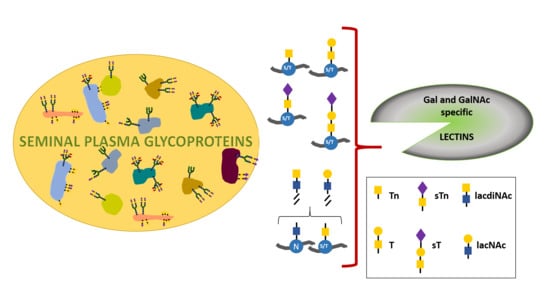
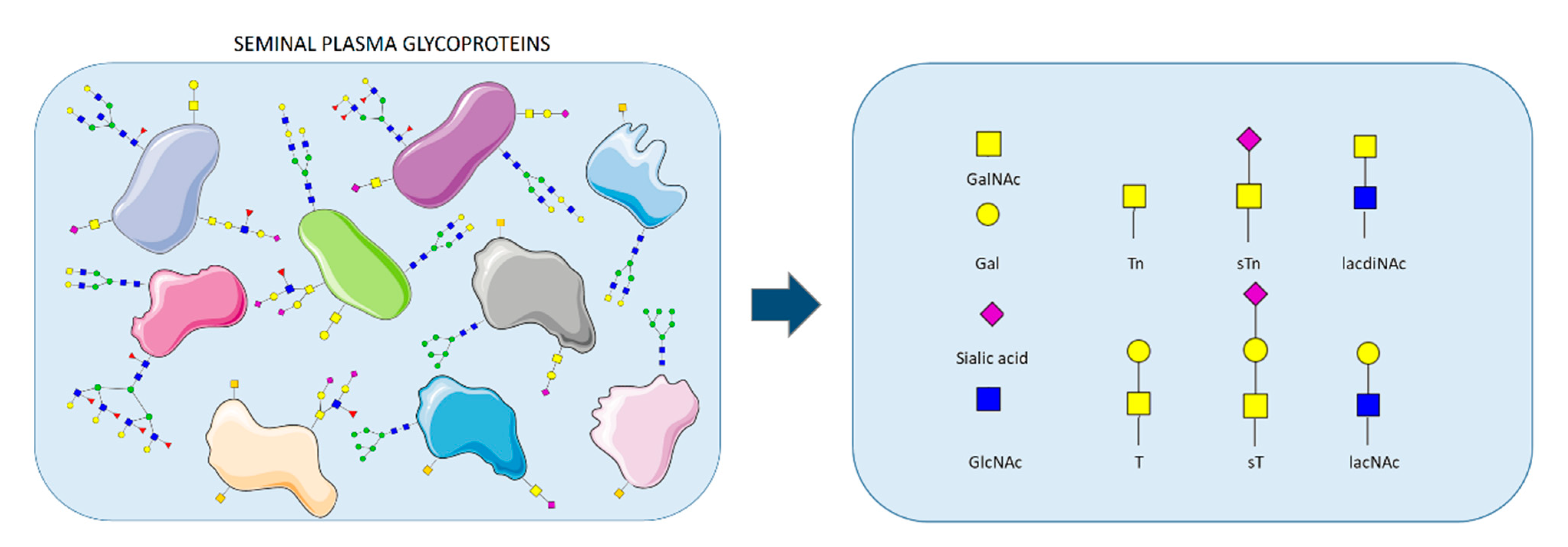
Glycoproteins Presenting Galactose and N-Acetylgalactosamine in Human Seminal Plasma as Potential Players Involved in Immune Modulation in the Fertilization Process
Abstract
:1. Introduction
2. Results
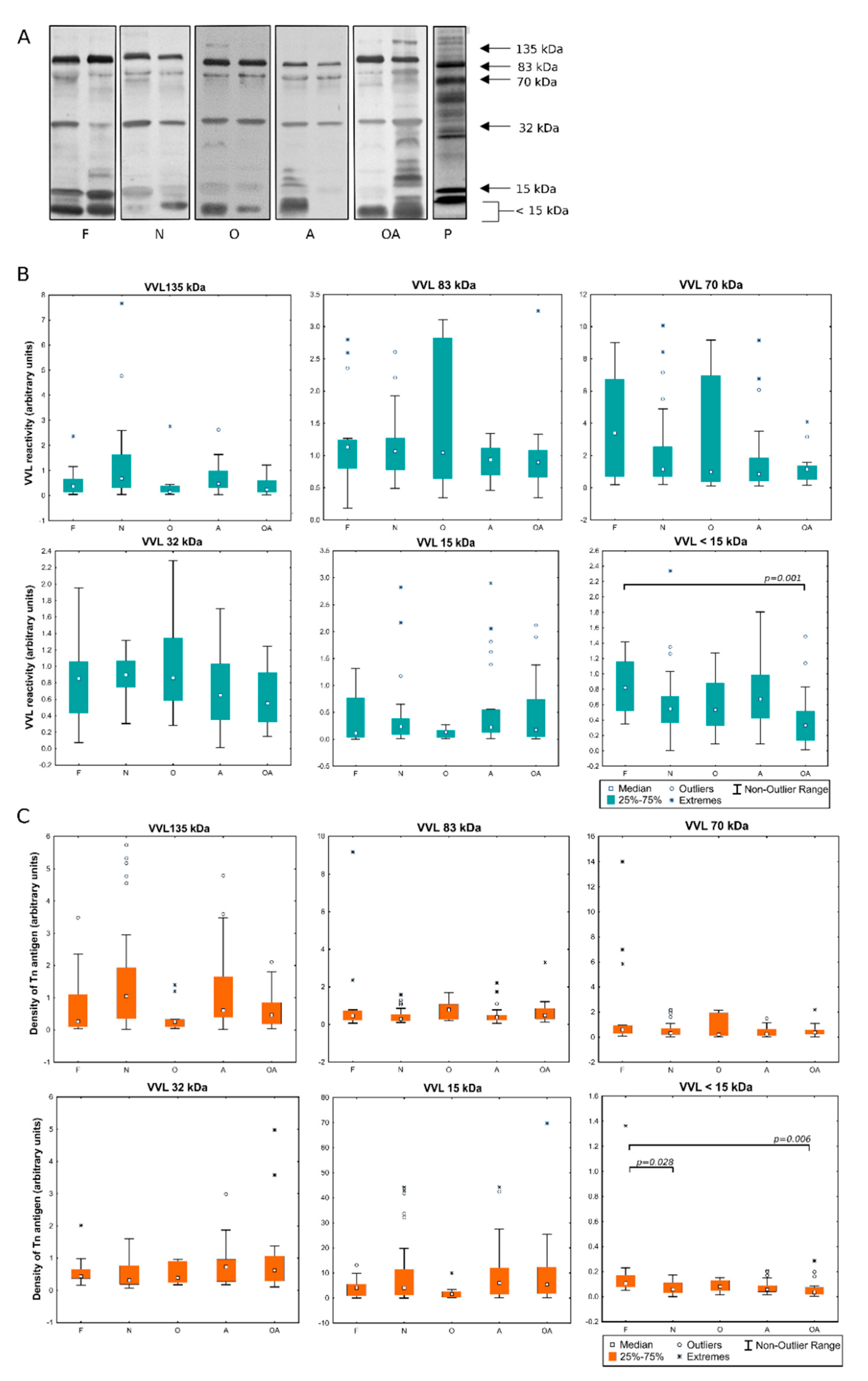
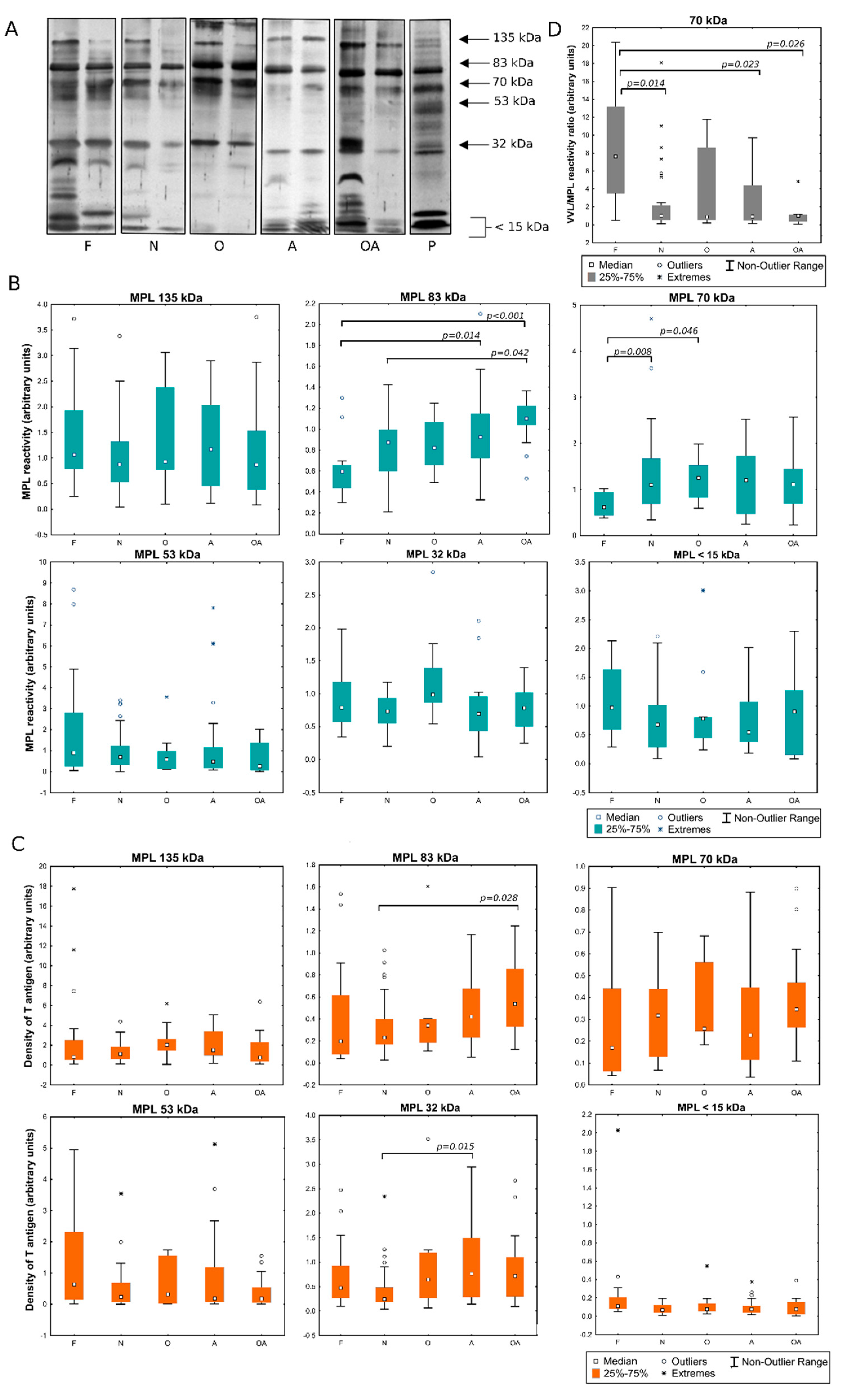
