How Far Are Non-Viral Vectors to Come of Age and Reach Clinical Translation in Gene Therapy?
Abstract
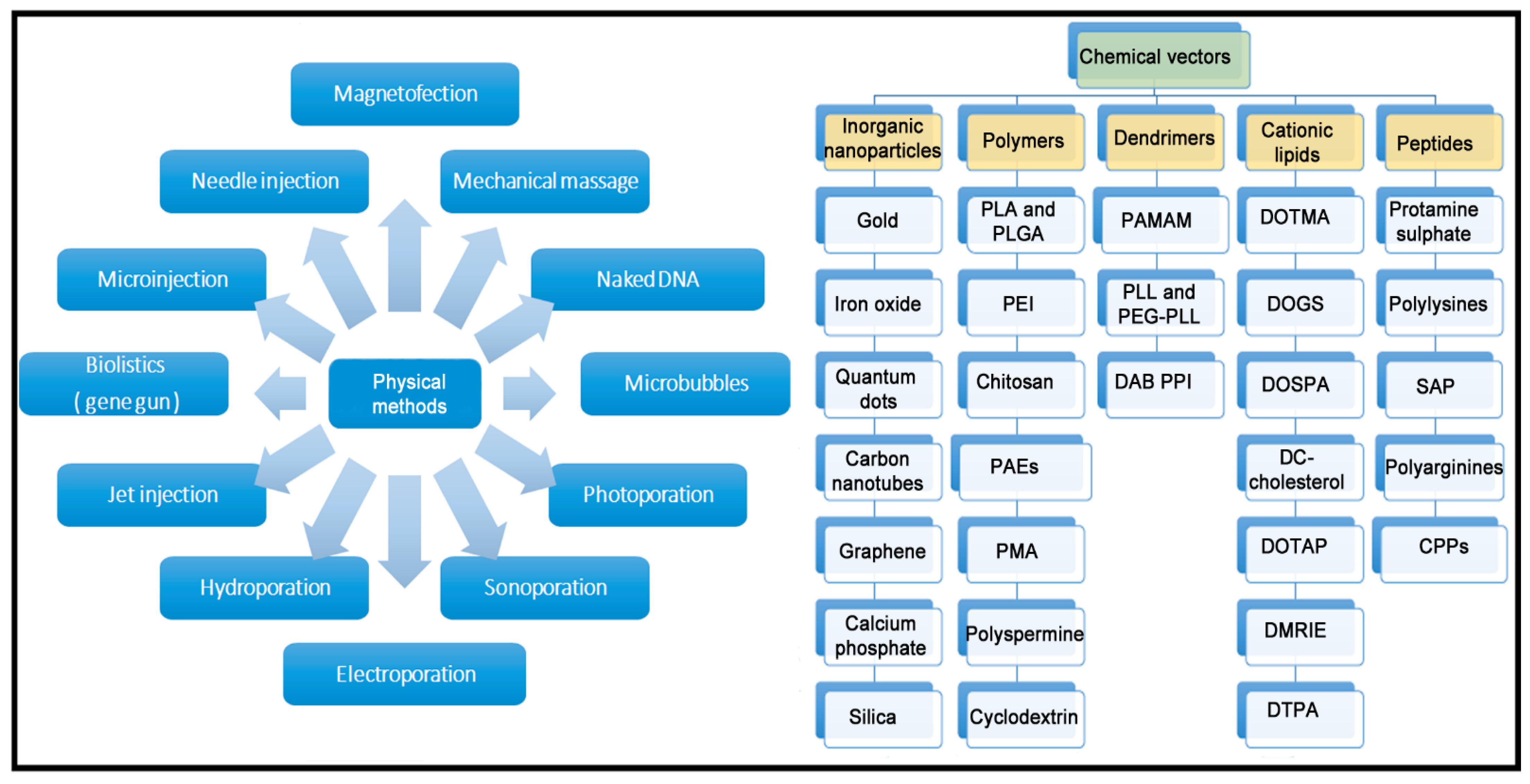
1. Introduction
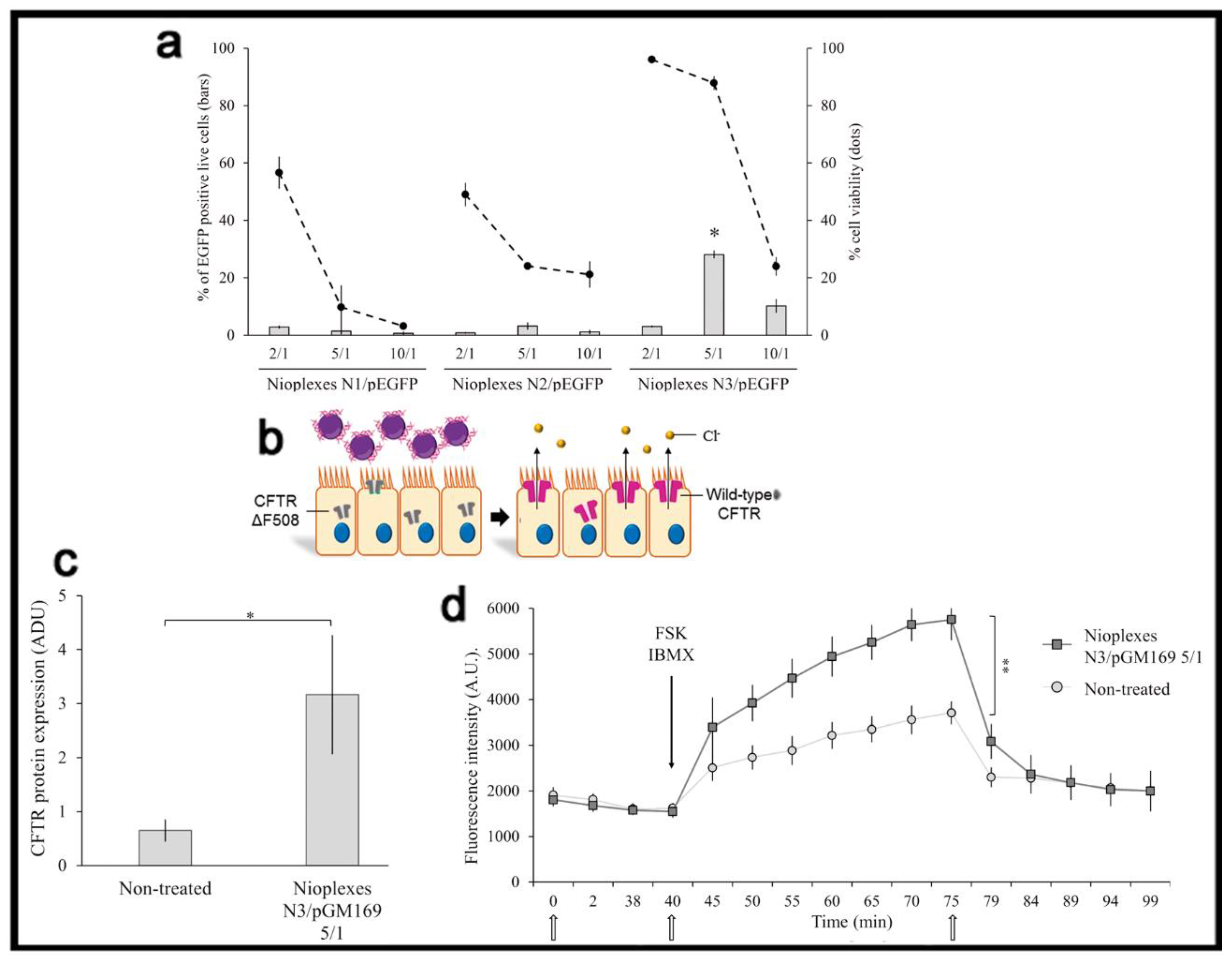
2. Gene Delivery Efficiency
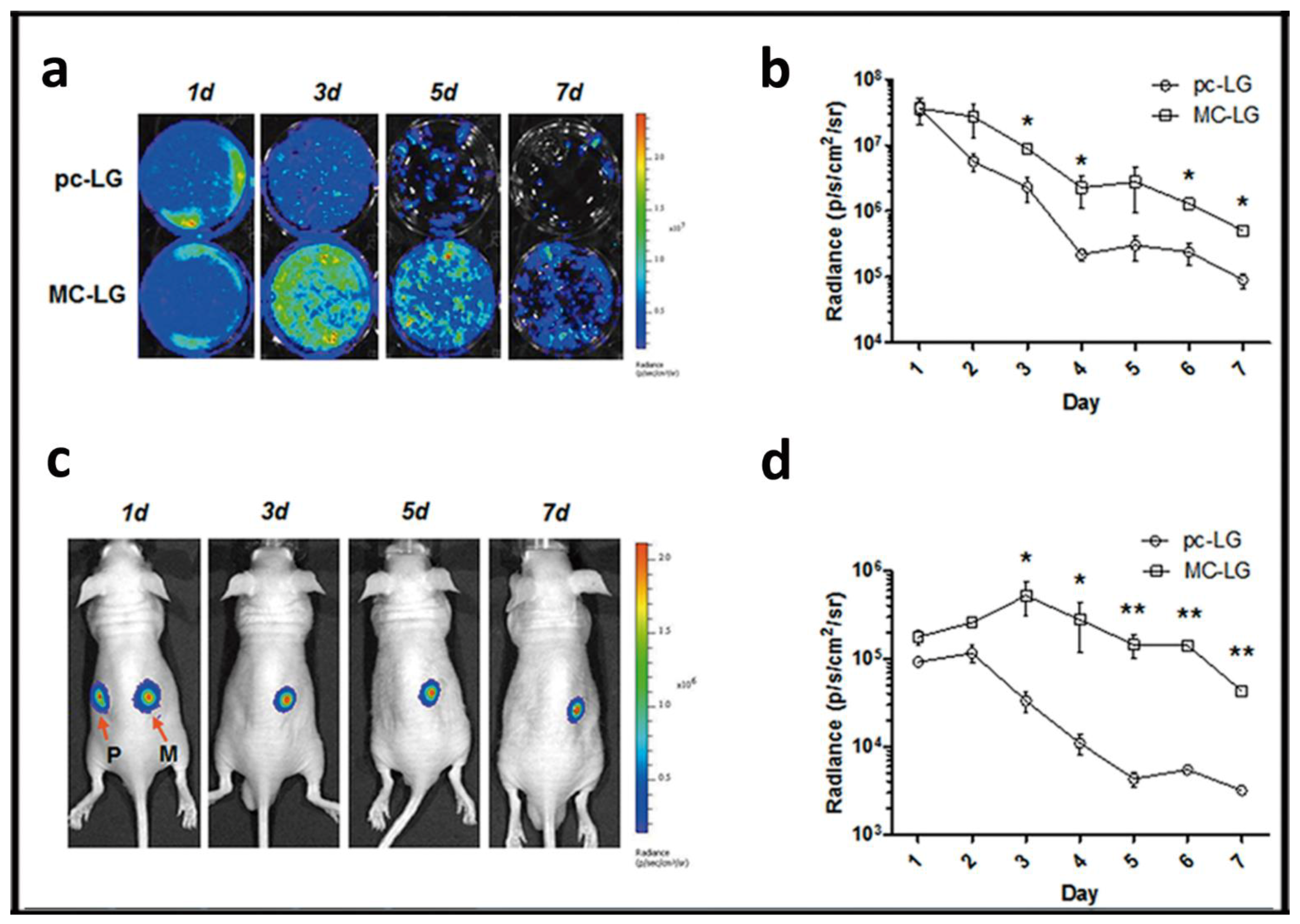
3. Duration of Gene Expression
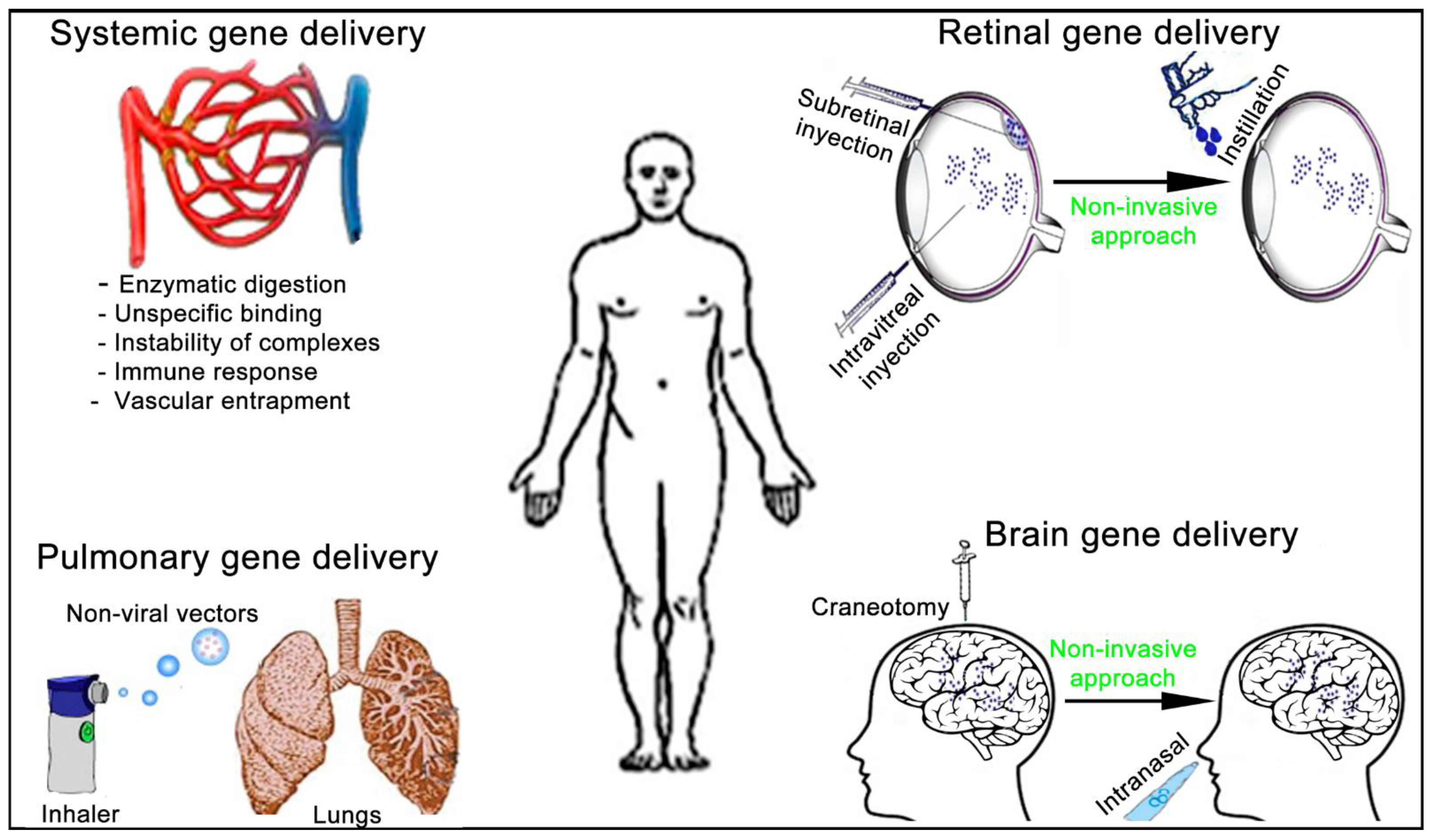
4. Administration Route
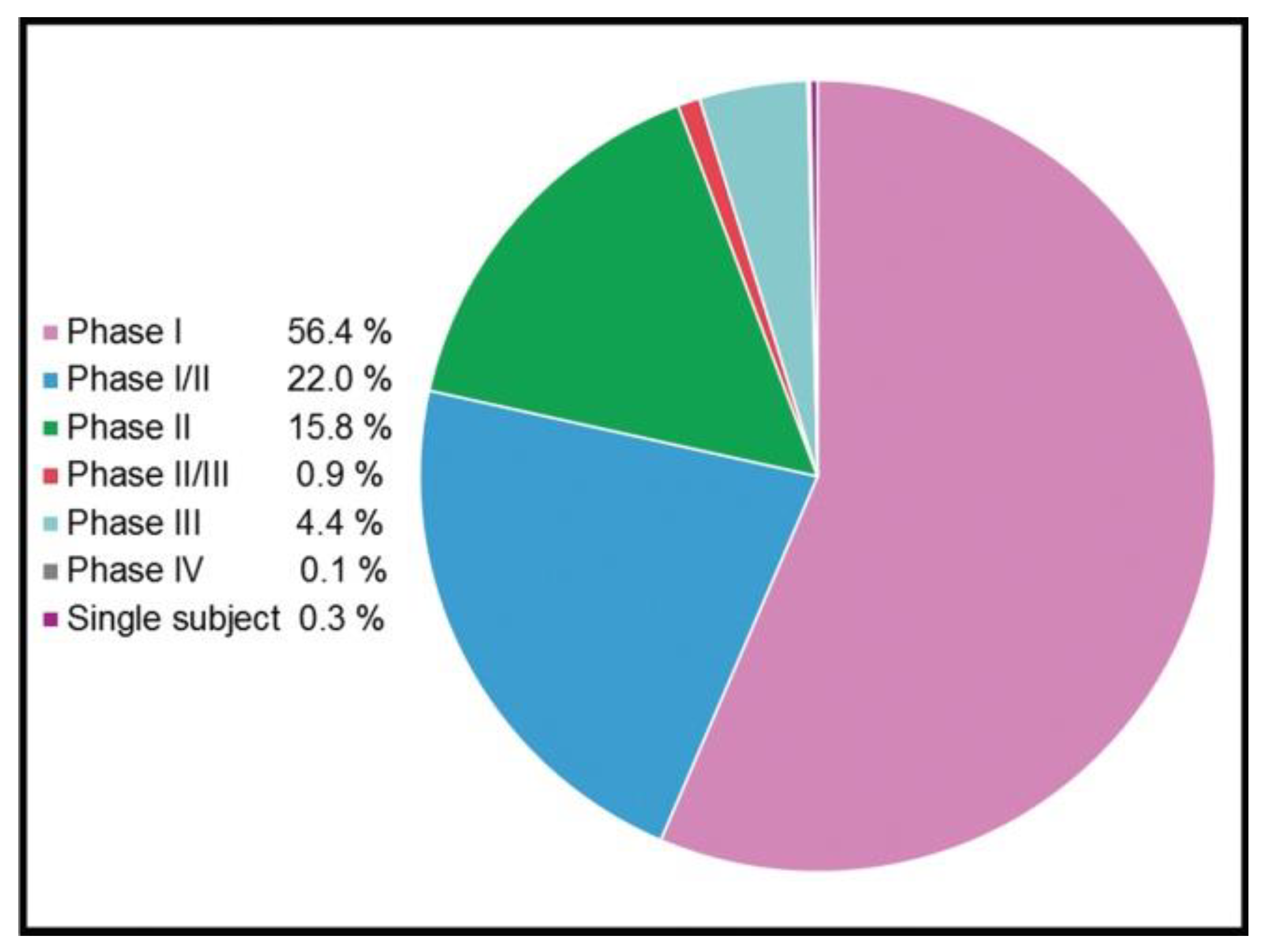
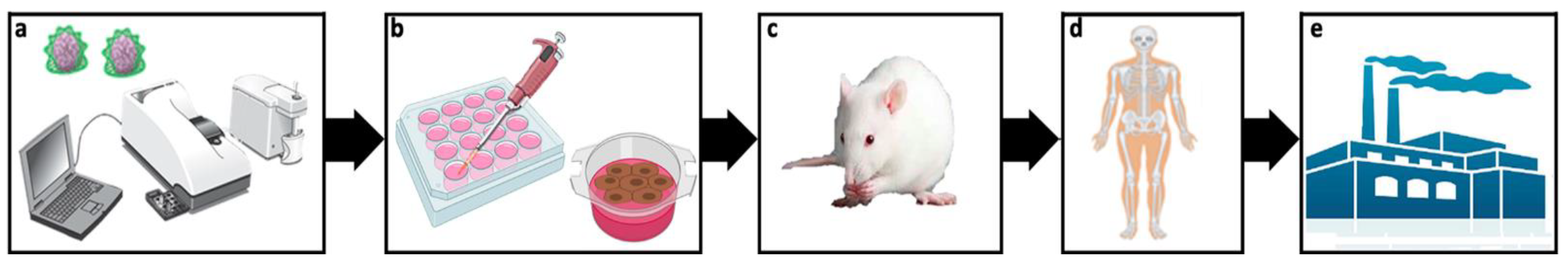
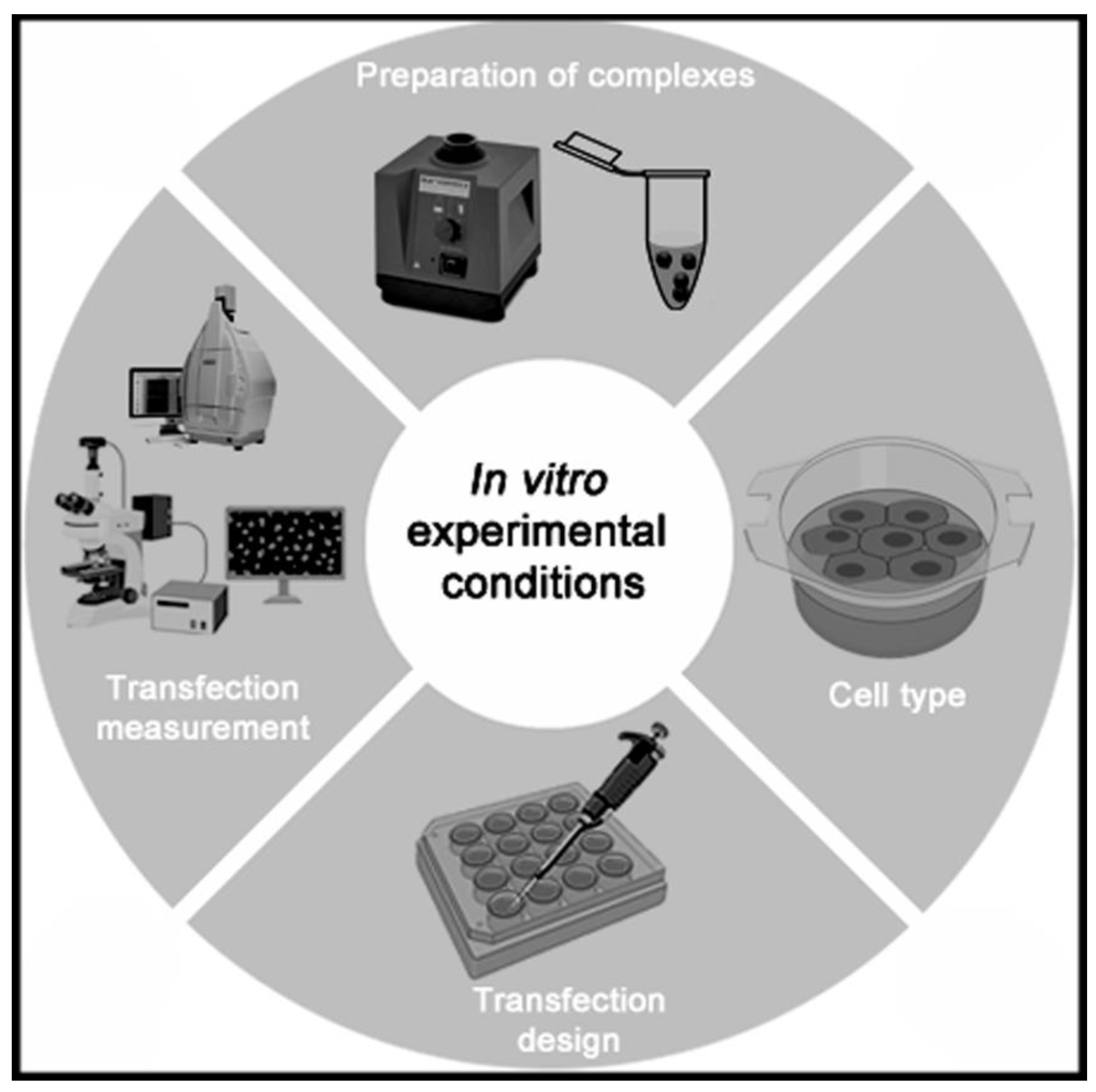
5. Design of Experimental Conditions
6. Commercialization Process
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ziccardi, L.; Cordeddu, V.; Gaddini, L.; Matteucci, A.; Parravano, M.; Malchiodi-Albedi, F.; Varano, M. Gene Therapy in Retinal Dystrophies. Int. J. Mol. Sci. 2019, 20, 5722. [Google Scholar] [CrossRef]
- Cooney, A.L.; McCray, J.P.B.; Sinn, P.L. Cystic Fibrosis Gene Therapy: Looking Back, Looking Forward. Genes 2018, 9, 538. [Google Scholar] [CrossRef] [PubMed]
- Hibbitts, A.; O’Connor, A.M.; McCarthy, J.; Forde, É.B.; Hessman, G.; O’Driscoll, C.M.; Cryan, S.-A.; Devocelle, M. Poly(Ethylene Glycol)-Based Peptidomimetic “PEGtide” of Oligo-Arginine Allows for Efficient siRNA Transfection and Gene Inhibition. ACS Omega 2019, 4, 10078–10088. [Google Scholar] [CrossRef]
- Utz, V.M.; Beight, C.D.; Marino, M.J.; Hagstrom, S.A.; Traboulsi, E.I. Autosomal Dominant Retinitis Pigmentosa Secondary to Pre-mRNA Splicing-Factor Gene PRPF31 (RP11): Review of Disease Mechanism and Report of a Family with a Novel 3-Base Pair Insertion. Ophthalmic Genet. 2013, 34, 183–188. [Google Scholar] [CrossRef]
- Jiang, F.; Doudna, J.A. CRISPR-Cas9 Structures and Mechanisms. Annu. Rev. Biophys. 2017, 46, 505–529. [Google Scholar] [CrossRef]
- Ou, Z.; Niu, X.; He, W.; Chen, Y.; Song, B.; Xian, Y.; Fan, D.; Tang, D.; Sun, X. The Combination of CRISPR/Cas9 and iPSC Technologies in the Gene Therapy of Human Beta-Thalassemia in Mice. Sci. Rep. 2016, 6, 32463. [Google Scholar] [CrossRef]
- Schmeer, M.; Buchholz, T.; Schleef, M. Plasmid DNA Manufacturing for Indirect and Direct Clinical Applications. Hum. Gene Ther. 2017, 28, 856–861. [Google Scholar] [CrossRef]
- Gracey Maniar, L.E.; Maniar, J.M.; Chen, Z.Y.; Lu, J.; Fire, A.Z.; Kay, M.A. Minicircle DNA Vectors Achieve Sustained Expression Reflected by Active Chromatin and Transcriptional Level. Mol. Ther. 2013, 21, 131–138. [Google Scholar] [CrossRef]
- Jia, F.; Wilson, K.D.; Sun, N.; Gupta, D.M.; Huang, M.; Li, Z.; Panetta, N.J.; Chen, Z.Y.; Robbins, R.C.; Kay, M.A.; et al. A Nonviral Minicircle Vector for Deriving Human iPS Cells. Nat. Methods 2010, 7, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Gigante, A.; Li, M.; Junghänel, S.; Hirschhäuser, C.; Knauer, S.; Schmuck, C. Non-Viral Transfection Vectors: Are Hybrid Materials the Way Forward? MedChemComm 2019, 10, 1692–1718. [Google Scholar] [CrossRef] [PubMed]
- Keller, M. Lipidic Carriers of RNA/DNA Oligonucleotides and Polynucleotides: What a Difference a Formulation Makes! J. Control. Release 2005, 103, 537–540. [Google Scholar] [CrossRef]
- Yamamoto, A.; Kormann, M.; Rosenecker, J.; Rudolph, C. Current Prospects for mRNA Gene Delivery. Eur. J. Pharm. Biopharm. 2009, 71, 484–489. [Google Scholar] [CrossRef]
- Puras, G.; Navarrete, G.M.; Mashal, M.; Zarate, J.; Agirre, M.; Ojeda, E.; Grijalvo, S.; Eritja, R.; Diaz-Tahoces, A.; Avilés-Trigueros, M.; et al. Protamine/DNA/Niosome Ternary Nonviral Vectors for Gene Delivery to the Retina: The Role of Protamine. Mol. Pharm. 2015, 12, 3658–3671. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Ragi, S.D.; Tsang, S.H. Therapy in Rhodopsin-Mediated Autosomal Dominant Retinitis Pigmentosa. Mol. Ther. 2020, 28, 2139–2149. [Google Scholar] [CrossRef] [PubMed]
