Functional Properties of Polyurethane Ureteral Stents with PLGA and Papaverine Hydrochloride Coating
Abstract
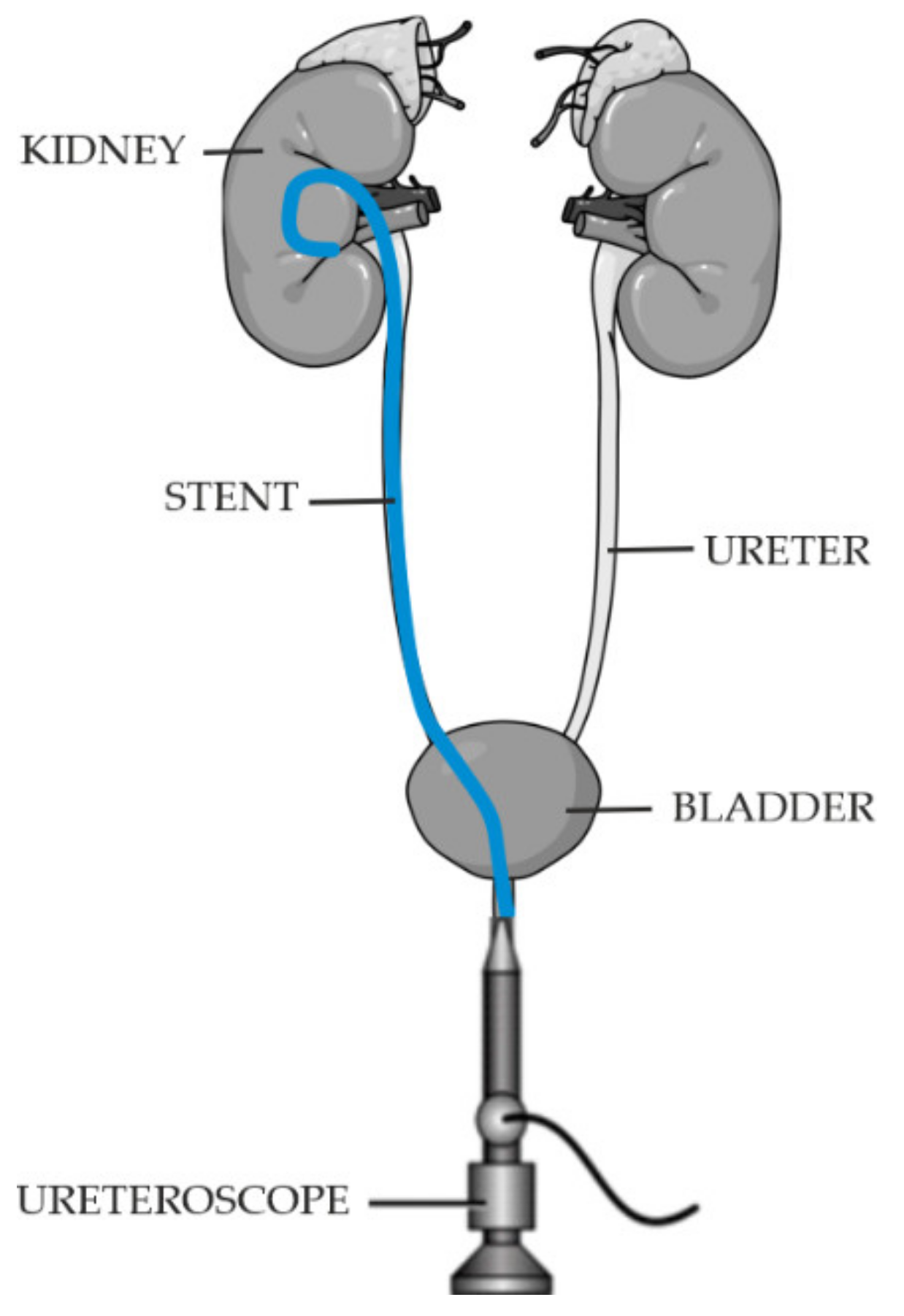
1. Introduction
- Ease of implantation and removal,
- Position stability after implantation,
- Good visibility during imaging,
- Good biocompatibility, and biodegradable after the treatment process is completed,
- Resistance to incrustation.
