Mercury Toxicity and Neurogenesis in the Mammalian Brain
Abstract
1. Introduction
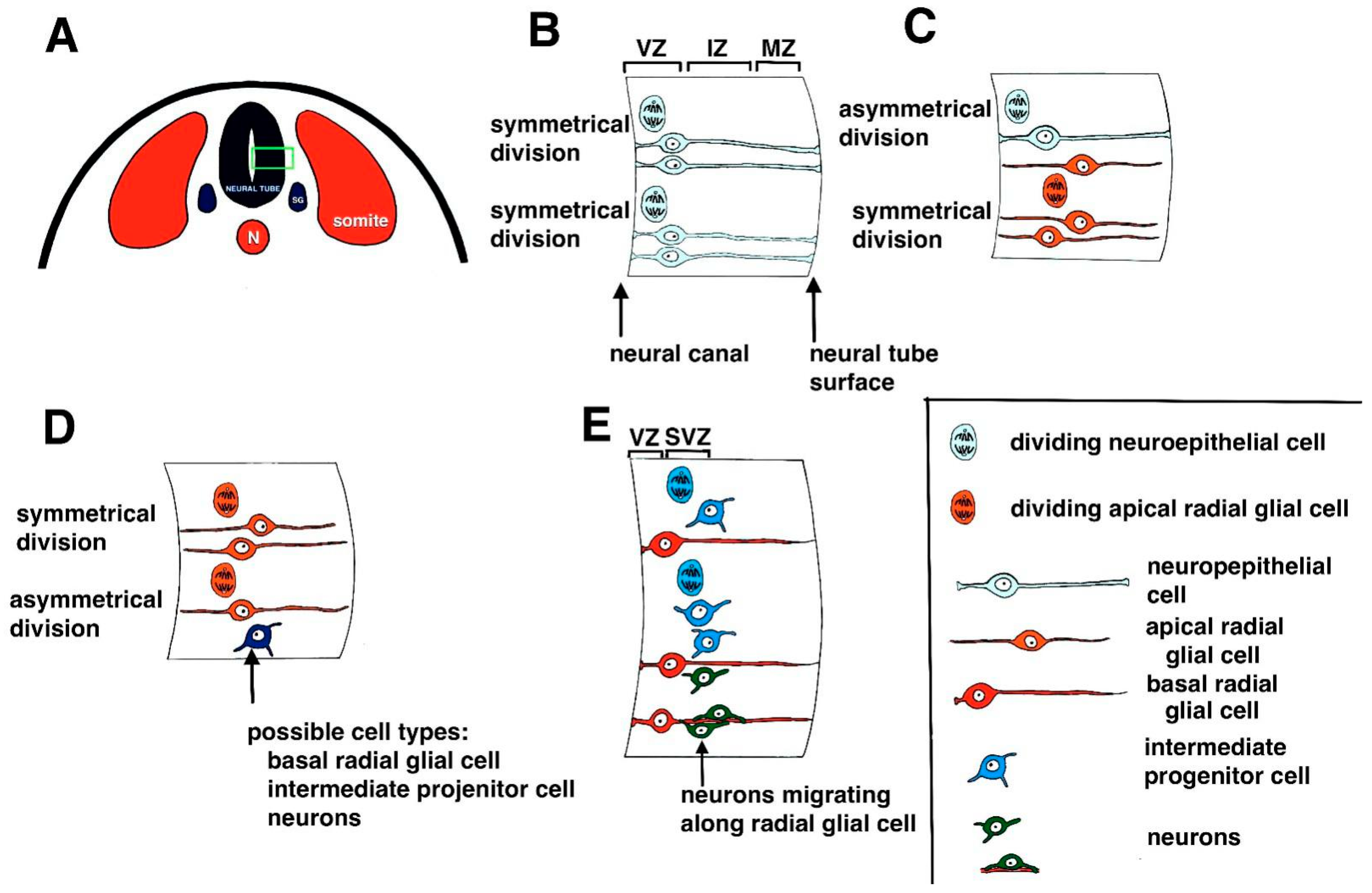
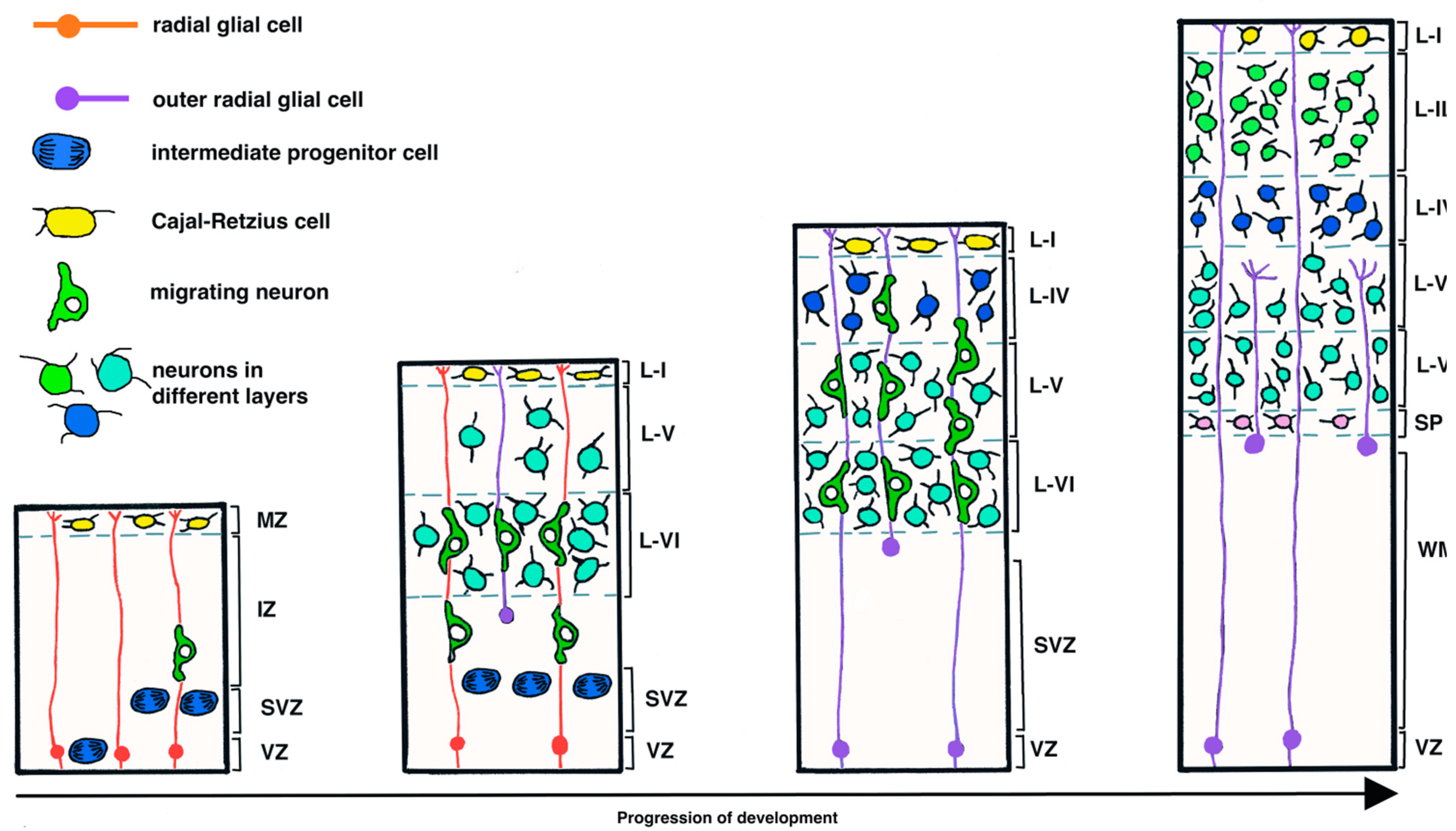
1.1. Neurogenesis during Development
1.2. Mercury in the Environment
2. Models Used to Study Developmental Mercury Toxicity
3. Theories of the Pathogenesis of Mercury Toxicity in Neurogenesis
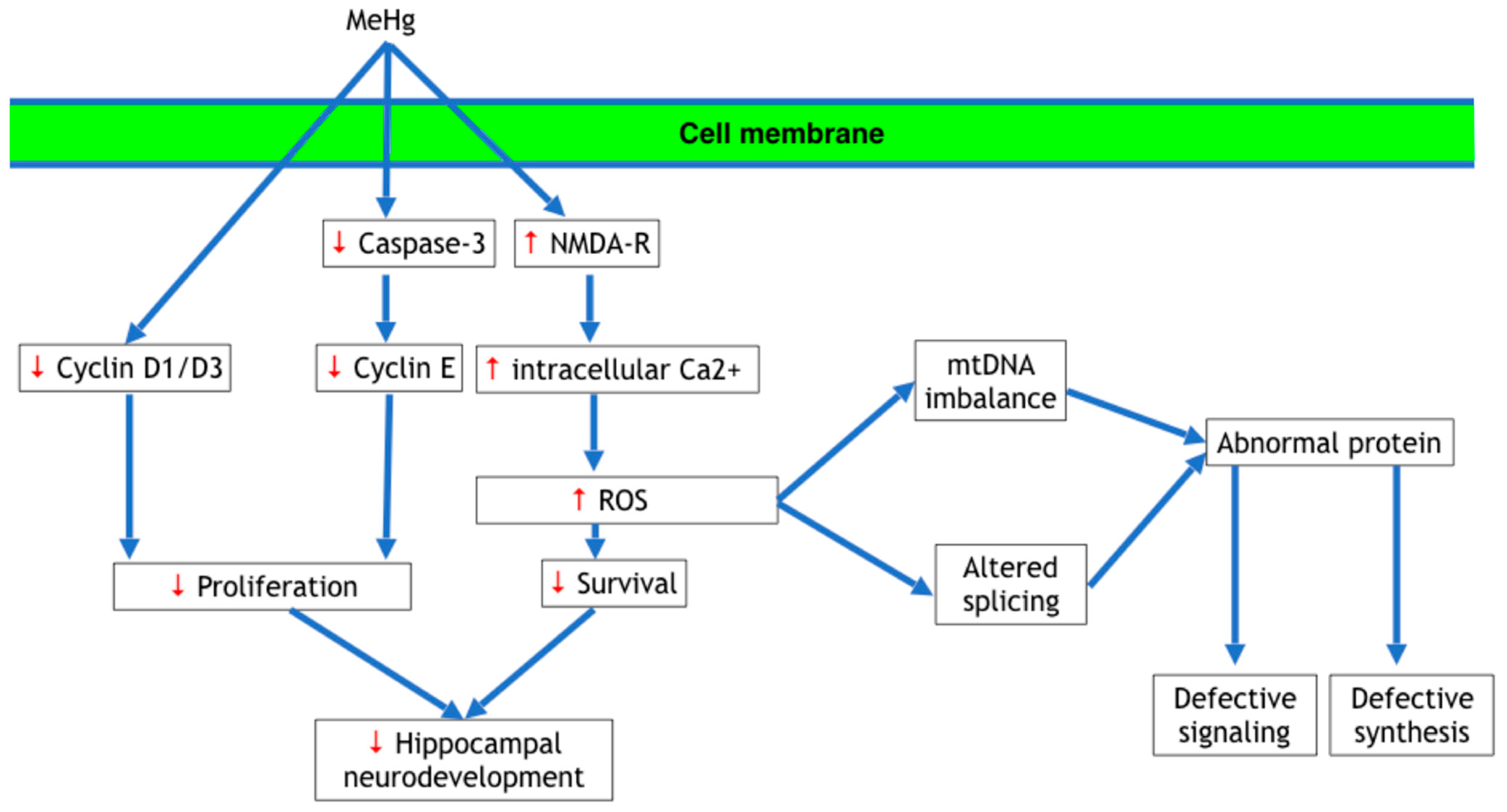
3.1. Disruption of Cell Proliferation
3.2. Disruption of Gene Expression, Cell Signaling Pathways, and Protein Phosphorylation
3.3. Oxidative Stress
3.4. Disruptions in Intracellular Calcium Ion (Ca2+) Homeostasis
3.5. Disruptions in Migration
3.6. Long-Lasting Effects of Mercury Exposure
4. Possible Treatments to Reduce the Toxic Effects of Mercury on Developing Neurons
5. Limitations
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nawarawong, N.N.; Nickell, C.G.; Hopkins, D.M.; Pauly, J.R.; Nixon, K. Functional Activation of Newborn Neurons Following Alcohol-Induced Reactive Neurogenesis. Brain Sci. 2021, 11, 499. [Google Scholar] [CrossRef]
- Kovach, C.; Dixit, R.; Li, S.; Mattar, P.; Wilkinson, G.; Elsen, G.E.; Kurrasch, D.M.; Hevner, R.F.; Schuurmans, C. Neurog2 simultaneously activates and represses alternative gene expression programs in the developing neocortex. Cereb. Cortex 2013, 23, 1884–1900. [Google Scholar] [CrossRef]
- Mira, H.; Morante, J. Neurogenesis from embryos to adult—Lessons from flies and mice. Front. Cell Dev. Biol. 2020, 8, 533. [Google Scholar] [CrossRef] [PubMed]
- Obernier, K.; Alvarez-Buylla, A. Neural stem cells: Origin, heterogeneity and regulation in the adult mammalian brain. Development 2019, 146, dev156059. [Google Scholar] [CrossRef]
- Cardenas, A.; Borrell, V. Molecular and cellular evolution of corticogenesis in amniotes. Cell Mol. Life Sci. 2019, 77, 1435–1460. [Google Scholar] [CrossRef]
- Franchini, L.M. Genetic Mechanisms Underlying Cortical Evolution in Mammals. Front. Cell Dev. Biol. 2021, 9, 591017. [Google Scholar] [CrossRef] [PubMed]
- Florio, M.; Huttner, W.B. Neural progenitors, neurogenesisand the evolution of the neocortex. Development 2014, 141, 2182–2194. [Google Scholar] [CrossRef] [PubMed]
- Goffinet, A.M. The evolution of cortical development: The synapsid-diapsid divergence. Development 2017, 144, 4061–4077. [Google Scholar] [CrossRef]
- Haubensak, W.; Attardo, A.; Denk, W.; Huttner, W.B. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: A major site of neurogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 3196–3201. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Kawaguchi, A.; Saito, K.; Kawano, M.; Muto, T.; Ogawa, M. Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development 2004, 131, 3133–3145. [Google Scholar] [CrossRef] [PubMed]
- Noctor, S.C.; Martinez-Cerdeno, V.; Ivic, L.; Krigstein, A.R. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 2004, 7, 136–144. [Google Scholar] [CrossRef]
- Kalebic, N.; Huttner, W.B. Basal Progenitor Morphology and Neocortex Evolution. Trends Neurosci. 2020, 43, 843–853. [Google Scholar] [CrossRef]
- Smart, I.H.M. Proliferative characteristics of the ependymal layer during the early development of the mouse diencephalon, as revealed by recording the number, location, and plane of cleavage of mitotic figures. J. Anat. 1972, 113, 109–1296. [Google Scholar]
- Smart, I.H.M.; Dehay, C.; Giroud, P.; Berland, M.; Kennedy, H. Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb. Cortex 2002, 12, 37–53. [Google Scholar] [CrossRef]