- Chitkara, D.; Singh, S.; Mittal, A. Nanocarrier-Based Co-Delivery of Small Molecules and siRNA/miRNA for Treatment of Cancer. Ther. Deliv. 2016, 7, 245–255. [Google Scholar] [CrossRef]
- Lok, C.N.; Viazovkina, E.; Min, K.L.; Nagy, E.; Wilds, C.J.; Damha, M.J.; Parniak, M.A. Potent Gene-Specific Inhibitory Properties of Mixed-Backbone Antisense Oligonucleotides Comprised of 2′-Deoxy-2′-Fluoro-D-Arabinose and 2′-Deoxyribose Nucleotides. Biochemistry 2002, 41, 3457–3467. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; La Russa, M.; Qi, L.S. CRISPR/Cas9 in Genome Editing and Beyond. Annu. Rev. Biochem. 2016, 85, 227–264. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hu, S.; Chen, X. Non-Viral Delivery Systems for CRISPR/Cas9-Based Genome Editing: Challenges and Opportunities. Biomaterials 2018, 171, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Glass, Z.; Lee, M.; Li, Y.; Xu, Q. Engineering the Delivery System for CRISPR-Based Genome Editing. Trends Biotechnol. 2018, 36, 173–185. [Google Scholar] [CrossRef]
- Kaneda, Y. Gene Therapy: A Battle against Biological Barriers. Curr. Mol. Med. 2001, 1, 493–499. [Google Scholar] [CrossRef]
- Pezzoli, D.; Chiesa, R.; De Nardo, L.; Candiani, G. We Still Have a Long Way to Go to Effectively Deliver Genes! J. Appl. Biomater. Funct. Mater. 2012, 10, 82–91. [Google Scholar] [CrossRef]
- Shahryari, A.; Jazi, M.S.; Mohammadi, S.; Nikoo, H.R.; Nazari, Z.; Hosseini, E.S.; Burtscher, I.; Mowla, S.J.; Lickert, H. Development and Clinical Translation of Approved Gene Therapy Products for Genetic Disorders. Front. Genet. 2019, 10, 868. [Google Scholar] [CrossRef] [PubMed]
- Al Qtaish, N.; Gallego, I.; Villate-Beitia, I.; Sainz-Ramos, M.; Lopez-Mendez, T.B.; Grijalvo, S.; Eritja, R.; Soto-Sánchez, C.; Martínez-Navarrete, G.; Fernandez, E.; et al. Niosome-Based Approach for In Situ Gene Delivery to Retina and Brain Cortex as Immune-Privileged Tissues. Pharmaceutics 2020, 12, 198. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.N.; Everett, J.K.; Kafle, S.; Roche, A.M.; Raymond, H.E.; Leiby, J.; Wood, C.; Assenmacher, C.-A.; Merricks, E.P.; Long, C.T.; et al. A Long-Term Study of AAV Gene Therapy in Dogs with Hemophilia A Identifies Clonal Expansions of Transduced Liver Cells. Nat. Biotechnol. 2020, 39, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Venditti, C.P. Safety Questions for AAV Gene Therapy. Nat. Biotechnol. 2021, 39, 24–26. [Google Scholar] [CrossRef]
- Orkin, S.H.; Reilly, P. Paying for Future Success in Gene Therapy. Science 2016, 352, 1059–1061. [Google Scholar] [CrossRef]
- Patil, S.; Gao, Y.-G.; Lin, X.; Li, Y.; Dang, K.; Tian, Y.; Zhang, W.-J.; Jiang, S.-F.; Qadir, A.; Qian, A.-R. The Development of Functional Non-Viral Vectors for Gene Delivery. Int. J. Mol. Sci. 2019, 20, 5491. [Google Scholar] [CrossRef] [PubMed]
- Shim, G.; Kim, D.; Park, G.T.; Jin, H.; Suh, S.-K.; Oh, Y.-K. Therapeutic Gene Editing: Delivery and Regulatory Perspectives. Acta Pharmacol. Sin. 2017, 38, 738–753. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorth, M.; Narvekar, A. Non Viral Vectors in Gene Therapy—An Overview. J. Clin. Diagn. Res. 2015, 9, GE01–GE06. [Google Scholar] [CrossRef]
- Crunkhorn, S. Towards Improved Viral Vectors for Gene Therapy. Nat. Rev. Drug Discov. 2021. [Google Scholar] [CrossRef]
- Kay, M.A.; He, C.-Y.; Chen, Z.-Y. A robust system for production of minicircle DNA vectors. Nat. Biotechnol. 2010, 28, 1287–1289. [Google Scholar] [CrossRef]
