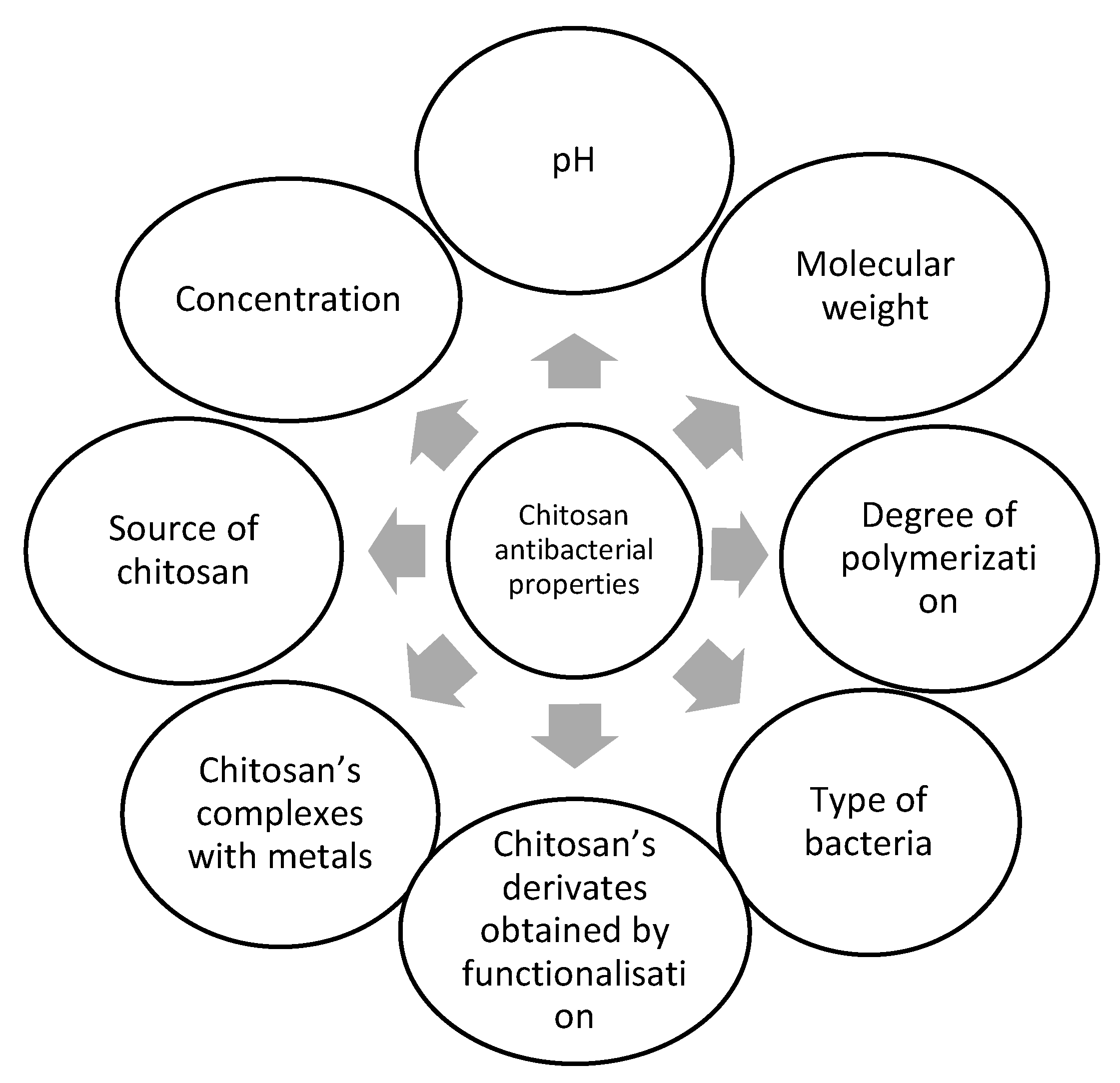
Factors Influencing the Antibacterial Activity of Chitosan and Chitosan Modified by Functionalization
Abstract
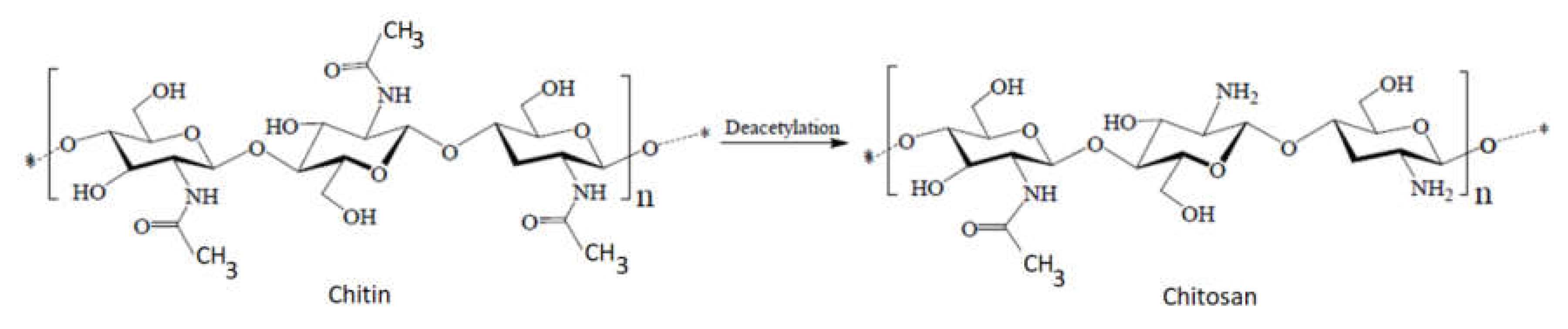
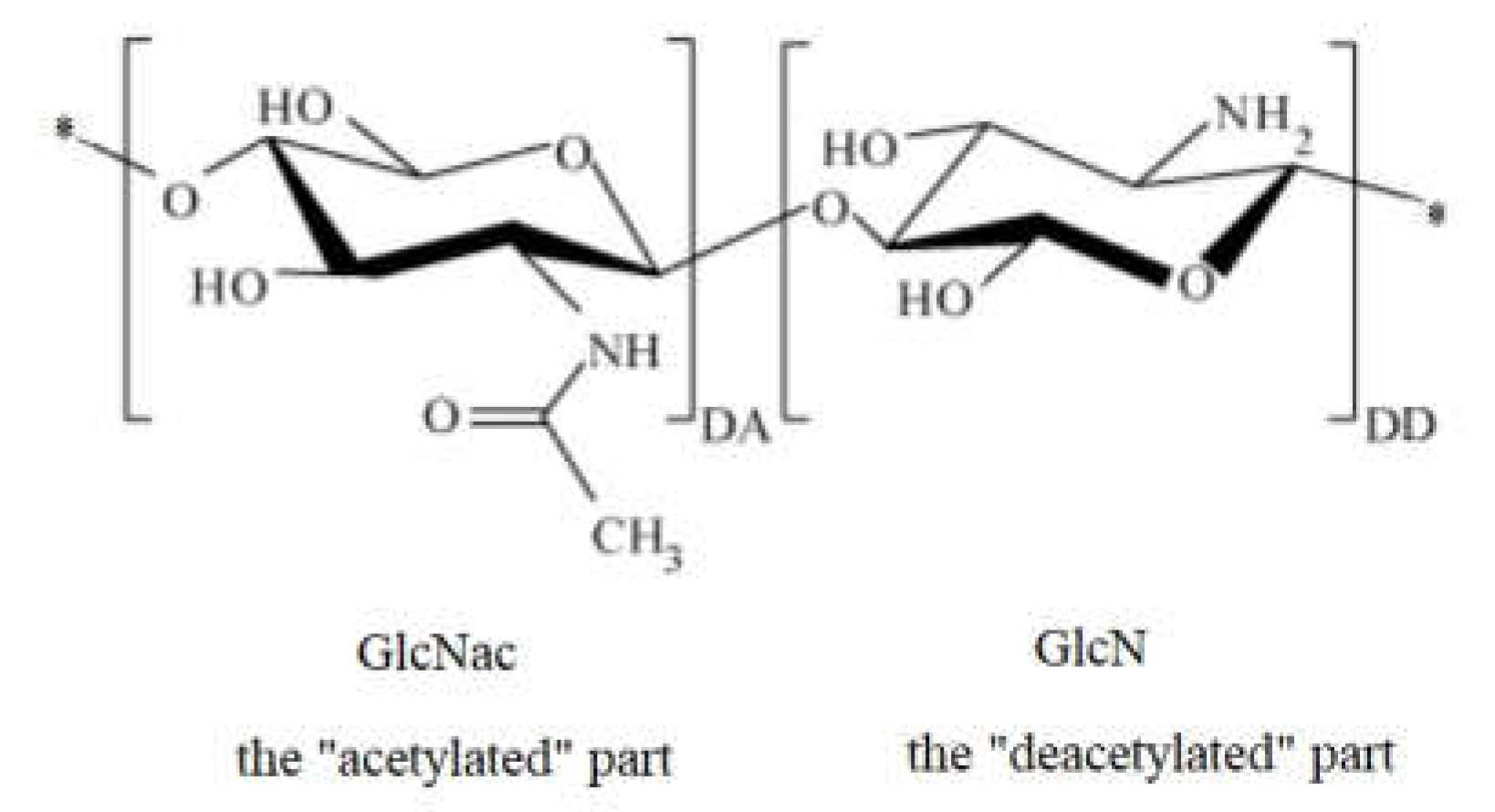
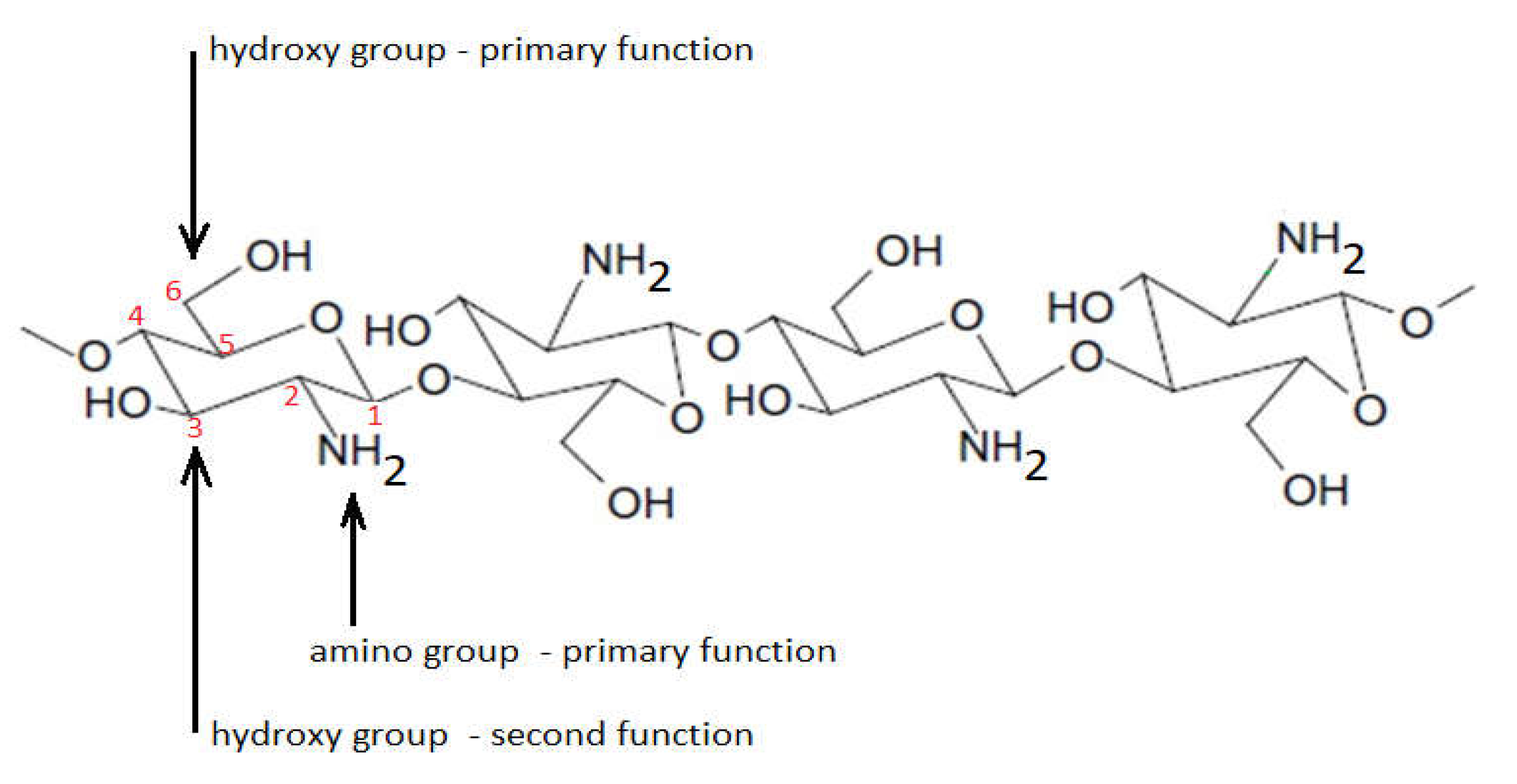
1. Introduction
- −
- low molecular weight chitosan, with a mass lower than 100 kDa;
- −
- medium molecular weight chitosan, with a mass between 100 and 1000 kDa;
- −
- high molecular weight chitosan, with a mass higher than 1000 kDa.
