Freeze-Dried Secretome (Lyosecretome) from Mesenchymal Stem/Stromal Cells Promotes the Osteoinductive and Osteoconductive Properties of Titanium Cages
Abstract
:1. Introduction
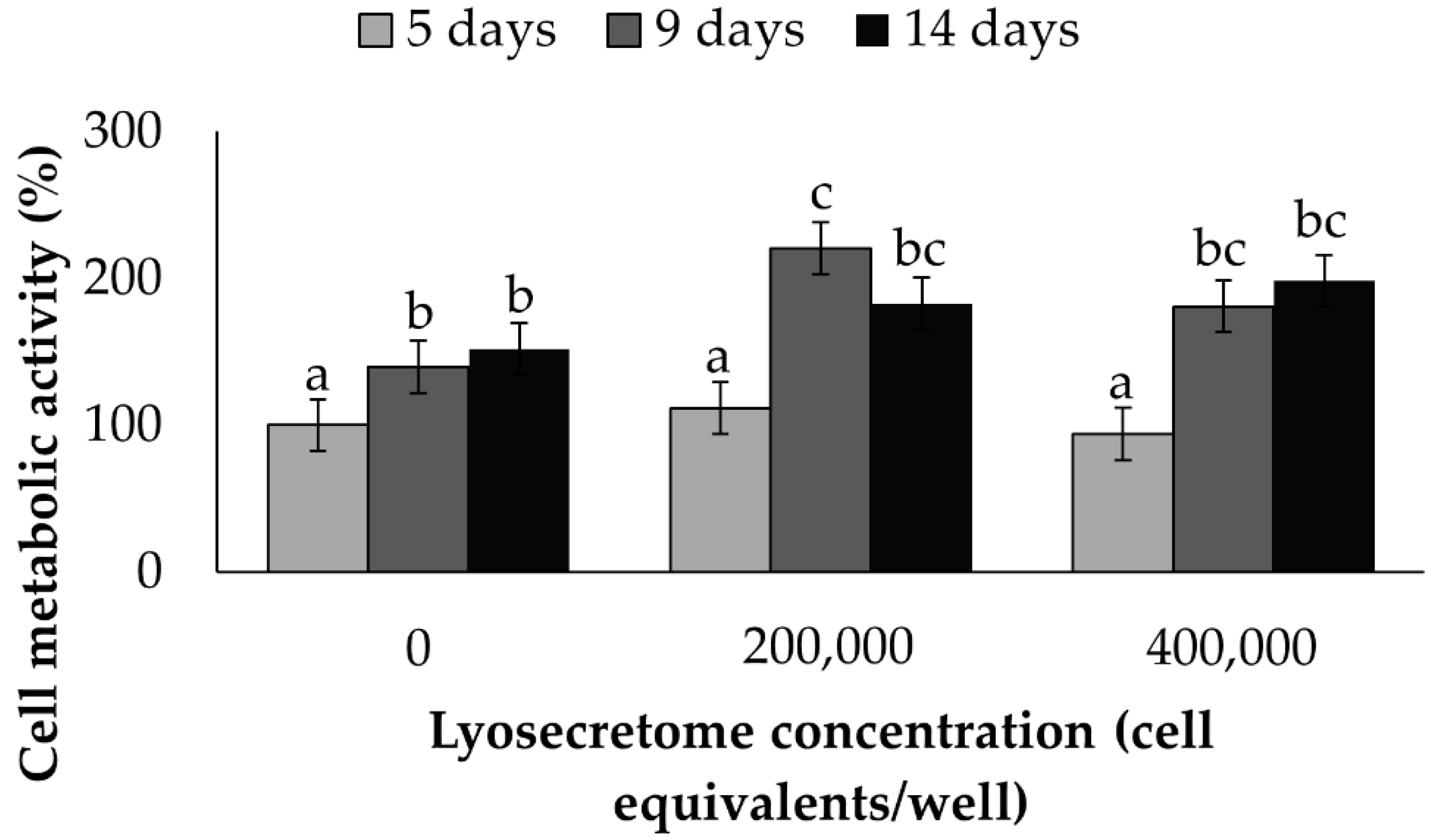
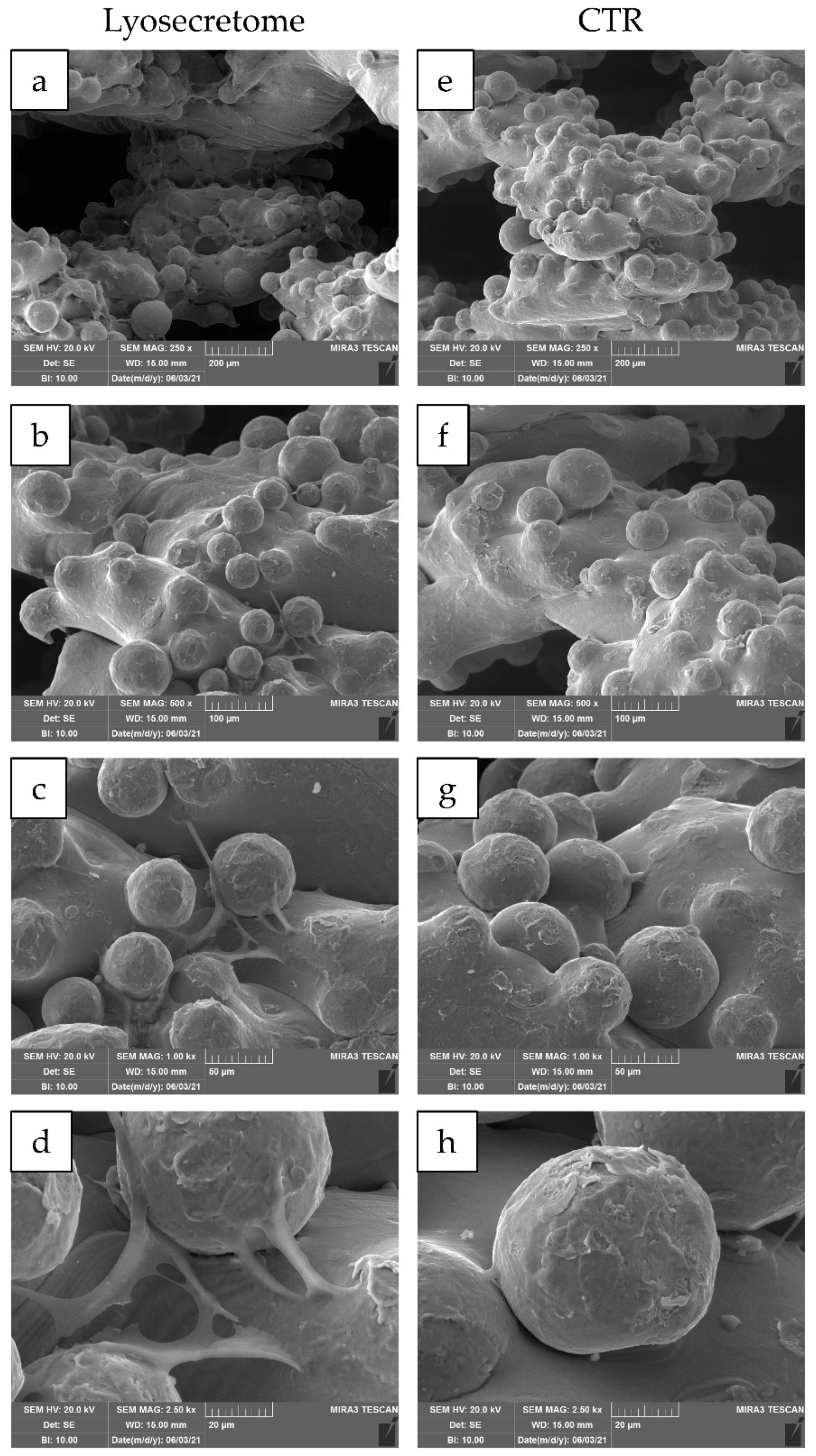
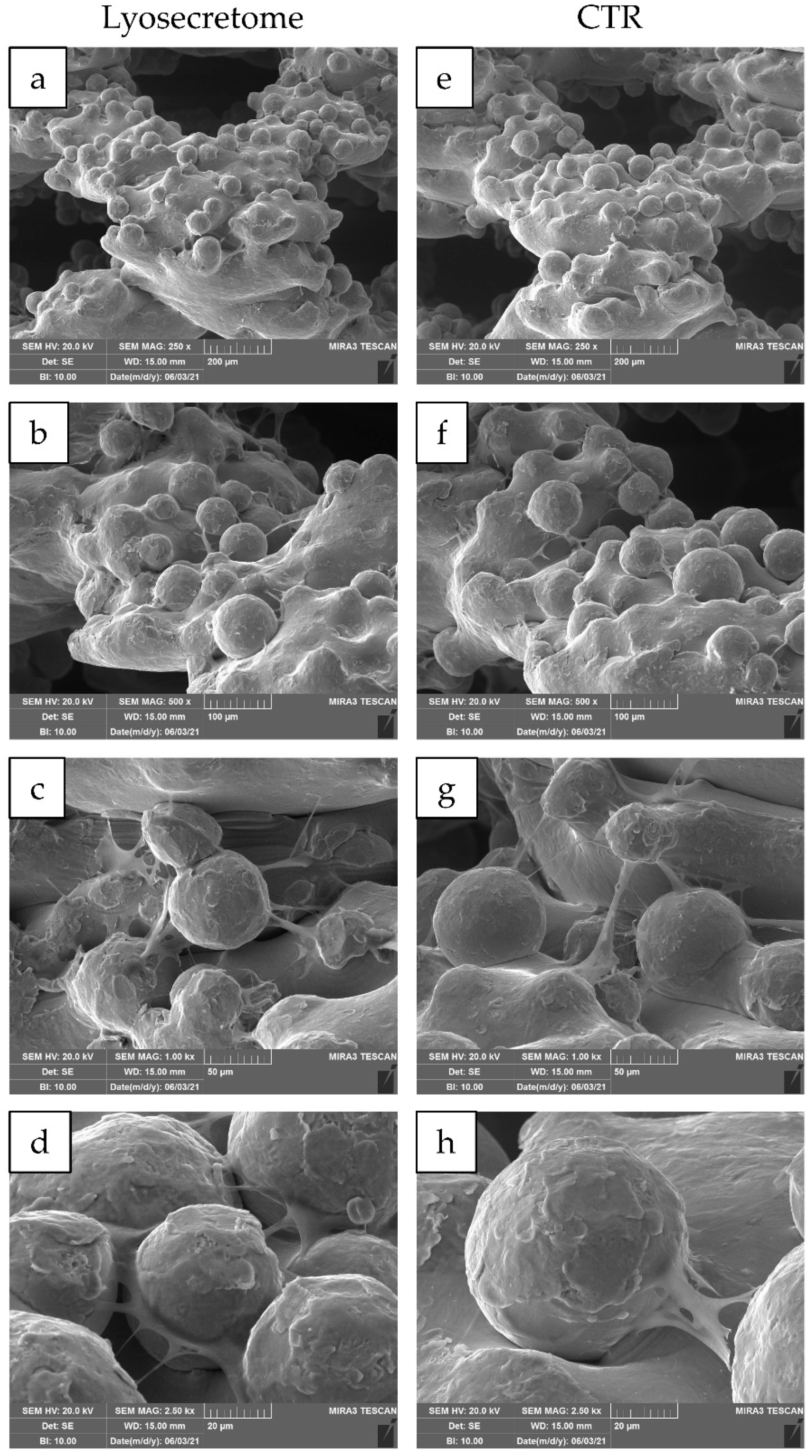
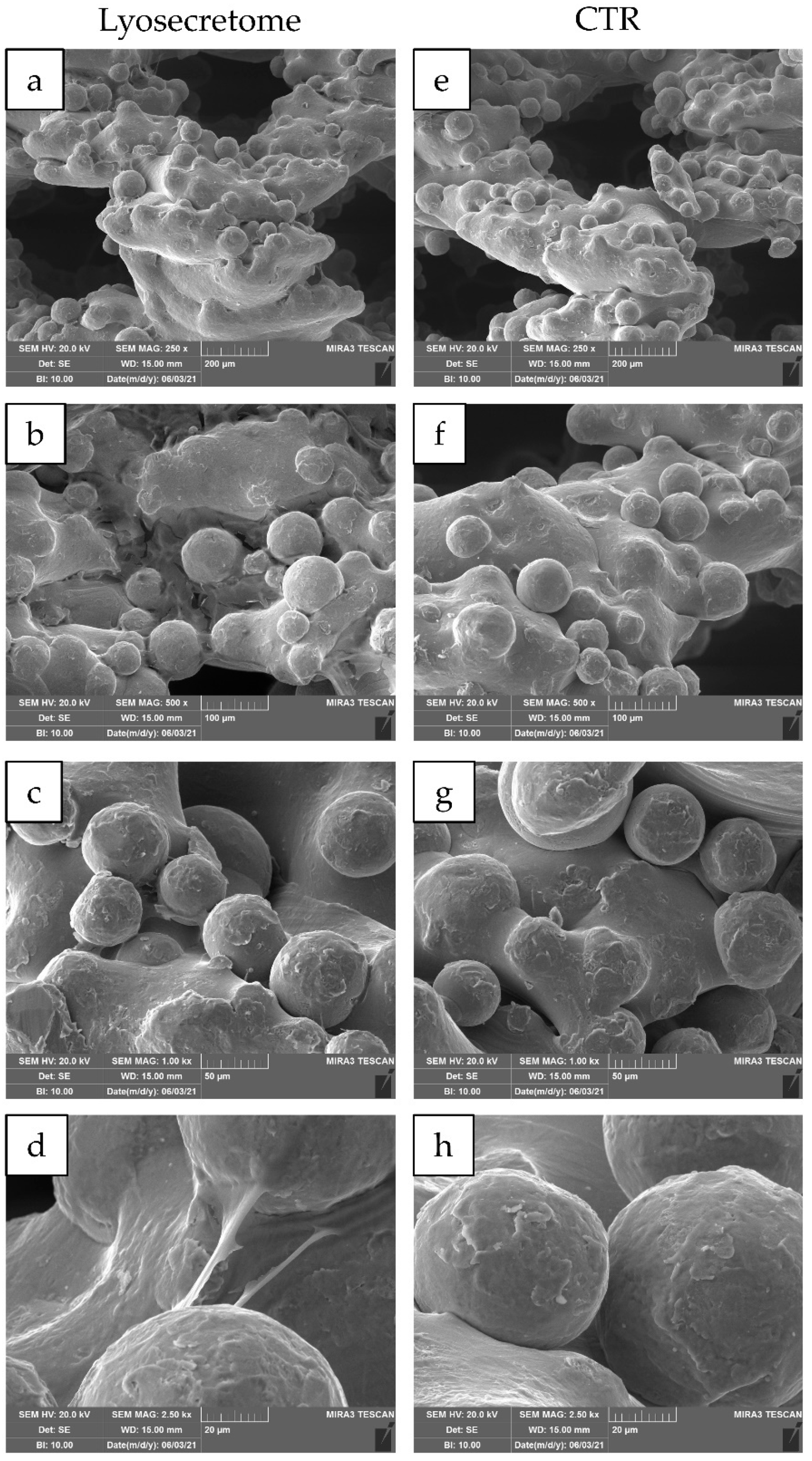
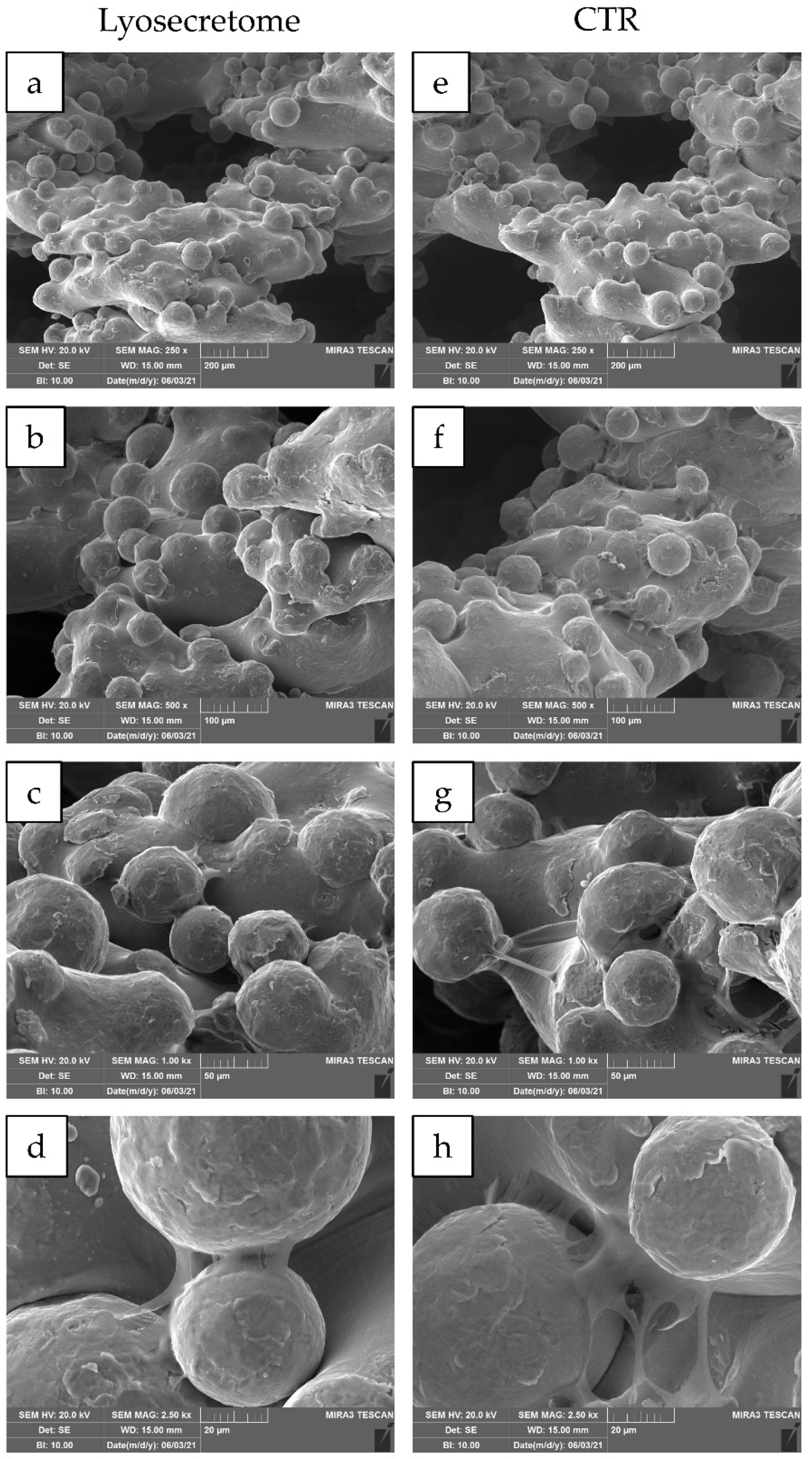
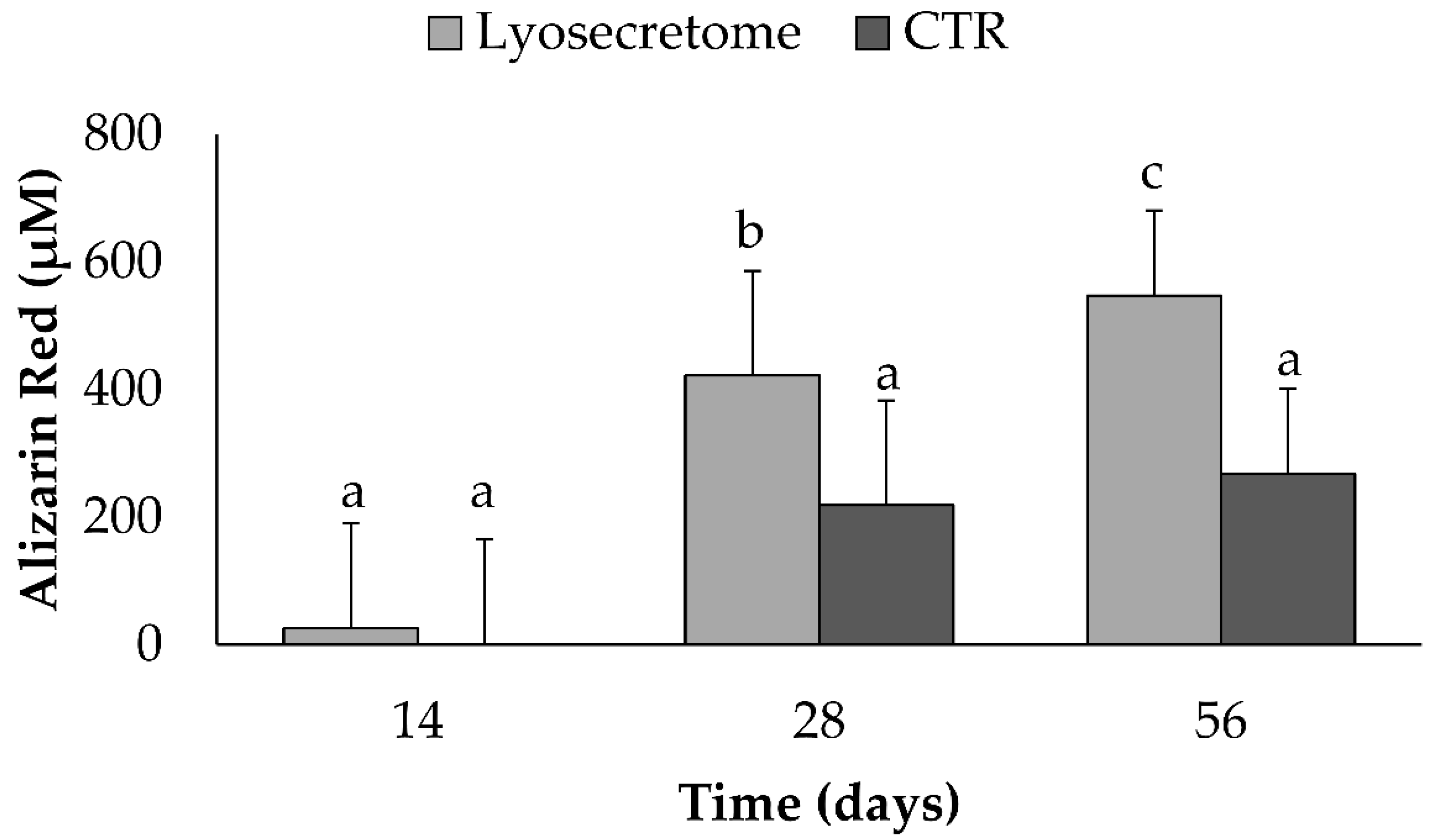
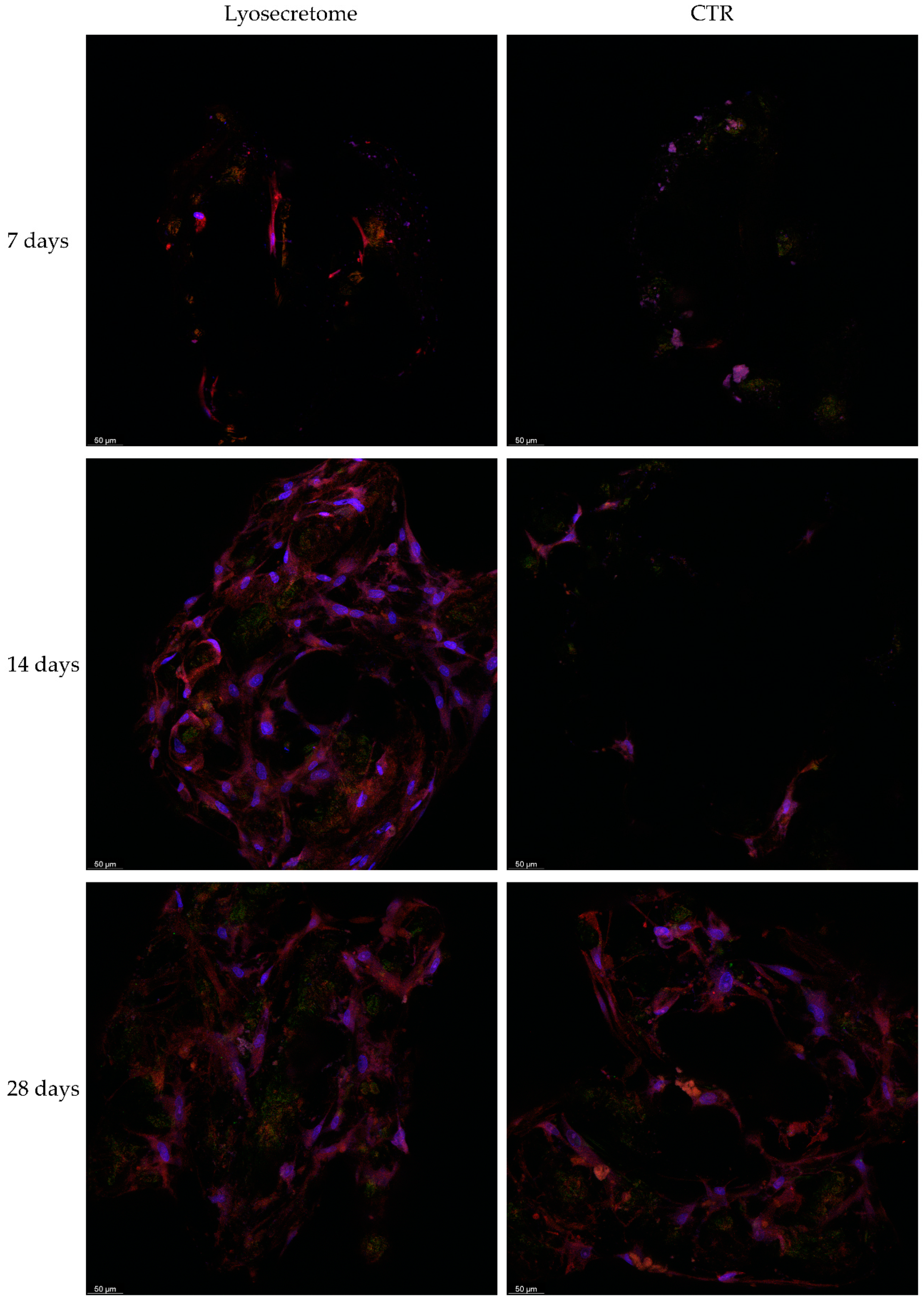
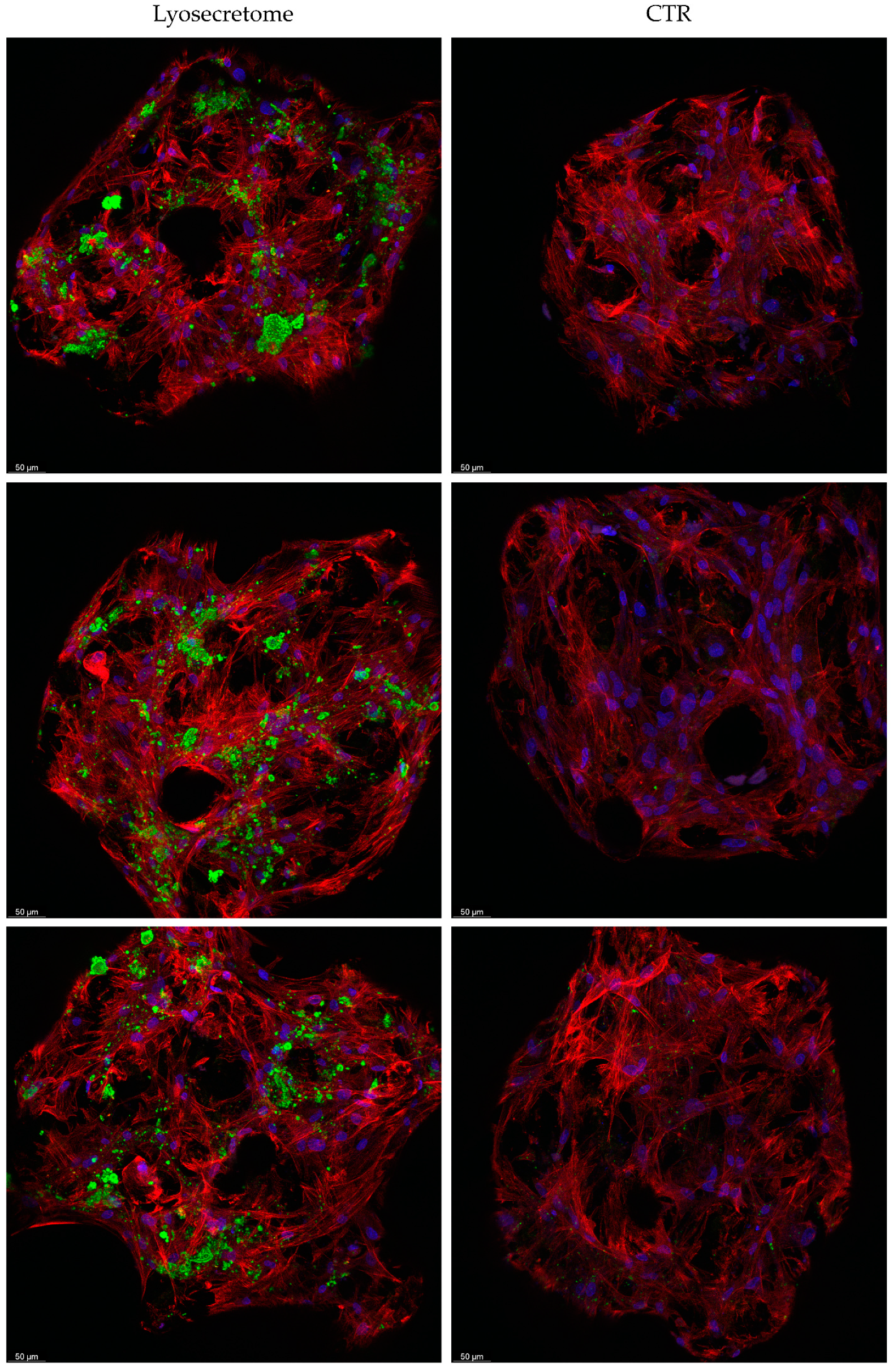
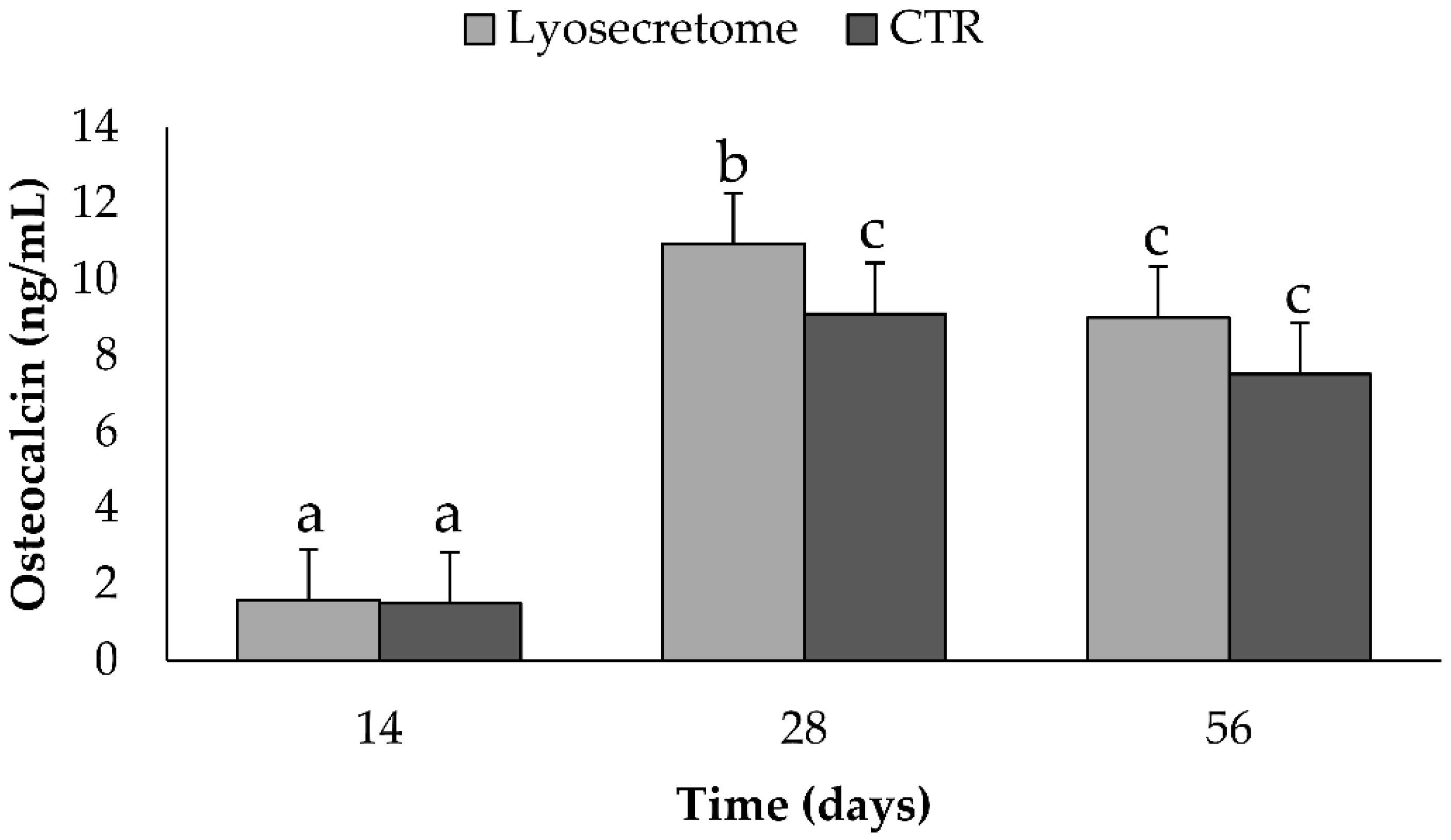
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Isolation and Expansion of Human AD-MSCs
3.3. Lyosecretome Preparation and Characterization
3.3.1. Total Protein and Lipid Content
3.3.2. EV Particle Size and Concentration
3.3.3. Microbiological Controls
3.4. Titanium Cages
3.5. Seeding of AD-MSCs
3.6. Evaluation of Cell Proliferation
3.7. MSCs Culture and Osteogenic Differentiation
3.8. Scanning Electron Microscopy (SEM)
3.9. Alizarin Red Assay
3.10. Confocal Microscopy
3.11. Dosage of Osteocalcin (OCN) by ELISA
3.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tzioupis, C.; Giannoudis, P.V. Prevalence of long-bone non-unions. Inj. Int. J. Care Inj. 2007, 38, S3–S9. [Google Scholar] [CrossRef]
- Gomez-Barrena, E.; Rosset, P.; Lozano, D.; Stanovici, J.; Ermthaller, C.; Gerbhard, F. Bone fracture healing: Cell therapy in delayed unions and nonunions. Bone 2015, 70, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.-Z.; Lee, J.H. Mesenchymal Stem Cell Therapy for Bone Regeneration. Clin. Orthop. Surg. 2018, 10, 271–278. [Google Scholar] [CrossRef]
- Bruder, S.P.; Fink, D.J.; Caplan, A.I. Mesenchymal Stem-cells in bone-development, bone repair, and skeletal regeneration therapy. J. Cell. Biochem. 1994, 56, 283–294. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Eslaminejad, M.B. Role of Mesenchymal Stem Cells in Bone Regenerative Medicine: What Is the Evidence? Cells Tissues Organs 2017, 204, 59–83. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Cao, W.; Shi, Y. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat. Immunol. 2014, 15, 1009–1016. [Google Scholar] [CrossRef]