- Dehay, C.; Kennedy, H.; Kosik, K.S. The outer subventricular zone and primate-specific cortical complexification. Neuron 2015, 85, 683–694. [Google Scholar] [CrossRef]
- Uzquiano, A.; Gladwyn-Ng, I.; Nguyen, L.; Reiner, O.; Gotz, M.; Matsuzaki, F.; Francis, F. Cortical progenitor biology: Key features mediating proliferation versus differentiation. J. Neurochem. 2018, 146, 500–525. [Google Scholar] [CrossRef]
- Rakic, P. Evolution of the neocortex: A perspective from developmental biology. Nat. Rev. Neurosci. 2009, 10, 724–735. [Google Scholar] [CrossRef]
- Germain, N.; Banda, E.; Grabel, L. Embryonic stem cell neurogenesis and neural specification. J. Cell Biochem. 2010, 111, 535–542. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, R.D.; Burkhalter, A. A Laminar Organization for Selective Cortico-Cortical Communication. Front. Neuroanat. 2017, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Hasegawa, K.; Liu, M.; Song, W.-J. Comparison of the Upper Marginal Neurons of Cortical Layer 2 with Layer 2/3 Pyramidal Neurons in Mouse Temporal Cortex. Front. Neuroanat. 2017, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Pfisterer, U.; Khodosevich, K. Neuronal survival in the brain: Neuron type-specific mechanisms. Cell Death Dis. 2017, 8, e2643. [Google Scholar] [CrossRef]
- Park, S.Y.; Han, J.S. Phospholipase D1 Signaling: Essential Roles in Neural Stem Cell Differentiation. J. Mol. Neurosci. 2018, 64, 333–340. [Google Scholar] [CrossRef]
- Fuentealba, L.C.; Rompani, S.B.; Parraguez, J.I.; Obernier, K.; Romero, R.; Cepko, C.L.; Alvarez-Buylla, A. Embryonic origin of postnatal neural stem cells. Cell 2015, 161, 1644–1655. [Google Scholar] [CrossRef] [PubMed]
- Furutachi, S.; Miya, H.; Watanabe, T.; Kawai, H.; Yamasaki, N.; Harada, Y.; Imayoshi, I.; Nelson, M.; Nakayama, K.I.; Hirabayashi, Y.; et al. Slowly dividing neural progenitors are an embryonic origin of adult neural stem cells. Nat. Neurosci. 2015, 18, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Bond, A.M.; Berg, D.A.; Lee, S.; Garcia-Epelboim, A.S.; Adusumilli, V.S.; Ming, G.L.; Song, H. Differential Timing and Coordination of Neurogenesis and Astrogenesis in Developing Mouse Hippocampal Subregions. Brain Sci. 2020, 10, 909. [Google Scholar] [CrossRef] [PubMed]
- Gotz, M.; Stoykova, A.; Gruss, P. Pax6 controls radial glia differentiation in the cerebral cortex. Neuron 1998, 21, 1031–1044. [Google Scholar] [CrossRef]
- Englund, C.; Fink, A.; Lau, C.; Pham, D.; Daza, R.A.; Bulfone, A.; Kowalczyk, T.; Hevner, R.F. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J. Neurosci. 2005, 25, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Mihalas, A.B.; Elsen, G.E.; Bedogni, F.; Daza, R.A.M.; Ramos-Laguna, K.A.; Arnold, S.J.; Hevner, R.F. Intermediate progenitor cohorts differentially generate cortical layers and require tbr2 for timely acquisition of neuronal subtype identity. Cell Rep. 2016, 16, 92–105. [Google Scholar] [CrossRef]
- Braga, M.C.; Shaw, G.; Lester, J.N. Mercury modeling to predict contamination and bioaccumulation in aquatic ecosystems. Rev. Environ. Contam. Toxicol. 2020, 164, 69–92. [Google Scholar]
- ATSDR—US Department of Health and Human Services, Public Health Service (ATSDR). Toxicological Profile for Mercury; US Department of Health and Human Services: Atlanta, GA, USA, 2012; pp. 1–600. Available online: https://www.atsdr.cdc.gov/mercury/docs/11-229617-E-508_HealthEffects.pdf (accessed on 1 May 2021).
