Angiopoietin-like Proteins in Colorectal Cancer—A Literature Review
Abstract
:1. Colorectal Cancer
2. Angiopoietin-like Proteins
3. Angiopoietin-like Proteins in Colorectal Cancer
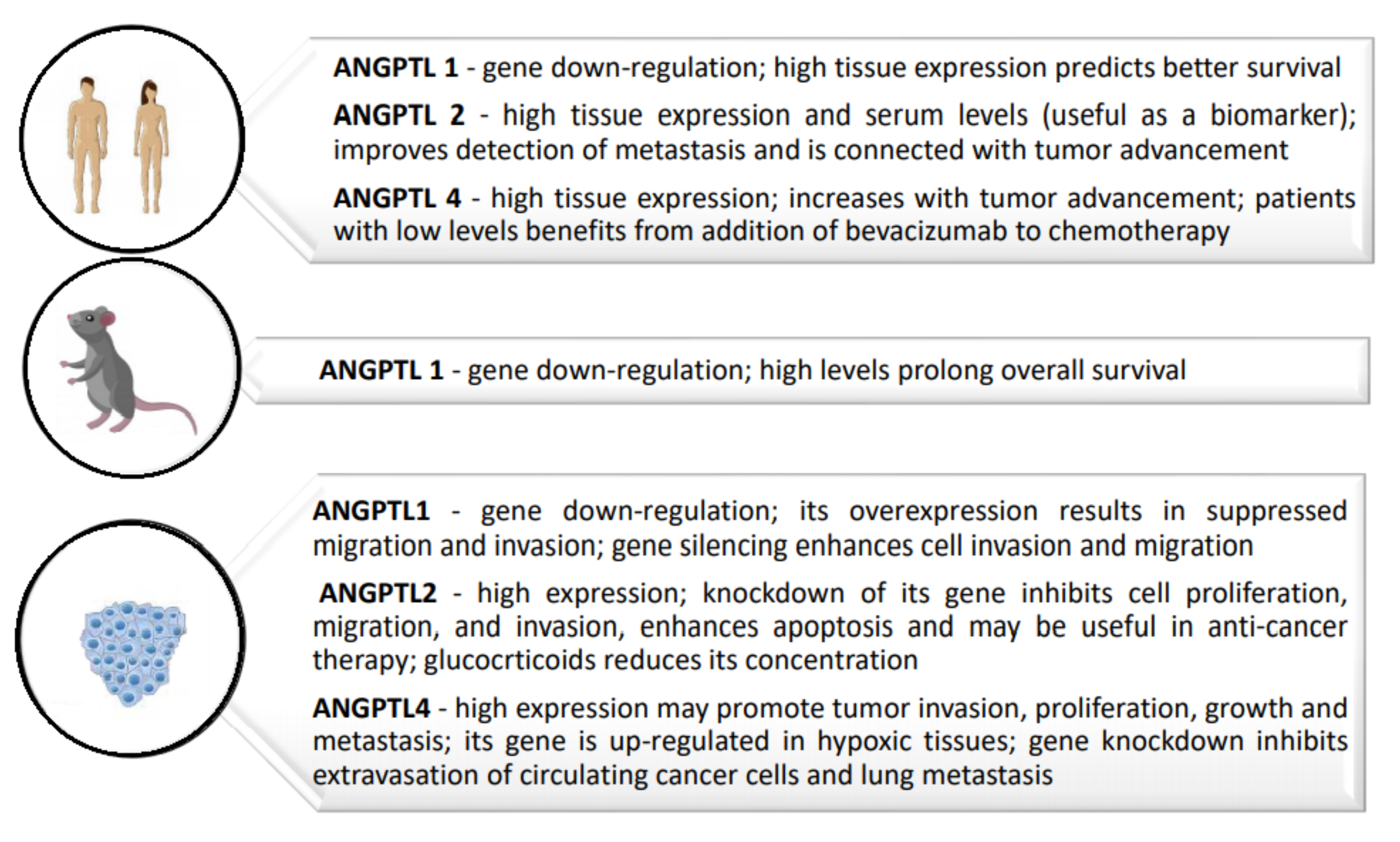
3.1. Angiopoietin-like Protein 1
3.2. Angiopoietin-like Protein 2
3.3. Angiopoietin-like Protein 4
3.4. Angiopoietin-like Protein 5
3.5. Angiopoietin-like Protein 6
3.6. Angiopoietin-like Protein 7
4. Summary
5. Conclusions
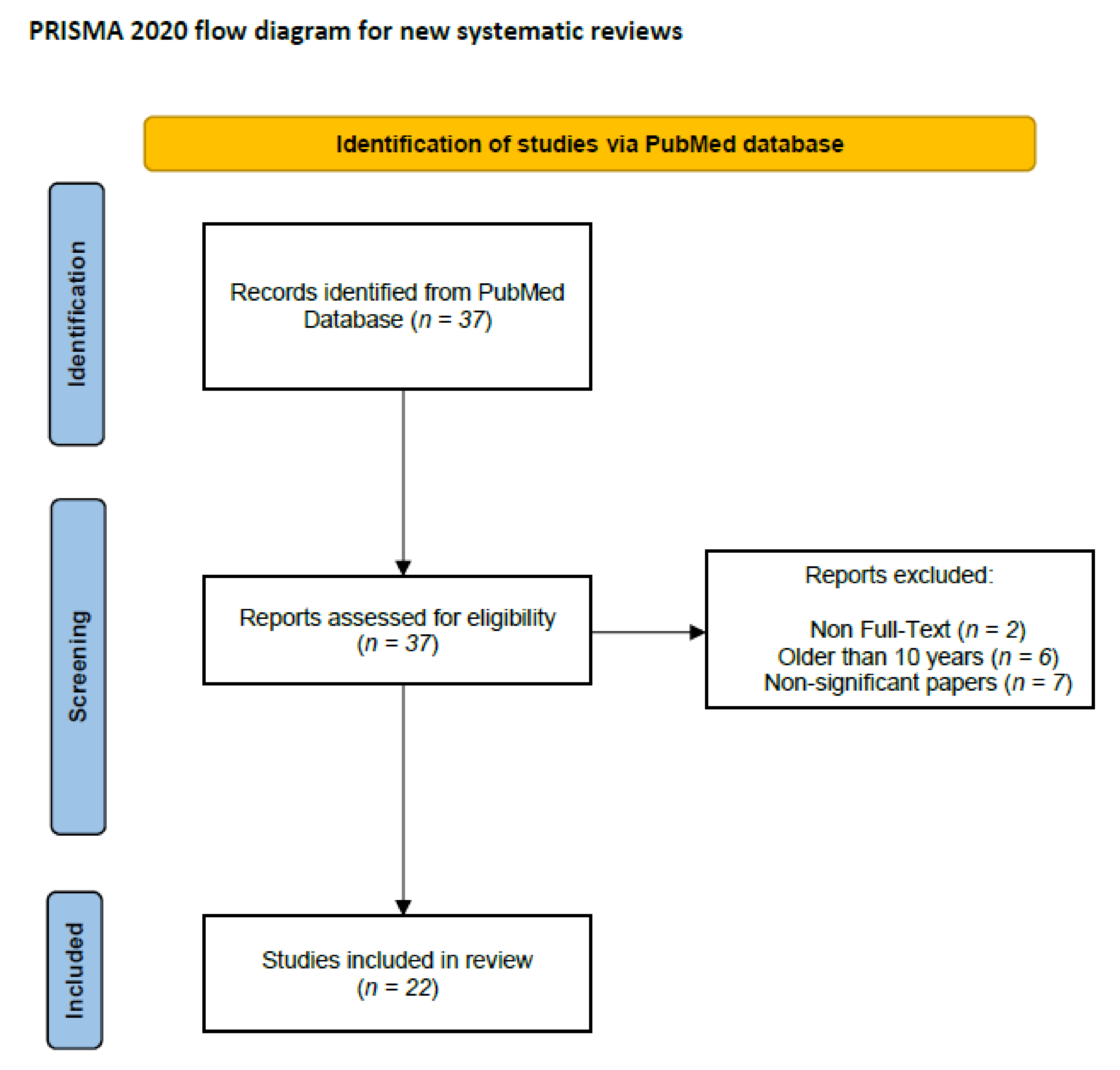
6. Literature Search and Data Extraction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Grant, W.B. Review of Recent Advances in Understanding the Role of Vitamin D in Reducing Cancer Risk: Breast, Colorectal, Prostate, and Overall Cancer. Anticancer Res. 2019, 40, 491–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Chen, C.; Zhong, Y.N.; Zhao, F.; Hao, Z.; Xu, Y.; Lai, R.; Shen, G.; Yin, X. Effect and mechanism of vitamin D on the development of colorectal cancer based on intestinal flora disorder. J. Gastroenterol. Hepatol. 2019, 35, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Komarova, N.L.; Boland, C.R.; Goel, A.; Wodarz, D. Aspirin and the chemoprevention of cancers: A mathematical and evolutionary dynamics perspective. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1487. [Google Scholar] [CrossRef]
- Shiels, M.S.; Katki, H.A.; Freedman, N.D.; Purdue, M.; Wentzensen, N.; Trabert, B.; Kitahara, C.M.; Furr, M.; Li, Y.; Kemp, T.J.; et al. Cigarette Smoking and Variations in Systemic Immune and Inflammation Markers. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Balchen, V.; Simon, K. Colorectal cancer development and advances in screening. Clin. Interv. Aging 2016, 11, 967–976. [Google Scholar] [CrossRef] [Green Version]
- Nee, J.; Chippendale, R.Z.; Feuerstein, J.D. Screening for Colon Cancer in Older Adults: Risks, Benefits, and When to Stop. Mayo Clin. Proc. 2020, 95, 184–196. [Google Scholar] [CrossRef] [Green Version]
- Das, V.; Kalita, J.; Pal, M. Predictive and prognostic biomarkers in colorectal cancer: A systematic review of recent advances and challenges. Biomed. Pharmacother. 2017, 87, 8–19. [Google Scholar] [CrossRef]
- Oike, Y.; Yasunaga, K.; Suda, T. Angiopoietin-Related/Angiopoietin-like Proteins Regulate Angiogenesis. Int. J. Hematol. 2004, 80, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.L.; Dumont, D.J. The angiopoietins and Tie2/Tek: Adding to the complexity of cardiovascular development. Semin. Cell Dev. Biol. 2002, 13, 19–27. [Google Scholar] [CrossRef]