- Bari, E.; Di Silvestre, D.; Mastracci, L.; Grillo, F.; Grisoli, P.; Marrubini, G.; Nardini, M.; Mastrogiacomo, M.; Sorlini, M.; Rossi, R.; et al. GMP-compliant sponge-like dressing containing MSC lyo-secretome: Proteomic network of healing in a murine wound model. Eur. J. Pharm. Biopharm. 2020, 155, 37–48. [Google Scholar] [CrossRef]
- Estrada, R.; Li, N.; Sarojini, H.; An, J.; Lee, M.-J.; Wang, E. Secretome From Mesenchymal Stem Cells Induces Angiogenesis Via Cyr61. J. Cell. Physiol. 2009, 219, 563–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kehl, D.; Generali, M.; Mallone, A.; Heller, M.; Uldry, A.-C.; Cheng, P.; Gantenbein, B.; Hoerstrup, S.P.; Weber, B. Proteomic analysis of human mesenchymal stromal cell secretomes: A systematic comparison of the angiogenic potential. NPJ Regen. Med. 2019, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bari, E.; Ferrarotti, I.; Di Silvestre, D.; Grisoli, P.; Barzon, V.; Balderacchi, A.; Torre, M.L.; Rossi, R.; Mauri, P.; Corsico, A.G.; et al. Adipose Mesenchymal Extracellular Vesicles as Alpha-1-Antitrypsin Physiological Delivery Systems for Lung Regeneration. Cells 2019, 8, 965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bari, E.; Ferrarotti, I.; Saracino, L.; Perteghella, S.; Torre, M.; Corsico, A. Mesenchymal Stromal Cell Secretome for Severe COVID-19 Infections: Premises for the Therapeutic Use. Cells 2020, 9, 924. [Google Scholar] [CrossRef] [Green Version]
- Bari, E.; Ferrarotti, I.; Saracino, L.; Perteghella, S.; Torre, M.L.; Richeldi, L.; Corsico, A.G. Mesenchymal Stromal Cell Secretome for Post-COVID-19 Pulmonary Fibrosis: A New Therapy to Treat the Long-Term Lung Sequelae? Cells 2021, 10, 1203. [Google Scholar] [CrossRef]
- Basalova, N.; Sagaradze, G.; Arbatskiy, M.; Evtushenko, E.; Kulebyakin, K.; Grigorieva, O.; Akopyan, Z.; Kalinina, N.; Efimenko, A. Secretome of Mesenchymal Stromal Cells Prevents Myofibroblasts Differentiation by Transferring Fibrosis-Associated microRNAs within Extracellular Vesicles. Cells 2020, 9, 1272. [Google Scholar] [CrossRef]
- Driscoll, J.; Patel, T. The mesenchymal stem cell secretome as an acellular regenerative therapy for liver disease. J. Gastroenterol. 2019, 54, 763–773. [Google Scholar] [CrossRef] [Green Version]