- Sharma, B.J.; Sanka, O.; Kalina, J.; Scheringer, M. An overview of worldwide and regional time trends in total mercury levels in human blood and breast milk from 1966 to 2015 and their associations with health effects. Environ. Internat. 2019, 125, 300–319. [Google Scholar] [CrossRef]
- UNEP (United Nations Environment Programme). Mercury—Time to Act; Kirby, A., Rucevska, I., Yemelin, V., Cooke, C., Simonett, O., Novikov, V., Hughes, G., Eds.; United Nations Environment Programme: Paris, France, 2013. [Google Scholar]
- Gochfeld, M. Cases of mercury exposure, bioavailability, and absorption. Ecotoxicol. Environ. Saf. 2003, 56, 174–179. [Google Scholar] [CrossRef]
- Syversen, T.; Kaur, P. The toxicology of mercury and its compounds. J. Trace Elem. Med. Biol. 2012, 26, 215–226. [Google Scholar] [CrossRef]
- Raposo, R.S.; Pinto, D.V.; Moreira, R.; Dias, R.P.; Fontes-Ribeiro, C.A.; Oriá, R.B.; Malva, J.O. Methylmercury Impact on Adult Neurogenesis: Is theWorst Yet to Come From Recent Brazilian Environmental Disasters? Front. Aging Neurosci. 2020, 12, 591–601. [Google Scholar] [CrossRef]
- Murti, R.; Shukla, G.S. Mercuric chloride intoxication in freshwater prawn. I. Effect on carbohydrate metabolism. Ecotoxicol. Environ. Saf. 1984, 8, 284–288. [Google Scholar] [CrossRef]
- Stohs, S.J.; Bagchi, D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef]
- Carratu, M.R.; Signorile, A. Methyl mercury injury to CNS: Mitochondrial at the core of the matter? Open Acc. Toxicol. 2015, 1, 1–6. [Google Scholar] [CrossRef]
- Farina, M.; Rocha, J.B.T.; Aschner, M. Mechanism of methylmercury induced neurotoxicity: Evidence from experimental studies. Life Sci. 2012, 89, 555–563. [Google Scholar] [CrossRef]
- Castoldi, A.F.; Onishchenko, N.; Johansson, C.; Coccini, T.; Roda, E.; Vahter, M.; Ceccatelli, S.; Manzo, L. Neurodevelopmental toxicity of methylmercury: Laboratory animal data and their contribution to human risk assessment. Regul. Toxicol. Pharmacol. 2008, 51, 215–229. [Google Scholar] [CrossRef]
- Myers, G.J.; Thurston, S.W.; Pearson, A.T.; Davidson, P.W.; Cox, C.; Shamlaye, C.F.; Cernichiari, E.; Clarkson, T.W. Postnatal exposure to methyl mercury from fish consumption: A review and new data from the Seychelles Child Development Study. Neurotoxicology 2009, 30, 338–349. [Google Scholar] [CrossRef]
- Yu, X.; Robinson, J.F.; Sidhu, J.S.; Hong, S.; Faustman, E.M. A system-based comparison of gene expression reveals alterations in oxidative stress, disruption of ubiquitin-proteasome system and altered cell cycle regulation after exposure to cadmium and methylmercury in mouse embryonic fibroblast. Toxicol. Sci. 2010, 114, 356–377. [Google Scholar] [CrossRef]
- Rand, M.D.; Dao, J.C.; Clason, T.A. Methylmercury disruption of embryonic neural development in Drosophila. Neurotoxicology 2009, 30, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Antunes Dos Santos, A.; Appel Hort, M.; Culbreth, M.; López-Granero, C.; Farina, M.; Rocha, J.B.; Aschner, M. Methylmercury and brain development: A review of recent literature. J. Trace Elem. Med. Biol. 2016, 38, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, T.A.; Pierce, C.H.; Pingree, S.D.; Hong, S.; Faustman, E.M. Methylmercury Distribution in the Pregnant Rat and Embryo During Early Midbrain Organogenesis. Teratology 2002, 66, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Faustman, E.M.; Gohlke, J.; Judd, N.L.; Lewandowski, T.A.; Bartelll, S.M.; Griffith, W.C. Modeling developmental processes in animals: Applications in neurodevelopment toxicology. Environ. Tox. Pharmacol. 