- Grijalvo, S.; Puras, G.; Zarate, J.; Sainz-Ramos, M.; Al Qtaish, N.; Lopez-Mendez, T.B.; Mashal, M.; Attia, N.; Díaz, D.D.; Pons, R.; et al. Cationic Niosomes as Non-Viral Vehicles for Nucleic Acids: Challenges and Opportunities in Gene Delivery. Pharmaceutics 2019, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Puras, G.; Mashal, M.; Zarate, J.; Agirre, M.; Ojeda, E.; Grijalvo, S.; Eritja, R.; Diaz-Tahoces, A.; Navarrete, G.M.; Avilés-Trigueros, M.; et al. A Novel Cationic Niosome Formulation for Gene Delivery to the Retina. J. Control. Release 2014, 174, 27–36. [Google Scholar] [CrossRef]
- Murphy, E.A.; Waring, A.J.; Murphy, J.C.; Willson, R.C.; Longmuir, K.J. Development of an Effective Gene Delivery System: A Study of Complexes Composed of a Peptide-Based Amphiphilic DNA Compaction Agent and Phospholipid. Nucleic. Acids Res. 2001, 29, 3694–3704. [Google Scholar] [CrossRef][Green Version]
- van Gaal, E.V.; van Eijk, R.; Oosting, R.S.; Kok, R.J.; Hennink, W.E.; Crommelin, D.J.; Mastrobattista, E. How to Screen Non-Viral Gene Delivery Systems In Vitro? J. Control. Release 2011, 154, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Marjanović, I.; Kandušer, M.; Miklavčič, D.; Keber, M.M.; Pavlin, M. Comparison of Flow Cytometry, Fluorescence Microscopy and Spectrofluorometry for Analysis of Gene Electrotransfer Efficiency. J. Membr. Biol. 2014, 247, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Puras, G.; Zarate, J.; Díaz-Tahoces, A.; Avilés-Trigueros, M.; Fernández, E.; Pedraz, J. Oligochitosan Polyplexes as Carriers for Retinal Gene Delivery. Eur. J. Pharm. Sci. 2013, 48, 323–331. [Google Scholar] [CrossRef]
- Sainz-Ramos, M.; Villate-Beitia, I.; Gallego, I.; Qtaish, N.A.L.; Lopez-Mendez, T.B.; Eritja, R.; Grijalvo, S.; Puras, G.; LuisPedraz, J. Non-Viral Mediated Gene Therapy in Human Cystic Fibrosis Airway Epithelial Cells Recovers Chloride Channel Functionality. Int. J. Pharm. 2020, 588, 119757. [Google Scholar] [CrossRef]
- Mashal, M.; Attia, N.; Puras, G.; Martínez-Navarrete, G.; Fernández, E.; Pedraz, J.L. Retinal Gene Delivery Enhancement by Lycopene Incorporation into Cationic Niosomes Based on DOTMA and Polysorbate 60. J. Control. Release Off. J. Control. Release Soc. 2017, 254, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wagner, E. History of Polymeric Gene Delivery Systems. Top. Curr. Chem. 2017, 375, 26. [Google Scholar] [CrossRef]
- Agirre, M.; Zarate, J.; Puras, G.; Ojeda, E.; Pedraz, J.L. Improving Transfection Efficiency of Ultrapure Oligochitosan/DNA Polyplexes by Medium Acidification. Drug Deliv. 2015, 22, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Chen, H.; Jin, R.; Weng, T.; Ho, J.K.; You, C.; Zhang, L.; Wang, X.; Han, C. Non-Viral Gene Delivery Systems for Tissue Repair and Regeneration. J. Transl. Med. 2018, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Yew, N.S.; Zhao, H.; Wu, I.-H.; Song, A.; Tousignant, J.D.; Przybylska, M.; Cheng, S.H. Reduced Inflammatory Response to Plasmid DNA Vectors by Elimination and Inhibition of Immunostimulatory CpG Motifs. Mol. Ther. 2000, 1, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Gallego, I.; Villate-Beitia, I.; Martinez-Navarrete, G.; Menendez, M.; Lopez-Mendez, T.; Soto-Sanchez, C.; Zárate, J.; Puras, G.; Fernández, E.; Pedraz, J.L. Non-Viral Vectors Based on Cationic Niosomes and Minicircle DNA Technology Enhance Gene Delivery Efficiency for Biomedical Applications in Retinal Disorders. Nanomedicine 2019, 17, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, E.; Puras, G.; Agirre, M.; Zarate, J.; Grijalvo, S.; Pons, R.; Eritja, R.; Navarrete, G.M.; Soto-Sanchez, C.; Fernández, E.; et al. Niosomes Based on Synthetic Cationic Lipids for Gene Delivery: The Influence of Polar Head-Groups on the Transfection Efficiency in HEK-293, ARPE-19 and MSC-D1 Cells. Org. Biomol. Chem. 2014, 13, 1068–1081. [Google Scholar] [CrossRef]
