Cancer Cachexia: Its Mechanism and Clinical Significance
Abstract
:1. Introduction
2. Definition and Diagnostic Criteria for Cachexia
2.1. Definition and Diagnostic Criteria
2.2. Difficulties for the Use of Cachexia in Daily Clinical Practice
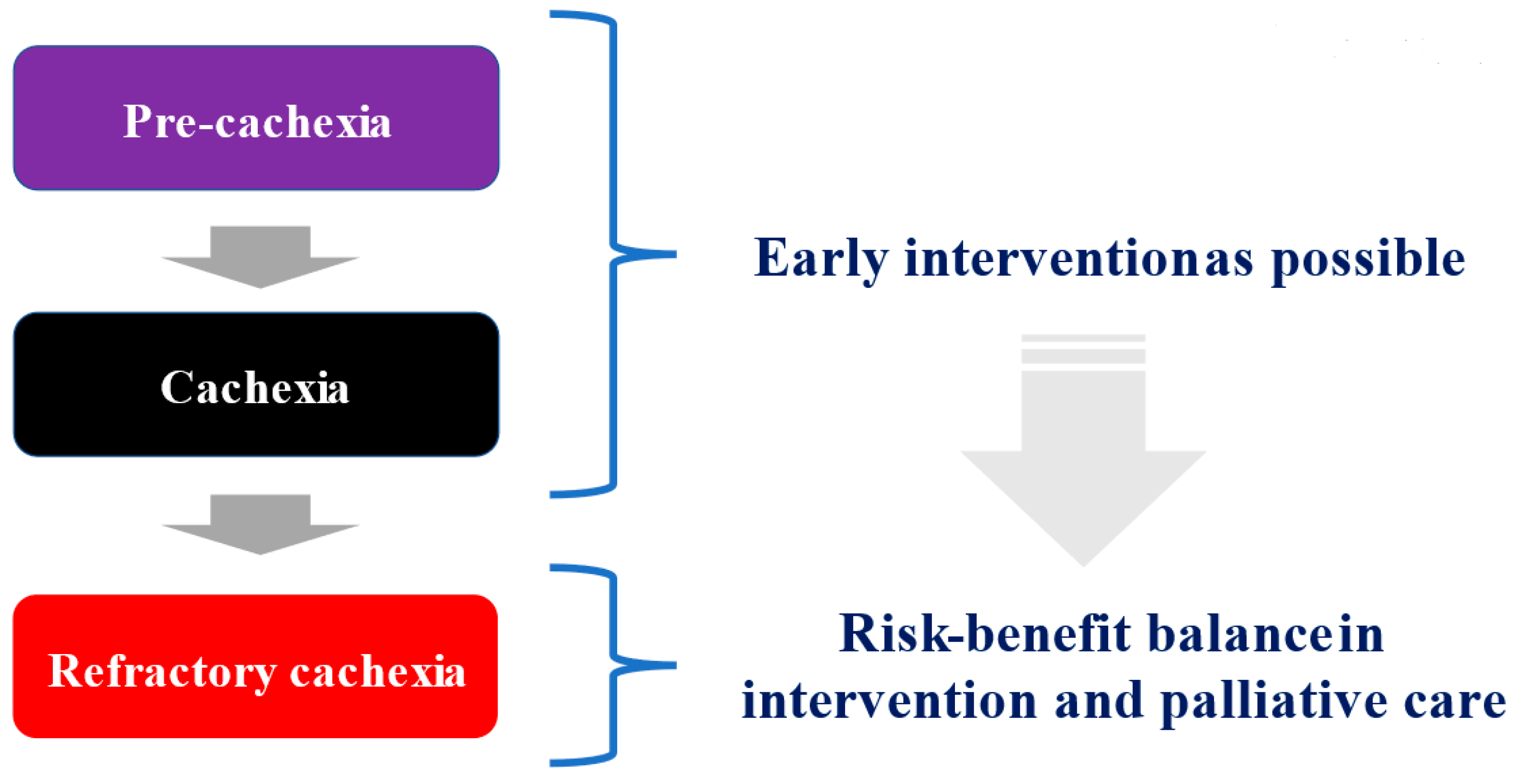
3. Three Stages in Cancer Cachexia
3.1. Pre-Cachexia, Cachexia, and Refractory Cachexia
3.2. Treatment Strategies
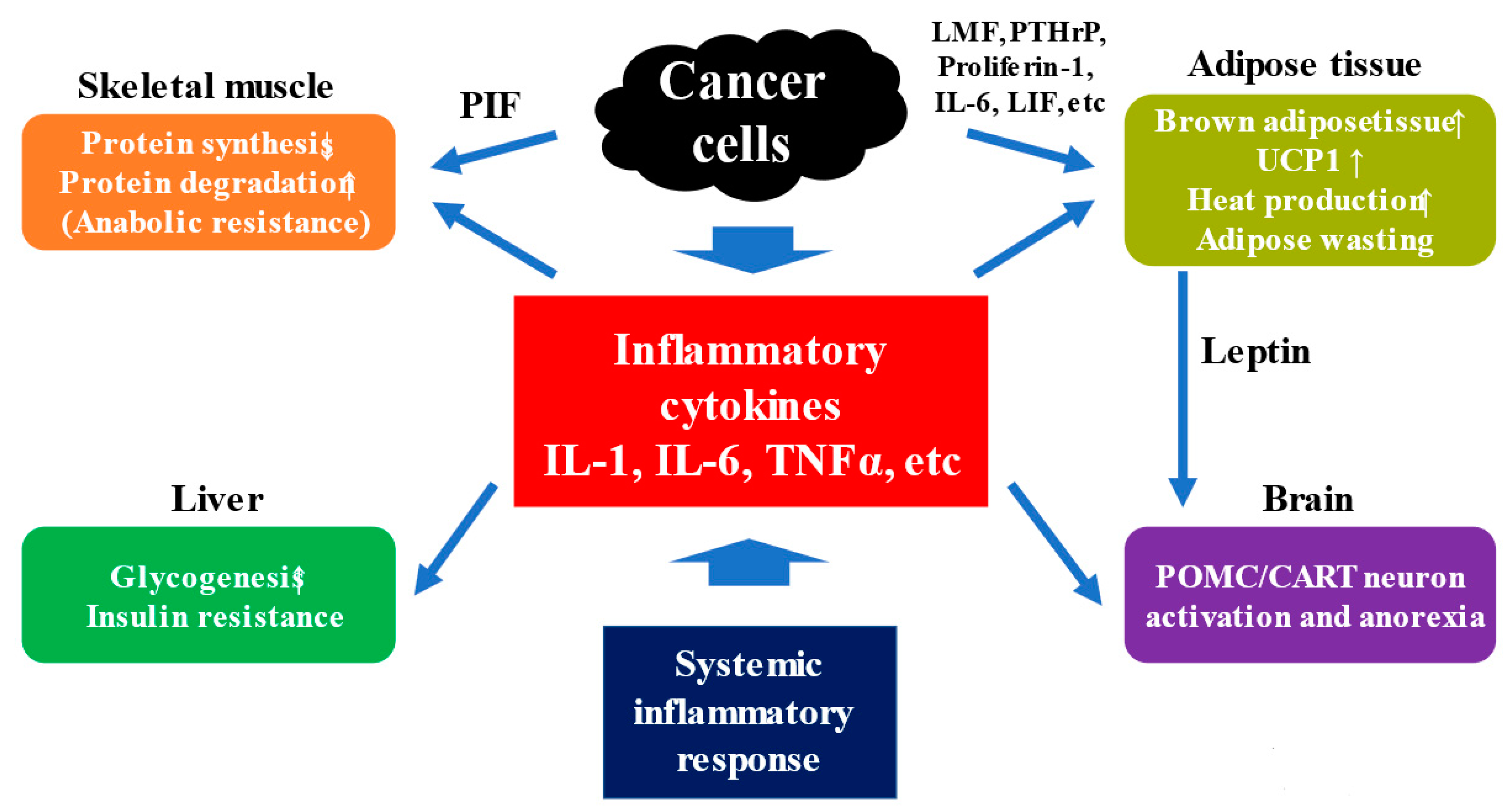
4. Anabolic Resistance and Mechanism for Cachexia
4.1. Clinical Features for Anabolic Resistance
4.2. Mechanism for Cachexia
4.3. Anorexia and Appetite-Related Hormones in Cancer Cachexia
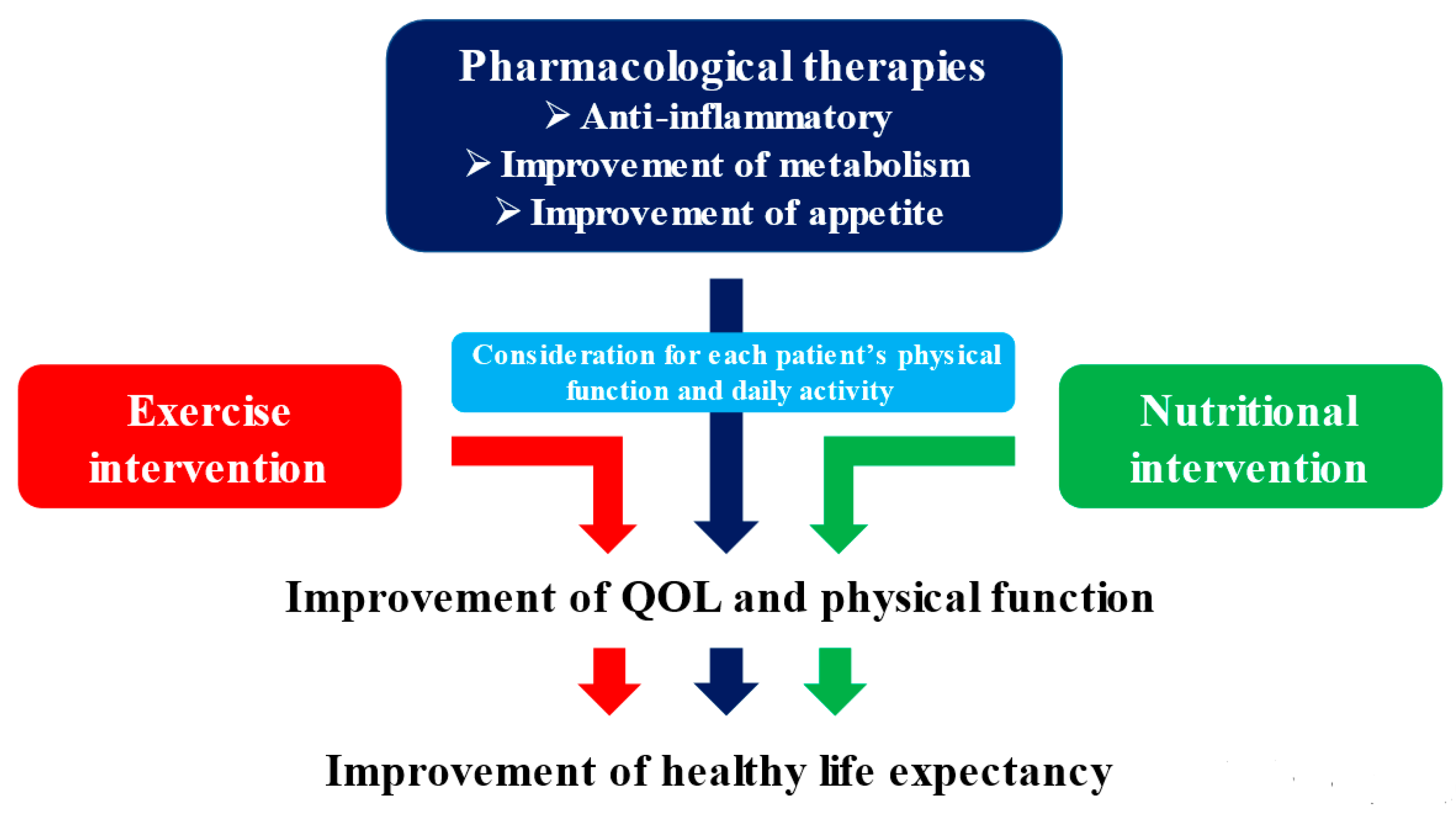
5. Non-Pharmacological Therapies for Cancer Cachexia
5.1. Clinical Evidence for the Treatment of Cancer Cachexia
5.2. Energy Metabolism and Nutritional Support in Cancer Cachexia
6. Pharmacological Therapies for Cancer Cachexia
6.1. Anamorelin
6.2. Enobasarm, MABp1, and Others
7. Multidisciplinary Intervention Model
8. Final Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| REE | resting energy expenditure |
| BMI | body mass index |
| ESPEN | European Society of Clinical Nutrition and Metabolism |
| CSS | cachexia staging score |
| ESMO | European Society of Medical Oncology |
| CRP | C-reactive protein |
| GPS | Glasgow prognostic score |
| PTHrP | parathyroid hormone-related protein |
| UCP1 | uncoupling protein 1 |
| NPY | neuropeptide Y |
| AgRP | agouti gene-related protein |
| POMC | proopiomelanocortin |
| CART | cocaine and amphetamine-regulated transcript |
| RCTs | randomized controlled trials |
| TEE | total energy expenditure |
| AEE | activity energy expenditure |
| BEE | basal energy expenditure |
| DIE | diet-induced thermogenesis |
| ONS | oral nutritional support |
| LBM | lean body mass |
| GH | growth hormone |
References
- Bennani-Baiti, N.; Walsh, D. What is cancer anorexia-cachexia syndrome? A historical perspective. J. R. Coll. Physicians Edinb. 2009, 39, 257–262. [Google Scholar]
- Muscaritoli, M.; Anker, S.D.; Argilés, J.; Aversa, Z.; Bauer, J.M.; Biolo, G.; Boirie, Y.; Bosaeus, I.; Cederholm, T.; Costelli, P.; et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin. Nutr. 2010, 29, 154–159. [Google Scholar] [CrossRef]
- Argiles, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef]