- Ensure continuity of urine flow,
- Not cause reflux,
- Not cause peri-implant reactions in the patient,
- Not cause irritation.
2. Materials and Methods
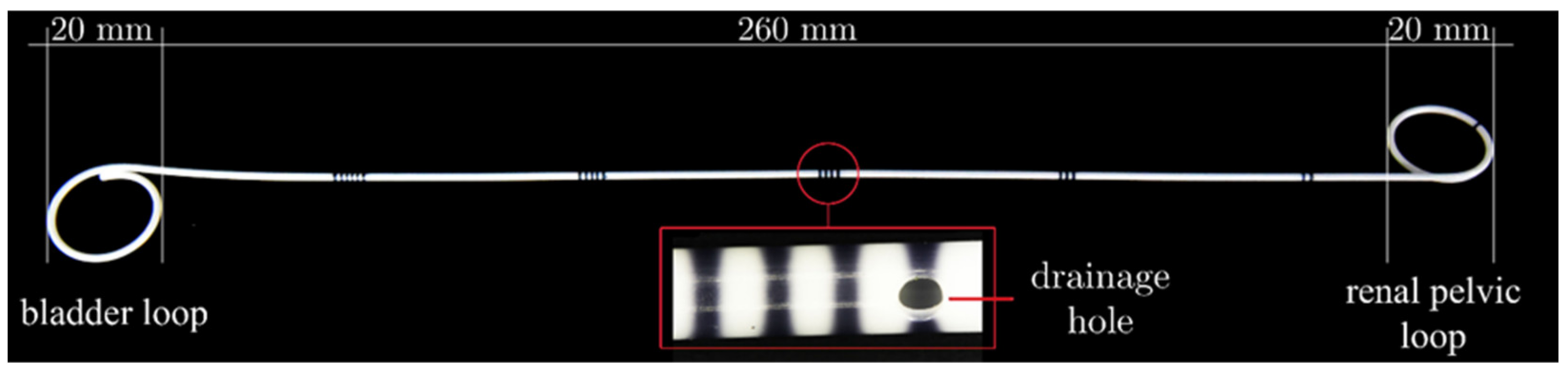
2.1. Modification of Ureteral Stents
2.2. Modification of Ureteral Stents
2.3. Stent Pretreatment Procedures In Vitro
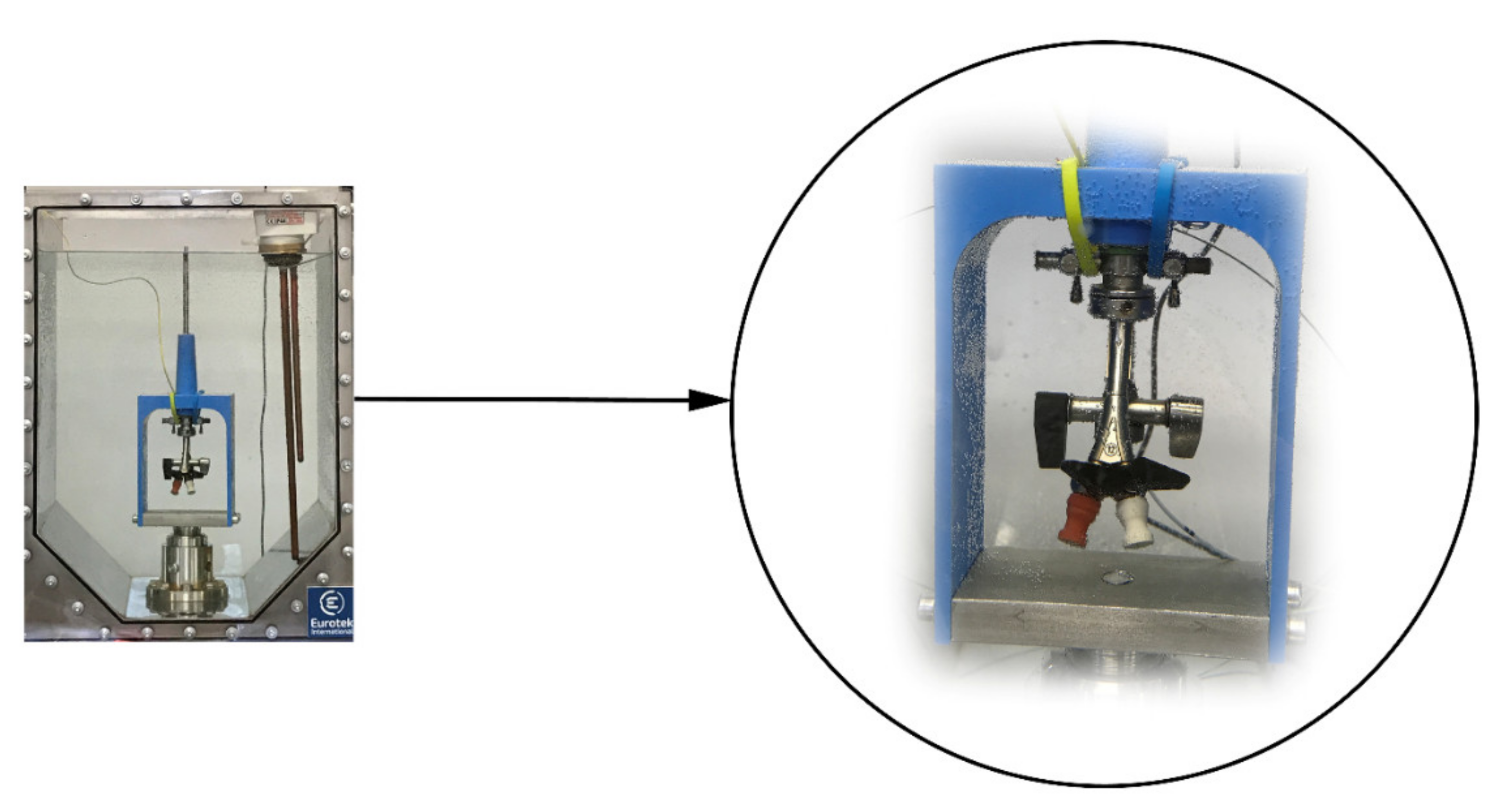
2.4. Mechanical Properties of Ureteral Stents
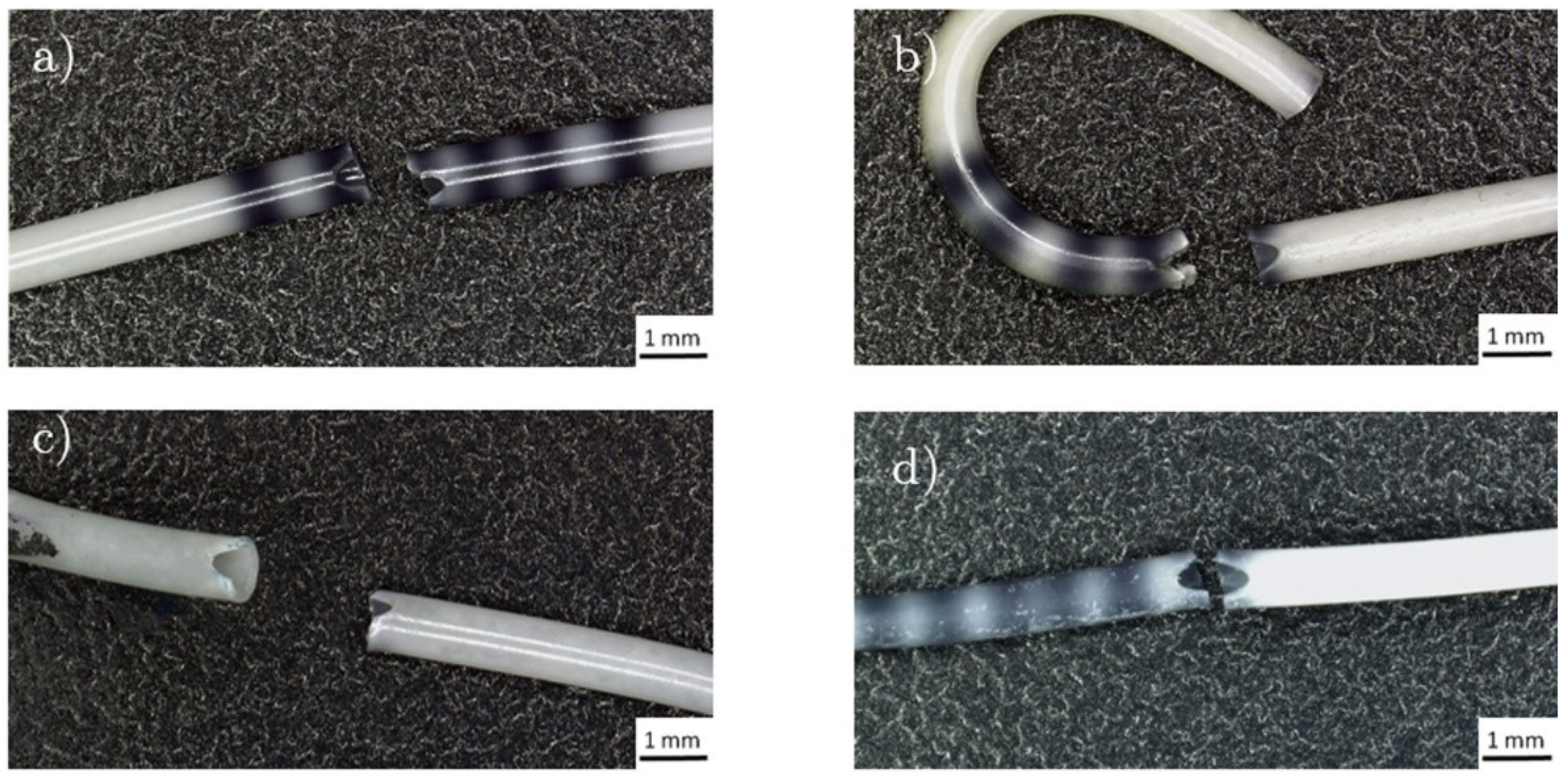
2.4.1. Break Strength
2.4.2. Retention Strength
2.4.3. Dynamic Frictional Force
2.4.4. Radiopacity
2.4.5. Statistical Analysis
3. Results
3.1. Break Strength
3.2. Retention Strength Test
3.3. Dynamic Frictional Force
3.4. Radiopacity
4. Discussion
5. Conclusions
- Increase in its strength properties, in dry and soaked condition,
- Quadruple reduction of dynamic friction force,
- lowering the value of the retention strength of the stent tips: proximal and distal, in a dry and soaked state.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Uthappa, M.C.; Cowan, N.C. Retrograde or Antegrade Double-Pigtail Stent Placement for Malignant Ureteric Obstruction? Clin. Radiol. 2005, 60, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Ilie, V.G.; Ilie, V.I. Ureteric Stent Use-Part of the Solution and Part of the Problem. Curr. Urol. 2017, 11, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Al-Aown, A.; Kyriazis, I.; Kallidonis, P.; Kraniotis, P.; Rigopoulos, C.; Karnabatidis, D. Ureteral Stents: New Ideas, New Designs. Ther. Adv. Urol. 2010, 2, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Denstedt, J.D.; Reid, G.; Sofer, M. Advances in Ureteral Stent Technology. World. J. Urol. 2000, 18, 237–242. [Google Scholar] [CrossRef]
- Stoller, M.L.; Meng, M.V. Urinary Stone Disease. The Practical Guide to Medical and Surgical Management, 2007th ed.; Humana Press: Totowa, NJ, USA, 2007. [Google Scholar]
- Dyer, R.B.; Chen, M.Y.; Zagoria, R.J.; Regan, J.D.; Hood, C.G.; Kavanagh, P.V. Complications of Ureteral Stent Placement. Radiographics 2002, 22, 1005–1022. [Google Scholar] [CrossRef]