- Deng, R.; Yue, Y.; Jin, F.; Chen, Y.; Kung, H.-F.; Lin, M.C.; Wu, C. Revisit the Complexation of PEI and DNA—How to Make Low Cytotoxic and Highly Efficient PEI Gene Transfection Non-Viral Vectors with a Controllable Chain Length and Structure? J. Control. Release 2009, 140, 40–46. [Google Scholar] [CrossRef]
- Tripathi, S.K.; Goyal, R.; Kumar, P.; Gupta, K.C. Linear Polyethylenimine-Graft-Chitosan Copolymers as Efficient DNA/siRNA Delivery Vectors In Vitro and In Vivo. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 337–345. [Google Scholar] [CrossRef]
- Agirre, M.; Zárate, J.; Ojeda, E.; Puras, G.; Rojas, L.A.; Alemany, R.; Pedraz, J.L. Delivery of an Adenovirus Vector Plasmid by Ultrapure Oligochitosan Based Polyplexes. Int. J. Pharm. 2015, 479, 312–319. [Google Scholar] [CrossRef]
- Ochoa, G.P.; Sesma, J.Z.; Díez, M.A.; Díaz-Tahoces, A.; Avilés-Trigeros, M.; Grijalvo, S.; Eritja, R.; Fernández, E.; Pedraz, J.L. A Novel Formulation Based on 2,3-Di(tetradecyloxy)propan-1-amine Cationic Lipid Combined with Polysorbate 80 for Efficient Gene Delivery to the Retina. Pharm. Res. 2014, 31, 1665–1675. [Google Scholar] [CrossRef]
- Ojeda, E.; Puras, G.; Agirre, M.; Zarate, J.; Grijalvo, S.; Eritja, R.; Digiacomo, L.; Caracciolo, G.; Pedraz, J.-L. The role of helper lipids in the intracellular disposition and transfection efficiency of niosome formulations for gene delivery to retinal pigment epithelial cells. Int. J. Pharm. 2016, 503, 115–126. [Google Scholar] [CrossRef]
- Del Pozo-Rodriguez, A.; Solinis, M.A.; Rodriguez-Gascon, A. Applications of Lipid Nanoparticles in Gene Therapy. Eur. J. Pharm. Biopharm. 2016, 109, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Thayumanavan, S. Noncationic Material Design for Nucleic Acid Delivery. Adv. Ther. 2020, 3. [Google Scholar] [CrossRef]
- Dincer, S.; Türk, M.; Piskin, E. Intelligent Polymers as Nonviral Vectors. Gene Ther. 2005, 12, S139–S145. [Google Scholar] [CrossRef]
- Hinrichs, W.; Schuurmans-Nieuwenbroek, N.; van de Wetering, P.; Hennink, W.; Hinrichs, W.; Schuurmans-Nieuwenbroek, N.; van de Wetering, P.; Hennink, W. Thermosensitive Polymers as Carriers for DNA Delivery. J. Control. Release 1999, 60, 249–259. [Google Scholar] [CrossRef]
- Van Gaal, E.V.; Hennink, W.E.; Crommelin, D.J.; Mastrobattista, E. Plasmid Engineering for Controlled and Sustained Gene Expression for Nonviral Gene Therapy. Pharm. Res. 2006, 23, 1053–1074. [Google Scholar] [CrossRef]
- Kotterman, M.A.; Chalberg, T.W.; Schaffer, D.V. Viral Vectors for Gene Therapy: Translational and Clinical Outlook. Annu. Rev. Biomed. Eng. 2015, 17, 63–89. [Google Scholar] [CrossRef]
- Desfarges, S.; Ciuffi, A. Viral Integration and Consequences on Host Gene Expression. In Viruses: Essential Agents of Life; Springer Science and Business Media LLC: Dordrecht, The Netherlands, 2012; pp. 147–175. [Google Scholar]
- Naso, M.F.; Tomkowicz, B.; Perry, W.L., 3rd; Strohl, W.R. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs 2017, 31, 317–334. [Google Scholar] [CrossRef]
- Jager, R.D.; Aiello, L.P.; Patel, S.C.; Cunningham, E.T., Jr. Risks of Intravitreous Injection: A Comprehensive Review. Retina 2004, 24, 676–698. [Google Scholar] [CrossRef]
- Mun, J.-Y.; Shin, K.K.; Kwon, O.; Lim, Y.T.; Oh, D.-B. Minicircle Microporation-Based Non-Viral Gene Delivery Improved the Targeting of Mesenchymal Stem Cells to an Injury Site. Biomaterials 2016, 101, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Kisseljova, N.P.; Dmitriev, P.; Katargin, A.; Kim, E.; Ezeriņa, D.; Markozashvili, D.; Malysheva, D.; Planche, E.; Lemmers, R.J.L.F.; Van Der Maarel, S.M.; et al. DNA Polymorphism and Epigenetic Marks Modulate the Affinity of a Scaffold/Matrix Attachment Region to the Nuclear Matrix. Eur. J. Hum. Genet. 2014, 22, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, A.; Haase, R.; Schepers, A.; Deutsch, M.J.; Lipps, H.J.; Baiker, A. Episomal Vectors for Gene Therapy. Curr. Gene Ther. 2008, 8, 147–161. [Google Scholar] [CrossRef]
