Structural Identification of the Pacemaker Cells and Expression of Hyperpolarization-Activated Cyclic Nucleotide-Gated (HCN) Channels in the Heart of the Wild Atlantic Cod, Gadus morhua (Linnaeus, 1758)
Abstract
1. Introduction
2. Results
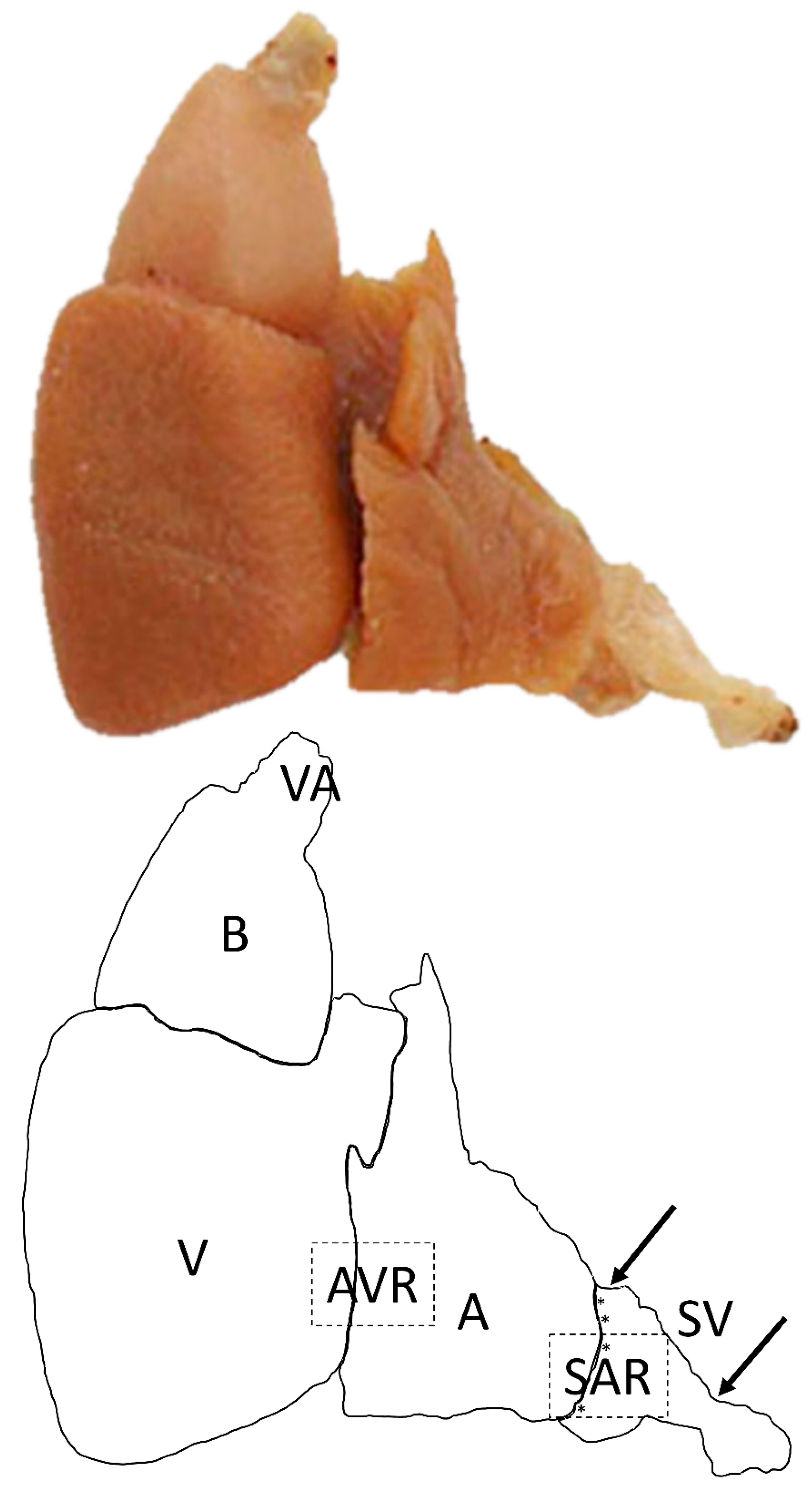
2.1. Morphological Characterization of the Pacemaker in Atlantic Cod
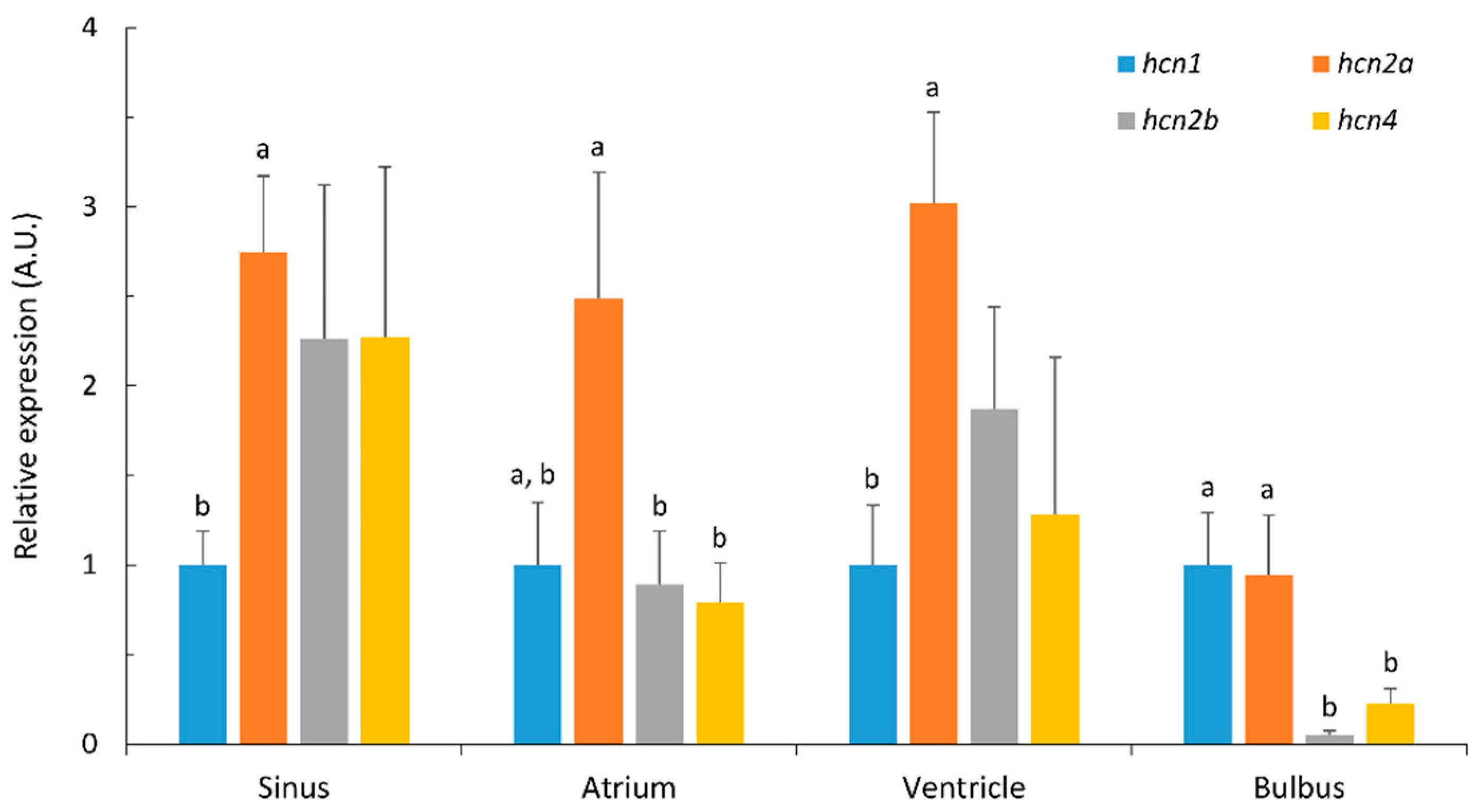
2.2. Differential Expression of Hcn Paralogues in Heart
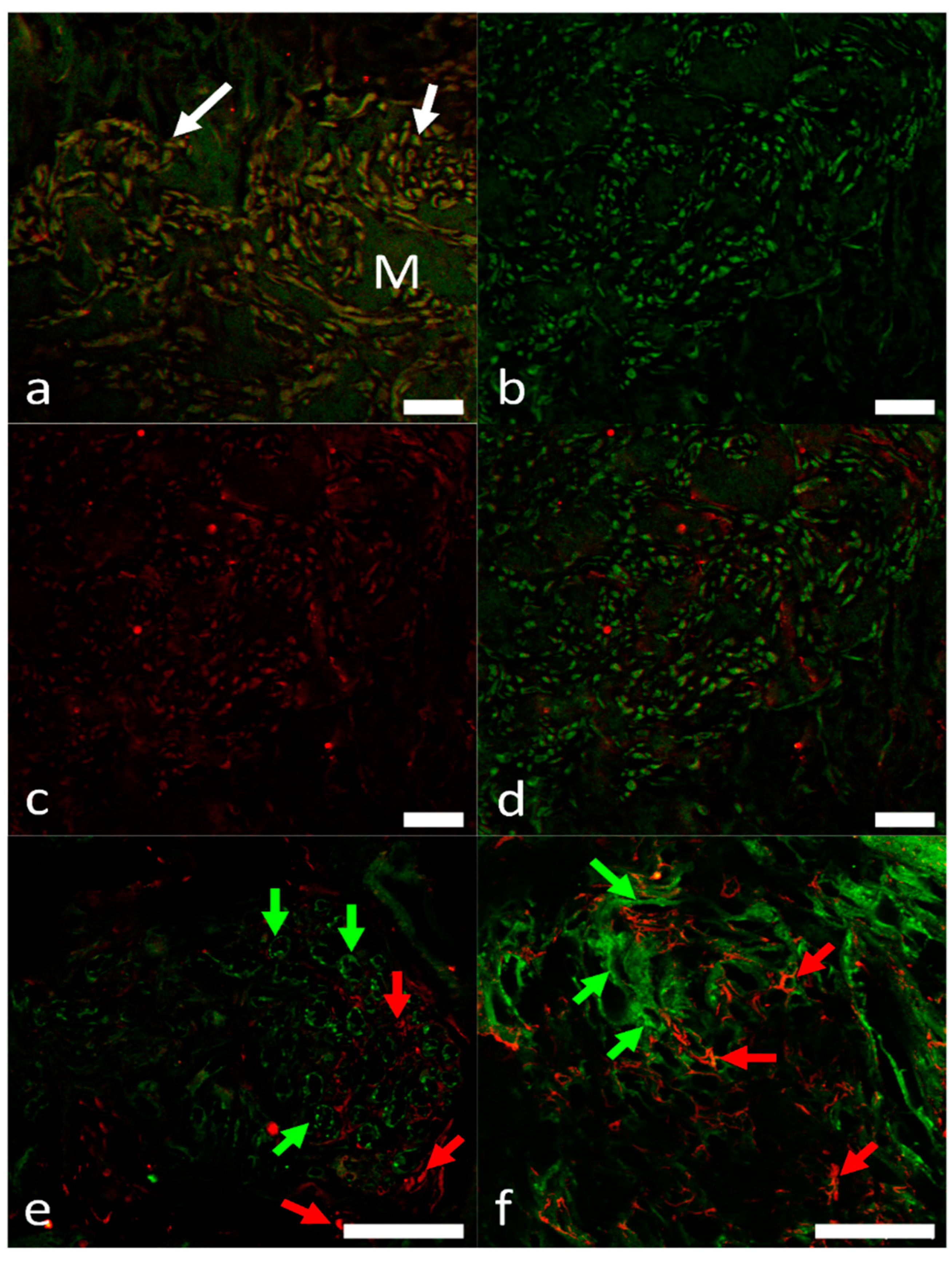
2.3. Overview of the Innervation Pattern
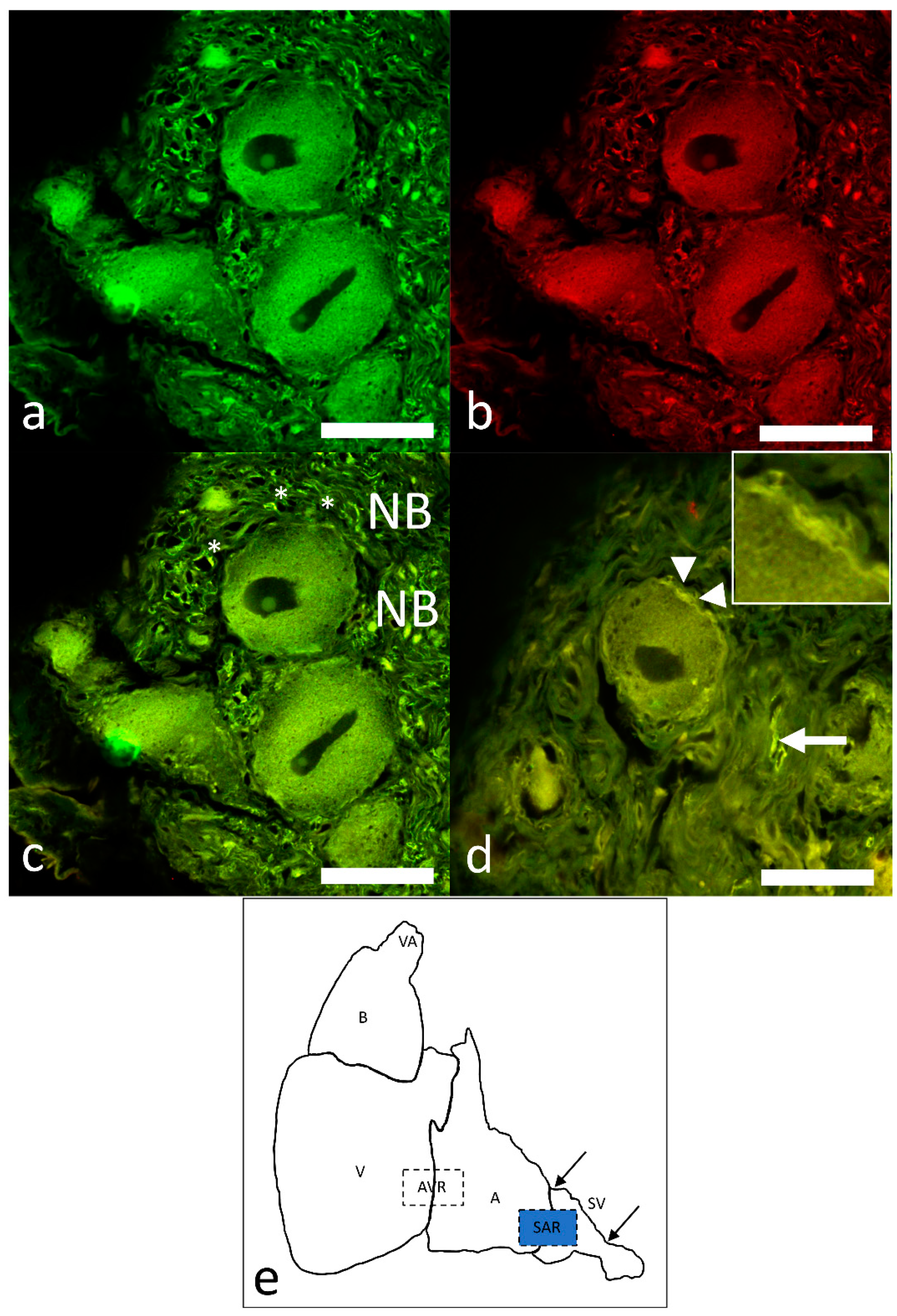
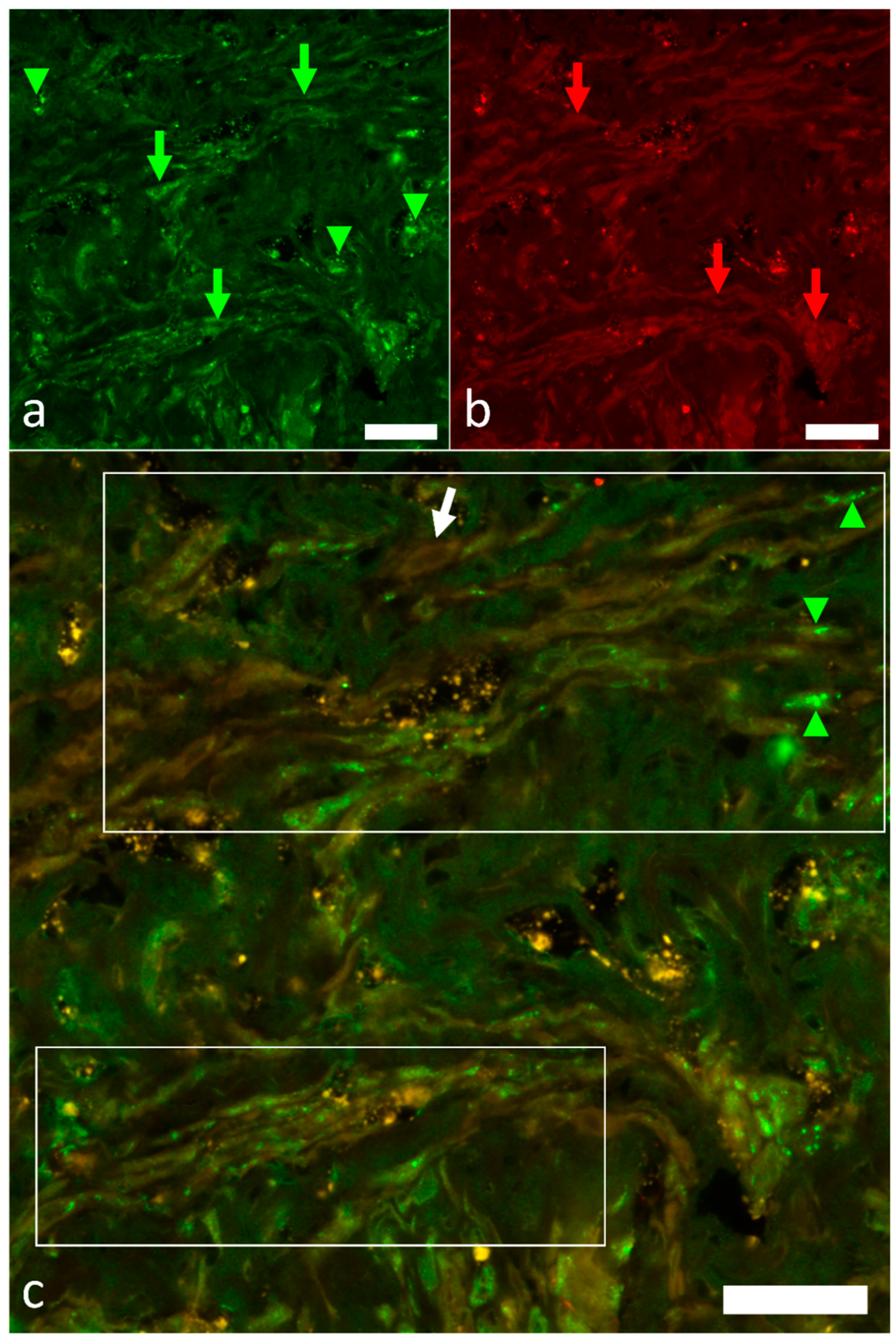
2.4. Identification of Pacemaker Cells
3. Discussion
4. Materials and Methods
4.1. Collection of Samples
4.2. Immunohistochemistry
4.2.1. General and Neurotransmitter-Specific Labeling
4.2.2. Primary Antibodies and Antibody Specificity
4.2.3. Pacemaker Cell Immunohistochemistry
4.2.4. Analysis and Imaging
4.3. Semi-Thin Sections and Transmission Electron Microscopy (TEM)
4.4. RNA Extraction and cDNA Synthesis
4.5. Real-Time PCR (qPCR) and Quantification of Gene Expression
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Icardo, J.M. Heart Morphology and Anatomy. In Fish Physiology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 36. [Google Scholar]
- Yao, Y.; Marra, A.N.; Yelon, D. Pathways Regulating Establishment and Maintenance of Cardiac Chamber Identity in Zebrafish. J. Cardiovasc. Dev. Dis. 2021, 8, 13. [Google Scholar] [CrossRef]
- MacDonald, E.A.; Stoyek, M.R.; Rose, R.A.; Quinn, T.A. Intrinsic regulation of sinoatrial node function and the zebrafish as a model of stretch effects on pacemaking. Prog. Biophys. Mol. Biol. 2017, 130, 198–211. [Google Scholar] [CrossRef]
- Tessadori, F.; van Weerd, J.H.; Burkhard, S.B.; Verkerk, A.O.; de Pater, E.; Boukens, B.J.; Vink, A.; Christoffels, V.M.; Bakkers, J. Identification and Functional Characterization of Cardiac Pacemaker Cells in Zebrafish. PLoS ONE 2012, e47644. [Google Scholar] [CrossRef]