- Zagzag, J.; Hu, M.I.; Fisher, S.B.; Perrier, N.D. Hypercalcemia and cancer: Differential diagnosis and treatment. Cancer J. Clin. 2018, 68, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Tisdale, M.J. Pathogenesis of cancer cachexia. J. Support. Oncol. 2003, 1, 159–168. [Google Scholar]
- Fearon, K.C.; Glass, D.J.; Guttridge, D.C. Cancer cachexia: Mediators, signaling, and metabolic pathways. Cell. Metab. 2012, 16, 153–166. [Google Scholar] [CrossRef] [Green Version]
- Petruzzelli, M.; Wagner, E.F. Mechanisms of metabolic dysfunction in cancer-associated cachexia. Genes Dev. 2016, 30, 489–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, I.H. Summary comments. Am. J. Clin. Nutr. 1989, 50, 1121–1235. [Google Scholar] [CrossRef]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Moriya, K.; Hino, K.; Nishiguchi, S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016, 46, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sar-copenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J.; Morley, J.E.; Argilés, J.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G.; et al. Cachexia: A new definition. Clin. Nutr. 2008, 27, 793–799. [Google Scholar] [CrossRef] [PubMed]
- ter Beek, L.; Vanhauwaert, E.; Slinde, F.; Orrevall, Y.; Henriksen, C.; Johansson, M.; Vereecken, C.; Rothenberg, E.; Jager-Wittenaar, H. Unsatisfactory knowledge and use of terminology regarding malnutrition, starvation, cachexia and sarcopenia among dietitians. Clin. Nutr. 2016, 35, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Arai, H.; Inui, A. The regulatory approval of anamorelin for treatment of cachexia in patients with non-small cell lung cancer, gastric cancer, pancreatic cancer, and colorectal cancer in Japan: Facts and numbers. J. Cachexia Sarcopenia Muscle 2021, 12, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Rausch, V.; Sala, V.; Penna, F.; Porporato, P.E.; Ghigo, A. Understanding the common mechanisms of heart and skeletal muscle wasting in cancer cachexia. Oncogene 2021, 10, 1–13. [Google Scholar] [CrossRef]
- Roeland, E.J.; Bohlke, K.; Baracos, V.E.; Bruera, E.; Del Fabbro, E.; Dixon, S.; Fallon, M.; Herrstedt, J.; Lau, H.; Platek, M.; et al. Management of Cancer Cachexia: ASCO Guideline. J. Clin. Oncol. 2020, 38, 2438–2453. [Google Scholar] [CrossRef]