- Bari, E.; Perteghella, S.; Catenacci, L.; Sorlini, M.; Croce, S.; Mantelli, M.; Avanzini, M.A.; Sorrenti, M.; Torre, M.L. Freeze-dried and GMP-compliant pharmaceuticals containing exosomes for acellular mesenchymal stromal cell immunomodulant therapy. Nanomedicine 2019, 14, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Palama, M.E.F.; Shaw, G.M.; Carluccio, S.; Reverberi, D.; Sercia, L.; Persano, L.; Pisignano, D.; Cortese, K.; Barry, F.P.; Murphy, J.M.; et al. The Secretome Derived from Mesenchymal Stromal Cells Cultured in a Xeno-Free Medium Promotes Human Cartilage Recovery in vitro. Front. Bioeng. Biotechnol. 2020, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Jahandideh, S.; Khatami, S.; Far, A.E.; Kadivar, M. Anti-inflammatory effects of human embryonic stem cell-derived mesenchymal stem cells secretome preconditioned with diazoxide, trimetazidine and MG-132 on LPS-induced systemic inflammation mouse model. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1178–1187. [Google Scholar] [CrossRef] [Green Version]
- van Buul, G.M.; Villafuertes, E.; Bos, P.K.; Waarsing, J.H.; Kops, N.; Narcisi, R.; Weinans, H.; Verhaar, J.A.N.; Bernsen, M.R.; van Osch, G.J.V.M. Mesenchymal stem cells secrete factors that inhibit inflammatory processes in short-term osteoarthritic synovium and cartilage explant culture. Osteoarthr. Cartil. 2012, 20, 1186–1196. [Google Scholar] [CrossRef] [Green Version]
- Bari, E.; Roato, I.; Perale, G.; Rossi, F.; Genova, T.; Mussano, F.; Ferracini, R.; Sorlini, M.; Torre, M.L.; Perteghella, S. Biohybrid Bovine Bone Matrix for Controlled Release of Mesenchymal Stem/Stromal Cell Lyosecretome: A Device for Bone Regeneration. Int. J. Mol. Sci. 2021, 22, 4064. [Google Scholar] [CrossRef] [PubMed]
- Eichholz, K.F.; Woods, I.; Johnson, G.P.; Shen, N.; Corrigan, M.; Labour, M.-N.; Wynne, K.; Lowery, M.C.; O’Driscoll, L.; Hoey, D.A.; et al. Human bone marrow stem/stromal cell osteogenesis is regulated via mechanically activated osteocyte-derived extracellular vesicles. Stem Cells Transl. Med. 2020, 9, 1431–1447. [Google Scholar] [CrossRef] [PubMed]
- Bari, E.; Perteghella, S.; Di Silvestre, D.; Sorlini, M.; Catenacci, L.; Sorrenti, M.; Marrubini, G.; Rossi, R.; Tripodo, G.; Mauri, P.; et al. Pilot Production of Mesenchymal Stem/Stromal Freeze-Dried Secretome for Cell-Free Regenerative Nanomedicine: A Validated GMP-Compliant Process. Cells 2018, 7, 190. [Google Scholar] [CrossRef] [Green Version]