- Stoyek, M.R.; Croll, R.P.; Smith, F.M. Intrinsic and extrinsic innervation of the heart in zebrafish (Danio rerio). J. Comp. Neurol. 2015, 523, 1683–1700. [Google Scholar] [CrossRef]
- Stoyek, M.R.; Quinn, T.A.; Croll, R.P.; Smith, F.M. Zebrafish heart as a model to study the integrative autonomic control of pacemaker function. Am. J. Physiol. Hearth Circ. Physiol. 2016, 311, H676–H688. [Google Scholar] [CrossRef]
- Santer, R.M.; Cobb, J.L.S. The fine structure of the heart of the teleost, Pleuronectes platessa L. Z. Zellforsch. Mikrosk. Anat. 1972, 131, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, A.; Fujimaki, Y.; Yokota, R. Fine structural studies of the sino-auricular nodal tissue in the heart of a teleost fish, misgurnus, with particular reference to the cardiac internuncial cell. Am. J. Anat. 1973, 138, 407–429. [Google Scholar] [CrossRef] [PubMed]
- Laurent, P. Contribution a L’étude Morphologique et Physiologique de Innervation du Coeur des Teleosteens; Masson: Moulineaux, France, 1962. [Google Scholar]
- Yamauchi, A.; Burnstock, G. An electron microscopic study on the innervation of the trout heart. J. Comp. Neurol. 1968, 132, 567–587. [Google Scholar] [CrossRef] [PubMed]
- Cobb, J.L.S.; Santer, R.M. Electrophysiology of cardiac function in teleosts: Cholinergically mediated inhibition and rebound excitation. J. Physiol. 1973, 230, 561–573. [Google Scholar] [CrossRef]
- Wilson, C.M.; Stecyk, J.A.W.; Couturier, C.S.; Nilsson, G.E.; Farrell, A.P. Phylogeny and effects of anoxia on hyperpolarization- activated cyclic nucleotidegated channel gene expression in the heart of a primitive chordate, the pacific hagfish (Eptatretus stoutii). J. Exp. Biol. 2013, 216, 4462–4472. [Google Scholar] [CrossRef]
- Vornanen, M.; Hassinen, M. Zebrafish heart as a model for human cardiac electrophysiology. Channels 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Hassinen, M.; Haverinen, J.; Vornanen, M. Small functional If current in sinoatrial pacemaker cells of the brown trout (Salmo trutta fario) heart despite strong expression of HCN channel transcripts. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- DiFrancesco, D. Pacemaker mechanisms in cardiac tissue. Annu. Rev. Physiol. 1993, 55, 455–472. [Google Scholar] [CrossRef]