- Farkas, J.; von Haehling, S.; Kalantar-Zadeh, K.; Morley, J.E.; Anker, S.D.; Lainscak, M. Cachexia as a major public health problem: Frequent, costly, and deadly. J. Cachex-Sarcopenia Muscle 2013, 4, 173–178. [Google Scholar] [CrossRef]
- Caillet, P.; Liuu, E.; Simon, A.R.; Bonnefoy, M.; Guerin, O.; Berrut, G.; Lesourd, B.; Jeandel, C.; Ferry, M.; Rolland, Y.; et al. Association between cachexia, chemotherapy and outcomes in older cancer patients: A systematic review. Clin. Nutr. 2017, 36, 1473–1482. [Google Scholar] [CrossRef]
- Nishikawa, H.; Kita, R.; Kimura, T.; Endo, M.; Ohara, Y.; Sakamoto, A.; Saito, S.; Nishijima, N.; Nasu, A.; Komekado, H.; et al. Proposal of the performance status combined Japan Integrated Staging system in hepatocellular carcinoma complicated with cirrhosis. Int. J. Oncol. 2015, 46, 2371–2379. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.; Senesse, P.; Gioulbasanis, I.; Antoun, S.; Bozzetti, F.; Deans, C.; Strasser, F.; Thoresen, L.; Jagoe, R.T.; Chasen, M.; et al. Diagnostic Criteria for the Classification of Cancer-Associated Weight Loss. J. Clin. Oncol. 2015, 33, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Chasen, M.R.; Bhargava, R. A descriptive review of the factors contributing to nutritional compromise in patients with head and neck cancer. Support. Care Cancer 2009, 17, 1345–1351. [Google Scholar] [CrossRef]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Wang, B.; Liu, H.; Yang, K.; Thapa, S.; Zhang, H.; Li, L.; Yu, S. Development and validation of a clinically applicable score to classify cachexia stages in advanced cancer patients. J. Cachex-Sarcopenia Muscle 2018, 9, 306–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malmstrom, T.K.; Miller, D.K.; Simonsick, E.M.; Ferrucci, L.; Morley, J.E. SARC-F: A symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J. Cachex-Sarcopenia Muscle 2015, 7, 28–36. [Google Scholar] [CrossRef]
- Arends, J.; Strasser, F.; Gonella, S.; Solheim, T.S.; Madeddu, C.; Ravasco, P.; Buonaccorso, L.; de van der Schueren, M.A.E.; Baldwin, C.; Chasen, M.; et al. Cancer Cachexia in Adult Patients: ESMO Clinical Practice Guidelines. Available online: https://www.esmo.org/guidelines/supportive-and-palliative-care/cancer-cachexia-in-adult-patients (accessed on 2 June 2021).