1.1. Chitosan Sources and Their Contribution to the Antibacterial Activity of Chitosan
1.2. Influence of Chitosan Concentration on the Antibacterial Effect
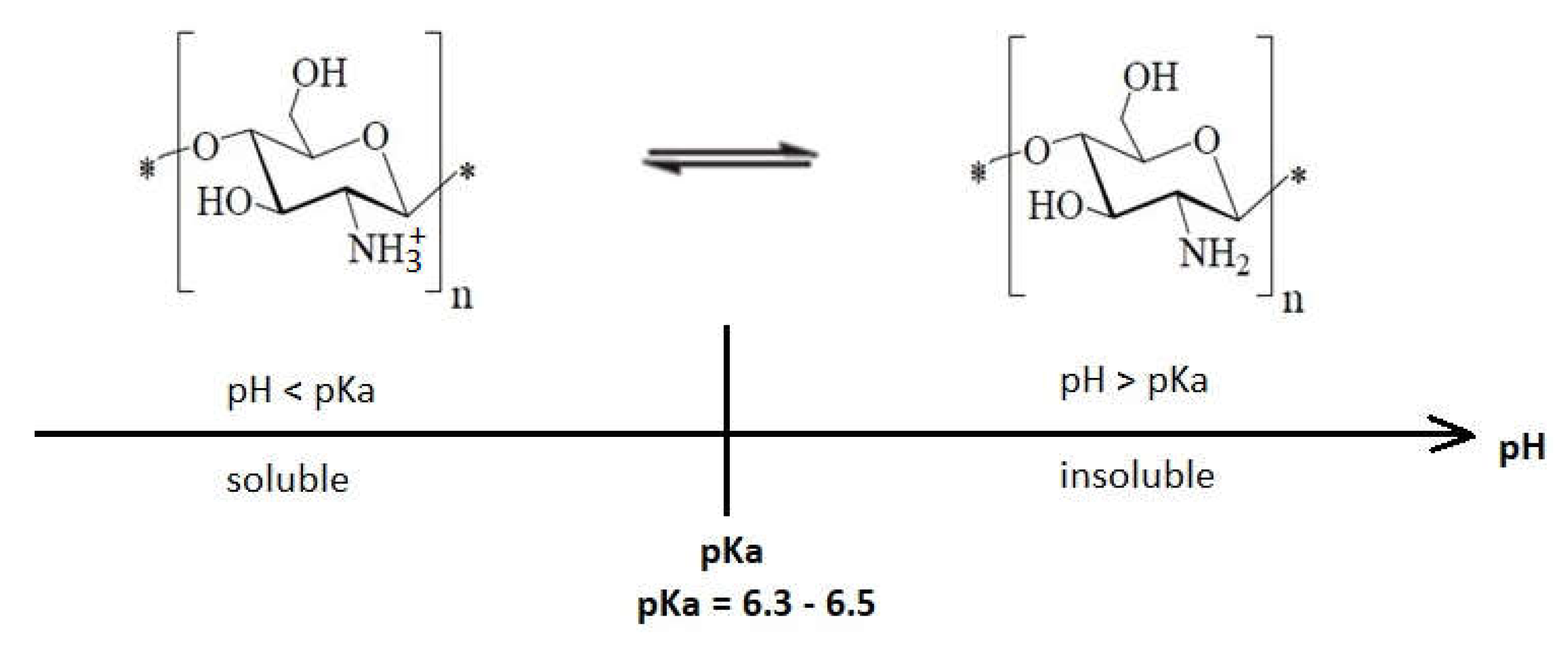
1.3. Environment pH Influence on the Antibacterial Effect of Chitosan
1.4. The Molecular Weight Contribution to the Antibacterial Effect of Chitosan
- ✓
- low molecular weight (LMw) chitosan, named also “oligo-chitosan” or “short chain chitosan” (molecular weight < 50 kDa);