- Ringel, A.; Richter, S.; Shalev, M.; Nissenkorn, I. Late Complications of Ureteral Stents. Eur. Urol. 2000, 38, 41–44. [Google Scholar] [CrossRef]
- Finney, R.P. Experience with New Double J Ureteral Catheter stent. J. Urol. 1978, 120, 678–681. [Google Scholar] [CrossRef]
- Solari, V.; Piotrowska, A.P.; Puri, P. Altered expression of interstitial cells of Cajal in congenital ureteropelvic junction obstruction. J. Urol. 2003, 1, 2420–2422. [Google Scholar] [CrossRef]
- Lammers, W.J.; Ahmad, H.R.; Arafat, K. Spatial and temporal variations in pacemaking and conduction in the isolated renal pelvis. Am. J. Physiol. Renal. Physiol. 1996, 270, 567–574. [Google Scholar] [CrossRef]
- Nakagawa, Y. Mechanical Property of The Human Ureter. Jpn. J. Urol. 1989, 80, 1481–1488. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rassoli, A.; Shafigh, M.; Seddighi, A.; Seddighi, A.; Daneshhparvar, H.; Fatouraee, N. Biaxial Mechanical Properties of Human Ureter Under Tension. Urol. J. 2014, 11, 1678–1686. [Google Scholar]
- Nagele, U.; Anastasiadis, A.G.; Amend, B.; Schilling, D.; Kuczyk, M.; Stenzl, A.; Sievert, K.D. Steerable antegrade stenting: A new trick of the trade. Int. Braz. J. Urol. 2007, 33, 389–394. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lange, D.; Bidnur, S.; Hoag, N.; Chew, B.H. Ureteral stent-associated complications-where we are and where we are going. Nat. Rev. Urol. 2015, 12, 17–25. [Google Scholar] [CrossRef]
- He, F.; Man, L.B.; Li, G.Z.; Liu, N. Efficacy of α-Blocker in Improving Ureteral Stent-Related Symptoms: A Meta-Analysis of Both Direct And Indirect Comparison. Drug Des. Dev. Ther. 2016, 10, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Golzari, S.; Soleimanpour, H.; Rahmani, F.; Mehr, N.Z.; Safari, S.; Heshmat, Y.; Bakhtavar, H.E. Therapeutic Approaches for Renal colic in the Emergency Department: A Review Article. Anest. Pain Med. 2014, 4, 1–12. [Google Scholar] [CrossRef]