- Bari, E.; Scocozza, F.; Perteghella, S.; Sorlini, M.; Auricchio, F.; Torre, M.L.; Conti, M. 3D Bioprinted Scaffolds Containing Mesenchymal Stem/Stromal Lyosecretome: Next Generation Controlled Release Device for Bone Regenerative Medicine. Pharmaceutics 2021, 13, 515. [Google Scholar] [CrossRef]
- Uehara, S.; Kurita, H.; Shimane, T.; Sakai, H.; Kamata, T.; Teramoto, Y.; Yamada, S. Predictability of staged localized alveolar ridge augmentation using a micro titanium mesh. Oral Maxillofac. Surg. Heidelb. 2015, 19, 411–416. [Google Scholar] [CrossRef]
- Mounir, M.; Shalash, M.; Mounir, S.; Nassar, Y.; El Khatib, O. Assessment of three dimensional bone augmentation of severely atrophied maxillary alveolar ridges using prebent titanium mesh vs customized poly-ether-ether-ketone (PEEK) mesh: A randomized clinical trial. Clin. Implant Dent. Relat. Res. 2019, 21, 960–967. [Google Scholar] [CrossRef]
- Sagheb, K.; Schiegnitz, E.; Moergel, M.; Walter, C.; Al-Nawas, B.; Wagner, W. Clinical outcome of alveolar ridge augmentation with individualized CAD-CAM-produced titanium mesh. Int. J. Implant Dent. 2017, 3, 36. [Google Scholar] [CrossRef] [Green Version]
- Ragni, E.; Perucca Orfei, C.; Bidossi, A.; De Vecchi, E.; Francaviglia, N.; Romano, A.; Maestretti, G.; Tartara, F.; de Girolamo, L. Superior Osteo-Inductive and Osteo-Conductive Properties of Trabecular Titanium vs. PEEK Scaffolds on Human Mesenchymal Stem Cells: A Proof of Concept for the Use of Fusion Cages. Int. J. Mol. Sci. 2021, 22, 2379. [Google Scholar] [CrossRef]
- Wang, W.; Poh, C.K. Titanium Alloys in Orthopaedics. In Titanium Alloys—Advances in Properties Control; IntechOpen: London, UK, 2013. [Google Scholar]
- Provaggi, E.; Capelli, C.; Leong, J.J.H.; Kalaskar, D.M. A UK-based pilot study of current surgical practice and implant preferences in lumbar fusion surgery. Medicine 2018, 97, e11169. [Google Scholar] [CrossRef]
- Svehla, M.; Morberg, P.; Zicat, B.; Bruce, W.; Sonnabend, D.; Walsh, W.R. Morphometric and mechanical evaluation of titanium implant integration: Comparison of five surface structures. J. Biomed. Mater. Res. 2000, 51, 15–22. [Google Scholar] [CrossRef]