- Zaccone, G.; Marino, F.; Zaccone, D. Design and Physiology of the heart|Intracardiac Neurons and Neurotransmitters in Fish. In Encyclopedia of Fish Physiology; Farrell, A., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 1067–1072. ISBN 978-0-08-092323-9. [Google Scholar]
- Zaccone, G.; Mauceri, A.; Maisano, M.; Fasulo, S. Innervation of lung and heart in the ray-finned fish, bichirs. Acta Histochem. 2009, 111, 217–229. [Google Scholar] [CrossRef]
- Inoue, J.; Sato, Y.; Sinclair, R.; Tsukamoto, K.; Nishida, M. Rapid genome reshaping by multiple-gene loss after whole-genome duplication in teleost fish suggested by mathematical modeling. Proc. Natl. Acad. Sci. USA 2015, 112, 14918–14923. [Google Scholar] [CrossRef] [PubMed]
- Volff, J.N. Genome evolution and biodiversity in teleost fish. Heredity 2005, 94, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Burkhard, S.; van Eif, V.; Garric, L.; Christoffels, V.; Bakkers, J. On the Evolution of the Cardiac Pacemaker. J. Cardiovasc. Dev. Dis. 2017, 4, 4. [Google Scholar] [CrossRef]
- Jackson, H.A.; Marshall, C.R.; Accili, E.A. Evolution and structural diversification of hyperpolarization-activated cyclic nucleotide-gated channel genes. Physiol. Genom. 2007, 29, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Laurent, P.; Holmgren, S.; Nilsson, S. Nervous and humoral control of the fish heart: Structure and function. Comp. Biochem. Physiol. Part A Physiol. 1983, 76, 525–542. [Google Scholar] [CrossRef]
- Bleeker, W.K.; Mackaay, A.J.; Masson-Pévet, M.; Bouman, L.N.; Becker, A.E. Functional and morphological organization of the rabbit sinus node. Circ. Res. 1980, 46, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Monfredi, O.; Tsutsui, K.; Ziman, B.; Stern, M.D.; Lakatta, E.G.; Maltsev, V.A. Electrophysiological heterogeneity of pacemaker cells in the rabbit intercaval region, including the SA node: Insights from recording multiple ion currents in each cell. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H403–H414. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S. Comparative anatomy of the autonomic nervous system. Auton. Neurosci. Basic Clin. 2011, 165, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Santer, R.M. Morphology and Innervation of the Fish Heart; Springer: Berlin/Heidelberg, Germany, 1985. [Google Scholar]
- Newton, C.M.; Stoyek, M.R.; Croll, R.P.; Smith, F.M. Regional innervation of the heart in the goldfish, Carassius auratus: A confocal microscopy study. J. Comp. Neurol. 2014, 522, 456–478. [Google Scholar] [CrossRef] [PubMed]
- Zaccone, G.; Mauceri, A.; Maisano, M.; Giannetto, A.; Parrino, V.; Fasulo, S. Postganglionic nerve cell bodies and neurotransmitter localization in the teleost heart. Acta Histochem. 2010, 112, 328–336. [Google Scholar] [CrossRef]
- Yamauchi, A. Fine structure of the fish heart. In Heart and Heart- Like Organs; Bourne, G., Ed.; Academic Press: New York, NY, USA, 1980; Volume 1, pp. 119–148. [Google Scholar]
- Satchell, G.H. Physiology and Form of Fish Circulation; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar] [CrossRef]
- Farrell, A.P.; Jones, D.R. The Heart. In Fish Physiology; Elsevier: Amsterdam, The Netherlands, 1992. [Google Scholar]
- Sun, Y.; Liang, X.; Najafi, N.; Cass, M.; Lin, L.; Cai, C.L.; Chen, J.; Evans, S.M. Islet 1 is expressed in distinct cardiovascular lineages, including pacemaker and coronary vascular cells. Dev. Biol. 2007, 232, 98–104. [Google Scholar] [CrossRef]
- Itoh, M.; Kaizuka, T.; Hayashi, T. Evolutionary acquisition and divergence of vertebrate HCN2 palmitoylation. Neurotransmitter 2017, 4, e1603. [Google Scholar] [CrossRef][Green Version]
- Sutcliffe, R.L.; Li, S.; Gilbert, M.J.H.; Schulte, P.M.; Miller, K.M.; Farrell, A.P. A rapid intrinsic heart rate resetting response with thermal acclimation in rainbow trout, Oncorhynchus mykiss. J. Exp. Biol. 2020, 223, jeb215210. [Google Scholar] [CrossRef]