- Yencilek, F.; Aktas, C.; Goktas, C.; Yilmaz, C.; Yilmaz, U.; Sarica, K. Role of Papaverine Hydrochloride Administration in Patients with Intractable Renal Colic: Randomized Prospective Trial. Urology 2008, 72, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.D.; Sur, R.L.; Springhart, W.P.; Marguet, C.G.; Mathias, B.J.; Pietrow, P.K. A Prospective, Randomize, Double-Blinded Placebo-Controlled Comparison of Extended Release Oxybutynin Versus Phenazopyridine for The Management of Postoperative Ureteral Stent Discomfort. J. Urol. 2008, 71, 792–795. [Google Scholar]
- Beddingfield, R.; Pedro, R.N.; Hinck, B.; Kreidberg, C.; Feia, K.; Monga, M. Alfuzosin To Relieve Ureteral Stent Discomfort: A Prospective, Randomized, Placebo Controlled Study. J. Urol. 2009, 181, 170–176. [Google Scholar] [CrossRef]
- Mardis, H.K.; Kroeger, R.M.; Hepperlen, T.W. Polyethylene double-pigtail ureteral stents. Urol. Clin. N. Am. 1982, 9, 95–101. [Google Scholar] [CrossRef]
- Ray, R.P.; Mahapatra, R.S.; Modnal, P.P.; Pal, D.K. Long-term complications of JJ stent and its management: A 5 year review. Urol. Ann. 2015, 7, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Ahallal, Y.; Khannouk, A.; El Fassi, M.J.; Farih, M.H. Risk Factor Analysis and Management of Ureteral Double-J stent complications. Rev. Urol. 2010, 12, 147–151. [Google Scholar]
- Kandemir, A.; Sonmez, M.G. Treatment of fragmented and severely encustrated ureteral double-J stent forgotten for 11 years through multimodal endourological methods. Urol. Ann. 2019, 11, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Farshid, S.; Sharifi-Aghdas, F.; Varyani, M. Fragmented ureteral stent extraction by antegrade and retrograde access: Using ureteroscope and nephroscope. Urol. Case Rep. 2019, 24, 100871. [Google Scholar] [CrossRef] [PubMed]