- Kim, I.; Kwak, H.J.; Ahn, J.E.; So, J.-N.; Liu, M.; Koh, K.N.; Koh, G.Y. Molecular cloning and characterization of a novel angiopoietin family protein, angiopoietin-3. FEBS Lett. 1999, 443, 353–356. [Google Scholar] [CrossRef] [Green Version]
- Santulli, G. Angiopoietin-like Proteins: A Comprehensive Look. Front. Endocrinol. 2014, 5, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endo, M. The Roles of ANGPTL Families in Cancer Progression. J. UOEH 2019, 41, 317–325. [Google Scholar] [CrossRef] [Green Version]
- Stitziel, N.O.; Khera, A.V.; Wang, X.; Bierhals, A.J.; Vourakis, C.; Sperry, A.E.; Natarajan, P.; Klarin, D.; Emdin, C.A.; Zekavat, S.; et al. ANGPTL3 Deficiency and Protection Against Coronary Artery Disease. J. Am. Coll. Cardiol. 2017, 69, 2054–2063. [Google Scholar] [CrossRef] [PubMed]
- Dewey, F.E.; Gusarova, V.; Dunbar, R.; O’Dushlaine, C.; Schurmann, C.; Gottesman, O.; McCarthy, S.; Van Hout, C.V.; Bruse, S.; Dansky, H.M.; et al. Genetic and Pharmacologic Inactivation of ANGPTL3 and Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Myocardial Infarction Genetics and CARDIoGRAM Exome Consortia Investigators Coding Variation in ANGPTL4, LPL and SVEP1and the Risk of Coronary Disease. N. Engl. J. Med. 2016, 374, 1134–1144. [CrossRef] [PubMed] [Green Version]
- Davies, B.S.J. Can targeting ANGPTL proteins improve glucose tolerance? Diabetologia 2018, 61, 1277–1281. [Google Scholar] [CrossRef] [Green Version]
- Robciuc, M.R.; Naukkarinen, J.; Ortega-Alonso, A.; Tyynismaa, H.; Raivio, T.; Rissanen, A.; Kaprio, J.; Ehnholm, C.; Jauhiainen, M.; Pietiläinen, K. Serum angiopoietin-like 4 protein levels and expression in adipose tissue are inversely correlated with obesity in monozygotic twins. J. Lipid Res. 2011, 52, 1575–1582. [Google Scholar] [CrossRef] [Green Version]
- Noto, D.; Cefalu, A.B.; Valenti, V.; Fayer, F.; Pinotti, E.; Ditta, M.; Spina, R.; Vigna, G.; Yue, P.; Kathiresan, S.; et al. Prevalence of ANGPTL3 and APOB Gene Mutations in Subjects With Combined Hypolipidemia. Arter. Thromb. Vasc. Biol. 2012, 32, 805–809. [Google Scholar] [CrossRef] [Green Version]