- Van Craenenbroeck, K.; Vanhoenacker, P.; Haegeman, G. Episomal Vectors for Gene Expression in Mammalian Cells. Eur. J. Biochem. 2000, 267, 5665–5678. [Google Scholar] [CrossRef]
- Allo, M.; Kornblihtt, A.R. Gene Silencing: Small RNAs Control RNA Polymerase II Elongation. Curr. Biol. 2010, 20, R704–R707. [Google Scholar] [CrossRef] [PubMed]
- Andreollo, N.A.; Santos, E.F.; Araujo, M.R.; Lopes, L.R. Rat’s Age Versus Human’s Age: What is the Relationship? Arq. Bras. Cir. Dig. 2012, 25, 49–51. [Google Scholar] [CrossRef]
- Conceição, M.; Mendonça, L.; Nóbrega, C.; Gomes, C.; Costa, P.; Hirai, H.; Moreira, J.N.; de Lima, M.P.; Manjunath, N.; de Almeida, L.P. Intravenous Administration of Brain-Targeted Stable Nucleic Acid Lipid Particles Alleviates Machado-Joseph Disease Neurological Phenotype. Biomaterials 2016, 82, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, D.; Rejman, J.; Wasungu, L.; Shi, F.; Zuhorn, I. Gene Delivery by Cationic Lipids: In and Out of an Endosome. Biochem. Soc. Trans. 2007, 35, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Aggarwal, P.; Hall, J.B.; McNeil, S.E. Preclinical Studies to Understand Nanoparticle Interaction with the Immune System and Its Potential Effects on Nanoparticle Biodistribution. Mol. Pharm. 2008, 5, 487–495. [Google Scholar] [CrossRef]
- Al-Dosari, M.S.; Gao, X. Nonviral Gene Delivery: Principle, Limitations, and Recent Progress. AAPS J. 2009, 11, 671–681. [Google Scholar] [CrossRef]
- Delgado, D.; del Pozo-Rodriguez, A.; Solinis, M.A.; Rodríguez-Gascón, A. Understanding the Mechanism of Protamine in Solid Lipid Nanoparticle-Based Lipofection: The Importance of the Entry Pathway. Eur. J. Pharm. Biopharm. 2011, 79, 495–502. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.S.; Lee, Y.K.; Koo, K.H.; Park, Y.S.; Kim, H.S.; Kim, J.S.; Lee, Y.K.; Koo, K.H.; Park, Y.S. An Efficient Liposomal Gene Delivery Vehicle Using Sendai F/HN Proteins and Protamine. Cancer Gene Ther. 2008, 15, 214–224. [Google Scholar] [CrossRef][Green Version]
- Moghimi, S. Chemical Camouflage of Nanospheres with a Poorly Reactive Surface: Towards Development of Stealth and Target-Specific Nanocarriers. Biochim. Biophys. Acta Bioenerg. 2002, 1590, 131–139. [Google Scholar] [CrossRef]
- Mishra, S.; Webster, P.; Davis, M.E. PEGylation Significantly Affects Cellular Uptake and Intracellular Trafficking of Non-Viral Gene Delivery Particles. Eur. J. Cell Biol. 2004, 83, 97–111. [Google Scholar] [CrossRef]
- Meyer, O.; Kirpotin, D.; Hong, K.; Sternberg, B.; Park, J.W.; Woodle, M.C.; Papahadjopoulos, D. Cationic Liposomes Coated with Polyethylene Glycol as Carriers for Oligonucleotides. J. Biol. Chem. 1998, 273, 15621–15627. [Google Scholar] [CrossRef]
- Allon, N.; Saxena, A.; Chambers, C.; Doctor, B.P. A New Liposome-Based Gene Delivery System Targeting Lung Epithelial Cells Using Endothelin Antagonist. J. Control. Release 2012, 160, 217–224. [Google Scholar] [CrossRef]
- Lengsfeld, C.; Anchordoquy, T.; Lengsfeld, C.; Anchordoquy, T. Shear-Induced Degradation of Plasmid DNA. J. Pharm. Sci. 2002, 91, 1581–1589. [Google Scholar] [CrossRef]