- Tao, G.; Wu, G.; Yang, L.; Wang, F.; Han, C.; Liu, F.; Yuan, J. Fragmentation of Severely encrusted ureteral stent indwelled for 4 years in a boy. Urol. Case Rep. 2017, 12, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Goel, H.K.; Kundu, A.K.; Maji, T.K.; Pal, D.K. Retained fragmented double J ureteric stent: A report of four cases with review of the literature. Saudi J. Kidney Dis. Transpl. 2015, 26, 747–750. [Google Scholar] [CrossRef]
- Barros, A.A.; Oliviera, C.; Lima, E.; Duarte, A.R.C.; Reis, R.L. Gelatin-based biodegradable ureteral stents with enhanced mechanical properties. App. Mater. Today 2016, 5, 9–18. [Google Scholar] [CrossRef]
- Wen, C.; Lu, L.; Li, X. Mechanically Robust Gelatin-Alginate IPN Hydrogels IPN hydrogels by a combination of enzymatic and ionic crosslinking approaches. Macromol. Mater. Eng. 2013, 299, 504–513. [Google Scholar] [CrossRef]
- Shan, H.; Cao, Z.; Chi, C.; Wang, J.; Wang, X.; Tian, J.; Yu, B. Advances in drug delivery via biodegradable ureteral stent for the treatment of upper tract urothelial carcinoma. Front. Pharmacol. 2020, 11, 224. [Google Scholar] [CrossRef]
- El-Rehewy, M.S.K.; El-Feky, M.A.; Hassan, M.A.; Abolella, H.A.; Abolyosr, A.; El-Baky, R.M.A.; Gad, G.F. In Vitro Efficacy of Ureteral Catheters Impregnated with Ciprofloxacin, N-Acetylcysteine and Their Combinations on Microbial Adherence. Clin. Med. Urol. 2009, 3, 1–8. [Google Scholar]
- Yang, L.; Whiteside, S.; Cadieux, P.A.; Denstedt, J.D. Ureteral stent technology: Drug-eluting stents and stent coatings. Asian J. Urol. 2015, 2, 194–201. [Google Scholar] [CrossRef]
- Elayarajah, E.; Rajendran, R.; Venkatrajah, B.; Sreekumar, S. Biopolymer Tocopherol Acetate as A Drug Carrier to Prevent Bacterial Biofilm Formation on Silicone Ureteral Stents. Int. J. Pharm. Sci. Rev. Res. 2011, 7, 96–103. [Google Scholar]
- Jin, S.; Xia, X.; Huang, J.; Yuan, C.; Zuo, Y.; Li, Y.; Li, J. Recent advances in PLGA-based biomaterials for bone tissue regeneration. Acta Biomateri. 2021, 127, 56–79. [Google Scholar] [CrossRef]