- ✓
- medium molecular weight (MMw) chitosan, with molecular weight between 50 kDa and 250 kDa;
- ✓
1.5. The Contribution of the Degree of Polymerization to the Antibacterial Effect of Chitosan
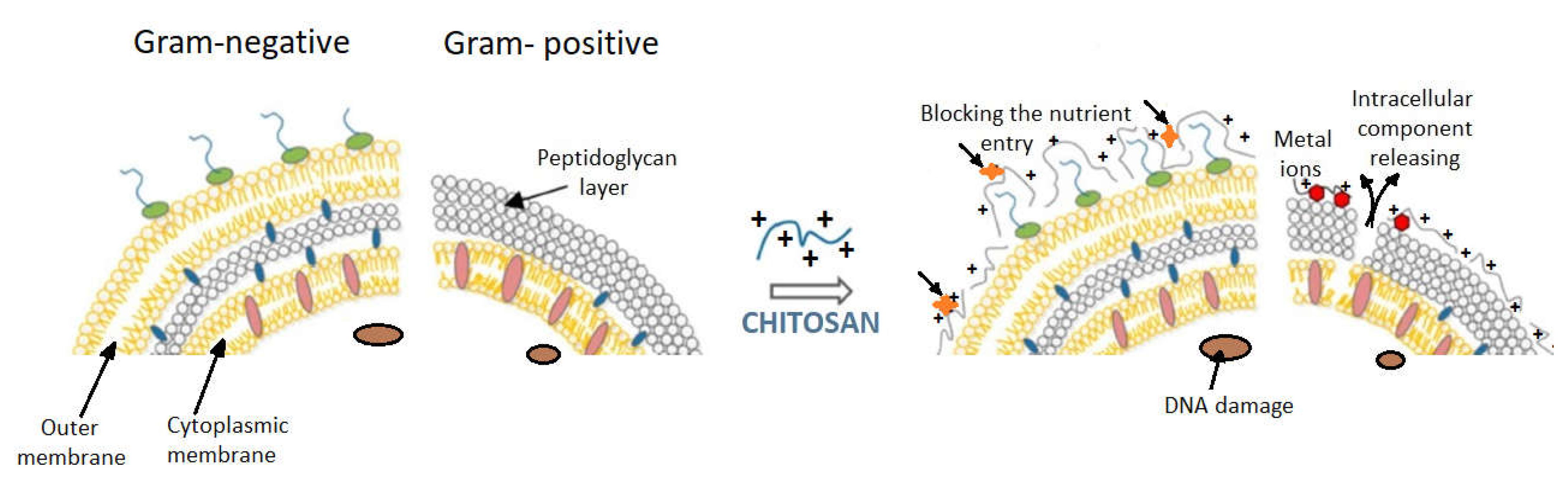
1.6. The Contribution of the Type of Bacteria to the Antibacterial Effect of Chitosan
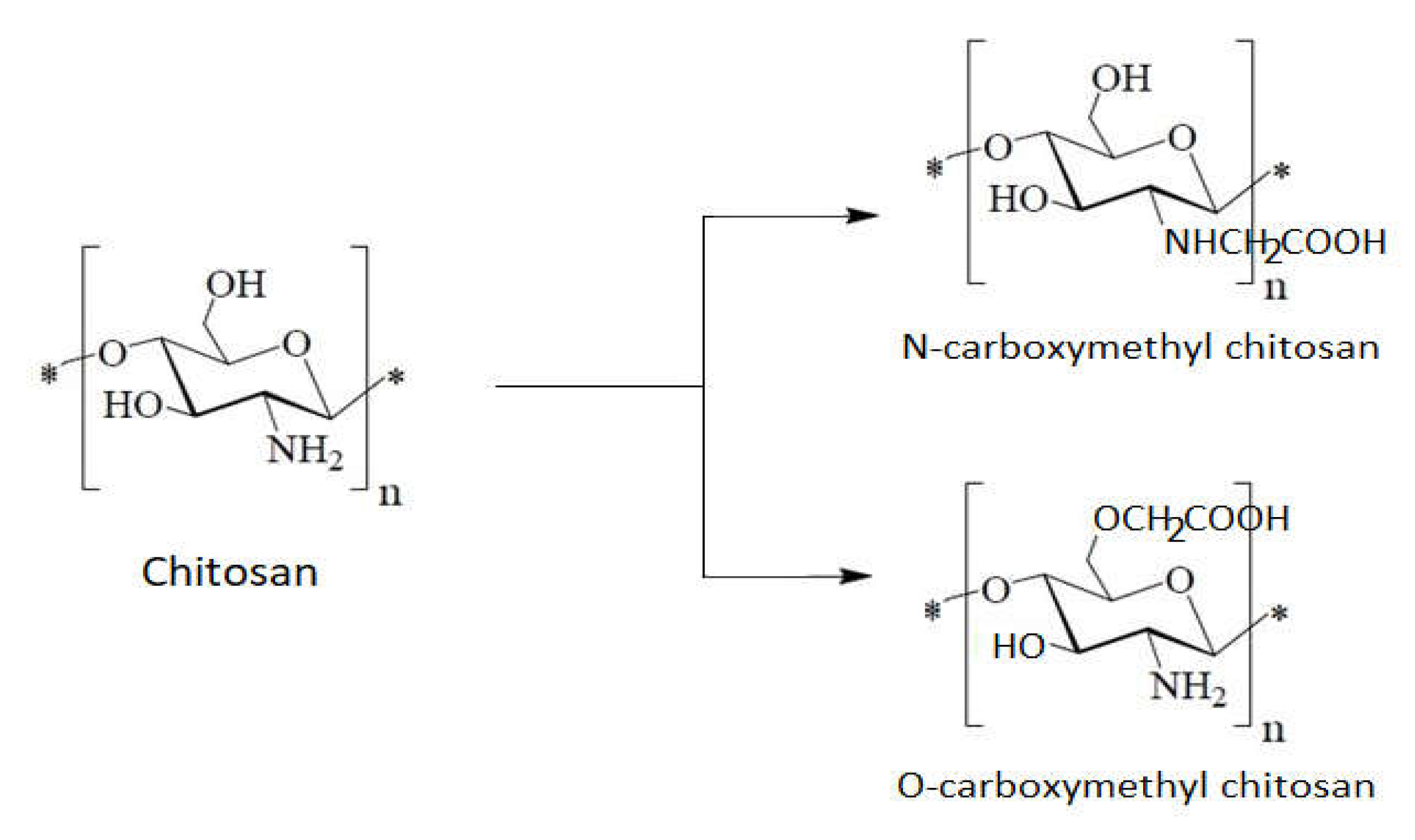
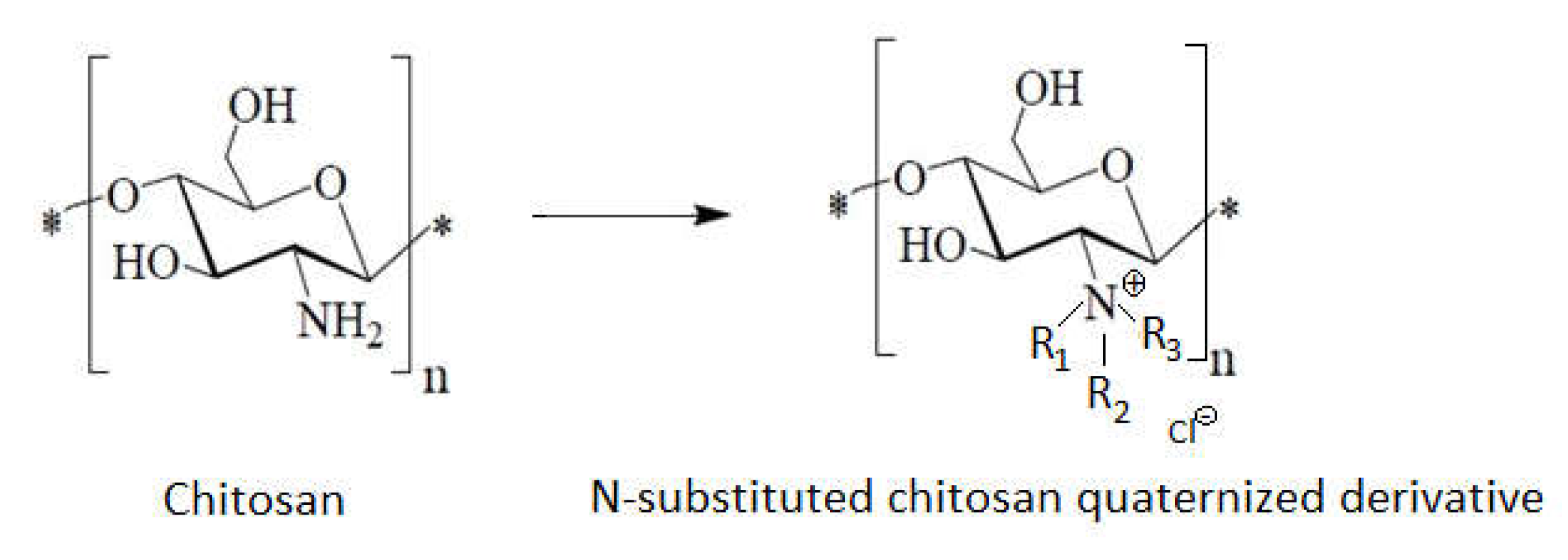
1.7. The Chitosan Derivatives’ Contributions to the Antibacterial Effect