- Cederholm, T.; Jensen, G.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2018, 38, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishikawa, H.; Enomoto, H.; Iwata, Y.; Nishimura, T.; Iijima, H.; Nishiguchi, S. Clinical utility of bioimpedance analysis in liver cirrhosis. J. Hepato-Biliary-Pancreat. Sci. 2017, 24, 409–416. [Google Scholar] [CrossRef]
- Nishikawa, H.; Enomoto, H.; Nishiguchi, S.; Iijima, H. Sarcopenic Obesity in Liver Cirrhosis: Possible Mechanism and Clinical Impact. Int. J. Mol. Sci. 2021, 22, 1917. [Google Scholar] [CrossRef]
- Aapro, M.; Arends, J.; Bozzetti, F.; Fearon, K.; Grunberg, S.M.; Herrstedt, J.; Hopkinson, J.; Jacquelin-Ravel, N.; Jatoi, A.; Kaasa, S.; et al. Early recognition of malnutrition and cachexia in the cancer patient: A position paper of a European School of Oncology Task Force. Ann. Oncol. 2014, 25, 1492–1499. [Google Scholar] [CrossRef]
- Kasvis, P.; Vigano, M.; Vigano, A. Health-related quality of life across cancer cachexia stages. Ann. Palliat. Med. 2019, 8, 33–42. [Google Scholar] [CrossRef]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.; Deutz, N.; Erickson, N.; Laviano, A.; Lisanti, M.; Lobo, D.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef] [Green Version]
- Penafuerte, C.A.; Gagnon, B.; Sirois, J.; Murphy, J.; MacDonald, N.; Tremblay, M.L. Identification of neutrophil-derived pro-teases and angiotensin II as biomarkers of cancer cachexia. Br. J. Cancer 2016, 114, 680–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuhls, H.; Marinova, M.; Kaasa, S.; Stieber, C.; Conrad, R.; Radbruch, L.; Mücke, M. A systematic review on the role of vitamins, minerals, proteins, and other supplements for the treatment of cachexia in cancer: A European Palliative Care Research Centre cachexia project. J. Cachexia Sarcopenia Muscle 2017, 8, 25–39. [Google Scholar]
- Montalvo, R.N.; Hardee, J.P.; Vanderveen, B.N.; Carson, J. Resistance Exercise’s Ability to Reverse Cancer-Induced Anabolic Resistance. Exerc. Sport Sci. Rev. 2018, 46, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Antoun, S.; Raynard, B. Muscle protein anabolism in advanced cancer patients: Response to protein and amino acids support, and to physical activity. Ann. Oncol. 2018, 29, ii10–ii17. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, T.; Terracina, K.P.; Raza, A.; Matsubara, H.; Takabe, K. Cancer cachexia, mechanism and treatment. World J. Gastrointest. Oncol. 2015, 7, 17–29. [Google Scholar] [CrossRef]
- Deans, C.; Wigmore, S.J. Systemic inflammation, cachexia and prognosis in patients with cancer. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 265–269. [Google Scholar] [CrossRef]
- McMillan, D. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat. Rev. 2013, 39, 534–540. [Google Scholar] [CrossRef]
- Tong, T.; Guan, Y.; Xiong, H.; Wang, L.; Pang, J. A Meta-Analysis of Glasgow Prognostic Score and Modified Glasgow Prognostic Score as Biomarkers for Predicting Survival Outcome in Renal Cell Carcinoma. Front. Oncol. 2020, 10, 1541. [Google Scholar] [CrossRef]
- Tisdale, M.J. Mechanisms of Cancer Cachexia. Physiol. Rev. 2009, 89, 381–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirza, K.; Tisdale, M.J. Functional identity of receptors for proteolysis-inducing factor on human and murine skeletal muscle. Br. J. Cancer 2014, 111, 903–908. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Lu, J.-B.; Wu, B.; Hao, L.-Y. Expression and Clinicopathologic Significance of Proteolysis-Inducing Factor in Non–Small-Cell Lung Cancer: An Immunohistochemical Analysis. Clin. Lung Cancer 2010, 11, 346–351. [Google Scholar] [CrossRef]
- Argilés, J.M.; López-Soriano, F.J.; Busquets, S. Mediators of cachexia in cancer patients. Nutrition 2019, 66, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Aniort, J.; Stella, A.; Philipponnet, C.; Poyet, A.; Polge, C.; Claustre, A.; Combaret, L.; Béchet, D.; Attaix, D.; Boisgard, S.; et al. Muscle wasting in patients with end-stage renal disease or ear-ly-stage lung cancer: Common mechanisms at work. J. Cachexia Sarcopenia Muscle 2019, 10, 323–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akash, M.S.H.; Rehman, K.; Liaqat, A. Tumor Necrosis Factor-Alpha: Role in Development of Insulin Resistance and Patho-genesis of Type 2 Diabetes Mellitus. J. Cell Biochem. 2018, 119, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.F. Hypercalcemia Associated with Cancer. N. Engl. J. Med. 2005, 352, 373–379. [Google Scholar] [CrossRef]
- Zhang, R.; Li, J.; Assaker, G.; Camirand, A.; Sabri, S.; Karaplis, A.C.; Kremer, R. Parathyroid Hormone-Related Protein (PTHrP): An Emerging Target in Cancer Progression and Metastasis. Adv. Exp. Med. Biol. 2019, 1164, 161–178. [Google Scholar] [CrossRef]