- Davies, L.A.; Nunez-Alonso, G.A.; McLachlan, G.; Hyde, S.C.; Gill, D.R. Aerosol Delivery of DNA/Liposomes to the Lung for Cystic Fibrosis Gene Therapy. Hum. Gene Ther. Clin. Dev. 2014, 25, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Gomes Dos Reis, L.; Svolos, M.; Hartwig, B.; Windhab, N.; Young, P.M.; Traini, D. Inhaled Gene Delivery: A Formulation and Delivery Approach. Expert. Opin. Drug Deliv. 2017, 14, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Cho, M.-H.; Kim, S. Recent Advances in Aerosol Gene Delivery Systems Using Non-Viral Vectors for Lung Cancer Therapy. Expert Opin. Drug Deliv. 2019, 16, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Sanders, N.; Rudolph, C.; Braeckmans, K.; De Smedt, S.; Demeester, J. Extracellular Barriers in Respiratory Gene Therapy. Adv. Drug Deliv. Rev. 2009, 61, 115–127. [Google Scholar] [CrossRef]
- King, M.; Rubin, B.K. Pharmacological Approaches to Discovery and Development of New Mucolytic Agents. Adv. Drug Deliv. Rev. 2002, 54, 1475–1490. [Google Scholar] [CrossRef]
- Foldvari, M.; Chen, D.-W.; Nafissi, N.; Calderon, D.; Narsineni, L.; Rafiee, A. Non-Viral Gene Therapy: Gains and Challenges of Non-Invasive Administration Methods. J. Control. Release 2016, 240, 165–190. [Google Scholar] [CrossRef]
- Mathias, N.R.; Hussain, M.A. Non-Invasive Systemic Drug Delivery: Developability Considerations for Alternate Routes of Administration. J. Pharm. Sci. 2010, 99, 1–20. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, A.M.; Godinho, B.M.D.C.; Cryan, J.F.; O’Driscoll, C.M. Non-Viral Nanosystems for Gene and Small Interfering RNA Delivery to the Central Nervous System: Formulating the Solution. J. Pharm. Sci. 2013, 102, 3469–3484. [Google Scholar] [CrossRef] [PubMed]
- Ying, L.; Tahara, K.; Takeuchi, H. Drug Delivery to the Ocular Posterior Segment Using Lipid Emulsion via Eye Drop Administration: Effect of Emulsion Formulations and Surface Modification. Int. J. Pharm. 2013, 453, 329–335. [Google Scholar] [CrossRef]
- Lajunen, T.; Hisazumi, K.; Kanazawa, T.; Okada, H.; Seta, Y.; Yliperttula, M.; Urtti, A.; Takashima, Y. Topical Drug Delivery to Retinal Pigment Epithelium with Microfluidizer Produced Small Liposomes. Eur. J. Pharm. Sci. 2014, 62, 23–32. [Google Scholar] [CrossRef]
- Harmon, B.T.; Aly, A.E.; Padegimas, L.; Sesenoglu-Laird, O.; Cooper, M.J.; Waszczak, B.L. Intranasal Administration of Plasmid DNA Nanoparticles Yields Successful Transfection and Expression of a Reporter Protein in Rat Brain. Gene Ther. 2014, 21, 514–521. [Google Scholar] [CrossRef]
- Attia, N.; Mashal, M.; Grijalvo, S.; Eritja, R.; Zárate, J.; Puras, G.; Pedraz, J.L. Stem Cell-Based Gene Delivery Mediated by Cationic Niosomes for Bone Regeneration. Nanomed. Nanotechnolog. Biol. Med. 2018, 14, 521–531. [Google Scholar] [CrossRef]
- Santos-Carballal, B.; Fernández, E.F.; Goycoolea, F.M. Chitosan in Non-Viral Gene Delivery: Role of Structure, Characterization Methods, and Insights in Cancer and Rare Diseases Therapies. Polymers 2018, 10, 444. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Sandberg, A.; Heckert, E.; Self, W.; Seal, S. Protein Adsorption and Cellular Uptake of Cerium Oxide Nanoparticles as a Function of Zeta Potential. Biomaterials 2007, 28, 4600–4607. [Google Scholar] [CrossRef]
- Malm, A.V.; Corbett, J.C.W. Improved Dynamic Light Scattering Using an Adaptive and Statistically Driven Time Resolved Treatment of Correlation Data. Sci. Rep. 2019, 9, 74. [Google Scholar] [CrossRef]
- Teulon, J.-M.; Godon, C.; Chantalat, L.; Moriscot, C.; Cambedouzou, J.; Odorico, M.; Ravaux, J.; Podor, R.; Gerdil, A.; Habert, A.; et al. On the Operational Aspects of Measuring Nanoparticle Sizes. Nanomaterials 2018, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Tan, J.; Wang, Y.-F.; Qian, C.; Zhang, M. Chitosan Nanoparticles as Non-Viral Gene Delivery Vehicles Based on Atomic force Microscopy Study. Acta Biochim. Biophys. Sin. 2009, 41, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Sze, A.; Erickson, D.; Ren, L.; Li, D. Zeta-Potential Measurement Using the Smoluchowski Equation and the Slope of the Current–Time Relationship in Electroosmotic Flow. J. Colloid Interface Sci. 2003, 261, 402–410. [Google Scholar] [CrossRef]