- Brioschi, C.; Micheloni, S.; Tellez, J.O.; Pisoni, G.; Longhi, R.; Moroni, P.; Billeter, R.; Barbuti, A.; Dobrzynski, H.; Boyett, M.R.; et al. Distribution of the pacemaker HCN4 channel mRNA and protein in the rabbit sinoatrial node. J. Mol. Cell. Cardiol. 2009, 47, 221–227. [Google Scholar] [CrossRef]
- Hammelmann, V.; Stieglitz, M.S.; Hülle, H.; Meur, K.L.; Kass, J.; Brümmer, M.; Gruner, C.; Rötzer, R.D.; Fenske, S.; Hartmann, J.; et al. Abolishing cAMP sensitivity in HCN2 pacemaker channels induces generalized seizures. JCI Insight 2019, 4, e126418. [Google Scholar] [CrossRef]
- Nolan, M.F.; Malleret, G.; Lee, K.H.; Gibbs, E.; Dudman, J.T.; Santoro, B.; Yin, D.; Thompson, R.F.; Siegelbaum, S.A.; Kandel, E.R.; et al. The Hyperpolarization-Activated HCN1 Channel Is Important for Motor Learning and Neuronal Integration by Cerebellar Purkinje Cells. Cell 2003, 115, 551–564. [Google Scholar] [CrossRef]
- Biel, M.; Wahl-Schott, C.; Michalakis, S.; Zong, X. Hyperpolarization-activated cation channels: From genes to function. Physiol. Rev. 2009, 1, 433–451. [Google Scholar] [CrossRef]
- Jones, D.R.; Braun, M.H. Design and physiology of the heart|The Outflow Tract from the Heart. In Encyclopedia of Fish Physiology; Academic Press: San Diego, CA, USA, 2011; ISBN 9780080923239. [Google Scholar]
- Zaccone, G.; Lauriano, E.R.; Kuciel, M.; Capillo, G.; Pergolizzi, S.; Alesci, A.; Ishimatsu, A.; Ip, Y.K.; Icardo, J.M. Identification and distribution of neuronal nitric oxide synthase and neurochemical markers in the neuroepithelial cells of the gill and the skin in the giant mudskipper, Periophthalmodon schlosseri. Zoology 2017, 125, 51–52. [Google Scholar] [CrossRef]
- Zaccone, G.; Cupello, C.; Capillo, G.; Kuciel, M.; Nascimento, A.L.R.; Gopesh, A.; Germanà, G.P.; Spanò, N.; Guerrera, M.C.; Aragona, M.; et al. Expression of Acetylcholine- and G protein coupled Muscarinic receptor in the Neuroepithelial cells (NECs) of the obligated air-breathing fish, Arapaima gigas (Arapaimatidae: Teleostei). Zoology 2020, 139. [Google Scholar] [CrossRef]