- Mocchi, M.; Grolli, S.; Dotti, S.; Di Silvestre, D.; Villa, R.; Berni, P.; Conti, V.; Passignani, G.; Brambilla, F.; Bue, M.D.; et al. Equine Mesenchymal Stem/Stromal Cells Freeze-Dried Secretome (Lyosecretome) for the Treatment of Musculoskeletal Diseases: Production Process Validation and Batch Release Test for Clinical Use. Pharmaceuticals 2021, 14, 553. [Google Scholar] [CrossRef]
- Eirin, A.; Zhu, X.-Y.; Puranik, A.S.; Woollard, J.R.; Tang, H.; Dasari, S.; Lerman, A.; van Wijnen, A.J.; Lerman, L.O. Integrated transcriptomic and proteomic analysis of the molecular cargo of extracellular vesicles derived from porcine adipose tissue-derived mesenchymal stem cells. PLoS ONE 2017, 12, e0174303. [Google Scholar] [CrossRef]
- Hsiao, S.T.-F.; Asgari, A.; Lokmic, Z.; Sinclair, R.; Dusting, G.J.; Lim, S.Y.; Dilley, R.J. Comparative Analysis of Paracrine Factor Expression in Human Adult Mesenchymal Stem Cells Derived from Bone Marrow, Adipose, and Dermal Tissue. Stem Cells Dev. 2012, 21, 2189–2203. [Google Scholar] [CrossRef] [Green Version]
- Illario, M.; Cavallo, A.L.; Monaco, S.; Di Vito, E.; Mueller, F.; Marzano, L.A.; Troncone, G.; Fenzi, G.; Rossi, G.; Vitale, M. Fibronectin-induced proliferation in thyroid cells is mediated by alpha v beta 3 integrin through Ras/Raf-1/MEK/ERK and calcium/CaMKII signals. J. Clin. Endocrinol. Metab. 2005, 90, 2865–2873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.W.; Roman, J. Fibronectin induces cell proliferation and inhibits apoptosis in human bronchial epithelial cells: Pro-oncogenic effects mediated by PI3-kinase and NF-kappa B. Oncogene 2006, 25, 4341–4349. [Google Scholar] [CrossRef] [Green Version]
- Fan, Z.; Qin, J.; Wang, D.; Geng, S. Complement C3a promotes proliferation, migration and stemness in cutaneous squamous cell carcinoma. J. Cell. Mol. Med. 2019, 23, 3097–3107. [Google Scholar] [CrossRef] [Green Version]
- Bonacci, G.R.; Caceres, L.C.; Sanchez, M.C.; Chiabrando, G.A. Activated alpha(2)-macroglobulin induces cell proliferation and mitogen-activated protein kinase activation by LRP-1 in the J774 macrophage-derived cell line. Arch. Biochem. Biophys. 2007, 460, 100–106. [Google Scholar] [CrossRef]