- Minicocci, I.; Santini, S.; Cantisani, V.; Stitziel, N.; Kathiresan, S.; Arroyo, J.A.; Martí, G.; Pisciotta, L.; Noto, D.; Cefalu, A.B.; et al. Clinical characteristics and plasma lipids in subjects with familial combined hypolipidemia: A pooled analysis. J. Lipid Res. 2013, 54, 3481–3490. [Google Scholar] [CrossRef] [Green Version]
- Di Costanzo, A.; Di Leo, E.; Noto, D.; Cefalù, A.B.; Minicocci, I.; Polito, L.; D’Erasmo, L.; Cantisani, V.; Spina, R.; Tarugi, P.; et al. Clinical and biochemical characteristics of individuals with low cholesterol syndromes: A comparison between familial hypobetalipoproteinemia and familial combined hypolipidemia. J. Clin. Lipidol. 2017, 11, 1234–1242. [Google Scholar] [CrossRef]
- Ruge, T.; Sukonina, V.; Kroupa, O.; Makoveichuk, E.; Lundgren, M.; Svensson, M.K.; Olivecrona, G.; Eriksson, J.W. Effects of hyperinsulinemia on lipoprotein lipase, angiopoietin-like protein 4, and glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein 1 in subjects with and without type 2 diabetes mellitus. Metababolism 2012, 61, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Janssen, A.W.F.; Katiraei, S.; Bartosinska, B.; Eberhard, D.; Van Dijk, K.W.; Kersten, S. Loss of angiopoietin-like 4 (ANGPTL4) in mice with diet-induced obesity uncouples visceral obesity from glucose intolerance partly via the gut microbiota. Diabetologia 2018, 61, 1447–1458. [Google Scholar] [CrossRef] [Green Version]
- Vatner, D.F.; Goedeke, L.; Camporez, J.-P.G.; Lyu, K.; Nasiri, A.R.; Zhang, D.; Bhanot, S.; Murray, S.F.; Still, C.D.; Gerhard, G.S.; et al. Angptl8 antisense oligonucleotide improves adipose lipid metabolism and prevents diet-induced NAFLD and hepatic insulin resistance in rodents. Diabetologia 2018, 61, 1435–1446. [Google Scholar] [CrossRef] [Green Version]
- Tuuri, A.L.; Jauhiainen, M.S.; Ehnholm, C.P.; Tikkanen, M.J.; Nicholls, M.G.; Kaaja, R.J. Elevated serum angiopoietin-like protein 6 in women with subsequent pregnancy-induced hypertension: A preliminary study. Hypertens. Pregnancy 2013, 32, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Carbone, C.; Piro, G.; Merz, V.; Simionato, F.; Santoro, R.; Zecchetto, C.; Tortora, G.; Melisi, D. Angiopoietin-like Proteins in Angiogenesis, Inflammation and Cancer. Int. J. Mol. Sci. 2018, 19, 431. [Google Scholar] [CrossRef] [Green Version]
- Viallard, C.; Larrivée, B. Tumor angiogenesis and vascular normalization: Alternative therapeutic targets. Angiogenesis 2017, 20, 409–426. [Google Scholar] [CrossRef]
