010, 47, 640–642. [Google Scholar] [CrossRef]
- Vezain, M.; Saugier-Veber, P.; Goina, E.; Touraine, R.; Manel, V.; Toutain, A.; Fehrenbach, S.; Frebourg, T.; Pagani, F.; Tosi, M.; et al. A rare SMN2 variant in a previously unrecognized composite splicing regulatory element induces exon 7 inclusion and reduces the clinical severity of spinal muscular atrophy. Hum. Mutat 2010, 31, E1110–E1125. [Google Scholar] [CrossRef] [PubMed]
- Prior, T.W.; Krainer, A.R.; Hua, Y.; Swoboda, K.J.; Snyder, P.C.; Bridgeman, S.J.; Burghes, A.H.; Kissel, J.T. A positive modifier of spinal muscular atrophy in the SMN2 gene. Am. J. Hum. Genet. 2009, 85, 408–413. [Google Scholar] [CrossRef]
- Wu, X.; Wang, S.H.; Sun, J.; Krainer, A.R.; Hua, Y.; Prior, T.W. A-44G transition in SMN2 intron 6 protects patients with spinal muscular atrophy. Hum. Mol. Genet. 2017, 26, 2768–2780. [Google Scholar] [CrossRef] [PubMed]
- Cobben, J.M.; van der Steege, G.; Grootscholten, P.; de Visser, M.; Scheffer, H.; Buys, C.H. Deletions of the survival motor neuron gene in unaffected siblings of patients with spinal muscular atrophy. Am. J. Hum. Genet. 1995, 57, 805–808. [Google Scholar]
- Di Donato, C.J.; Ingraham, S.E.; Mendell, J.R.; Prior, T.W.; Lenard, S.; Moxley, R.T., 3rd; Florence, J.; Burghes, A.H. Deletion and conversion in spinal muscular atrophy patients: Is there a relationship to severity? Ann. Neurol. 1997, 41, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Cusco, I.; Barcelo, M.J.; Rojas-Garcia, R.; Illa, I.; Gamez, J.; Cervera, C.; Pou, A.; Izquierdo, G.; Baiget, M.; Tizzano, E.F. SMN2 copy number predicts acute or chronic spinal muscular atrophy but does not account for intrafamilial variability in siblings. J. Neurol. 2006, 253, 21–25. [Google Scholar] [CrossRef]
- Jedrzejowska, M.; Borkowska, J.; Zimowski, J.; Kostera-Pruszczyk, A.; Milewski, M.; Jurek, M.; Sielska, D.; Kostyk, E.; Nyka, W.; Zaremba, J.; et al. Unaffected patients with a homozygous absence of the SMN1 gene. Eur. J. Hum. Genet. 2008, 16, 930–934. [Google Scholar] [CrossRef][Green Version]
- Oprea, G.E.; Krober, S.; McWhorter, M.L.; Rossoll, W.; Muller, S.; Krawczak, M.; Bassell, G.J.; Beattie, C.E.; Wirth, B. Plastin 3 is a protective modifier of autosomal recessive spinal muscular atrophy. Science 2008, 320, 524–527. [Google Scholar] [CrossRef]
- Pane, M.; Lapenta, L.; Abiusi, E.; de Sanctis, R.; Luigetti, M.; Palermo, C.; Ranalli, D.; Fiori, S.; Tiziano, F.D.; Mercuri, E. Longitudinal assessments in discordant twins with SMA. Neuromuscul. Disord. NMD 2017, 27, 890–893. [Google Scholar] [CrossRef]
- Bernal, S.; Also-Rallo, E.; Martinez-Hernandez, R.; Alias, L.; Rodriguez-Alvarez, F.J.; Millan, J.M.; Hernandez-Chico, C.; Baiget, M.; Tizzano, E.F. Plastin 3 expression in discordant spinal muscular atrophy (SMA) siblings. Neuromuscul. Disord. 2011, 21, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, B.; Krober, S.; Torres-Benito, L.; Borgmann, A.; Peters, M.; Hosseini Barkooie, S.M.; Tejero, R.; Jakubik, M.; Schreml, J.; Milbradt, J.; et al. Plastin 3 ameliorates spinal muscular atrophy via delayed axon pruning and improves neuromuscular junction functionality. Hum. Mol. Genet. 2013, 22, 1328–1347. [Google Scholar] [CrossRef] [PubMed]