2005, 19, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Tasneem, S.; Farrell, K.; Lee, M.-Y.; Kothapalli, C.R. Sensitivity of Neural Stem Cell Survival, Differentiation and Neurite Outgrowth within 3D Hydrogels to Environmental Heavy Metals. Toxicol. Lett. 2015, 242, 9–22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yuan, X.; Wang, J.; Chan, H.M. Sub-Nanomolar Methylmercury Exposure Promotes Premature Differentiation of Murine Embryonic Neural Precursor at the Expense of Their Proliferation. Toxics 2018, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- Faustman, E.M.; Ponce, R.A.; Ou, Y.C.; Mendoza, M.A.C.; Lewandowski, T.; Kavanagh, T. Investigations of Methylmercury-Induced Alterations in Neurogenesis. Environ. Health Perspect. 2002, 110 (Suppl. 5), 859–864. [Google Scholar] [CrossRef]
- Bose, R.; Onishchenko, N.; Edoff, K.; Lang, A.M.J.; Ceccatelli, S. Inherited Effects of Low-Dose Exposure to Methylmercury in Neural Stem Cells. Toxicol. Sci. 2012, 130, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Ceccatelli, S.; Dare, E.; Moors, M. Methylmercury-induced neurotoxicity and apoptosis. Chem. Biol. Interact. 2010, 188, 301–308. [Google Scholar] [CrossRef]
- Nat, R.; Dechant, G. Milestones of directed differentiation of mouse and human embryonic stem cells into telencephalic neurons based on neural development in vivo. Stem Cells Dev. 2011, 20, 947–958. [Google Scholar] [CrossRef]
- Attoff, K.; Gliga, A.; Lundqvist, J.; Norinder, U.; Forsby, A. Whole genome microarray analysis of neural progenitor C17.2 cells during differentiation and validation of 30 neural mRNA biomarkers for estimation of developmental neurotoxicity. PLoS ONE 2017, 12, e0190066. [Google Scholar] [CrossRef]
- Ke, T.; Tsatsakis, A.; Santamaría, A.; Soare, F.A.A.; Tinkov, A.A.; Docea, A.O.; Skalny, A.; Bowman, A.B.; Aschner, M. Chronic exposure to methylmercury induces puncta formation in cephalic dopaminergic neurons in Caenorhabditis elegans. Neurotoxicology 2020, 77, 105–113. [Google Scholar] [CrossRef]
- Caito, S.W.; Aschner, M. NAD+ Supplementation Attenuates Methylmercury Dopaminergic and Mitochondrial Toxicity in Caenorhabditis elegans. Toxicol. Sci. 2016, 151, 139–149. [Google Scholar] [CrossRef]
- Vanduyn, N.; Settivari, R.; Wong, G.; Nass, R. SKN-1/Nrf2 inhibits dopamine neuron degeneration in a Caenorhabditis elegans model of methylmercury toxicity. Toxicol. Sci. 2010, 118, 613–624. [Google Scholar] [CrossRef]
- Schetinger, M.R.C.; Peres, T.V.; Arantes, L.P.; Carvalho, F.; Dressler, V.; Heidrich, G.; Aaron BBowman, A.B.; Aschner, M. Combined exposure to methylmercury and manganese during L1 larval stage causes motor dysfunction, cholinergic and monoaminergic up-regulation and oxidative stress in L4 Caenorhabditis elegans. Toxicology 2019, 411, 154–162. [Google Scholar] [CrossRef] [PubMed]
- NRC (National Research Council). Toxicological Effects of Methylmercury; National Academy Press: Washington, DC, USA, 2000. [Google Scholar]