- Babilotte, J.; Martin, B.; Guduric, V.; Bareille, R.; Agniel, R. Development and characterization of a PLGA-HA composite material to fabricate 3D-printed scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2021, 118, 111334–111347. [Google Scholar] [CrossRef] [PubMed]
- Shadman, B.; Asadi, A.; Zahri, S.; Barzegar, A.; Marzban, A. Design of PLGA-based Scaffolds for Developing and Differentiating Mesenchymal Stem Cells (MSCs). Biointerface Res. Appl. Chem. 2021, 11, 12732–12742. [Google Scholar]
- Chlopek, J.; Morawska-Chochol, A.; Paluszkiewicz, C.; Jaworska, J.; Kasperczyk, J.; Dobrzyński, P. FTIR and NMR study of poly(lactide-co-glycolide) and hydroxyapatite implant degradation under in vivo conditions. Polym. Degrad. Stab. 2009, 94, 1479–1485. [Google Scholar] [CrossRef]
- Fredenberg, C.S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanism of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems-a review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef]
- Cauda, F.; Cauda, V.; Fiori, C.; Onida, B.; Garrone, E. Heparin coating on ureteral Double J stents prevents encrustations: An in vivo case study. J Endourol. 2008, 22, 465–472. [Google Scholar] [CrossRef] [PubMed]
- McManamon, C.; de Silva, J.P.; Delaney, P.; Morris, M.A.; Cross, G.L.W. Characteristics, interactions and coating adherence of heterogeneous polymer/drug coatings for biomedical devices. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 102–108. [Google Scholar] [CrossRef]
- Jaworska, J.; Jelonek, K.; Kajzer, W.; Szewczenko, J.; Kaczmarczyk, B.; Marcinkowski, A.; Janeczek, H.; Pastusiak, M.; Basiaga, M.; Kasperczyk, J. Comparison of biodegradable poly(glycolide-epsilon-caprolactone) and poly(glycolide-epsilon-caprolactone-D,L-lactide) coatings enriched with ciprofloxacin formed on Ti6Al4V alloy. Polym. Degrad. Stab. 2018, 155, 136–144. [Google Scholar] [CrossRef]
- Kazek-Kesik, A.; Jaworska, A.; Krok-Borkowicz, J.; Golda-Cepa, M.; Pastusiak, M.; Brzychczy-Wloch, M.; Pamula, M.; Kotarba, E.; Simka, W. Hybrid oxide-polymer layer formed on Ti-15Mo alloy surface enhancing antibacterial and osseointegration functions. Surf. Coat. Technol. 2016, 302, 158–165. [Google Scholar] [CrossRef]