- de Girolamo, L.; Lucarelli, E.; Alessandri, G.; Avanzini, M.A.; Bernardo, M.E.; Biagi, E.; Brini, A.T.; D’Amico, G.; Fagioli, F.; Ferrero, I.; et al. Mesenchymal Stem/Stromal Cells: A New “Cells as Drugs” Paradigm. Efficacy and Critical Aspects in Cell Therapy. Curr. Pharm. Des. 2013, 19, 2459–2473. [Google Scholar] [CrossRef] [Green Version]
- Li, J.P.; Habibovic, P.; van den Doel, M.; Wilson, C.E.; de Wijn, J.R.; van Blitterswijk, C.A.; de Groot, K. Bone ingrowth in porous titanium implants produced by 3D fiber deposition. Biomaterials 2007, 28, 2810–2820. [Google Scholar] [CrossRef]
- Staines, K.A.; Zhu, D.; Farquharson, C.; MacRae, V.E. Identification of novel regulators of osteoblast matrix mineralization by time series transcriptional profiling. J. Bone Miner. Metab. 2014, 32, 240–251. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, A.; Dohi, Y.; Akahane, M.; Ohgushi, H.; Nakajima, H.; Funaoka, H.; Takakura, Y. Osteocalcin Secretion as an Early Marker of In Vitro Osteogenic Differentiation of Rat Mesenchymal Stem Cells. Tissue Eng. Part C Methods 2009, 15, 169–180. [Google Scholar] [CrossRef]
- Lin, K.; Xia, L.; Gan, J.; Zhang, Z.; Chen, H.; Jiang, X.; Chang, J. Tailoring the Nanostructured Surfaces of Hydroxyapatite Bioceramics to Promote Protein Adsorption, Osteoblast Growth, and Osteogenic Differentiation. ACS Appl. Mater. Interfaces 2013, 5, 8008–8017. [Google Scholar] [CrossRef]
- Buyuksungur, S.; Hasirci, V.; Hasirci, N. 3D printed hybrid bone constructs of PCL and dental pulp stem cells loaded GelMA. J. Biomed. Mater. Res. Part A. 2021, 1–13. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, L.; Gao, Z.; Chen, G.; Zhang, C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci. Rep. 2016, 6, 21961. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, J.; Yuan, H.; Xu, Z.; Li, Q.; Niu, X.; Hu, B.; Wang, Y.; Li, X. Exosomes Secreted by Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Repair Critical-Sized Bone Defects through Enhanced Angiogenesis and Osteogenesis in Osteoporotic Rats. Int. J. Biol. Sci. 2016, 12, 836–849. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, X.; Li, H.; Chen, C.; Hu, B.; Niu, X.; Li, Q.; Zhao, B.; Xie, Z.; Wang, Y. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res. Ther. 2016, 7, 136. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Liu, H.X.; Li, S.H.; Gong, Y.S.; Zhou, M.W.; Zhang, J.H.; Zhu, G.Y. Effects of human umbilical cord mesenchymal stem cells-derived exosomes on fracture healing in rats through the Wnt signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4954–4960. [Google Scholar] [PubMed]