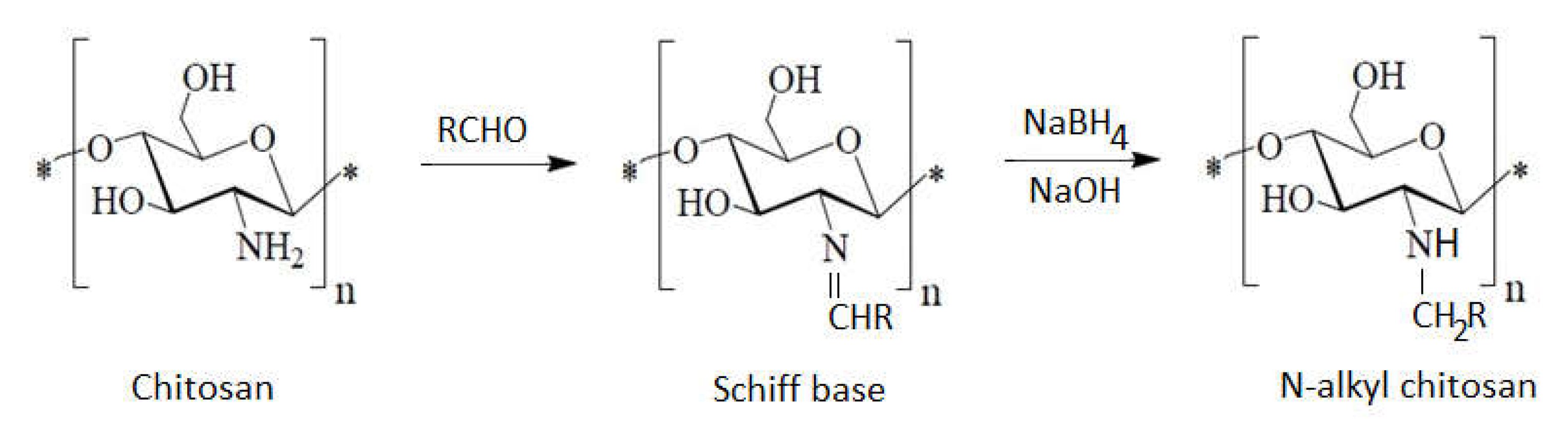
1.8. Chitosan Containing Alkyl Groups
1.9. Chitosan Containing Aromatic Groups
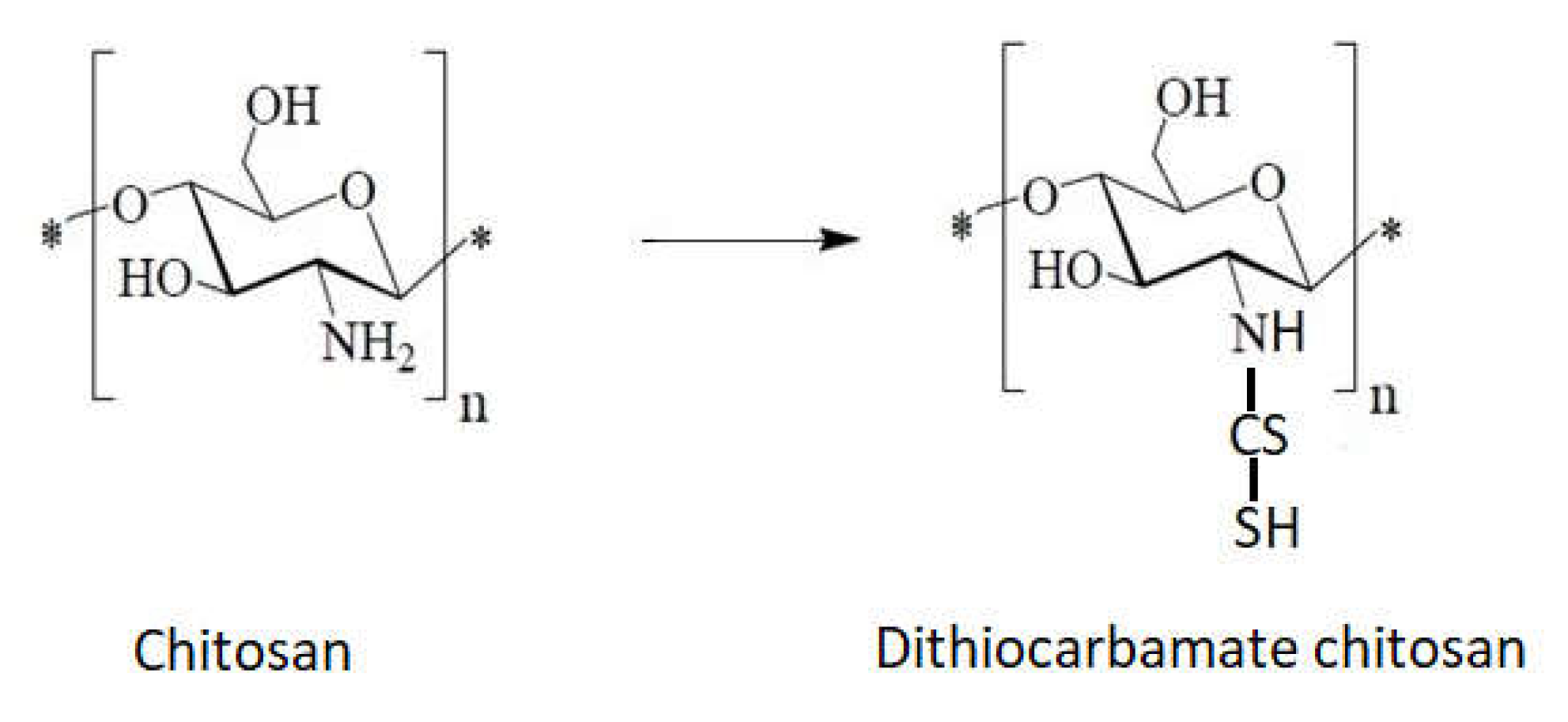
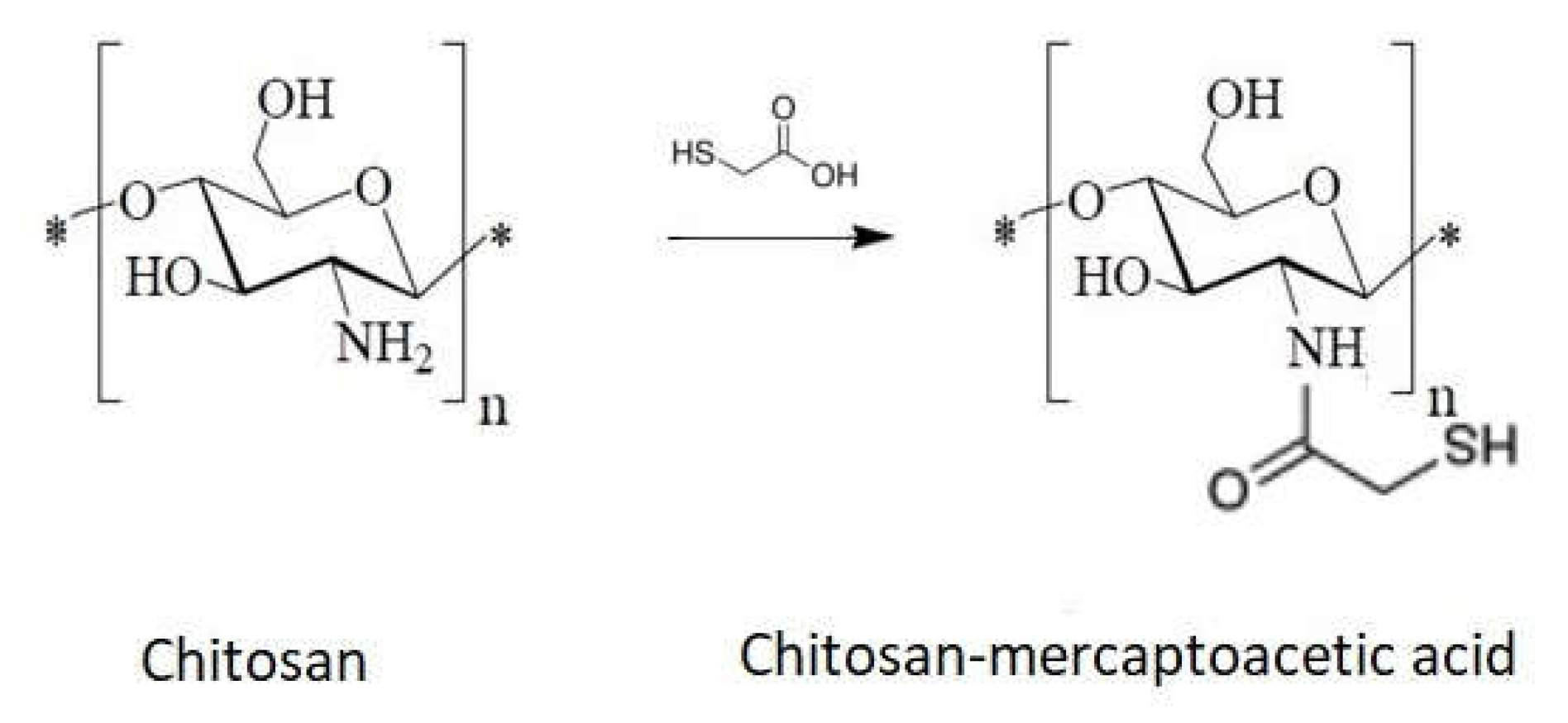
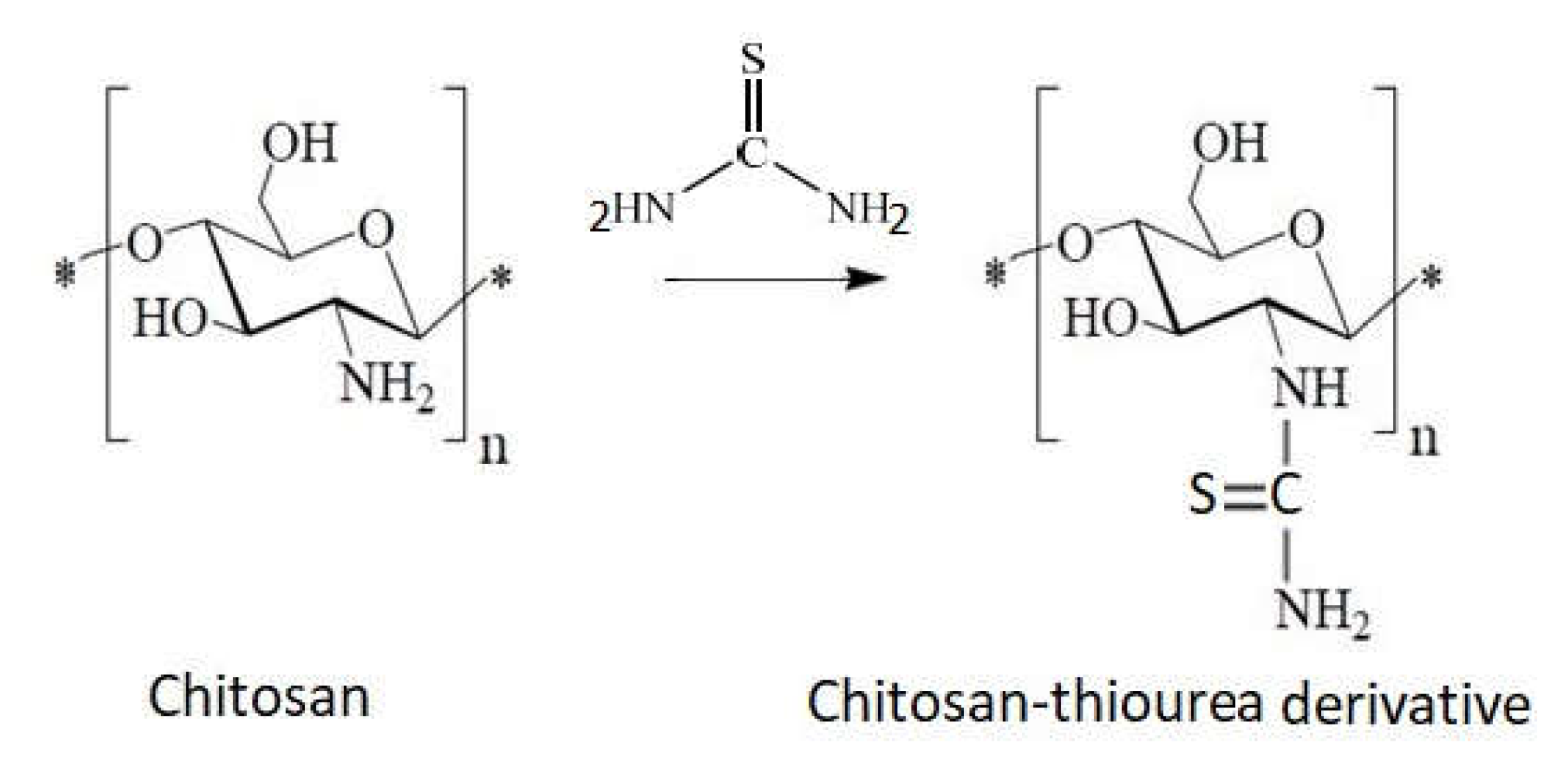
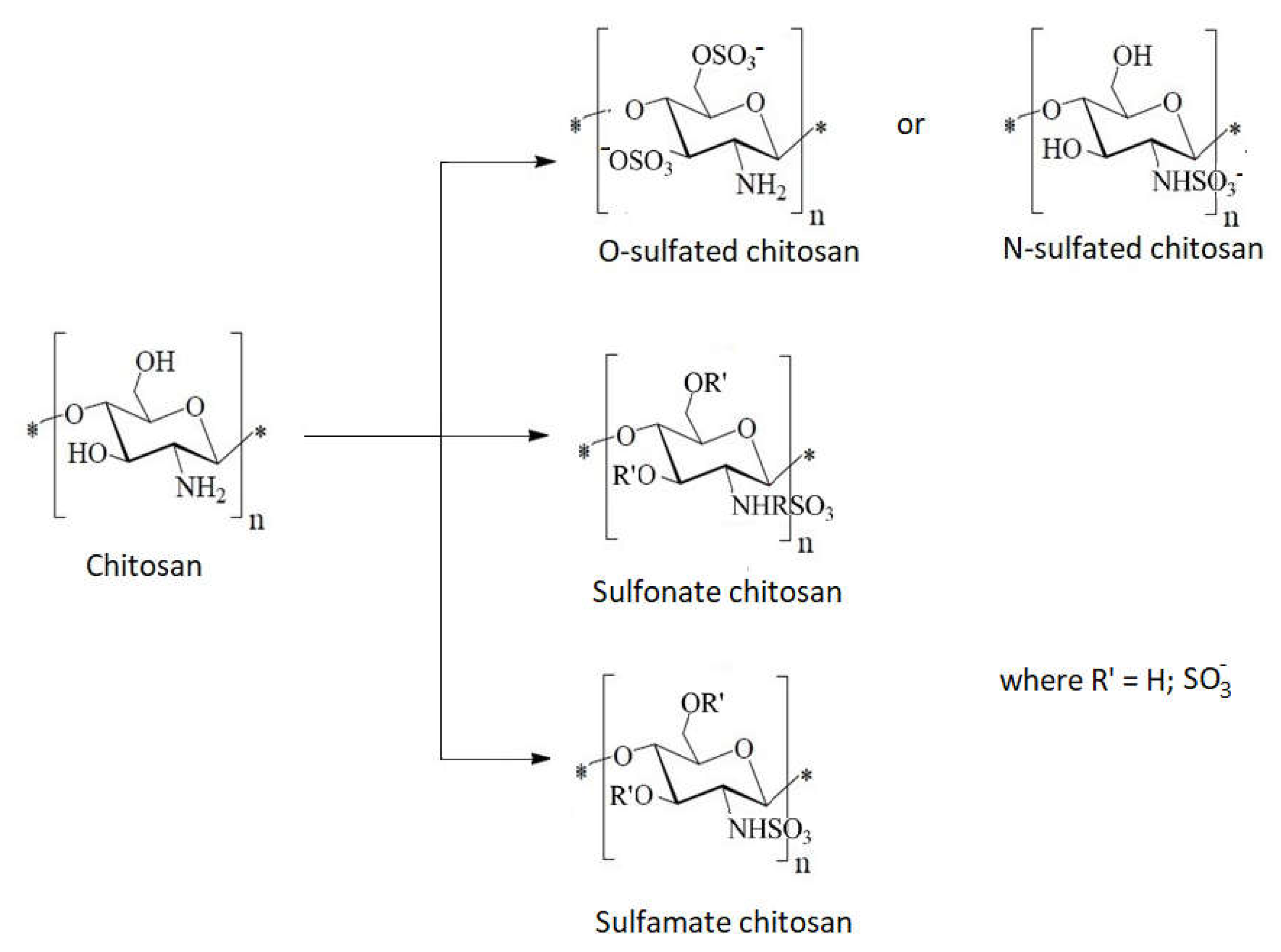
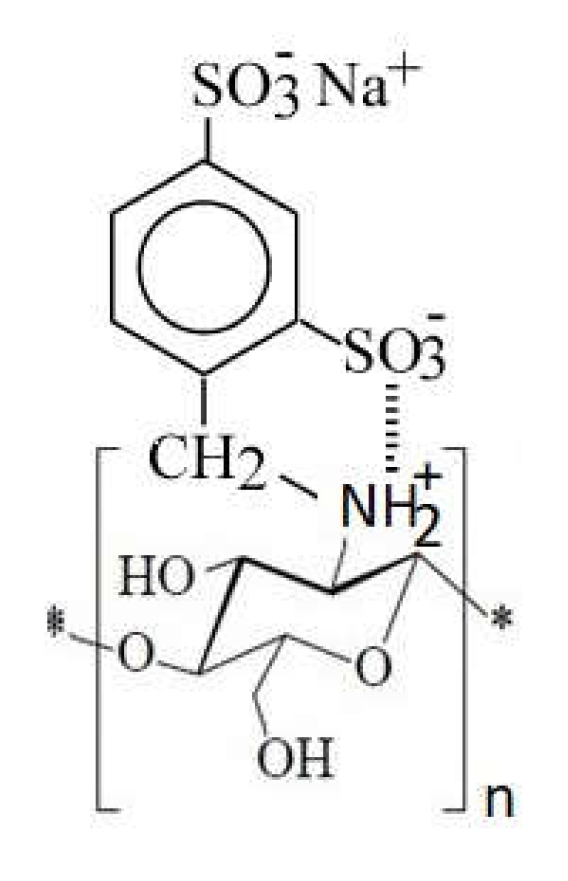
1.10. Chitosan Derivative with Sulfur as Heteroatom
- Direct reaction of chitosan with carbon sulfur (Figure 13).