- Zaccone, G.; Lauriano, E.R.; Silvestri, G.; Kenaley, C.; Icardo, J.M.; Pergolizzi, S.; Alesci, A.; Sengar, M.; Kuciel, M.; Gopesh, A. Comparative neurochemical features of the innervation patterns of the gut of the basal actinopterygian, Lepisosteus oculatus, and the euteleost, Clarias batrachus. Acta Zool. 2015, 96, 127–139. [Google Scholar] [CrossRef]
- Zaccone, D.; Dabrowski, K.; Lauriano, E.R.; De Pasquale, A.; Macrì, D.; Satora, L.; Lanteri, G. The simultaneous presence of neuroepithelial cells and neuroepithelial bodies in the respiratory gas bladder of the longnose gar, Lepisosteus osseus, and the spotted gar, L. oculatus. Acta Histochem. 2012. [Google Scholar] [CrossRef] [PubMed]
- Olsson, C.; Holbrook, J.D.; Bompadre, G.; Jönsson, E.; Hoyle, C.H.V.; Sanger, G.J.; Holmgren, S.; Andrews, P.L.R. Identification of genes for the ghrelin and motilin receptors and a novel related gene in fish, and stimulation of intestinal motility in zebrafish (Danio rerio) by ghrelin and motilin. Gen. Comp. Endocrinol. 2008, 155, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Uyttebroek, L.; Shepherd, I.T.; Harrisson, F.; Hubens, G.; Blust, R.; Timmermans, J.P.; van Nassauw, L. Neurochemical coding of enteric neurons in adult and embryonic zebrafish (Danio rerio). J. Comp. Neurol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Pauza, D.H.; Rysevaite, K.; Inokaitis, H.; Jokubauskas, M.; Pauza, A.G.; Brack, K.E.; Pauziene, N. Innervation of sinoatrial nodal cardiomyocytes in mouse. A combined approach using immunofluorescent and electron microscopy. J. Mol. Cell. Cardiol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Zaccone, G.; Maina, J.; Germanà, A.; Montalbano, G.; Capillo, G.; Aragona, L.; Kuciel, M.J.; Lauriano, E.R.; Icardo, J.M. First demonstration of the neuroepithelial cells and their chemical code in the accessory respiratory organ and the gill of the sharptooth catfish, Clarias gariepinus: A preliminary study. Acta Zool. 2019, 100, 160–166. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002. [Google Scholar] [CrossRef]