- Tian, Z.; Miyata, K.; Tazume, H.; Sakaguchi, H.; Kadomatsu, T.; Horio, E.; Takahashi, O.; Komohara, Y.; Araki, K.; Hirata, Y.; et al. Perivascular adipose tissue-secreted angiopoietin-like protein 2 (Angptl2) accelerates neointimal hyperplasia after endovascular injury. J. Mol. Cell. Cardiol. 2013, 57, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, J.H.; Kim, J.-H.; Martinus, R.; Park, S.H. Angiopoietin-like protein 2, a chronic inflammatory mediator, is a new target induced by TGF-β1 through a Smad3-dependent mechanism. Biochem. Biophys. Res. Commun. 2013, 430, 981–986. [Google Scholar] [CrossRef]
- Khoury, M.; Drake, A.; Chen, Q.; Dong, D.; Leskov, I.B.; Fragoso, M.F.; Li, Y.; Iliopoulou, B.P.; Hwang, W.Y.K.; Lodish, H.F.; et al. Mesenchymal Stem Cells Secreting Angiopoietin-like-5 Support Efficient Expansion of Human Hematopoietic Stem Cells Without Compromising Their Repopulating Potential. Stem Cells Dev. 2011, 20, 1371–1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comes, N.; Buie, L.K.; Borrás, T. Evidence for a role of angiopoietin-like 7 (ANGPTL7) in extracellular matrix formation of the human trabecular meshwork: Implications for glaucoma. Genes Cells 2010, 16, 243–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhanabal, M.; LaRochelle, W.J.; Jeffers, M.; Herrmann, J.; Rastelli, L.; McDonald, W.F.; A Chillakuru, R.; Yang, M.; Boldog, F.L.; Padigaru, M.; et al. Angioarrestin: An antiangiogenic protein with tumor-inhibiting properties. Cancer Res. 2002, 62, 3834–3841. [Google Scholar]
- Chen, H.; Xiao, Q.; Hu, Y.; Chen, L.; Jiang, K.; Tang, Y.; Tan, Y.; Hu, W.; Wang, Z.; He, J.; et al. ANGPTL1 attenuates colorectal cancer metastasis by up-regulating microRNA-138. J. Exp. Clin. Cancer Res. 2017, 36, 1–13. [Google Scholar] [CrossRef]
- Fan, H.; Huang, L.; Zhuang, X.; Ai, F.; Sun, W. Angiopoietin-like protein 1 inhibits epithelial to mesenchymal transition in colorectal cancer cells via suppress Slug expression. Cytotechnology 2019, 71, 35–44. [Google Scholar] [CrossRef]
- Yoshinaga, T.; Shigemitsu, T.; Nishimata, H.; Kitazono, M.; Hori, E.; Tomiyoshi, A.; Takei, T.; Yoshida, M. Angiopoietin-like protein 2 as a potential biomarker for colorectal cancer. Mol. Clin. Oncol. 2015, 3, 1080–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toiyama, Y.; Inoue, Y.; Shimura, T.; Fujikawa, H.; Saigusa, S.; Hiro, J.; Kobayashi, M.; Ohi, M.; Araki, T.; Tanaka, K.; et al. Serum Angiopoietin-like Protein 2 Improves Preoperative Detection of Lymph Node Metastasis in Colorectal Cancer. Anticancer Res. 2015, 35, 2849–2856. [Google Scholar]