- 2.
- Direct reaction with mercaptoacetic acid (Figure 14)
- 3.
- 4.
- Grafting of sulfonic groups onto chitosan (Figure 16)
1.11. Chitosan Derivative with Phosphorus as Heteroatom
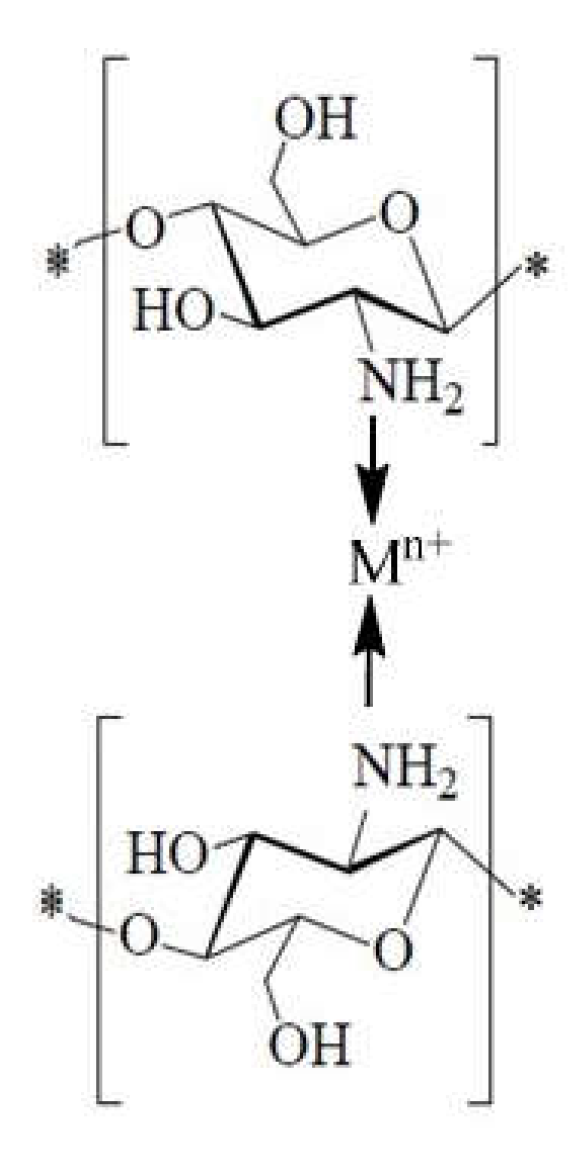
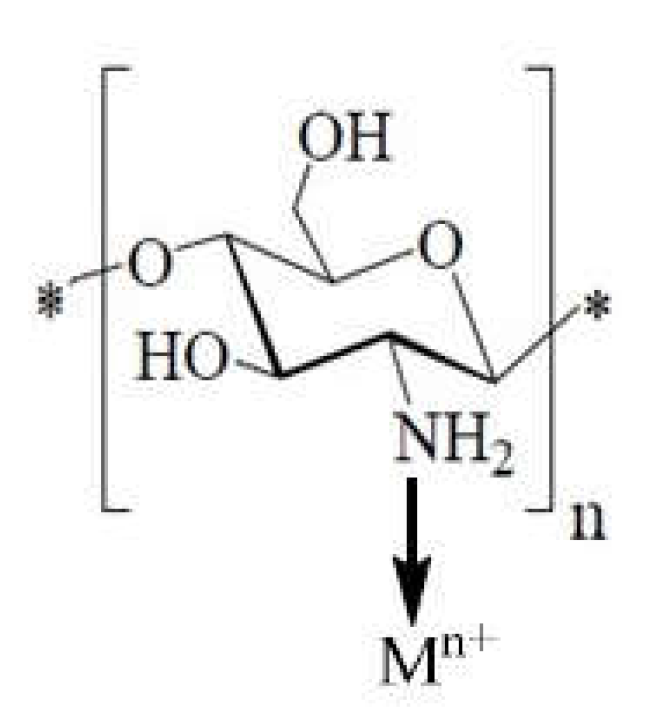
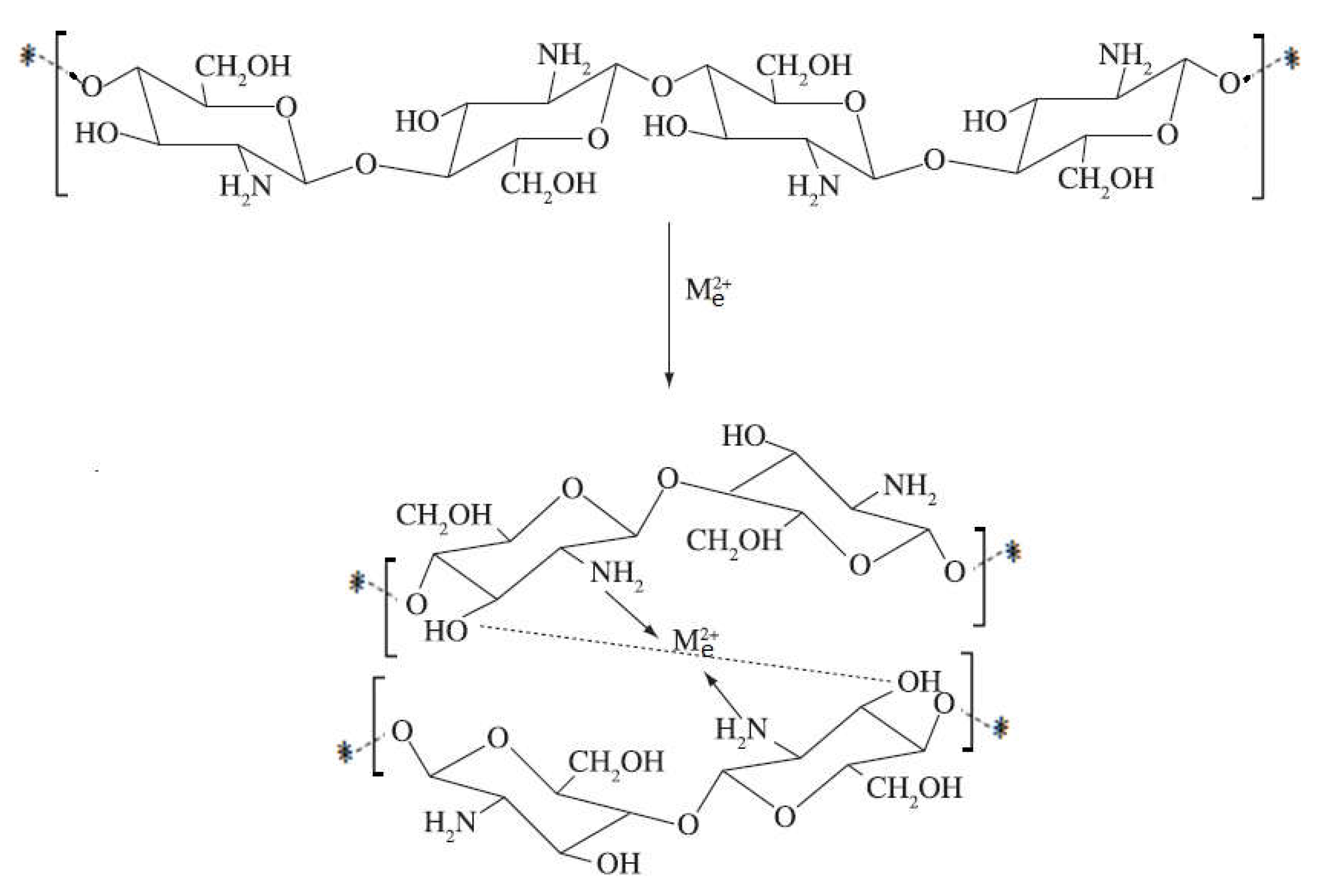
1.12. The Complexes with Metal Ions Contribution to the Antibacterial Effect of Chitosan
1.12.1. The Case of Adsorption
1.12.2. The Case of Ion Exchanges
1.12.3. The Case of Chelates with Metal Ions
- 2.
2. Conclusions
- ✓
- Being a cationic polyamine, it is a biocompatible product.
- ✓
- When it is positively charged, when the molecule is protonated, it adheres to negatively charged surfaces, so it displays its bioadhesion property.
- ✓
- Due to the possibility of chitosan to form salts with organic and inorganic acids, it is a biodegradable product.
- ✓
- Chitosan, because of its positively charged amino groups and its high charge density in acidic conditions, is able to interact spontaneously with anionic polymers to form a polyelectrolyte complex. The more monomer units there are, the higher the molecular weight of chitosan and the greater its potential to form polyelectrolytes complexes. Because the polyelectrolyte complex is generally obtained by self-assembly of oppositely charged polymers in aqueous medium without using organic solvents or toxic cross-linkers, it is considered to be safe and nontoxic.
- ✓
- Due to the possibility of having a different degree of viscosity, it is a hemostatic, bacteriostatic, and fungistatic compound.