- Jaworska, J.; Jelonek, K.; Jaworska-Kik, M.; Musiał-Kulik, M.; Marcinkowski, A.; Szewczenko, J.; Kajzer, W.; Pastusiak, M.; Kasperczyk, J. Development of antibacterial, ciprofloxacin-eluting biodegradable coatings on Ti6Al7Nb implants to prevent peri-implant infections. J. Biomed. Mater. Res. A 2020, 108, 1006–1015. [Google Scholar] [CrossRef]
- Szewczenko, J.; Kajzer, W.; Kajzer, A.; Basiaga, M.; Kaczmarek, M.; Antonowicz, M.; Nowińska, K.; Jaworska, J.; Jelonek, K.; Kasperczyk, J. Biodegradable polymer coating on Ti6Al7Nb alloy. Acta Bioeng. Bomech. 2019, 21, 83–92. [Google Scholar] [CrossRef]
- Golda-Cepa, M.; Chorylek, A.; Chytrosz, P.; Brzychczy–Wloch, M.; Jaworska, J.; Kasperczyk, J.; Hakkarainen, M.; Engvall, K.; Kotarba, A. Multifunctional PLGA/Parylene C Coating for Implant Materials-an Integral Approach for Biointerface Optimization. ACS Appl. Mater. Interfaces 2016, 8, 22093–22105. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyński, P.; Bero, M.; Kasperczyk, J. Sposob wytwarzania bioresorbowalnych polimerow. Standard 2000, 191846, B1. [Google Scholar]
- Burns, J.R.; Finlayson, B. Proposal for a standard reference artificial urine in in vitro urolithiasis experiments. Investig. Urol. 1980, 18, 167–169. [Google Scholar]
- Standard Specification for Ureteral Stents; ASTM F1828-97; ASTM International: West Conshohocken, PA, USA, 1997.
- Tworzywa Sztuczne-Oznaczanie Właściwości Mechanicznych przy Statycznym Rozciąganiu; PN-EN ISO 527:2012; Politechnika Wroclawska: Wrocław, Poland, 2012.
- Evaluation of Measurement Data-Guide to the Expression of Uncertainty in Measurement; JCGM 100:2008; Bureau International des Poids et Mesures: Sèvres, France, 2008.
- Characteristics Card; PAN-ICSD:98-003-3730. University of Cambridge: Cambridge, UK. Available online: https://www.crystallography.net/cod/archives/2021/PANalytical/ (accessed on 14 July 2021).