- Fernandes, J.M.O.; Mommens, M.; Hagen, Ø.; Babiak, I.; Solberg, C. Selection of suitable reference genes for real-time PCR studies of Atlantic halibut development. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, K.; Giannetto, A.; Fernandes, J.M.O. Photoperiod influences growth and MLL (mixed-lineage leukaemia) expression in Atlantic cod. PLoS ONE 2012, 7, e36908. [Google Scholar] [CrossRef] [PubMed][Green Version]


| Primary Antibody | Clonality | Manufacturer | Catalogue Number | Dilution | ID Number |
|---|---|---|---|---|---|
| Monoclonal anti-tubulin, acetylated antibody (AcT) | Monoclonal | Sigma-Aldrich | T6793 | 1:1000 | AB_477585 |
| Mouse anti-human HuC/HuD neuronal protein (Hu) | Monoclonal | Molecular Probes | A21271 | 1:250 | AB_221448 |
| Choline acetyltransferase (ChAT) | Polyclonal | Atlas Antibodies | HPA048547 | 1:50 | AB_2680437 |
| Anti-vesicular acetylcholine transporter (VAChT) | Monoclonal | Millipore | AB1588 | 1:100 | AB_2187981 |
| Anti-vesicular acetylcholine transporter (VAChT) | Polyclonal | Sigma-Aldrich | V5387 | 1:500 | AB_261875 |
| Neuronal nitric oxide synthase (nNOS) | Monoclonal | Abcam | ab67002 | 1:25 | AB_1141642 |
| Neuronal nitric oxide synthase (nNOS) | Polyclonal | Santa Cruz Biotechnology | sc-648 | 1:500 | AB_630935 |
| Anti-HCN1 | Polyclonal | Alomone Labs | APC-056 | 1:100 | AB_2039900 |
| Anti-HCN2 | Polyclonal | Alomone Labs | APC-030 | 1:100 | AB_2313726 |
| Anti-HCN4 | Polyclonal | Alomone Labs | APC-052 | 1:100 | AB_2039906 |
| Islet-1 | Monoclonal | DSHB | 39.4D5 | 1:100 | AB_2314683 |
| Gene | Primer Sequence (5′–3′) | Amplicon Size (bp) | Ta (°C) | E (%) | Accession or Reference |
|---|---|---|---|---|---|
| hcn1 | AGCGATTTTAGGTTCTACTGGG GGAAGATGGTGTCCGAGGC | 146 | 58 | 82 | XM_030342287.1 |
| hcn2a | GACGTGAGGCAGAAGATCCA GTCGCTCAGCTCTCCCAGG | 90 | 62 | 72 | XM_030370466.1 |
| hcn2b | ACAGTGACTTCAGCAGGTTCTAC AAGTTGAGCACCAGGTCCAT | 173 | 62 | 78 | ENSGMOG00000011849 |
| hcn4 | GTGATTTCAGGTTCTACTGGGAC CAGGTCCAGGAGGAAGAAGG | 155 | 62 | 75 | ENSGMOG00000001132 |