- Petruzzelli, M.; Schweiger, M.; Schreiber, R.; Campos-Olivas, R.; Tsoli, M.; Allen, J.; Swarbrick, M.; Rose-John, S.; Rincon, M.; Robertson, G.; et al. A Switch from White to Brown Fat Increases Energy Expenditure in Cancer-Associated Cachexia. Cell Metab. 2014, 20, 433–447. [Google Scholar] [CrossRef] [Green Version]
- Scheele, C.; Wolfrum, C. Brown Adipose Crosstalk in Tissue Plasticity and Human Metabolism. Endocr. Rev. 2019, 41, 53–65. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Cheng, Q.; Wang, Y.; Leung, P.S.; Mak, K.K. Hedgehog signaling in bone regulates whole-body energy metabolism through a bone–adipose endocrine relay mediated by PTHrP and adiponectin. Cell Death Differ. 2016, 24, 225–237. [Google Scholar] [CrossRef] [Green Version]
- Argiles, J.M.; Stemmler, B.; López-Soriano, F.J.; Busquets, S. Inter-tissue communication in cancer cachexia. Nat. Rev. Endocrinol. 2018, 15, 9–20. [Google Scholar] [CrossRef]
- Iyengar, N.M.; Gucalp, A.; Dannenberg, A.J.; Hudis, C.A. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J. Clin. Oncol. 2016, 34, 4270–4276. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Miyatake, Y.; Yoshida, T.; Kawahara, H.; Hanayama, R. Tumor-secreted proliferin-1 regulates adipogenesis and lipolysis in cachexia. Int. J. Cancer 2020, 148, 1982–1992. [Google Scholar] [CrossRef]
- Rupert, J.E.; Narasimhan, A.; Jengelley, D.H.; Jiang, Y.; Liu, J.; Au, E.; Silverman, L.M.; Sandusky, G.; Bonetto, A.; Cao, S.; et al. Tumor-derived IL-6 and trans-signaling among tumor, fat, and muscle mediate pancreatic cancer cachexia. J. Exp. Med. 2021, 218. [Google Scholar] [CrossRef]
- Sun, X.; Feng, X.; Wu, X.; Lu, Y.; Chen, K.; Ye, Y. Fat Wasting Is Damaging: Role of Adipose Tissue in Cancer-Associated Cachexia. Front. Cell Dev. Biol. 2020, 8, 33. [Google Scholar] [CrossRef] [Green Version]
- Marceca, G.P.; Londhe, P.; Calore, F. Management of Cancer Cachexia: Attempting to Develop New Pharmacological Agents for New Effective Therapeutic Options. Front. Oncol. 2020, 10, 298. [Google Scholar] [CrossRef] [Green Version]
- Tural, U.; Iosifescu, D.V. Neuropeptide Y in PTSD, MDD, and chronic stress: A systematic review and meta-analysis. J. Neurosci. Res. 2020, 98, 950–963. [Google Scholar] [CrossRef]
- Nogueiras, R.; Sabio, G. Brain JNK and metabolic disease. Diabetologia 2020, 64, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Razolli, D.; De Araújo, T.M.; Ana, M.R.S.A.; Kirwan, P.; Cintra, D.; Merkle, F.T.; Velloso, L. Proopiomelanocortin Processing in the Hypothalamus Is Directly Regulated by Saturated Fat: Implications for the Development of Obesity. Neuroendocrinology 2019, 110, 92–104. [Google Scholar] [CrossRef]
- Muller, P.A.; Matheis, F.; Schneeberger, M.; Kerner, Z.; Jové, V.; Mucida, D. Microbiota-modulated CART+ enteric neurons autonomously regulate blood glucose. Science 2020, 370, 314–321. [Google Scholar] [CrossRef]
- Amitani, M.; Asakawa, A.; Amitani, H.; Inui, A. Control of food intake and muscle wasting in cachexia. Int. J. Biochem. Cell Biol. 2013, 45, 2179–2185. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.S.; Yennurajalingam, S. Prokinetics and ghrelin for the management of cancer cachexia syndrome. Ann. Palliat. Med. 2019, 8, 80–85. [Google Scholar] [CrossRef]
- Blum, D.; de Wolf-Linder, S.; Oberholzer, R.; Brändle, M.; Hundsberger, T.; Strasser, F. Natural ghrelin in advanced cancer patients with cachexia, a case series. J. Cachex-Sarcopenia Muscle 2021, 12, 506–516. [Google Scholar] [CrossRef]
- Lin, T.-C.; Hsiao, M. Leptin and Cancer: Updated Functional Roles in Carcinogenesis, Therapeutic Niches, and Developments. Int. J. Mol. Sci. 2021, 22, 2870. [Google Scholar] [CrossRef] [PubMed]
- Blauwhoff-Buskermolen, S.; de van der Schueren, M.A.; Verheul, H.M.; Langius, J.A. ‘Pre-cachexia’: A non-existing phenomenon in cancer? Ann. Oncol. 2014, 25, 1668–1669. [Google Scholar] [CrossRef]
- Borg, J.J.; Anker, S.D.; Rosano, G.; Serracino-Inglott, A.; Strasser, F. Multimodal management as requirement for the clinical use of anticachexia drugs—A regulatory and a clinical perspective. Curr. Opin. Support. Palliat. Care 2015, 9, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Arends, J.; Baracos, V. Understanding the mechanisms and treatment options in cancer cachexia. Nat. Rev. Clin. Oncol. 2012, 10, 90–99. [Google Scholar] [CrossRef]
- Bland, K.A.; Harrison, M.; Zopf, E.M.; Sousa, M.S.; Currow, D.C.; Ely, M.; Agar, M.; Butcher, B.E.; Vaughan, V.; Dowd, A.; et al. Quality of Life and Symptom Burden Improve in Patients Attending a Multidisciplinary Clinical Service for Cancer Cachexia: A Retrospective Observational Review. J. Pain Symptom Manag. 2021. [Google Scholar] [CrossRef]