- Nimesh, S.; Thibault, M.M.; Lavertu, M.; Buschmann, M. Enhanced Gene Delivery Mediated by Low Molecular Weight Chitosan/DNA Complexes: Effect of pH and Serum. Mol. Biotechnol. 2010, 46, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Falconer, R.J. Applications of Isothermal Titration Calorimetry—The Research and Technical Developments from 2011 to 2015. J. Mol. Recognit. 2016, 29, 504–515. [Google Scholar] [CrossRef]
- Hill, A.B.; Chen, M.; Chen, C.-K.; Pfeifer, B.A.; Jones, C.H. Overcoming Gene-Delivery Hurdles: Physiological Considerations for Nonviral Vectors. Trends Biotechnol. 2016, 34, 91–105. [Google Scholar] [CrossRef]
- Ojeda, E.; Puras, G.; Agirre, M.; Zarate, J.; Grijalvo, S.; Eritja, R.; Navarrete, G.M.; Soto-Sánchez, C.; Diaz-Tahoces, A.; Avilés-Trigueros, M.; et al. The Influence of the Polar Head-Group of Synthetic Cationic Lipids on the Transfection Efficiency Mediated by Niosomes in Rat Retina and Brain. Biomaterials 2016, 77, 267–279. [Google Scholar] [CrossRef]
- Medawar, P.B. Immunity to Homologous Grafted Skin; the Fate of Skin Homografts Transplanted to the Brain, to Subcutaneous Tissue, and to the Anterior Chamber of the Eye. Br. J. Exp. Pathol. 1948, 29, 58–69. [Google Scholar]
- Isaacson, A.; Swioklo, S.; Connon, C.J. 3D Bioprinting of a Corneal Stroma Equivalent. Exp. Eye Res. 2018, 173, 188–193. [Google Scholar] [CrossRef]
- Jaffe, A.; Prasad, S.A.; Larcher, V.; Hart, S. Gene Therapy for Children with Cystic Fibrosis—Who has the Right to Choose? J. Med. Ethic. 2006, 32, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Seville, P.C.; Kellaway, I.W.; Birchall, J.C. Preparation of Dry Powder Dispersions for Non-Viral Gene Delivery by Freeze-Drying and Spray-Drying. J. Gene Med. 2002, 4, 428–437. [Google Scholar] [CrossRef]
- Kuo, J.-H.S.; Hwang, R. Preparation of DNA Dry Powder for Non-Viral Gene Delivery by Spray-Freeze Drying: Effect of Protective Agents (Polyethyleneimine and Sugars) on the Stability of DNA. J. Pharm. Pharmacol. 2010, 56, 27–33. [Google Scholar] [CrossRef]
- Bertoni, S.; Dolci, L.S.; Albertini, B.; Passerini, N. Spray Congealing: A Versatile Technology for Advanced Drug-Delivery Systems. Ther. Deliv. 2018, 9, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Villate-Beitia, I.; Zarate, J.; Puras, G.; Pedraz, J.L. Gene Delivery to the Lungs: Pulmonary Gene Therapy for Cystic Fibrosis. Drug Dev. Ind. Pharm. 2017, 43, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Davies, L.A.; Nunez-Alonso, G.A.; Hebel, H.L.; Scheule, R.K.; Cheng, S.H.; Hyde, S.C.; Hyde, S.C.; Gill, D.R. A Novel Mixing Device for the Reproducible Generation of Nonviral Gene Therapy Formulations. BioTechniques 2010, 49, 666–668. [Google Scholar] [CrossRef] [PubMed]
- Hirota, S.; De Ilarduya, C.T.; Barron, L.G.; Szoka, F.C. Simple Mixing Device to Reproducibly Prepare Cationic Lipid-DNA Complexes (Lipoplexes). Biotechniques 1999, 27, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Darrow, J.J. Luxturna: FDA Documents Reveal the Value of a Costly Gene Therapy. Drug Discov. Today 2019, 24, 949–954. [Google Scholar] [CrossRef]
- Watanabe, N.; Yano, K.; Tsuyuki, K.; Okano, T.; Yamato, M. Re-Examination of Regulatory Opinions in Europe: Possible Contribution for the Approval of the First Gene Therapy Product Glybera. Mol. Ther. Methods Clin. Dev. 2015, 2, 14066. [Google Scholar] [CrossRef]