- ✓
- The property of forming chelates with various metal ions makes it a suitable compound for wastewater treatment and in applications that require antibacterial effects.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grégorio, C. Historical review on chitin and chitosan biopolymers. Environ. Chem. Lett. 2019, 17, 1623–1643. [Google Scholar]
- Dimzon, I.K.D.; Ebert, J.; Knepper, T.P. The interaction of chitosan and olive oil: Effects of degree of deacetylation and degree of polymerization. Carbohydr. Polym. 2013, 92, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Hadwiger, L.A.; Chinag, C.C.; Victory, S.; Horovitz, D. Chitin and Chitosan; Elsevier: London, UK, 1989; p. 138. [Google Scholar]
- Se-Kwon, K. (Ed.) Chitin, Chitosan, Oligosaccharides and Their Derivatives: Biological Activities and Applications; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Prashanth, K.V.H.; Tharanathan, R.N. Chitin/chitosan: Modifications and their unlimited application—an overview. Trends Food Sci. Technol. 2007, 18, 117–131. [Google Scholar] [CrossRef]
- De Farias, B.S.; Grundmann, D.D.R.; Rizzi, F.Z.; Martins, N.S.S.; Sant’Anna Cadaval, T.R., Jr.; de Almeida Pinto, L.A. Production of low molecular weight chitosan by acid and oxidative pathways: Effect on physicochemical properties. Food Res. Int. 2019, 123, 88–94. [Google Scholar] [CrossRef]
- Alvarenga, E. Characterization and Properties of Chitosan. In Biotechnology of Biopolymers; InTech: Rijeka, Croatia, 2011; Volume 24. [Google Scholar]
- Barbosa, H.F.G.; Attjioui, M.; Leitao, A.; Moerschbacher, B.M.; Cavalheiro, E.T.G. Characterization, solubility and biological activity of amphihilic biopolymeric Schiff bases synthesized using chitosans. Carbohydr. Polym. 2019, 220, 1–11. [Google Scholar] [CrossRef]
- Anan, N.A.; Hassan, S.M.; Saad, E.M.; Butler, I.S.; Mostafa, S.I. Preparation, characterization and pH-metric measurements of 4-hydroxysalicylidenechitosan Schiff-base complexes of Fe(III), Co(II), Ni(II), Cu(II), Zn(II), Ru(III), Rh(III), Pd(II) and Au(III). Carbohydr. Res. 2011, 346, 775–793. [Google Scholar] [CrossRef]
- Vadivel, T.; Dhamodaran, M. Synthesis, characterization and antibacterial studies of ruthenium(III) complexes derived from chitosan schiff base. Int. J. Biol. Macromol. 2016, 90, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Hafdani, F.; Sadeghinia, N. A review on application of chitosan as a natural antimicrobial. World Acad. Sci. Eng. Technol. 2011, 74, 257–261. [Google Scholar]
- Kim, S. Competitive Biological Activities of Chitosan and Its Derivatives: Antimicrobial, Antioxidant, Anticancer, and Anti-Inflammatory Activities. Int. J. Polym. Sci. 2018. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.W. Preparation of sulfur nanoparticle-incorporated antimicrobial chitosan films. Food Hydrocoll. 2018, 82, 116–123. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef]
- Salama, H.E.; Saad, G.R.; Sabaa, M.W. Synthesis, characterization and biological activity of Schiff bases based on chitosan and arylpyrazole moiety. Int. J. Biol. Macromol. 2015, 79, 996–1003. [Google Scholar] [CrossRef]
- Helander, I.M.; Nurmiaho-Lassila, E.L.; Ahvenainen, R.; Rhoades, J.; Roller, S. Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. Int. J. Food Microbiol. 2001, 71, 235–244. [Google Scholar] [CrossRef]
- Duan, C.; Meng, X.; Meng, J.; Khan, M.I.H.; Dai, L.; Khan, A.; An, X.; Zhang, J.; Huq, T.; Ni, Y. Chitosan as A Preservative for Fruits and Vegetables: A Review on Chemistry and Antimicrobial Properties. J. Bioresour. Bioprod. 2019, 4, 11–21. [Google Scholar] [CrossRef]
- Belbekhouche, S.; Bousserrhine, N.; Alphonse, V.; Le Floch, F.; Mechiche, Y.C.; Menidjel, I.; Carbonnier, B. Chitosan based self-assembled nanocapsules as antibacterial agent. Colloid Surf. B Biointerfaces 2019, 181, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Periayah, M.; Halim, A.; Mat Saad, A. Biotechnology & Biomaterials Chitosan: A Promising Marine Polysaccharide for Biomedical Research. Biotechnol. Biomater. 2014, 4, 4. [Google Scholar]
- Bhaskara Reddy, M.V.; Arul, J.; Angers, P.; Couture, L. Chitosan Treatment of Wheat Seeds Induces Resistance to Fusarium graminearum and Improves Seed Quality. J. Agric. Food Chem. 1999, 47, 1208–1216. [Google Scholar] [CrossRef]
- Bhaskara Reddy, M.V.; Arul, J.; Essaid, A.B.; Angers, P.; Richard, C.; Castaigne, F. Effect of chitosan on growth and toxin production by Alternaria alternata f. sp. lycopersici. Biocontrol Sci. Technol. 1998, 8, 43. [Google Scholar]
- El Hadrami, A.; Adam, L.R.; El Hadrami, I.; Daayf, F. Chitosan in plant protection. Mar. Drugs 2010, 8, 968–987. [Google Scholar] [CrossRef]
- Rendina, N.; Nuzzaci, M.; Scopa, A.; Cuypers, A.; Sofo, A. Chitosan-elicited defense responses in Cucumber mosaic virus (CMV)-infected tomato plants. J. Plant Physiol. 2019, 234–235, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Beuchat, L.R. Natural Antimicrobial Sytstems and Food Preservation; CAB International: Wallingford, UK, 1994; p. 179. [Google Scholar]
- Cheung, R.C.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2018, 263, 131–194. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Tan, Y.F.; Wong, Y.S.; Liew, M.W.J.; Venkatraman, S. Recent Advances in Chitosan-Based Carriers for Gene Delivery. Mar. Drugs 2019, 17, 381. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Howard, K.A.; Dong, M.; Andersen, M.Ø.; Rahbek, U.L.; Johnsen, M.G.; Hansen, O.C.; Besenbacher, F.; Kjems, J. The influence of polymeric properties on chitosan/siRNA nanoparticle formulation and gene silencing. Biomaterials 2007, 28, 1280–1288. [Google Scholar] [CrossRef]
- Byun, S.M.; No, H.K.; Hong, J.-H.; Lee, S.I.; Prinyawiwatkul, W. Comparison of physicochemical, binding, antioxidant and antibacterial properties of chitosans prepared from ground and entire crab leg shells. Int. J. Food Sci. Technol. 2013, 48, 136–142. [Google Scholar] [CrossRef]
- Ai, H.; Wang, F.; Xia, Y.; Chen, X.; Lei, C. Antioxidant, antifungal and antiviral activities of chitosan from the larvae of housefly, Musca domestica L. Food Chem. 2012, 132, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.C.d.L.; Souza Neto, F.E.d.; Paiva, W.d.S. Review of fungal chitosan: Past, present and perspectives in Brazil. Polímeros Ciência Tecnol. 2018, 28, 275–283. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.-G.; Park, H.-J.; Liu, C.-G.; Liu, C.-S.; Meng, X.-H.; Yu, L.-J. Effect of MW and concentration of chitosan on antibacterial activity of Escherichia coli. Carbohydr. Polym. 2006, 64, 60–65. [Google Scholar] [CrossRef]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef]
- Vishu Kumar, A.B.; Varadaraj, M.C.; Lalitha, R.G.; Tharanathan, R.N. Low molecular weight chitosans: Preparation with the aid of papain and characterization. Biochim. Biophys. Acta 2004, 1670, 137–146. [Google Scholar] [CrossRef]
- Kulikov, S.N.; Chirkov, S.N.; Il’ina, A.V.; Lopatin, S.A.; Varlamov, V.P. Effect of the molecular weight of chitosan on its antiviral activity in plants. Appl. Biochem. Microbiol. 2006, 42, 200–203. [Google Scholar] [CrossRef]
- Younes, I.; Sellimi, S.; Rinaudo, M.; Jellouli, K.; Nasri, M. Influence of acetylation degree and molecular weight of homogeneous chitosans on antibacterial and antifungal activities. Int. J. Food Microbiol. 2014, 185, 57–63. [Google Scholar] [CrossRef]
- Cabrera, J.; Cutsem, P.V. Preparation of chitooligosaccharides with degree of polymerization higher than 6 by acid or enzymatic degradation of chitosan. Biochem. Eng. J. 2005, 25, 165–172. [Google Scholar] [CrossRef]
- Meng, X.; Xing, R.; Liu, S.; Yu, H.; Li, K.; Qin, Y.; Li, P. Molecular weight and pH effects of aminoethyl modified chitosan on antibacterial activity in vitro. Int. J. Biol. Macromol. 2012, 50, 918–924. [Google Scholar] [CrossRef]
- Jarmila, V.; Eva, V. Chitosan Derivatives with Antimicrobial, Antitumour and Antioxidant Activities—A Review. Curr. Pharm. Des. 2011, 17, 3596–3607. [Google Scholar] [PubMed]
- Chung, Y.C.; Su, Y.P.; Chen, C.C.; Jia, G.; Wang, H.I.; Wu, J.C.G.; Lin, J.G. Relationship between antibacterial activity of chitosan and surface characteristics of cell wall. Acta Pharmacol. Sin. 2004, 25, 932–936. [Google Scholar]
- Leuba, J.L.; Stossel, P. Chitin in Nature and Technology; Springer: Berlin/Heidelberg, Germany, 1986; pp. 215–222. [Google Scholar]
- Chien, R.C.; Yen, M.T.; Mau, J.L. Antimicrobial and antitumor activities of chitosan from shiitake stipes, compared to commercial chitosan from crab shells. Carbohydr. Polym. 2016, 138, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Errington, N.; Harding, S.E.; Varum, K.M.; Illum, L. Hydrodynamic characterization of chitosans varying in degree of acetylation. Int. J. Biol. Macromol. 1993, 15, 117. [Google Scholar] [CrossRef]
- Lim, S.H.; Hudson, S.M. Synthesis and antimicrobial activity of a water-soluble chitosan derivative with a fiber-reactive group. Carbohydr. Res. 2004, 339, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Varlamov, V.P.; Il’ina, A.V.; Shagdarova, B.T.; Lunkov, A.P.; Mysyakina, I.S. Chitin/Chitosan and Its Derivatives: Fundamental Problems and Practical Approaches. Biochemistry 2020, 85 (Suppl. 1), S154–S176. [Google Scholar] [CrossRef] [PubMed]
- Ristic, T.; Hribernik, S.; Fras-Zemljic, L. Electrokinetic properties of fibres functionalised by chitosan and chitosan nanoparticles. Cellulose 2015, 22, 3811–3823. [Google Scholar] [CrossRef]
- Qin, C.; Li, H.; Xiao, Q.; Liu, Y.; Zhu, J.; Du, Y. Water-solubility of chitosan and its antimicrobial activity. Carbohydr. Polym. 2006, 63, 367–374. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan Chemistry and Pharmaceutical Perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef] [PubMed]
- Côté, F.; Roberts, K.A.; Hahn, M.G. Identification of high-affinity binding sites for the hepta-β-glucoside elicitor in membranes of the model legumes Medicago truncatula and Lotus japonicus. Planta 2000, 211, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zall, R.R. Chitosan membranes for reverse osmosis application. J. Food Sci. 1984, 49, 91–93. [Google Scholar] [CrossRef]