- Baldwin, C.; Spiro, A.; McGough, C.; Norman, A.R.; Gillbanks, A.; Thomas, K.; Cunningham, D.; O’Brien, M.; Andreyev, H.J.N. Simple nutritional intervention in patients with advanced cancers of the gastrointestinal tract, non-small cell lung cancers or mesothelioma and weight loss receiving chemotherapy: A randomised controlled trial. J. Hum. Nutr. Diet. 2011, 24, 431–440. [Google Scholar] [CrossRef]
- Yennurajalingam, S.; Willey, J.S.; Palmer, J.L.; Allo, J.; Del Fabbro, E.; Cohen, E.N.; Tin, S.; Reuben, J.M.; Bruera, E. The role of thalidomide and placebo for the treatment of cancer-related anorexia-cachexia symptoms: Results of a double-blind place-bo-controlled randomized study. J. Palliat. Med. 2012, 15, 1059–1064. [Google Scholar] [CrossRef]
- Balstad, T.R.; Solheim, T.S.; Strasser, F.; Kaasa, S.; Bye, A. Dietary treatment of weight loss in patients with advanced cancer and cachexia: A systematic literature review. Crit. Rev. Oncol. 2014, 91, 210–221. [Google Scholar] [CrossRef] [Green Version]
- Grande, A.J.; Silva, V.; Maddocks, M. Exercise for cancer cachexia in adults: Executive summary of a Cochrane Collaboration systematic review. J. Cachex-Sarcopenia Muscle 2015, 6, 208–211. [Google Scholar] [CrossRef] [Green Version]
- Bourdel-Marchasson, I.; Blanc-Bisson, C.; Doussau, A.; Germain, C.; Blanc, J.F.; Dauba, J.; Lahmar, C.; Terrebonne, E.; Lecaille, C.; Ceccaldi, J.; et al. Nutritional advice in older patients at risk of malnutrition during treatment for chemotherapy: A two-year randomized con-trolled trial. PLoS ONE 2014, 9, e108687. [Google Scholar] [CrossRef]
- Oldervoll, L.M.; Loge, J.H.; Lydersen, S.; Paltiel, H.; Asp, M.B.; Nygaard, U.V.; Oredalen, E.; Frantzen, T.L.; Lesteberg, I.; Amundsen, L.; et al. Physical Exercise for Cancer Patients with Advanced Disease: A Randomized Controlled Trial. Oncologist 2011, 16, 1649–1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamsen, L.; Quist, M.; Andersen, C.; Møller, T.; Herrstedt, J.; Kronborg, D.; Baadsgaard, M.T.; Vistisen, K.; Midtgaard, J.; Christiansen, B.; et al. Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: Randomised controlled trial. BMJ 2009, 339, b3410. [Google Scholar] [CrossRef] [Green Version]
- Rummans, T.A.; Clark, M.M.; Sloan, J.A.; Frost, M.H.; Bostwick, J.M.; Atherton, P.J.; Johnson, M.E.; Gamble, G.; Richardson, J.; Brown, P.; et al. Impacting Quality of Life for Patients with Advanced Cancer with a Structured Multidisciplinary Intervention: A Randomized Controlled Trial. J. Clin. Oncol. 2006, 24, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Grote, M.; Maihöfer, C.; Weigl, M.; Davies-Knorr, P.; Belka, C. Progressive resistance training in cachectic head and neck cancer patients undergoing radiotherapy: A randomized controlled pilot feasibility trial. Radiat. Oncol. 2018, 13, 215. [Google Scholar] [CrossRef]
- Cheville, A.L.; Kollasch, J.; Vandenberg, J.; Shen, T.; Grothey, A.; Gamble, G.; Basford, J.R. A Home-Based Exercise Program to Improve Function, Fatigue, and Sleep Quality in Patients with Stage IV Lung and Colorectal Cancer: A Randomized Controlled Trial. J. Pain Symptom Manag. 2013, 45, 811–821. [Google Scholar] [CrossRef]
- Rutkowska, A.; Jastrzebski, D.; Rutkowski, S.; Żebrowska, A.; Stanula, A.; Szczegielniak, J.; Ziora, D.; Casaburi, R. Exercise Training in Patients With Non-Small Cell Lung Cancer During In-Hospital Chemotherapy Treatment: A randomized controlled trial. J. Cardiopulm. Rehabil. Prev. 2019, 39, 127–133. [Google Scholar] [CrossRef]
- Zimmer, P.; Trebing, S.; Timmers-Trebing, U.; Schenk, A.; Paust, R.; Bloch, W.; Rudolph, R.; Streckmann, F.; Baumann, F.T. Eight-week, multimodal exercise counteracts a progress of chemotherapy-induced peripheral neuropathy and improves bal-ance and strength in metastasized colorectal cancer patients: A randomized controlled trial. Support Care Cancer 2018, 26, 615–624. [Google Scholar] [CrossRef]
- Poort, H.; Peters, M.; Van Der Graaf, W.; Nieuwkerk, P.; Van De Wouw, A.; Der Sanden, M.N.-V.; Bleijenberg, G.; Verhagen, C.; Knoop, H. Cognitive behavioral therapy or graded exercise therapy compared with usual care for severe fatigue in patients with advanced cancer during treatment: A randomized controlled trial. Ann. Oncol. 2020, 31, 115–122. [Google Scholar] [CrossRef]
- Heywood, R.; McCarthy, A.L.; Skinner, T. Safety and feasibility of exercise interventions in patients with advanced cancer: A systematic review. Support Care Cancer 2017, 25, 3031–3050. [Google Scholar] [CrossRef]
- Uster, A.; Ruehlin, M.; Mey, S.; Gisi, D.; Knols, R.; Imoberdorf, R.; Pless, M.; Ballmer, P.E. Effects of nutrition and physical exercise intervention in palliative cancer patients: A randomized controlled trial. Clin. Nutr. 2018, 37, 1202–1209. [Google Scholar] [CrossRef]
- Solheim, T.S.; Laird, B.J.; Balstad, T.R.; Stene, G.B.; Bye, A.; Johns, N.; Pettersen, C.H.; Fallon, M.; Fayers, P.; Fearon, K.; et al. A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. J. Cachex-Sarcopenia Muscle 2017, 8, 778–788. [Google Scholar] [CrossRef]