- Falluel-Morel, A.; Sokolowski, K.; Sisti, H.M.; Zhou, X.F.; Shors, T.J.; DiCicco-Bloom, E. Developmental mercury exposure elicits acute hippocampal cell death, reductions in neurogenesis, and severe learning deficits during puberty. J. Neurochem. 2007, 103, 1968–1981. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.H.; Lapham, L.W.; Amin-Zaki, L.; Saleem, T. Abnormal neuronal migration, deranged cerebral cortical organization, and diffuse white matter astrocytosis of human fetal brain: A major effect of methylmercury poisoning in utero. J. Neuropathol. Exp. Neurol. 1978, 37, 719–733. [Google Scholar] [CrossRef] [PubMed]
- Faustman, E.M.; Ponce, R.A.; Seeley, M.S.; Whittaker, S.G. Experimental approaches to evaluate mechanisms of developmental toxicity. In Handbook of Developmental Toxicity; Hood, R., Ed.; CRC Press: Boca Raton, FL, USA, 1996; pp. 13–41. [Google Scholar]
- Mundy, W.R.; Freudenrich, T.M. Sensitivity of immature neurons in culture to metal-induced changes in reactive oxygen species and intracellular free calcium. Neurotoxicology 2000, 21, 1135–1144. [Google Scholar]
- Xu, M.Y.; Yan, C.H.; Tian, Y.; Yuan, X.B.; Shen, X.M. Effects of low level of methylmercury on proliferation of cortical progenitor cells. Brain Res. 2010, 1359, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Shackelford, R.E.; Kaufmann, W.K.; Paules, R.S. Cell cycle control, checkpoint mechanisms, and genotoxic stress. Environ. Health Perspect. 1999, 107, 5–24. [Google Scholar]
- Tamm, C.; Duckworth, J.; Hermanson, O.; Ceccatelli, S. High susceptibility of neural stem cells to methylmercury toxicity: Effects on cell survival and neuronal differentiation. J. Neurochem. 2006, 97, 69–78. [Google Scholar] [CrossRef]
- Fujimura, M.; Usuki, F. Low concentrations of methylmercury inhibit neural progenitor cell proliferation associated with up-regulation of glycogen synthase kinase 3 and subsequent degradation of cyclin E in rats. Toxicol. Appl. Pharmacol. 2015, 288, 19–25. [Google Scholar] [CrossRef]
- Trivedi, M.; Zhang, Y.; Lopez-Toledano, M.; Clarke, A.; Deth, R. Differential neurogenic effects of casein-derived opioid peptides on neuronal stem cells: Implications for redox-based epigenetic changes. J. Nutr. Biochem. 2017, 37, 39–46. [Google Scholar] [CrossRef]
- Tamm, C.; Duckworth, J.K.; Hermanson, O.; Ceccatelli, S. Methylmercury inhibits differentiation of rat neural stem cells via Notch signaling. Neuro Rep. 2008, 19, 339–343. [Google Scholar]
- Edoff, K.; Ceccatelli, S. Methylmercury and neural stem cells. In Methylmercury and Neurotoxicity; Ceccatelli, S., Aschner, M., Eds.; Springer: New York, NY, USA, 2012; pp. 287–302. [Google Scholar]
- Moors, M.; Rockel, T.D.; Abel, J.; Cline, J.E.; Gassmann, K.; Schreiber, T.; Schuwald, J.; Weinmann, N.; Fritsche, E. Human neurospheres as three-dimensional cellular systems for developmental neurotoxicity testing. Environ. Health Perspect. 2009, 117, 1131–1138. [Google Scholar] [CrossRef]
- Theunissen, P.T.; Schulpen, S.H.W.; van Dartel, D.A.M.; Hermsen, S.A.B.; van Schooten, F.J.; Piersma, A.H. An abbreviated protocol for multilineage neural differentiation of murine embryonic stem cells and its perturbation by methyl mercury. Reprod. Toxicol. 2010, 29, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Buzanska, L.; Sypecka, J.; Molteni, S.N.; Compagnoni, A.; Hogberg, H.T.; del Torchio, R.; Domanska-Janik, K.; Zimmer, J.; Coecke, S. A Human Stem Cell Based Model For Identifying Adverse Effects of Organic And Inorganic Chemicals On The Developing Nervous System. Stem Cells 2009, 27, 2591–2601. [Google Scholar] [CrossRef]