- Amro, N.A.; Kotra, L.P.; Wadu-Mesthrige, K.; Bulychev, A.; Mobashery, S.; Liu, G.Y. High-resolution atomic force microscopy studies of the Escherichia coli outer membrane: Structural basis for permeability. Langmuir 2000, 16, 2789–2796. [Google Scholar] [CrossRef]
- Honarkar, H.; Barikani, M. Applications of biopolymers I: Chitosan. Mon. Chem. 2009, 140, 1403–1420. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Kravanja, G.; Primožič, M.; Knez, Ž.; Leitgeb, M. Chitosan-Based (Nano)Materials for Novel Biomedical Applications. Molecules 2019, 24, 1960. [Google Scholar] [CrossRef] [PubMed]
- Ezati, P.; Rhim, J.W. pH-responsive chitosan-based film incorporated with alizarin for intelligent packaging applications. Food Hydrocoll. 2020, 102, 10. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Fundamentals and Applications of Chitosan. Sustain. Agric. Rev. 2019, 35, 49–123. [Google Scholar]
- Kulikov, S.N.; Tikhonov, V.E.; Bezrodnykh, E.A.; Lopatin, S.A.; Varlamov, V.P. Comparative evaluation of antimicrobial activity of oligochitosans against Klebsiella pneumoniae. Russ. J. Bioorg. Chem. 2015, 41, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Daly, W.H.; Manuszak-Guerrini, M.A. Biocidal Chitosan Derivatives for Cosmetics and Pharmaceuticals. U.S. Patent 6,306,835, 23 October 2001. [Google Scholar]
- Baba, Y.; Noma, H.; Nakayama, R.; Matsushita, Y. Preparation of chitosan derivatives containing methylthiocarbamoyl and phenylthiocarbamoyl groups and their selective adsorption of copper(II) over iron(III). Anal. Sci. 2002, 18, 359–361. [Google Scholar] [CrossRef]
- Martins, F.A.; Facchi, P.S.; Follmann, D.M.H.; Pereira, G.B.A.; Rubira, F.A.; Edvani, M.C. Antimicrobial Activity of Chitosan Derivatives Containing N-Quaternized Moieties in Its Backbone: A Review. Int. J. Mol. Sci. 2014, 15, 20800–20832. [Google Scholar] [CrossRef]
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef] [PubMed]
- Riaz Rajoka, M.S.; Mehwish, H.M.; Wu, Y.; Zhao, L.; Arfat, Y.; Majeed, K.; Anwaar, S. Chitin/chitosan derivatives and their interactions with microorganisms: A comprehensive review and future perspectives. Crit. Rev. Biotechnol. 2020, 40, 365–379. [Google Scholar] [CrossRef]
- Kaplan, S.; Aslan, S.; Ulusoy, S.; Oral, A. Natural-based polymers for antibacterial treatment of absorbent materials. J. Appl. Polym. Sci. 2020, 137, 12. [Google Scholar] [CrossRef]
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems. Funct. Chitosan 2020, 457–489. [Google Scholar] [CrossRef]
- Fernandez-Saiz, P.; Lagarón, J.M.; Ocio, M.J. Optimization of the film-forming and storage conditions of chitosan as an antimicrobial agent. J. Agric. Food Chem. 2009, 57, 3298–3307. [Google Scholar] [CrossRef]
- Peter, M.G. Applications and Environmental Aspects of Chitin and Chitosan. J. Macromol. Sci. Part A 1995, 32, 629–640. [Google Scholar] [CrossRef]
- Chirkov, S.N. The Antiviral Activity of Chitosan (Review). Appl. Biochem. Microbiol. 2002, 38, 1–8. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Raafat, D.; Sahl, H.-G. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Vishu Kumar, A.B.; Varadaraj, M.C.; Gowda, L.R.; Tharanathan, R.N. Characterization of chito-oligosaccharides prepared by chitosanolysis with the aid of papain and Pronase, and their bactericidal action against Bacillus cereus and Escherichia coli. Biochem. J. 2005, 391 Pt 2, 167–175. [Google Scholar] [CrossRef] [PubMed]
- El Ghaouth, A.; Arul, J.; Asselin, A.; Benhamou, N. Antifungal activity of chitosan on post harvest pathogens: Induction of morphological and cytological alterations in Rhizopus stolonifer. Mycol. Res. 1992, 96, 779. [Google Scholar] [CrossRef]
- Hadwiger, L.A.; Fristensky, B.W.; Riggleman, R. Advances in Chitin, Chitosan and Related Enzymes; Academic Press: Cambridge, MA, USA, 1984; p. 302. [Google Scholar]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr. E.V. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Coffin, D.R.; Fishman, M.L. Physical and mechanical properties of highly plasticized pectin/starch films. J. Appl. Polym. Sci. 1994, 54, 1320. [Google Scholar] [CrossRef]
- Hadwiger, L.A. Multiple effects of chitosan on plant systems: Solid science or hype. Plant Sci. 2013, 208, 42–49. [Google Scholar] [CrossRef]
- Allan, C.R.; Hadwiger, L.A. The fungicidal effect of chitosan on fungi of varying cell wall composition. Exp. Mycol. 1979, 3, 287. [Google Scholar] [CrossRef]
- Hassan, M.A.; Omer, A.M.; Abbas, E.; Baset, W.M.A.; Tamer, T.M. Preparation, physicochemical characterization and antimicrobial activities of novel two phenolic chitosan Schiff base derivatives. Sci. Rep. 2018, 8, 11416. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef]
- Pavinatto, A.; Souza, A.L.; Delezuk, J.A.M.; Pavinatto, F.J.; Campana-Filho, S.P.; Oliveira, O.N. Interaction of O-acylated chitosans with biomembrane models: Probing the effects from hydrophobic interactions and hydrogen bonding. Colloids Surf. B Biointerfaces 2014, 114, 53–59. [Google Scholar] [CrossRef]
- Goy, R.C.; de Britto, D.; Assis, O.B.G. A Review of the Antimicrobial Activity of Chitosan. Polim. Cienc. Tecnol. 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Eaton, P.; Fernandes, J.C.; Pereira, E.; Pintado, M.E.; Xavier Malcata, F. Atomic force microscopy study of the antibacterial effects of chitosans on Escherichia coli and Staphylococcus aureus. Ultramicroscopy 2008, 108, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, F.; Yang, R. Synthesis and characterization of chitosan derivatives with dual-antibacterial functional groups. Int. J. Biol. Macromol. 2015, 75, 378–387. [Google Scholar] [CrossRef]
- Severino, R.; Ferrari, G.; Vu, K.D.; Donsì, F.; Salmieri, S.; Lacroix, M. Antimicrobial effects of modified chitosan based coating containing nanoemulsion of essential oils, modified atmosphere packaging and gamma irradiation against Escherichia coli O157:H7 and Salmonella Typhimurium on green beans. Food Control 2015, 50, 215–222. [Google Scholar] [CrossRef]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Weckx, M.; Filippini, O. Removal of trace metal ions from industrial waters, nuclear effluents and drinking water, with the aid of cross-linked N-carboxymethyl chitosan. Carbohydr. Polym. 1989, 11, 306. [Google Scholar] [CrossRef]
- Krishnapriya, K.R.; Kandaswamy, M. Synthesis and characterization of a crosslinked chitosan derivative with a complexing agent and its adsorption studies toward metal(II) ions. Carbohydr. Res. 2009, 344, 1632–1638. [Google Scholar] [CrossRef]
- Mohd Yusof, H.; Mohamad, R.; Zaidan, U.H.; Abdul Rahman, N.A. Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: A review. J. Anim. Sci. Biotechnol. 2019, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Amidi, M.; Mastrobattista, E.; Jiskoot, W.; Hennink, W.E. Chitosan-based delivery systems for protein therapeutics and antigens. Adv. Drug Deliv. Rev. 2010, 62, 59–82. [Google Scholar] [CrossRef] [PubMed]
- Elshaarawy, R.F.M.; Refaee, A.A.; El-Sawi, E.A. Pharmacological performance of novel poly-(ionic liquid)-grafted chitosan-N-salicylidene Schiff bases and their complexes. Carbohydr. Polym. 2016, 146, 376–387. [Google Scholar] [CrossRef]
- Garoufis, A.; Hadjikakou, S.K.; Hadjiliadis, N. Palladium coordination compounds as anti-viral, anti-fungal, anti-microbial and anti-tumor agents. Coord. Chem. Rev. 2009, 253, 1384–1397. [Google Scholar] [CrossRef]
- Kurita, K.; Ikeda, H.; Yoshida, Y.; Shimojoh, M.; Harata, M. Chemoselective protection of the amino groups of chitosan by controlled phthaloylation: Facile preparation of a precursor useful for chemical modifications. Biomacromolecules 2002, 3, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Sahariah, P.; Gaware, V.S.; Lieder, R.; Jónsdóttir, S.; Hjálmarsdóttir, M.Á.; Sigurjonsson, O.E.; Másson, M. The effect of substituent, degree of acetylation and positioning of the cationic charge on the antibacterial activity of quaternary chitosan derivatives. Mar. Drugs 2014, 12, 4635–4658. [Google Scholar] [CrossRef] [PubMed]
- Kurita, K.; Hirakawa, M.; Kikuchi, S.; Yamanaka, H.; Yang, J. Trimethylsilylation of chitosan and some properties of the product. Carbohydr. Polym. 2004, 56, 333–337. [Google Scholar] [CrossRef]
- Rúnarsson, O.V.; Malainer, C.; Holappa, J.; Sigurdsson, S.T.; Másson, M. tert-Butyldimethylsilyl O-protected chitosan and chitooligosaccharides: Useful precursors for N-modifications in common organic solvents. Carbohydr. Res. 2008, 343, 2576–2582. [Google Scholar] [CrossRef]
- Prabaharan, M.; Mano, J.F. Chitosan-Based Particles as Controlled Drug Delivery Systems. Drug Deliv. 2004, 12, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Sajwan, M.; Alsuwayt, B.; Asif, M. Synthesis, characterization and anticoagulant activity of chitosan derivatives. Saudi Pharm. J. 2020, 28, 25–32. [Google Scholar] [CrossRef]
- Soliman, E.A.; El-Kousy, S.M.; Abd-Elbary, H.M.; Abou-Zeid, A.R. Low Molecular Weight Chitosan-based Schiff Bases: Synthesis, Characterization and Antibacterial Activity. Am. J. Food Technol. 2013, 8, 17–30. [Google Scholar] [CrossRef][Green Version]
- Mourya, V.K.; Inamdar, N.N. Chitosan-modifications and applications: Opportunities galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Lal, S.; Arora, S.; Sharma, C. Synthesis, thermal and antimicrobial studies of some Schiff bases of chitosan. J. Therm. Anal. Calorim. 2016, 124, 909–916. [Google Scholar] [CrossRef]
- Liu, W.G.; Zhang, X.; Sun, S.J.; Sun, G.J.; Yao, K.D.; Liang, D.C.; Guo, G.; Zhang, J.Y. N-alkylated chitosan as a potential nonviral vector for gene transfection. Bioconjug. Chem. 2003, 14, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Le, T.; Lacroix, M.; Ispas-Szabo, P.; Mateescu, M.A. N-acylated chitosan: Hydrophobic matrices for controlled drug release. J. Control. Release Off. J. Control. Release Soc. 2003, 93, 1–13. [Google Scholar]
- Jiao, T.F.; Zhou, J.; Zhou, J.; Gao, L.; Xing, Y.; Li, X. Synthesis and Characterization of Chitosan-Based Schiff Base Compounds with Aromatic Substituent Groups. Iran. Polym. J. 2011, 20, 123–136. [Google Scholar]
- Taghizadeh, M.; Bahadori, A. Preparation, characterization and adhesive properties of di- and tri-hydroxy benzoyl chitosan nanoparticles. Chin. J. Polym. Sci. 2013, 31, 649–659. [Google Scholar] [CrossRef]