- Toiyama, Y.; Tanaka, K.; Kitajima, T.; Shimura, T.; Kawamura, M.; Kawamoto, A.; Okugawa, Y.; Saigusa, S.; Hiro, J.; Inoue, Y.; et al. Elevated Serum Angiopoietin-like Protein 2 Correlates with the Metastatic Properties of Colorectal Cancer: A Serum Biomarker for Early Diagnosis and Recurrence. Clin. Cancer Res. 2014, 20, 6175–6186. [Google Scholar] [CrossRef] [Green Version]
- Horiguchi, H.; Endo, M.; Miyamoto, Y.; Sakamoto, Y.; Odagiri, H.; Masuda, T.; Kadomatsu, T.; Tanoue, H.; Motokawa, I.; Terada, K.; et al. Angiopoietin-like protein 2 renders colorectal cancer cells resistant to chemotherapy by activating spleen tyrosine kinase–phosphoinositide 3-kinase-dependent anti-apoptotic signaling. Cancer Sci. 2014, 105, 1550–1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Wang, J.; Wu, S.; Zhu, S.; Wang, S.; Zhou, H.; Tian, X.; Tang, N.; Nie, S. Angiopoietin-like protein 2 negatively regulated by microRNA-25 contributes to the malignant progression of colorectal cancer. Int. J. Mol. Med. 2014, 34, 1286–1292. [Google Scholar] [CrossRef]
- Carbone, C.; Piro, G.; Simionato, F.; Ligorio, F.; Cremolini, C.; Loupakis, F.; Alì, G.; Rossini, D.; Merz, V.; Santoro, R.; et al. Homeobox B9 Mediates Resistance to Anti-VEGF Therapy in Colorectal Cancer Patients. Clin. Cancer Res. 2017, 23, 4312–4322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drebert, Z.; Macaskill, M.; Doughty-Shenton, D.; De Bosscher, K.; Bracke, M.; Hadoke, P.W.; Beck, I.M. Colon cancer-derived myofibroblasts increase endothelial cell migration by glucocorticoid-sensitive secretion of a pro-migratory factor. Vasc. Pharmacol. 2017, 89, 19–30. [Google Scholar] [CrossRef]
- Kumara, H.M.C.S.; Kirchoff, D.; Herath, S.A.; Jang, J.H.; Yan, X.; Grieco, M.; Cekic, V.; Whelan, R.L. Plasma levels of angiopoietin-like protein 4 (ANGPTL4) are significantly lower preoperatively in colorectal cancer patients than in cancer-free patients and are further decreased during the first month after minimally invasive colorectal resection. Surg. Endosc. 2012, 26, 2751–2757. [Google Scholar] [CrossRef]