- Narayanan, R.; Huang, C.-C.; Ravindran, S. Hijacking the Cellular Mail: Exosome Mediated Differentiation of Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 3808674. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.-X.; Xu, L.-L.; Rui, Y.-F.; Huang, S.; Lin, S.-E.; Xiong, J.-H.; Li, Y.-H.; Lee, W.Y.-W.; Li, G. The Effects of Secretion Factors from Umbilical Cord Derived Mesenchymal Stem Cells on Osteogenic Differentiation of Mesenchymal Stem Cells. PLoS ONE 2015, 10, e0120593. [Google Scholar] [CrossRef] [Green Version]
- Moursi, A.M.; Damsky, C.H.; Lull, J.; Zimmerman, D.; Doty, S.B.; Aota, S.; Globus, R.K. Fibronectin regulates calvarial osteoblast differentiation. J. Cell Sci. 1996, 106, 1369–1380. [Google Scholar] [CrossRef]
- Gavish, H.; Bab, I.; Tartakovsky, A.; Chorev, M.; Mansur, N.; Greenberg, Z.; NamdarAttar, H.; Muhlrad, A. Human alpha(2)-macroglobulin is an osteogenic growth peptide-binding protein. Biochemistry 1997, 36, 14883–14888. [Google Scholar] [CrossRef]
- Blair, H.C.; Kalyvioti, E.; Papachristou, N.I.; Tourkova, I.L.; Syggelos, S.A.; Deligianni, D.; Orkoula, M.G.; Kontoyannis, C.G.; Karavia, E.A.; Kypreos, K.E.; et al. Apolipoprotein A-1 regulates osteoblast and lipoblast precursor cells in mice. Lab. Investig. 2016, 96, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Bonewald, L.F.; Mundy, G.R. Role of transforming growth factor-beta in bone remodeling. Clin. Orthop. Relat. Res. 1990, 250, 261–276. [Google Scholar] [CrossRef]
- Faustini, M.; Bucco, M.; Chlapanidas, T.; Lucconi, G.; Marazzi, M.; Tosca, M.C.; Gaetani, P.; Klinger, M.; Villani, S.; Ferretti, V.V.; et al. Nonexpanded Mesenchymal Stem Cells for Regenerative Medicine: Yield in Stromal Vascular Fraction from Adipose Tissues. Tissue Eng. Part C Methods 2010, 16, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Gaetani, P.; Torre, M.L.; Klinger, M.; Faustini, M.; Crovato, F.; Bucco, M.; Marazzi, M.; Chlapanidas, T.; Levi, D.; Tancioni, F.; et al. Adipose-derived stem cell therapy for intervertebral disc regeneration: An in vitro reconstructed tissue in alginate capsules. Tissue Eng. Part A 2008, 14, 1415–1423. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Bari, E.; Perteghella, S.; Marrubini, G.; Sorrenti, M.; Catenacci, L.; Tripodo, G.; Mastrogiacomo, M.; Mandracchia, D.; Trapani, A.; Farago, S.; et al. In vitro efficacy of silk sericin microparticles and platelet lysate for intervertebral disk regeneration. Int. J. Biol. Macromol. 2018, 118, 792–799. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bari, E.; Tartara, F.; Cofano, F.; di Perna, G.; Garbossa, D.; Perteghella, S.; Sorlini, M.; Mandracchia, D.; Giovannelli, L.; Gaetani, P.; et al. Freeze-Dried Secretome (Lyosecretome) from Mesenchymal Stem/Stromal Cells Promotes the Osteoinductive and Osteoconductive Properties of Titanium Cages. Int. J. Mol. Sci. 2021, 22, 8445. https://doi.org/10.3390/ijms22168445
Bari E, Tartara F, Cofano F, di Perna G, Garbossa D, Perteghella S, Sorlini M, Mandracchia D, Giovannelli L, Gaetani P, et al. Freeze-Dried Secretome (Lyosecretome) from Mesenchymal Stem/Stromal Cells Promotes the Osteoinductive and Osteoconductive Properties of Titanium Cages. International Journal of Molecular Sciences. 2021; 22(16):8445. https://doi.org/10.3390/ijms22168445
Chicago/Turabian StyleBari, Elia, Fulvio Tartara, Fabio Cofano, Giuseppe di Perna, Diego Garbossa, Sara Perteghella, Marzio Sorlini, Delia Mandracchia, Lorella Giovannelli, Paolo Gaetani, and et al. 2021. "Freeze-Dried Secretome (Lyosecretome) from Mesenchymal Stem/Stromal Cells Promotes the Osteoinductive and Osteoconductive Properties of Titanium Cages" International Journal of Molecular Sciences 22, no. 16: 8445. https://doi.org/10.3390/ijms22168445
APA StyleBari, E., Tartara, F., Cofano, F., di Perna, G., Garbossa, D., Perteghella, S., Sorlini, M., Mandracchia, D., Giovannelli, L., Gaetani, P., Torre, M. L., & Segale, L. (2021). Freeze-Dried Secretome (Lyosecretome) from Mesenchymal Stem/Stromal Cells Promotes the Osteoinductive and Osteoconductive Properties of Titanium Cages. International Journal of Molecular Sciences, 22(16), 8445. https://doi.org/10.3390/ijms22168445