- Yang, J.; Xie, Q.; Zhu, J.; Zou, C.; Chen, L.; Du, Y.; Li, D. Preparation and in vitro antioxidant activities of 6-amino-6-deoxychitosan and its sulfonated derivatives. Biopolymers 2015, 103, 539–549. [Google Scholar] [CrossRef]
- Ouerghemmi, S.; Dimassi, S.; Tabary, N.; Leclercq, L.; Degoutin, S.; Chai, F.; Pierlot, C.; Cazaux, F.; Ung, A.; Staelens, J.N.; et al. Synthesis and characterization of polyampholytic aryl-sulfonated chitosans and their in vitro anticoagulant activity. Carbohydr. Polym. 2018, 196, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Khattak, S.; Wahid, F.; Liu, L.-P.; Jia, S.-R.; Chu, L.-Q.; Xie, Y.-Y.; Li, Z.-X.; Zhong, C. Applications of cellulose and chitin/chitosan derivatives and composites as antibacterial materials: Current state and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 1989–2006. [Google Scholar] [CrossRef]
- Luan, F.; Wei, L.; Zhang, J.; Mi, Y.; Dong, F.; Li, Q.; Guo, Z. Antioxidant Activity and Antifungal Activity of Chitosan Derivatives with Propane Sulfonate Groups. Polymers 2018, 10, 395. [Google Scholar] [CrossRef]
- Sun, Z.; Shi, C.; Wang, X.; Fang, Q.; Huang, J. Synthesis, characterization, and antimicrobial activities of sulfonated chitosan. Carbohydr. Polym. 2017, 155, 321–328. [Google Scholar] [CrossRef]
- Varma, A.J.; Deshpande, S.V.; Kennedy, J.F. Metal complexation by chitosan and its derivatives: A review. Carbohydr. Polym. 2004, 55, 77–93. [Google Scholar] [CrossRef]
- Al-Saidi, H.M. Biosorption using chitosan thiourea polymer as an extraction and preconcentration technique for copper prior to its determination in environmental and food samples by flame atomic absorption spectrometry: Synthesis, characterization and analytical applications. Int. J. Biol. Macromol. 2016, 93, 390–401. [Google Scholar]
- Pestov, A.; Bratskaya, S. Chitosan and Its Derivatives as Highly Efficient Polymer Ligands. Molecules 2016, 21, 330. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef]
- Heise, K.; Hobisch, M.; Sacarescu, L.; Maver, U.; Hobisch, J.; Reichelt, T.; Sega, M.; Fischer, S.; Spirk, S. Low-molecular-weight sulfonated chitosan as template for anticoagulant nanoparticles. Int. J. Nanomed. 2018, 13, 4881–4894. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Nagahama, H.; Furuike, T.; Tamura, H. Synthesis of phosphorylated chitosan by novel method and its characterization. Int. J. Biol. Macromol. 2008, 42, 335–339. [Google Scholar] [CrossRef]
- Amaral, I.F.; Granja, P.L.; Barbosa, M.A. Chemical modification of chitosan by phosphorylation: An XPS, FT-IR and SEM study. J. Biomater. Sci. Polym. Ed. 2005, 16, 1575–1593. [Google Scholar] [CrossRef]
- Matevosyan, G.; Yukha, Y. Phosphorylation of Chitosan. Russ. J. Gen. Chem. 2003, 73, 1725–1728. [Google Scholar] [CrossRef]
- Suchyta, D.J.; Soto, R.J.; Schoenfisch, M.H. Selective monophosphorylation of chitosan via phosphorus oxychloride. Polym. Chem. 2017, 8, 2552–2558. [Google Scholar] [CrossRef] [PubMed]
- Pokhrel, S.; Yadav, P.N. Functionalization of chitosan polymer and their applications. J. Macromol. Sci. Part A 2019, 56, 450–475. [Google Scholar] [CrossRef]
- Hussein, M.; El-Hady, M.; Sayed, W.; Hefni, H. Preparation of some chitosan heavy metal complexes and study of its properties. Polym. Sci. Ser. A 2012, 54, 113–124. [Google Scholar] [CrossRef]
- Du, W.L.; Niu, S.S.; Xu, Y.L.; Xu, Z.R.; Fan, C.L. Antibacterial activity of chitosan tripolyphosphate nanoparticles loaded with various metal ions. Carbohydr. Polym. 2009, 75, 385–389. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, H.X.; Xue, F.; He, H.; Wang, S.F. Cellulose-based amphoteric adsorbent for the complete removal of low-level heavy metal ions via a specialization and cooperation mechanism. Chem. Eng. J. 2020, 385, 11. [Google Scholar] [CrossRef]
- Kalwar, K.; Bhutto, M.A.; Li, D.L.; Shan, D. Cellulose based nanofabrication; immobilization of silver nanoparticales and its size effect against Escherichia coli. Mater. Res. Express 2017, 4, 6. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Chung, Y.C.; Wang, H.L.; Chen, Y.M.; Li, S.L. Effect of abiotic factors on the antibacterial activity of chitosan against waterborne pathogens. Bioresour. Technol. 2003, 88, 179–184. [Google Scholar] [CrossRef]
- Jayakumar, R.; Reis, R.L.; Mano, J.F. Chemistry and Applications of Phosphorylated Chitin and Chitosan. e-Polymers 2006, 6, 35. [Google Scholar] [CrossRef]
- Krajewska, B. Diffusion of metal ions through gel chitosan membranes. React. Funct. Polym. 2001, 47, 37–47. [Google Scholar] [CrossRef]
- Bodek, K.H.; Kufelnicki, A. Interaction of microcrystalline chitosan with Ni(II) and Mn(II) ions in aqueous solution. J. Appl. Polym. Sci. 2005, 98, 2572–2577. [Google Scholar] [CrossRef]
- Sanpui, P.; Murugadoss, A.; Prasad, P.V.; Ghosh, S.S.; Chattopadhyay, A. The antibacterial properties of a novel chitosan-Ag-nanoparticle composite. Int. J. Food Microbiol. 2008, 124, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Potara, M.; Jakab, E.; Damert, A.; Popescu, O.; Canpean, V.; Astilean, S. Synergistic antibacterial activity of chitosan-silver nanocomposites on Staphylococcus aureus. Nanotechnology 2011, 22, 135101. [Google Scholar] [CrossRef] [PubMed]
- Davoodbasha, M.; Kim, S.C.; Lee, S.Y.; Kim, J.W. The facile synthesis of chitosan-based silver nano-biocomposites via a solution plasma process and their potential antimicrobial efficacy. Arch. Biochem. Biophys. 2016, 605, 49–58. [Google Scholar] [CrossRef]
- Titov, V.; Nikitin, D.; Naumova, I.; Losev, N.; Lipatova, I.; Kosterin, D.; Pleskunov, P.; Perekrestov, R.; Sirotkin, N.; Khlyustova, A.; et al. Dual-Mode Solution Plasma Processing for the Production of Chitosan/Ag Composites with the Antibacterial Effect. Materials 2020, 13, 4821. [Google Scholar] [CrossRef]
- Gerente, C.; Lee, V.K.C.; Le Cloirec, P.; McKay, G. Application of Chitosan for the Removal of Metals from Wastewaters by Adsorption—Mechanisms and Models Review. Crit. Rev. Environ. Sci. Technol. 2007, 37, 41–127. [Google Scholar] [CrossRef]
- Anbinder, P.S.; Macchi, C.; Amalvy, J.; Somoza, A. A study of the structural changes in a chitosan matrix produced by the adsorption of copper and chromium ions. Carbohydr. Polym. 2019, 222, 7. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Verma, M.; Brar, S.K. Biopolymer-Based Nanomaterials. Compr. Anal. Chem. 2012, 59, 91–129. [Google Scholar]
- Ismillayli, N.; Andayani, I.G.A.S.; Honiar, R.; Mariana, B.; Sanjaya, R.K.; Hermanto, D. Polyelectrolyte Complex (PEC) film based on chitosan as potential edible films and their antibacterial activity test. IOP Conf. Ser. Mater. Sci. Eng. 2020, 959, 012009. [Google Scholar] [CrossRef]
- Theapsak, S.; Watthanaphanit, A.; Rujiravanit, R. Preparation of Chitosan-Coated Polyethylene Packaging Films by DBD Plasma Treatment. ACS Appl. Mater. Interfaces 2012, 4, 2474–2482. [Google Scholar] [CrossRef] [PubMed]
- Zienkiewicz-Strzałka, M.; Deryło-Marczewska, A.; Skorik, Y.A.; Petrova, V.A.; Choma, A.; Komaniecka, I. Silver Nanoparticles on Chitosan/Silica Nanofibers: Characterization and Antibacterial Activity. Int. J. Mol. Sci. 2020, 21, 166. [Google Scholar] [CrossRef]
- Sibaja, B.; Culbertson, E.; Marshall, P.; Boy, R.; Broughton, R.M.; Solano, A.A.; Esquivel, M.; Parker, J.; De La Fuente, L.; Auad, M.L. Preparation of alginate-chitosan fibers with potential biomedical applications. Carbohydr. Polym. 2015, 134, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, K.; Afifi, A.M.; Jahangirian, H.; Webster, T.J. Biomedical applications of chitosan electrospun nanofibers as a green polymer—Review. Carbohydr. Polym. 2019, 207, 588–600. [Google Scholar] [CrossRef]
- Sophonvachiraporn, P.; Rujiravanit, R.; Sreethawong, T.; Tokura, S.; Chavadej, S. Surface Characterization and Antimicrobial Activity of Chitosan-Deposited DBD Plasma-Modified Woven PET Surface. Plasma Chem. Plasma Process. 2011, 31, 233–249. [Google Scholar] [CrossRef]
- Yorsaeng, S.; Pornsunthorntawee, O.; Rujiravanit, R. Preparation and Characterization of Chitosan-Coated DBD Plasma-Treated Natural Rubber Latex Medical Surgical Gloves with Antibacterial Activities. Plasma Chem. Plasma Process. 2012, 32, 1275–1292. [Google Scholar] [CrossRef]
- Nikitin, D.; Choukourov, A.; Titov, V.; Kuzmicheva, L.; Lipatova, I.; Mezina, E.; Aleksandriiskii, V.; Shelemin, A.; Khalakhan, I.; Slavinska, D.; et al. In situ coupling of chitosan onto polypropylene foils by an Atmospheric Pressure Air Glow Discharge with a liquid cathode. Carbohydr. Polym. 2016, 154, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Suganya, A.; Shanmugvelayutham, G.; Hidalgo-Carrillo, J. Plasma Surface Modified Polystyrene and Grafted with Chitosan Coating for Improving the Shelf Lifetime of Postharvest Grapes. Plasma Chem. Plasma Process. 2018, 38, 1151–1168. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ardean, C.; Davidescu, C.M.; Nemeş, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Musta, V. Factors Influencing the Antibacterial Activity of Chitosan and Chitosan Modified by Functionalization. Int. J. Mol. Sci. 2021, 22, 7449. https://doi.org/10.3390/ijms22147449
Ardean C, Davidescu CM, Nemeş NS, Negrea A, Ciopec M, Duteanu N, Negrea P, Duda-Seiman D, Musta V. Factors Influencing the Antibacterial Activity of Chitosan and Chitosan Modified by Functionalization. International Journal of Molecular Sciences. 2021; 22(14):7449. https://doi.org/10.3390/ijms22147449
Chicago/Turabian StyleArdean, Cristina, Corneliu Mircea Davidescu, Nicoleta Sorina Nemeş, Adina Negrea, Mihaela Ciopec, Narcis Duteanu, Petru Negrea, Daniel Duda-Seiman, and Virgil Musta. 2021. "Factors Influencing the Antibacterial Activity of Chitosan and Chitosan Modified by Functionalization" International Journal of Molecular Sciences 22, no. 14: 7449. https://doi.org/10.3390/ijms22147449
APA StyleArdean, C., Davidescu, C. M., Nemeş, N. S., Negrea, A., Ciopec, M., Duteanu, N., Negrea, P., Duda-Seiman, D., & Musta, V. (2021). Factors Influencing the Antibacterial Activity of Chitosan and Chitosan Modified by Functionalization. International Journal of Molecular Sciences, 22(14), 7449. https://doi.org/10.3390/ijms22147449