- Jones, C.H.; Hakansson, A.P.; Pfeifer, B.A. Biomaterials at the Interface of Nano- and Micro-Scale Vector-Cellular Interactions in Genetic Vaccine Design. J. Mater. Chem. B. 2014, 46, 8053–8068. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Curiel, D.T.; Berkland, C.J. The Era of Gene Therapy: From Preclinical Development to Clinical Application. Drug Discov. Today 2021. [Google Scholar] [CrossRef]
- Sheikh, O.; Yokota, T. Advances in Genetic Characterization and Genotype–Phenotype Correlation of Duchenne and Becker Muscular Dystrophy in the Personalized Medicine Era. J. Pers. Med. 2020, 10, 111. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hu, C.; El Achkar, C.M.; Black, L.E.; Douville, J.; Larson, A.; Pendergast, M.K.; Goldkind, S.F.; Lee, E.A.; Kuniholm, A.; et al. Patient-Customized Oligonucleotide Therapy for a Rare Genetic Disease. N. Engl. J. Med. 2019, 381, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
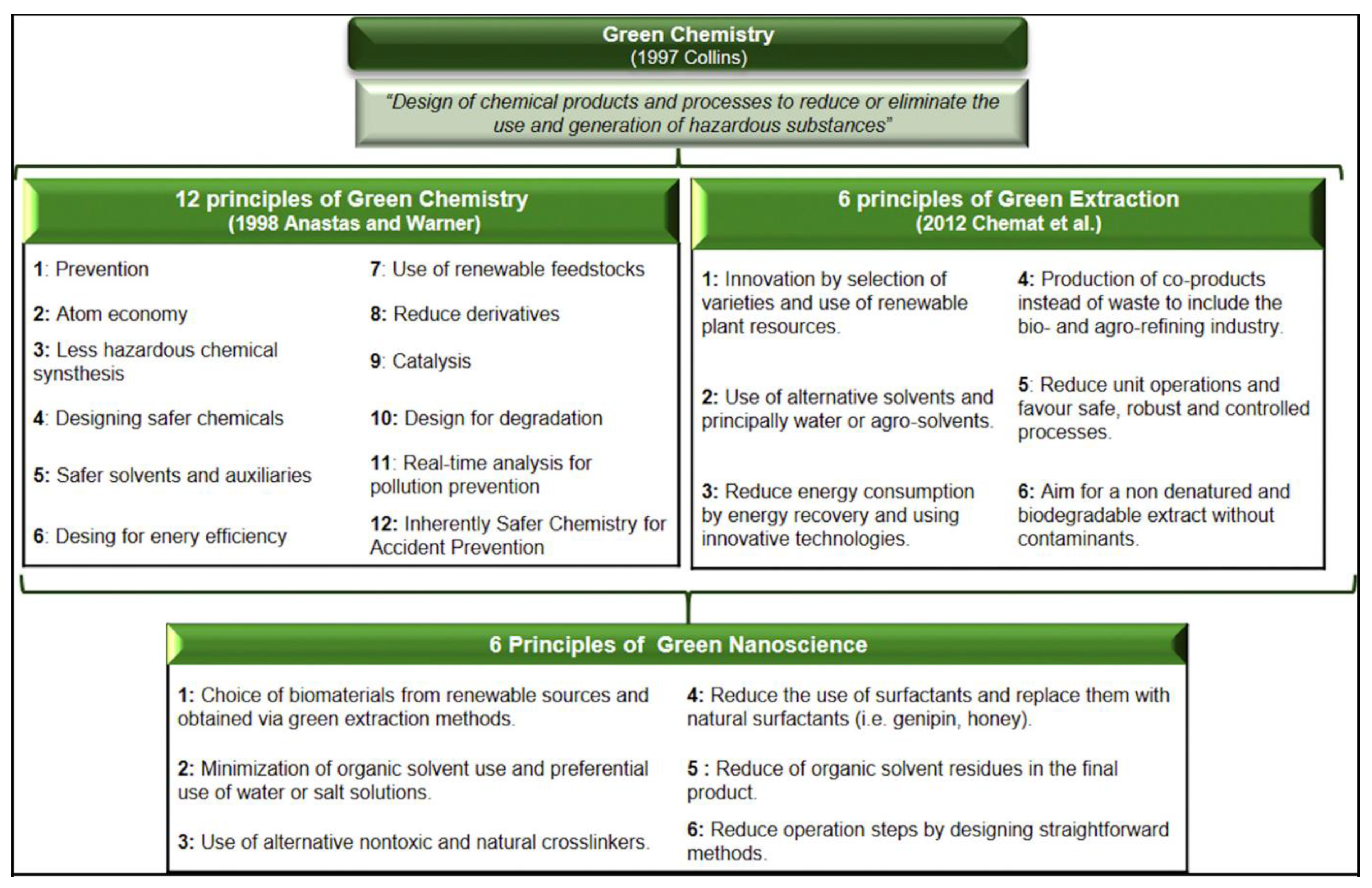
- Huguet-Casquero, A.; Gainza, E.; Pedraz, J.L. Towards Green Nanoscience: From Extraction to Nanoformulation. Biotechnol. Adv. 2021, 46, 107657. [Google Scholar] [CrossRef] [PubMed]
- Andres-Costa, M.J.; Rubio-Lopez, N.; Morales Suarez-Varela, M.; Pico, Y. Occurrence and Removal of Drugs of Abuse in Wastewater Treatment Plants of Valencia (Spain). Environ. Pollut. 2014, 194, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Jahangirian, H.; Lemraski, E.G.; Webster, T.J.; Rafiee-Moghaddam, R.; Abdollahi, Y. A Review of Drug Delivery Systems Based on Nanotechnology and Green Chemistry: Green Nanomedicine. Int. J. Nanomed. 2017, 12, 2957–2978. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Cui, Q.; Hernandez, R.; Mason, S.E.; Frauenheim, T.; Pedersen, J.; Geiger, F. Sustainable Nanotechnology: Opportunities and Challenges for Theoretical/Computational Studies. J. Phys. Chem. B 2016, 120, 7297–7306. [Google Scholar] [CrossRef]


| Mechanism of Action | Polynucleotide | Chemical Structure | Lasting Effect | Place of Action |
|---|---|---|---|---|
| Gene addition | Plasmid (dsDNA) |  | Transient | Nucleus |
| Gene addition | mRNA (ssRNA) |  | Transient | Cytoplasm |
| Gene inhibition | miRNA (ssRNA) |  | Transient | Cytoplasm |
| Gene inhibition | siRNA (dsRNA) |  | Transient | Cytoplasm |
| Gene inhibition | AON (ssDNA/RNA) |  | Transient | Cytoplasm/Nucleus |
| Genome editing | CRISPR/Cas9 Plasmid (dsDNA) |  | Permanent | Nucleus |
| Genome editing | CRISPR/Cas9 mRNA (ssRNA) |   | Permanent | Cytoplasm/Nucleus |
| Genome editing | CRISPR/Cas9 Ribonucleoprotein |  | Permanent | Nucleus |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sainz-Ramos, M.; Gallego, I.; Villate-Beitia, I.; Zarate, J.; Maldonado, I.; Puras, G.; Pedraz, J.L. How Far Are Non-Viral Vectors to Come of Age and Reach Clinical Translation in Gene Therapy? Int. J. Mol. Sci. 2021, 22, 7545. https://doi.org/10.3390/ijms22147545
Sainz-Ramos M, Gallego I, Villate-Beitia I, Zarate J, Maldonado I, Puras G, Pedraz JL. How Far Are Non-Viral Vectors to Come of Age and Reach Clinical Translation in Gene Therapy? International Journal of Molecular Sciences. 2021; 22(14):7545. https://doi.org/10.3390/ijms22147545
Chicago/Turabian StyleSainz-Ramos, Myriam, Idoia Gallego, Ilia Villate-Beitia, Jon Zarate, Iván Maldonado, Gustavo Puras, and Jose Luis Pedraz. 2021. "How Far Are Non-Viral Vectors to Come of Age and Reach Clinical Translation in Gene Therapy?" International Journal of Molecular Sciences 22, no. 14: 7545. https://doi.org/10.3390/ijms22147545
APA StyleSainz-Ramos, M., Gallego, I., Villate-Beitia, I., Zarate, J., Maldonado, I., Puras, G., & Pedraz, J. L. (2021). How Far Are Non-Viral Vectors to Come of Age and Reach Clinical Translation in Gene Therapy? International Journal of Molecular Sciences, 22(14), 7545. https://doi.org/10.3390/ijms22147545