- Chua, M.E.; Morales, M.L. Spontaneous Fracture of Indwelling Polyurethane Ureteral Stents: A Case Series and Review of Literature. Can. Urol. Assoc. J. 2012, 6, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Choi, Y.H.; Lee, S.B.; Baba, Y.; Kim, K.W.; Suh, S.H. Numerical Analysis of Urine Flow Through the Side Holes of a Double J Stent in A Ureteral Stenosis. Technol. Health Care 2017, 25, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Gorman, S.P.; Jones, D.S.; Bonner, M.C.; Akay, M.; Keane, F.P. Mechanical Performance of Polyurethane Ureteral Stents in vitro And ex vivo. Biomaterials 1997, 18, 1379–1383. [Google Scholar] [CrossRef]
- Beiko, D.T.; Knudsen, B.E.; Denstedt, J.D. Advances in Ureteral Stent Design. J. Endourol. 2003, 17, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Hendlin, K.; Dockendor, F.; Horn, C.; Pshon, N.; Lund, B.; Monga, M. Ureteral Stent: Coil Strength and Durometer. Urology 2006, 68, 42–45. [Google Scholar] [CrossRef]
- Kilciler, M.; Erdemir, F.; Bedir, S.; Coban, H.; Erten, K.; Guven, O.; Topac, H. Spontaneous Ureteral Stent Fragmentation: A Case Report and Review of the Literature. Kaohsiung J. Med. Sci. 2006, 22, 363–366. [Google Scholar] [CrossRef]
- Cauda, V.; Chiodoni, A.; Laurenti, M.; Canavese, G.; Tommasi, T. Ureteral Double-J Stents Performances Toward Encrustation after Long-Term Indwelling in a Dynamic In Vitro Model. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 2244–2253. [Google Scholar] [CrossRef] [PubMed]
- Linder, B.J.; Occhino, J.A. Cystoscopic Ureteral Stent Placement: Techniques and Tips. Int. Urogynecol. J. 2019, 30, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, F.C.; De, S.; Sarkissian, C.; Monga, M. Hydrophilic Guidewires: Evaluation and Comparison of Their Properties and Safety. Urology 2013, 82, 1182–1186. [Google Scholar] [CrossRef] [PubMed]


| DRY | SOAKED | |||
|---|---|---|---|---|
| Mechanical Properties | Polyurethane Stents | Stents with PLGA+PAP Coating | Polyurethane Stents | Stents with PLGA+PAP Coating |
| Young’s Modulus E [MPa] | 20.4(52) | 23.1(21) | 8.86(33) | 9.2(11) |
| max. Breaking Force Fmax [N] | 29.40(60) | 38.0(25) | 32.2(49) | 35.6(62) |
| Tensile strength Rm [MPa] | 22.10(50) | 28.7(19) | 24.3(37) | 26.9(47) |
| Elongation at break A [%] | 143(35) | 153(28) | 303(36) | 293(47) |
| SAMPLE | DRY | SOAKED | ||||||
|---|---|---|---|---|---|---|---|---|
| Polyurethane Stents | Stents with PLGA+PAP Coating | Polyurethane Stents | Stents with PLGA+PAP Coating | |||||
| End | proximal | distal | proximal | distal | proximal | distal | proximal | distal |
| Fmax [N] | 0.41 (13) | 0.38 (16) | 0.1370 (40) | 0.1290 (80) | 1.64 (18) | 1.742 (40) | 1.400 (46) | 1.354 (49) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonowicz, M.; Szewczenko, J.; Jaworska, J.; Jelonek, K.; Joszko, K.; Gzik-Zroska, B.; Nuckowski, P.M.; Bryniarski, P.; Paszenda, Z.; Nakonieczny, D.S.; et al. Functional Properties of Polyurethane Ureteral Stents with PLGA and Papaverine Hydrochloride Coating. Int. J. Mol. Sci. 2021, 22, 7705. https://doi.org/10.3390/ijms22147705
Antonowicz M, Szewczenko J, Jaworska J, Jelonek K, Joszko K, Gzik-Zroska B, Nuckowski PM, Bryniarski P, Paszenda Z, Nakonieczny DS, et al. Functional Properties of Polyurethane Ureteral Stents with PLGA and Papaverine Hydrochloride Coating. International Journal of Molecular Sciences. 2021; 22(14):7705. https://doi.org/10.3390/ijms22147705
Chicago/Turabian StyleAntonowicz, Magdalena, Janusz Szewczenko, Joanna Jaworska, Katarzyna Jelonek, Kamil Joszko, Bożena Gzik-Zroska, Paweł M. Nuckowski, Piotr Bryniarski, Zbigniew Paszenda, Damian S. Nakonieczny, and et al. 2021. "Functional Properties of Polyurethane Ureteral Stents with PLGA and Papaverine Hydrochloride Coating" International Journal of Molecular Sciences 22, no. 14: 7705. https://doi.org/10.3390/ijms22147705
APA StyleAntonowicz, M., Szewczenko, J., Jaworska, J., Jelonek, K., Joszko, K., Gzik-Zroska, B., Nuckowski, P. M., Bryniarski, P., Paszenda, Z., Nakonieczny, D. S., Barabaszová, K. Č., & Kasperczyk, J. (2021). Functional Properties of Polyurethane Ureteral Stents with PLGA and Papaverine Hydrochloride Coating. International Journal of Molecular Sciences, 22(14), 7705. https://doi.org/10.3390/ijms22147705