- Solheim, T.S.; Laird, B.J.; Balstad, T.R.; Bye, A.; Stene, G.; Baracos, V.; Strasser, F.; Griffiths, G.; Maddocks, M.; Fallon, M.; et al. Cancer cachexia: Rationale for the MENAC (Multimodal—Exercise, Nutrition and Anti-inflammatory medication for Cachexia) trial. BMJ Support. Palliat. Care 2018, 8, 258–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naito, T.; Mouri, T.; Morikawa, A.; Tatematsu, N.; Miura, S.; Okayama, T.; Omae, K.; Takayama, K. Promotion of Behavioral Change and the Impact on Quality of Life in Elderly Patients with Advanced Cancer: A Physical Activity Intervention of the Multimodal Nutrition and Exercise Treatment for Advanced Cancer Program. Asia-Pac. J. Oncol. Nurs. 2018, 5, 383–390. [Google Scholar] [CrossRef]
- Naito, T.; Mitsunaga, S.; Miura, S.; Tatematsu, N.; Inano, T.; Mouri, T.; Tsuji, T.; Higashiguchi, T.; Inui, A.; Okayama, T.; et al. Feasibility of early multi-modal interventions for elderly patients with advanced pancreatic and non-small-cell lung cancer. J. Cachexia Sarcopenia Muscle 2019, 10, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef]
- Bosaeus, I.; Daneryd, P.; Svanberg, E.; Lundholm, K. Dietary intake and resting energy expenditure in relation to weight loss in unselected cancer patients. Int. J. Cancer 2001, 93, 380–383. [Google Scholar] [CrossRef] [Green Version]
- Cao, D.X.; Wu, G.H.; Zhang, B.; Quan, Y.J.; Wei, J.; Jin, H.; Jiang, Y.; Yang, Z.A. Resting energy expenditure and body com-position in patients with newly detected cancer. Clin. Nutr. 2010, 29, 72–77. [Google Scholar] [CrossRef]
- Fukatsu, K.; Kudsk, K.A. Nutrition and gut immunity. Surg. Clin. N. Am. 2011, 91, 755–770. [Google Scholar] [CrossRef] [Green Version]
- Amano, K.; Maeda, I.; Ishiki, H.; Miura, T.; Hatano, Y.; Tsukuura, H.; Taniyama, T.; Matsumoto, Y.; Matsuda, Y.; Kohara, H.; et al. Effects of enteral nutrition and parenteral nutrition on survival in patients with advanced cancer cachexia: Analysis of a multicenter prospective cohort study. Clin. Nutr. 2021, 40, 1168–1175. [Google Scholar] [CrossRef]
- Bozzetti, F.; Bozzetti, V. Is the intravenous supplementation of amino acid to cancer patients adequate? A critical appraisal of literature. Clin. Nutr. 2013, 32, 142–146. [Google Scholar] [CrossRef]
- Deutz, N.; Safar, A.; Schutzler, S.; Memelink, R.; Ferrando, A.; Spencer, H.; van Helvoort, A.; Wolfe, R.R. Muscle protein synthesis in cancer patients can be stimulated with a specially formulated medical food. Clin. Nutr. 2011, 30, 759–768. [Google Scholar] [CrossRef] [Green Version]
- Fabi, A.; Bhargava, R.; Fatigoni, S.; Guglielmo, M.; Horneber, M.; Roila, F.; Weis, J.; Jordan, K.; Ripamonti, C. Cancer-related fatigue: ESMO Clinical Practice Guidelines for diagnosis and treatment. Ann. Oncol. 2020, 31, 713–723. [Google Scholar] [CrossRef]
- Solheim, T.S.; Fearon, K.C.H.; Blum, D.; Kaasa, S. Non-steroidal anti-inflammatory treatment in cancer cachexia: A systematic literature review. Acta Oncol. 2012, 52, 6–17. [Google Scholar] [CrossRef] [Green Version]
- Loprinzi, C.L.; Kugler, J.W.; Sloan, J.A.; Mailliard, J.A.; Krook, J.E.; Wilwerding, M.B.; Rowland, K.M., Jr.; Camoriano, J.K.; Novotny, P.J.; Christensen, B.J. Randomized comparison of megestrol acetate versus dexamethasone versus fluoxymesterone for the treatment of cancer anorexia/cachexia. J. Clin. Oncol. 1999, 17, 3299–3306. [Google Scholar] [CrossRef]
- Ruiz Garcia, V.; López-Briz, E.; Carbonell, S.R.; Gonzalvez Perales, J.L.; Bort-Marti, S. Megestrol acetate for treatment of ano-rexia-cachexia syndrome. Cochrane Database Syst Rev. 2013, 2013, CD004310. [Google Scholar]
- Strasser, F.; Lüftner, D.; Possinger, K.; Ernst, G.; Ruhstaller, T.; Meissner, W.; Ko, Y.-D.; Schnelle, M.; Reif, M.; Cerny, T. Comparison of Orally Administered Cannabis Extract and Delta-9-Tetrahydrocannabinol in Treating Patients with Cancer-Related Anorexia-Cachexia Syndrome: A Multicenter, Phase III, Randomized, Double-Blind, Placebo-Controlled Clinical Trial from the Cannabis-In-Cachexia-Study-Group. J. Clin. Oncol. 2006, 24, 3394–3400. [Google Scholar] [CrossRef]
- Dobs, A.S.; Boccia, R.V.; Croot, C.C.; Gabrail, N.Y.; Dalton, J.T.; Hancock, M.L.; Johnston, M.; Steiner, M.S. Effects of enobosarm on muscle wasting and physical function in patients with cancer: A double-blind, randomised controlled phase 2 trial. Lancet Oncol. 2013, 14, 335–345. [Google Scholar] [CrossRef] [Green Version]
- Advani, S.M.; Advani, P.G.; VonVille, H.M.; Jafri, S.H. Pharmacological management of cachexia in adult cancer patients: A systematic review of clinical trials. BMC Cancer 2018, 18, 1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temel, J.S.; Abernethy, A.P.; Currow, D.; Friend, J.; Duus, E.M.; Yan, Y.; Fearon, K.C. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): Results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 2016, 17, 519–531. [Google Scholar] [CrossRef]