- Akishima-Fukasawa, Y.; Ishikawa, Y.; Akasaka, Y.; Uzuki, M.; Inomata, N.; Yokoo, T.; Ishii, R.; Shimokawa, R.; Mukai, K.; Kiguchi, H.; et al. Histopathological predictors of regional lymph node metastasis at the invasive front in early colorectal cancer. Histopathology 2011, 59, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Tu, J.; Ding, S.; Lu, G.; Lin, Z.; Ding, Y.; Deng, B.; Zhang, Y.; Xiao, W.; Gong, W. High Expression of Angiopoietin-like Protein 4 in Advanced Colorectal Cancer and its Association with Regulatory T Cells and M2 Macrophages. Pathol. Oncol. Res. 2019, 26, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, T.; Shi, Q.; Li, J.; Cai, S.; Zhou, P.; Zhong, Y.; Yao, L. Angiopoietin-like 4 enhances metastasis and inhibits apoptosis via inducing bone morphogenetic protein 7 in colorectal cancer cells. Biochem. Biophys. Res. Commun. 2015, 467, 128–134. [Google Scholar] [CrossRef]
- He, C.; Huang, X.-F.; Han, J.; Hu, X.-T. Mechanisms involved in biological behavior changes associated with Angptl4 expression in colon cancer cell lines. Oncol. Rep. 2012, 27, 1541–1547. [Google Scholar] [CrossRef] [Green Version]
- Khong, T.L.; Thairu, N.; Larsen, H.; Dawson, P.M.; Kiriakidis, S.; Paleolog, E.M. Identification of the angiogenic gene signature induced by EGF and hypoxia in colorectal cancer. BMC Cancer 2013, 13, 518. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-H.; Park, Y.-Y.; Kim, S.-W.; Lee, J.-S.; Wang, D.; Dubois, R.N. ANGPTL4 Induction by Prostaglandin E2 under Hypoxic Conditions Promotes Colorectal Cancer Progression. Cancer Res. 2011, 71, 7010–7020. [Google Scholar] [CrossRef] [Green Version]
- Alex, S.; Lange, K.; Amolo, T.; Grinstead, J.S.; Haakonsson, A.K.; Szalowska, E.; Koppen, A.; Mudde, K.; Haenen, D.; Al-Lahham, S.; et al. Short-Chain Fatty Acids Stimulate Angiopoietin-like 4 Synthesis in Human Colon Adenocarcinoma Cells by Activating Peroxisome Proliferator-Activated Receptor. Mol. Cell. Biol. 2013, 33, 1303–1316. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.; Wang, F.-H.; Zhang, D.-S.; Li, C.; Jin, Y.; Wang, D.-S.; Chen, D.-L.; Qiu, M.-Z.; Luo, H.-Y.; Wang, Z.-Q.; et al. A plasma cytokine and angiogenic factor (CAF) analysis for selection of bevacizumab therapy in patients with metastatic colorectal cancer. Sci. Rep. 2015, 5, 17717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, C.-J.; Chang, K.-Y.; Lin, B.-W.; Lin, W.-T.; Su, C.-M.; Tsai, J.-P.; Liao, Y.-H.; Hung, L.-Y.; Chang, W.-C.; Chen, B.-K. Oleic acid-induced NOX4 is dependent on ANGPTL4 expression to promote human colorectal cancer metastasis. Theranostics 2020, 10, 7083–7099. [Google Scholar] [CrossRef] [PubMed]