- McGovern, V.L.; Massoni-Laporte, A.; Wang, X.; Le, T.T.; Le, H.T.; Beattie, C.E.; Rich, M.M.; Burghes, A.H. Plastin 3 Expression Does Not Modify Spinal Muscular Atrophy Severity in the 7 SMA Mouse. PLoS ONE 2015, 10, e0132364. [Google Scholar] [CrossRef] [PubMed]
- Delanote, V.; Vandekerckhove, J.; Gettemans, J. Plastins: Versatile modulators of actin organization in (patho)physiological cellular processes. Acta Pharm. Sin. 2005, 26, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Engqvist-Goldstein, A.E.; Drubin, D.G. Actin assembly and endocytosis: From yeast to mammals. Annu. Rev. Cell Dev. Biol. 2003, 19, 287–332. [Google Scholar] [CrossRef]
- Crooke, S.T.; Wang, S.; Vickers, T.A.; Shen, W.; Liang, X.H. Cellular uptake and trafficking of antisense oligonucleotides. Nat. Biotechnol. 2017, 35, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Szkandera, J.; Winder, T.; Stotz, M.; Weissmueller, M.; Langsenlehner, T.; Pichler, M.; Samonigg, H.; Renner, W.; Gerger, A.; Absenger, G. A common gene variant in PLS3 predicts colon cancer recurrence in women. Tumour. Biol. 2013, 34, 2183–2188. [Google Scholar] [CrossRef] [PubMed]
- Riessland, M.; Kaczmarek, A.; Schneider, S.; Swoboda, K.J.; Lohr, H.; Bradler, C.; Grysko, V.; Dimitriadi, M.; Hosseinibarkooie, S.; Torres-Benito, L.; et al. Neurocalcin Delta Suppression Protects against Spinal Muscular Atrophy in Humans and across Species by Restoring Impaired Endocytosis. Am. J. Hum. Genet. 2017, 100, 297–315. [Google Scholar] [CrossRef]
- Chang, H.C.; Dimlich, D.N.; Yokokura, T.; Mukherjee, A.; Kankel, M.W.; Sen, A.; Sridhar, V.; Fulga, T.A.; Hart, A.C.; Van Vactor, D.; et al. Modeling spinal muscular atrophy in Drosophila. PLoS ONE 2008, 3, e3209. [Google Scholar] [CrossRef]
- de Carlos Caceres, I.; Porto, D.A.; Gallotta, I.; Santonicola, P.; Rodriguez-Cordero, J.; Di Schiavi, E.; Lu, H. Automated screening of C. elegans neurodegeneration mutants enabled by microfluidics and image analysis algorithms. Integr. Biol. 2018, 10, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadi, M.; Sleigh, J.N.; Walker, A.; Chang, H.C.; Sen, A.; Kalloo, G.; Harris, J.; Barsby, T.; Walsh, M.B.; Satterlee, J.S.; et al. Conserved genes act as modifiers of invertebrate SMN loss of function defects. PLoS Genet. 2010, 6, e1001172. [Google Scholar] [CrossRef]
- Ning, K.; Drepper, C.; Valori, C.F.; Ahsan, M.; Wyles, M.; Higginbottom, A.; Herrmann, T.; Shaw, P.; Azzouz, M.; Sendtner, M. PTEN depletion rescues axonal growth defect and improves survival in SMN-deficient motor neurons. Hum. Mol. Genet. 2010, 19, 3159–3168. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Dimlich, D.N.; Guruharsha, K.G.; Kankel, M.W.; Hori, K.; Yokokura, T.; Brachat, S.; Richardson, D.; Loureiro, J.; Sivasankaran, R.; et al. Genetic circuitry of Survival motor neuron, the gene underlying spinal muscular atrophy. Proc. Natl. Acad. Sci. USA 2013, 110, E2371–E2380. [Google Scholar] [CrossRef]
- Chong, J.X.; Oktay, A.A.; Dai, Z.; Swoboda, K.J.; Prior, T.W.; Ober, C. A common spinal muscular atrophy deletion mutation is present on a single founder haplotype in the US Hutterites. Eur. J. Hum. Genet. 2011, 19, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).