- Bonni, A.; Sun, Y.; Nadal-Vicens, M.; Bhatt, A.; Frank, D.A.; Rozovsky, I.; Stahl, N.; Yancopoulos, G.D.; Greenberg, M.E. Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science 1997, 278, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Fan, G. DNA methylation controls the timing of astrogliogenesis through regulation of JAK-STAT signaling. Development 2005, 132, 3345–3356. [Google Scholar] [CrossRef] [PubMed]
- Jebbett, N.J.; Hamilton, J.W.; Rand, M.D.; Eckenstein, F. Low level Methylmercury enhances CNTF-evoked STAT3 signaling and glial differentiation in cultured cortical progenitor cells. Neurotoxicology 2013, 38, 91–100. [Google Scholar] [CrossRef]
- Ayensu, W.K.; Isokpehi, R.D.; Cohly, H.H.; Murray, J.M.; Webb, D.J.; Tchounwou, P.B. Altered GABAA Receptor Expression as Biomarker of Mercury Toxicity in Embryonic Neurogenesis. In Proceedings of the 1st Annual ORNL Biomedical Science and Engineering Conference, Oak Ridge, TN, USA, 18–19 March 2009; pp. 136–138. [Google Scholar]
- Monroe, R.K.; Halvorsen, S.W. Mercury abolishes neurotrophic factor-stimulated Jak-STAT signaling in nerve cells by oxidative stress. Toxicol. Sci. 2006, 94, 129–138. [Google Scholar] [CrossRef]
- Nerini-Molteni, S.; Mennecozzi, M.; Fabbri, M.; Sacco, M.G.; Vojnits, K.; Compagnoni, A.; Gribaldo, L.; Bremer-Hoffman, S. MicroRNA Profiling as a Tool for Pathway Analysis in a Human In Vitro Model for Neural Development. Curr. Med. Chem. 2012, 19, 6214–6223. [Google Scholar] [CrossRef]
- Pallocca, G.; Fabbri, M.; Nerini-Moalteni, S.; Pistollato, F.; Zagoura, D.; Sacco, M.G.; Gribaldo, L.; Bremer-Hoffmann, S.; Bal-Price, A. Changes in miRNA Expression Profiling during Nneuronal Differentiation and Methyl Mercury-induced Toxicity in Human In Vitro Models. Toxics 2014, 2, 443–463. [Google Scholar] [CrossRef]
- Oswald, M.C.W.; Garnham, N.; Sweeney, S.T.; Landgraf, M. Regulation of neuronal development and function by ROS. FEBS Lett. 2018, 592, 679–691. [Google Scholar] [CrossRef]
- Atchison, W.D.; Hare, M.F. Mechanisms of methylmercury-induced neurotoxicity. FASEB J. 1994, 8, 622–629. [Google Scholar] [CrossRef]
- Graff, R.D.; Falconer, M.M.; Brown, D.L.; Reuhl, K.R. Altered Sensitivity of Posttranslationally Modified Microtubules to Methylmercury in Differentiating Embryonal Carcinoma-Derived Neurons. Toxicol. Appl. Pharmacol. 1997, 144, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Marty, M.S.; Atchision, W.D. Pathways Mediating Ca2+/Entry in Rat Cerebellar Granule Cells Following In Vitro Exposure to Methyl Mercury. Toxicol. Appl. Pharmacol. 1997, 147, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Sarafian, T.A. Methyl mercury increases intracellular Ca2+ and inositol phosphate levels in cultured cerebellar granule neurons. J. Neurochem. 1993, 61, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Kakita, A.; Inenaga, C.; Sakamoto, M.; Takahashi, H. Neuronal migration disturbance and consequent cytoarchitecture in the cerebral cortex following transplacental administration of methylmercury. Acta Neuropath. 1992, 104, 409–417. [Google Scholar] [CrossRef]
- Bland, C.E.; Rand, M.R. Methylmercury induces activation of Notch signaling. Neurotoxicology 2006, 27, 982–991. [Google Scholar] [CrossRef]