- Marchiò, S.; Soster, M.; Cardaci, S.; Muratore, A.; Bartolini, A.; Barone, V.; Ribero, D.; Monti, M.; Bovino, P.; Sun, J.; et al. A complex of α 6 integrin and E-cadherin drives liver metastasis of colorectal cancer cells through hepatic angiopoietin-like 6. EMBO Mol. Med. 2012, 4, 1156–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parri, M.; Pietrovito, L.; Grandi, A.; Campagnoli, S.; De Camilli, E.; Bianchini, F.; Marchio’, S.; Bussolino, F.; Jin, B.; Sarmientos, P.; et al. Angiopoietin-like 7, a novel pro-angiogenetic factor over-expressed in cancer. Angiogenesis 2014, 17, 881–896. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Gordon-Weeks, A.; Allen, D.; Kersemans, V.; Beech, J.; Smart, S.; Muschel, R.J. Cd11b + myeloid cells support hepatic metastasis through down-regulation of angiopoietin-like 7 in cancer cells. Hepatology 2015, 62, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]


| Angiopoietin-like Protein | 1 | 2 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| Concentration/Expression | ↓ | ↑ | ↑ | ↓ | ↑ | ↑ | |
| Dependence On: | Cancer Stage | + | + | + | |||
| Tumor Type | + | + | |||||
| Hypoxia | + | + | |||||
| Angiogenesis | + | + | + | ||||
| Metastasis | + | + | + | + | |||
| Useful in Therapy | + | + | + | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zajkowska, M.; Mroczko, B. Angiopoietin-like Proteins in Colorectal Cancer—A Literature Review. Int. J. Mol. Sci. 2021, 22, 8439. https://doi.org/10.3390/ijms22168439
Zajkowska M, Mroczko B. Angiopoietin-like Proteins in Colorectal Cancer—A Literature Review. International Journal of Molecular Sciences. 2021; 22(16):8439. https://doi.org/10.3390/ijms22168439
Chicago/Turabian StyleZajkowska, Monika, and Barbara Mroczko. 2021. "Angiopoietin-like Proteins in Colorectal Cancer—A Literature Review" International Journal of Molecular Sciences 22, no. 16: 8439. https://doi.org/10.3390/ijms22168439
APA StyleZajkowska, M., & Mroczko, B. (2021). Angiopoietin-like Proteins in Colorectal Cancer—A Literature Review. International Journal of Molecular Sciences, 22(16), 8439. https://doi.org/10.3390/ijms22168439