- Katakami, N.; Uchino, J.; Yokoyama, T.; Naito, T.; Kondo, M.; Yamada, K.; Kitajima, H.; Yoshimori, K.; Sato, K.; Saito, H.; et al. Anamorelin (ONO-7643) for the treatment of patients with non-small cell lung cancer and cachexia: Results from a randomized, double-blind, placebo-controlled, multicenter study of Japanese patients (ONO-7643-04). Cancer 2017, 124, 606–616. [Google Scholar] [CrossRef]
- Hamauchi, S.; Furuse, J.; Takano, T.; Munemoto, Y.; Furuya, K.; Baba, H.; Takeuchi, M.; Choda, Y.; Higashiguchi, T.; Naito, T.; et al. A multicenter, open-label, single-arm study of anamorelin (ONO-7643) in advanced gastrointestinal cancer patients with cancer cachexia. Cancer 2019, 125, 4294–4302. [Google Scholar] [CrossRef] [Green Version]
- Crawford, J. Clinical results in cachexia therapeutics. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Hickish, T.; André, T.; Wyrwicz, L.; Saunders, M.; Sarosiek, T.; Kocsis, J.; Nemecek, R.; Rogowski, W.; Lesniewski-Kmak, K.; Petruzelka, L.; et al. MABp1 as a novel antibody treatment for advanced colorectal cancer: A randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2017, 18, 192–201. [Google Scholar] [CrossRef] [Green Version]
- Moyle, G.J.; Daar, E.S.; Gertner, J.M.; Kotler, D.P.; Melchior, J.-C.; O’Brien, F.; Svanberg, E. Serono 9037 Study Team Growth Hormone Improves Lean Body Mass, Physical Performance, and Quality of Life in Subjects With HIV-Associated Weight Loss or Wasting on Highly Active Antiretroviral Therapy. JAIDS J. Acquir. Immune Defic. Syndr. 2004, 35, 367–375. [Google Scholar] [CrossRef]
- Bindels, L.B.; Neyrinck, A.; Loumaye, A.; Catry, E.; Walgrave, H.; Cherbuy, C.; Leclercq, S.; Van Hul, M.; Plovier, H.; Pachikian, B.; et al. Increased gut permeability in cancer cachexia: Mechanisms and clinical relevance. Oncotarget 2018, 9, 18224–18238. [Google Scholar] [CrossRef] [Green Version]
- Di Renzo, L.; Gualtieri, P.; Romano, L.; Marrone, G.; Noce, A.; Pujia, A.; Perrone, M.A.; Aiello, V.; Colica, C.; De Lorenzo, A. Role of Personalized Nutrition in Chronic-Degenerative Diseases. Nutrients 2019, 11, 1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.L.C.; Leong, L.P.; Lim, S.L. Nutrition intervention approaches to reduce malnutrition in oncology patients: A systematic review. Support. Care Cancer 2015, 24, 469–480. [Google Scholar] [CrossRef] [Green Version]
- Tobberup, R.; Carus, A.; Rasmussen, H.H.; Falkmer, U.G.; Jorgensen, M.G.; Schmidt, E.B.; Jensen, N.A.; Mark, E.B.; Delekta, A.M.; Antoniussen, C.S.; et al. Feasibility of a multimodal intervention on malnutrition in patients with lung cancer during primary anti-neoplastic treatment. Clin. Nutr. 2021, 40, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Balstad, T.R.; Brunelli, C.; Pettersen, C.H.; Schønberg, S.A.; Skorpen, F.; Fallon, M.; Kaasa, S.; Bye, A.; Laird, B.J.A.; Stene, G.B.; et al. Power Comparisons and Clinical Meaning of Outcome Measures in Assessing Treatment Effect in Cancer Cachexia: Secondary Analysis from a Randomized Pilot Multimodal Intervention Trial. Front. Nutr. 2021, 7, 602775. [Google Scholar] [CrossRef] [PubMed]
- Avancini, A.; Trestini, I.; Tregnago, D.; Lanza, M.; Menis, J.; Belluomini, L.; Milella, M.; Pilotto, S. A multimodal approach to cancer-related cachexia: From theory to practice. Expert Rev. Anticancer Ther. 2021, 21, 819–826. [Google Scholar] [CrossRef] [PubMed]
| Cachexia | Starvation | |
|---|---|---|
| Body weight | ↓ | ↓ |
| Skeletal muscle | ↓ | → |
| Adipose tissue | ↓ | ↓ |
| Rest energy expenditure | ↑ | ↓ |
| Inflammatory protein | ↑ | → |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishikawa, H.; Goto, M.; Fukunishi, S.; Asai, A.; Nishiguchi, S.; Higuchi, K. Cancer Cachexia: Its Mechanism and Clinical Significance. Int. J. Mol. Sci. 2021, 22, 8491. https://doi.org/10.3390/ijms22168491
Nishikawa H, Goto M, Fukunishi S, Asai A, Nishiguchi S, Higuchi K. Cancer Cachexia: Its Mechanism and Clinical Significance. International Journal of Molecular Sciences. 2021; 22(16):8491. https://doi.org/10.3390/ijms22168491
Chicago/Turabian StyleNishikawa, Hiroki, Masahiro Goto, Shinya Fukunishi, Akira Asai, Shuhei Nishiguchi, and Kazuhide Higuchi. 2021. "Cancer Cachexia: Its Mechanism and Clinical Significance" International Journal of Molecular Sciences 22, no. 16: 8491. https://doi.org/10.3390/ijms22168491
APA StyleNishikawa, H., Goto, M., Fukunishi, S., Asai, A., Nishiguchi, S., & Higuchi, K. (2021). Cancer Cachexia: Its Mechanism and Clinical Significance. International Journal of Molecular Sciences, 22(16), 8491. https://doi.org/10.3390/ijms22168491