- Rand, M.D.; Bland, C.E.; Bond, J. Methylmercury activates enhancer-of-split and bearded complex genes independent of the notch receptor. Toxicol. Sci. 2008, 104, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Sass, J.B.; Haselow, D.T.; Silbergeld, E.K. Methylmercury-induced decrement in neuronal migration may involve cytokine-dependent mechanisms: A novel method to assess neuronal movement in vitro. Toxicol. Sci. 2001, 63, 74–81. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vogel, D.G.; Margolis, R.L.; Mottet, N.K. The effects of methyl mercury binding to microtubules. Toxicol. Appl. Pharmacol. 1985, 80, 473–486. [Google Scholar] [CrossRef]
- Huang, J.; Gan, Q.; Han, L.; Ki, J.; Zhang, H.; Sun, Y.; Zhang, Z.; Tong, T. SIRT1 overexpression antagonizes cellular senescence with activated ERK/S6k1 signaling in human diploid fibroblasts. PLoS ONE 2008, 3, e1710. [Google Scholar] [CrossRef] [PubMed]
- Ceccatelli, S.; Bose, R.; Edoff, K.; Onishchenko, N.; Spulber, S. Karolinska Institutet, Stockholm, Sweden) Long-lasting neurotoxic effects of exposure to methylmercury during development. (Review). J. Intern. Med. 2013, 273, 490–497. [Google Scholar] [CrossRef]
- Falluel-Morel, A.; Lin, L.; Sokolowski, K.; McCandish, E.; Buckley, B.; DiCicco-Bloom, E. N-acetyl cysteine (NAC) treatment reduces mercury-induced neurotoxicity in the developing rat hippocampus. J. Neurosci. Res. 2012, 90, 743–750. [Google Scholar] [CrossRef]
- Ballatori, N.; Lieberman, M.W.; Wang, W. N-Acetylcysteine as an Antidote in Methylmercury Poisoning. Environ. Health Perspect. 1998, 106, 267–271. [Google Scholar] [CrossRef]
- Park, B.Y.; Min, B.S.; Oh, S.R.; Kim, J.H.; Kim, T.J.; Kim, D.H.; Bae, K.H.; Lee, H.K. Isolation and anticomplement activity of compounds from Dendropanax morbifera. J. Ethnopharmacol. 2004, 90, 403–408. [Google Scholar] [CrossRef]
- Park, S.Y.; Karthivashan, G.; Ko, H.M.; Cho, D.Y.; Kim, J.; Cho, D.J.; Ganesan, P.; Su-Kim, I.; Choi, D.K. Aqueous extract of Dendropanax morbiferus leaves effectively alleviated neuroinflammation and behavioral impediments in MPTP-induced Parkinson’s mouse model. Oxid. Med. Cell Longev. 2018, 3175214. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; You, D.Y.; Jung, H.Y.; Kim, J.W.; Hahn, K.R.; Kwon, H.J.; You, M.; Lee, S.; Nam, S.M.; Yoon, Y.S.; et al. Leaf extracts from Dendropanax morbifera Léveille mitigate mercury-induced reduction of spatial memory, as well as cell proliferation, and neuroblast differentiation in rat dentate gyrus. BMC Compl. Alt. Med. 2019, 19, 94. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.-Y.; Chen, W.-W.; Cui, J.; Wang, H.; Chao, C.; Lu, Z.-Y.; Bi, A.-Y. Effect of Lycium bararum polysaccharides on methylmercury-induced abnormal differentiation of hippocampal stem cells. Experiment. Therepeut. Med. 2016, 12, 683–689. [Google Scholar] [CrossRef][Green Version]
- Yang, D.; Li, S.Y.; Yeung, C.M.; Chang, R.C.; So, K.F.; Wong, D.; Lo, A.C. Lycium barbarum extracts protect the brain from blood-brain barrier disruption and cerebral edema in experimental stroke. PLoS ONE 2012, 7, e33596. [Google Scholar]
- Cheng, W.; Cheng, X.; Chen, J.; Yi, X.; Nie, D.; Sun, X.; Qin, J.; Tian, M.; Jin, G.; Zhang, X. Lycium barbarum Polysaccharides Prevent Memory and Neurogenesis Impairments in Scopolamine-Treated Rats. PLoS ONE 2014, 9, e88076. [Google Scholar] [CrossRef]
- Day, J.J.; Reed, M.N.; Newland, M.C. Neuromotor deficits and mercury concentrations in rats exposed to methyl mercury and fish oil. Neurotoxicol. Teratol. 2005, 27, 629–641. [Google Scholar] [CrossRef]