| arp | ACGCACCAGCCAAGGTAGAG ATGTCGTCATCAGACTCCTCGG | 70 | 60 | 89 | EX741373.1 |
| eef1a | ATCGGCGGTATTGGAACAGT CATCTCAACGGACTTCACCTCA | 117 | 65 | 99 | EX721840.1 |
| ubi | GGCCGCAAAGATGCAGAT CTGGGCTCGACCTCAAGAGT | 69 | 60 | 96 | [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capillo, G.; Lauriano, E.R.; Icardo, J.M.; Siriyappagouder, P.; Kuciel, M.; Karapanagiotis, S.; Zaccone, G.; Fernandes, J.M.O. Structural Identification of the Pacemaker Cells and Expression of Hyperpolarization-Activated Cyclic Nucleotide-Gated (HCN) Channels in the Heart of the Wild Atlantic Cod, Gadus morhua (Linnaeus, 1758). Int. J. Mol. Sci. 2021, 22, 7539. https://doi.org/10.3390/ijms22147539
Capillo G, Lauriano ER, Icardo JM, Siriyappagouder P, Kuciel M, Karapanagiotis S, Zaccone G, Fernandes JMO. Structural Identification of the Pacemaker Cells and Expression of Hyperpolarization-Activated Cyclic Nucleotide-Gated (HCN) Channels in the Heart of the Wild Atlantic Cod, Gadus morhua (Linnaeus, 1758). International Journal of Molecular Sciences. 2021; 22(14):7539. https://doi.org/10.3390/ijms22147539
Chicago/Turabian StyleCapillo, Gioele, Eugenia R. Lauriano, Jose M. Icardo, Prabhugouda Siriyappagouder, Michal Kuciel, Stelios Karapanagiotis, Giacomo Zaccone, and Jorge M. O. Fernandes. 2021. "Structural Identification of the Pacemaker Cells and Expression of Hyperpolarization-Activated Cyclic Nucleotide-Gated (HCN) Channels in the Heart of the Wild Atlantic Cod, Gadus morhua (Linnaeus, 1758)" International Journal of Molecular Sciences 22, no. 14: 7539. https://doi.org/10.3390/ijms22147539
APA StyleCapillo, G., Lauriano, E. R., Icardo, J. M., Siriyappagouder, P., Kuciel, M., Karapanagiotis, S., Zaccone, G., & Fernandes, J. M. O. (2021). Structural Identification of the Pacemaker Cells and Expression of Hyperpolarization-Activated Cyclic Nucleotide-Gated (HCN) Channels in the Heart of the Wild Atlantic Cod, Gadus morhua (Linnaeus, 1758). International Journal of Molecular Sciences, 22(14), 7539. https://doi.org/10.3390/ijms22147539