- Engel, D.F.; Bobbo, V.; Solon, C.S.; Nogueira, G.A.; Moura-Assis, A.; Mendes, N.F.; Zanesco, A.M.; Papangelis, A.; Ulven, T.; Velloso, L.A. Activation of GPR40 induces hypothalamic neurogenesis through p38- and BDNF-dependent mechanisms. Sci. Rep. 2020, 10, 11047. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Qu, X.; Cui, L.; Wang, J.; Kang, J.X. Improved spatial learning performance of fat-1 mice is associated with enhanced neurogenesis and neuritogenesis by docosahexaenoic acid. Proc. Natl. Acad. Sci. USA 2009, 106, 11370–11375. [Google Scholar] [CrossRef]
- Basak, S.; Mallick, R.; Duttaroy, A.K. Maternal Docosahexaenoic Acid Status during Pregnancy and Its Impact on Infant Neurodevelopment. Nutrients 2020, 12, 3615. [Google Scholar] [CrossRef]
- Arantes, L.P.; Peres, T.V.; Chen, P.; Caito, S.; Aschner, M.; Soares, F.A. Guarana (Paullinia cupana Mart.) attenuates methylmercury-induced toxicity in Caenorhabditis elegans. Toxicol Res. 2016, 5, 1629–1638. [Google Scholar] [CrossRef]
- Espinola, E.B.; Dias, R.F.; Maattei, R.; Carlini, E.A. Pharmacological activity of Guarana (Paullinia cupana Mart.) in laboratory animals. J. Ethnophamacol. 1997, 53, 223–229. [Google Scholar] [CrossRef]
- Ke, T.; Goncalves, F.M.; Goncalves, C.L.; dos Santos, A.A.; Rocha, J.B.T.; Faria, M.; Skalny, A.; Tsatsakis, A.; Bowman, A.B.; Aschner, M. Post-translational modifications in MeHg-induced neurotoxicity. BBA Molc. Basis Dis. 2019, 1865, 2068–2081. [Google Scholar] [CrossRef] [PubMed]
| A | B | C | |
|---|---|---|---|
| North America | |||
| USA | 0–0.5 | 1–2.5 | 4–10 (Alaska) |
| Canada | 2.5–4 | 4–5.8 | 0.2–0.5 |
| Mexico | 0.5–2.5 | 1–1.7 | |
| South America | |||
| Brazil | 10–30 | 10–30 | 4–10 |
| Peru | 30–108 | ||
| Chile | 5.8–10 | ||
| Colombia | 4–5.8 | ||
| Venezuela | 10–30 | ||
| Ecuador | 5.8–10 | ||
| Suriname | 5.8–10 | ||
| Europe | 0–0.5 | ||
| Italy | 5.8–10 | 2.5–4 | 0.5–1 |
| Belgium | 5.8–10 | 5.8–10 | |
| Spain | 5.8–10 | 5.8–10 | |
| Germany | 1.7–3 | ||
| Sweden | 1.7–3 | ||
| United Kingdom | 2.5–4 | ||
| Denmark (Greenland) | 10–30 | 30–53.3 | |
| Finland | 5.8–10 | ||
| Asia | 5.8–10 | ||
| Russia | 0.5–2.5 | ||
| China | 2.5–4 | 2.5–4 | 1–1.7 |
| India | 30–108 | 4–10 | |
| Japan | 5.8–10 | 10–30 | 0.5–1 |
| Indonesia | 5.8–10 | 3–4 | |
| Philippines | 5.8–10 | 30–53.3 | 1.7–3 |
| Singapore | 30–108 | 30–53.3 | |
| Turkey | 0–0.5 | 0–0.5 | 10–30 |
| Iran | 2.5–4 | 1–2.5 | 2.5–4 |
| Africa | |||
| Egypt | 10–30 | ||
| Nigeria | 0.5–2.5 | 4–5.8 | 4–10 |
| Benin | 2.5–4 | ||
| Ghana | 30–108 | 4–10 | |
| Zimbabwe | 5.8–10 | ||
| Morocco | 4–5.8 | ||
| South Africa | 0–0.5 | 0–0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbott, L.C.; Nigussie, F. Mercury Toxicity and Neurogenesis in the Mammalian Brain. Int. J. Mol. Sci. 2021, 22, 7520. https://doi.org/10.3390/ijms22147520
Abbott LC, Nigussie F. Mercury Toxicity and Neurogenesis in the Mammalian Brain. International Journal of Molecular Sciences. 2021; 22(14):7520. https://doi.org/10.3390/ijms22147520
Chicago/Turabian StyleAbbott, Louise C., and Fikru Nigussie. 2021. "Mercury Toxicity and Neurogenesis in the Mammalian Brain" International Journal of Molecular Sciences 22, no. 14: 7520. https://doi.org/10.3390/ijms22147520
APA StyleAbbott, L. C., & Nigussie, F. (2021). Mercury Toxicity and Neurogenesis in the Mammalian Brain. International Journal of Molecular Sciences, 22(14), 7520. https://doi.org/10.3390/ijms22147520