2.1. Detection of MPL and VVL Ligands in Seminal Plasma
2.2. Evaluation of the Expression of T and Tn Glycoepitopes in Individual Seminal Plasma Samples
2.3. Identification of Proteins in Lectin-Reactive Bands
2.4. Isolation of Lectin-Reactive Glycoproteins
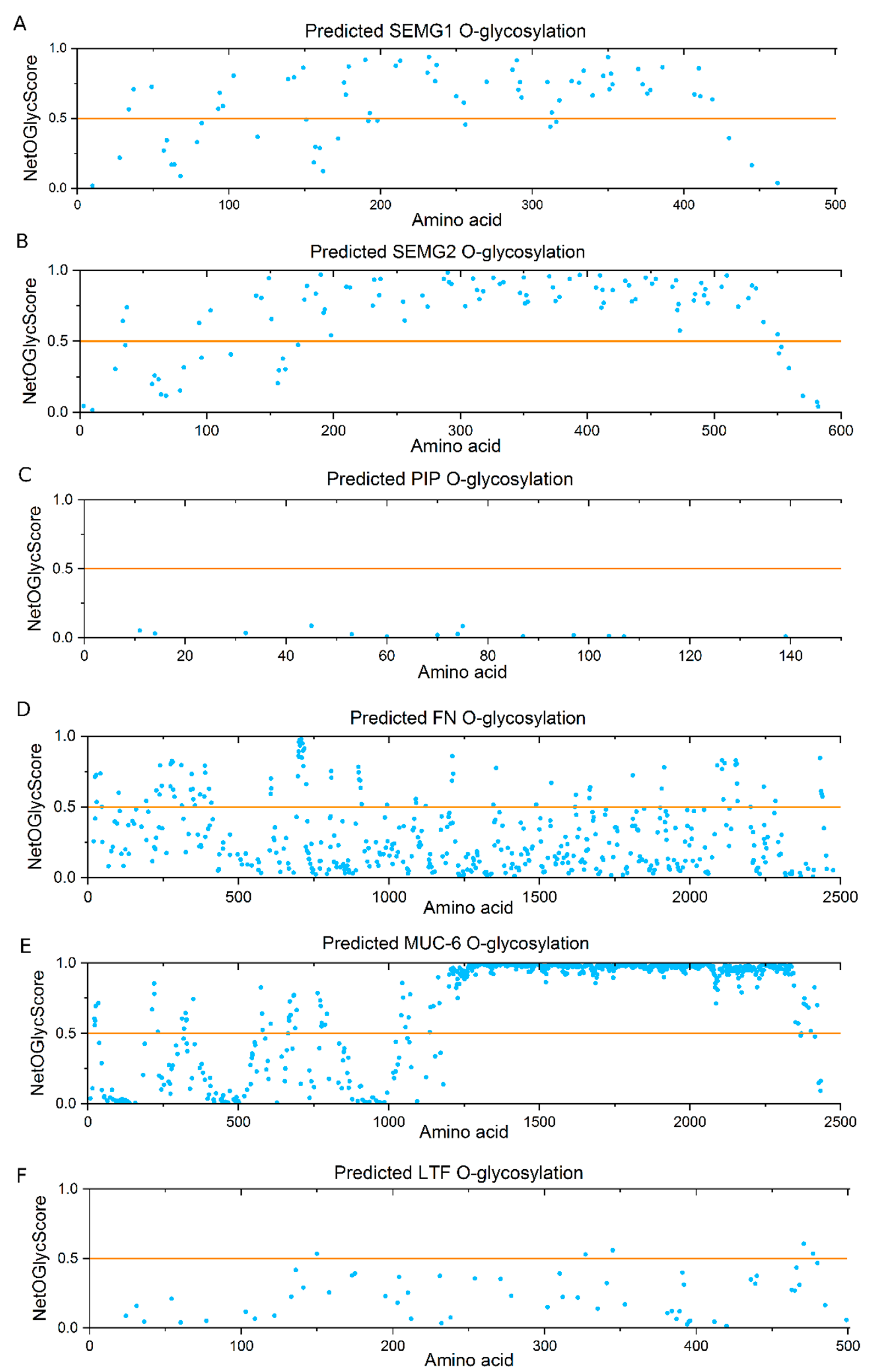
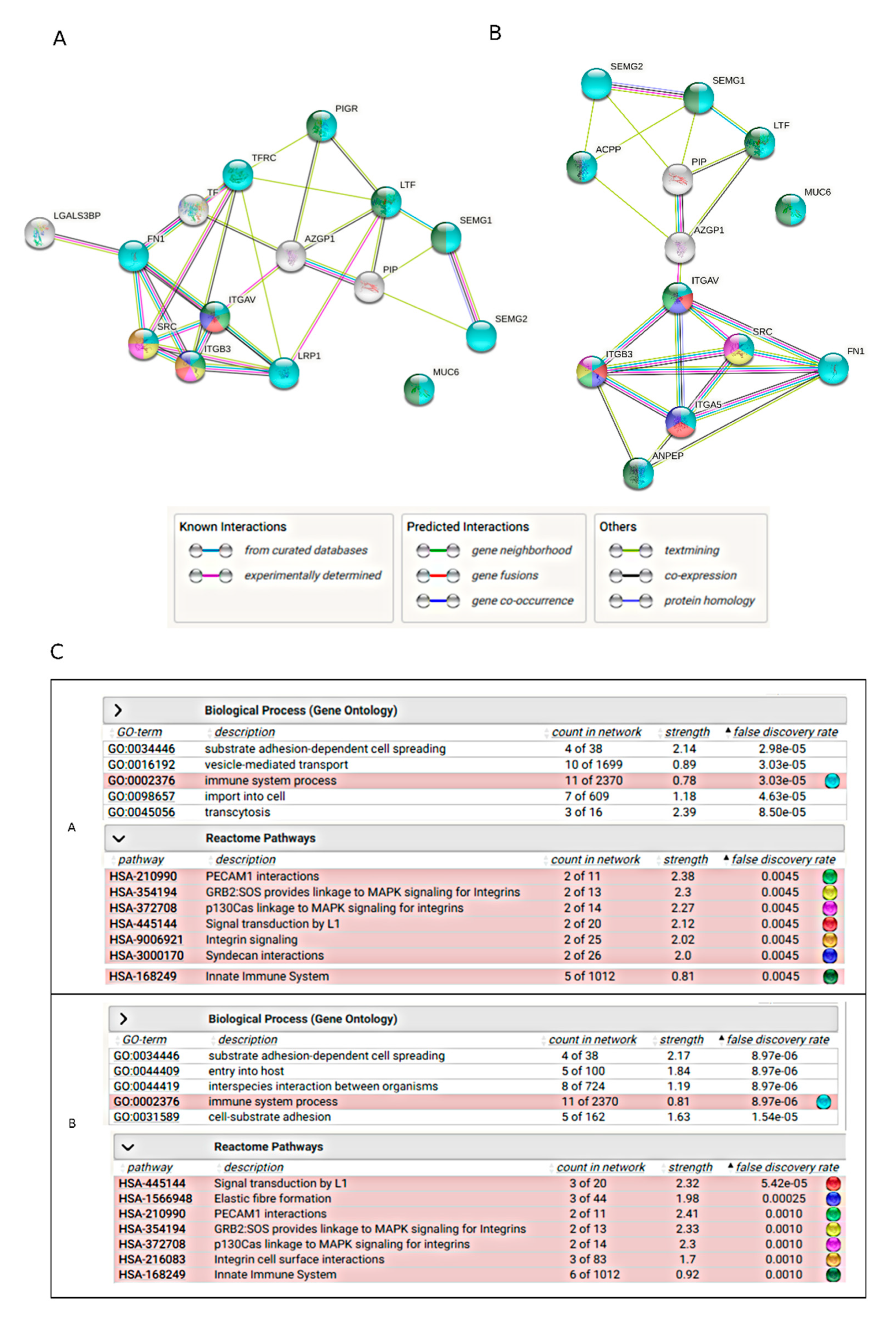
2.5. Bioinformatics Analysis
3. Discussion
4. Materials and Methods
4.1. Clinical Samples
4.2. SDS-PAGE
4.3. Lectin-Blotting
4.4. Densitometric Analysis
4.5. Affinity Chromatography
4.6. Liquid Chromatography–Mass Spectrometry (LC-MS) Identification of Glycoproteins
4.7. Reactivity with Antibodies
4.8. Bioinformatics Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szczykutowicz, J.; Kałuża, A.; Kaźmierowska-Niemczuk, M.; Ferens-Sieczkowska, M. The potential role of seminal plasma in the fertilization outcomes. BioMed Res. Int. 2019, 2019, 5397804. [Google Scholar] [CrossRef]
- Clark, G.F.; Schust, D.J. Manifestations of immune tolerance in the human female reproductive tract. Front. Immunol. 2013, 4, e26. [Google Scholar] [CrossRef] [Green Version]
- Clark, G.F. The role of glycans in immune evasion: The human fetoembryonic defence system hypothesis revisited. Mol. Hum. Reprod. 2014, 20, 185–199. [Google Scholar] [CrossRef] [Green Version]
- Pang, P.C.; Tissot, B.; Drobnis, E.Z.; Morris, H.R.; Dell, A.; Clark, G.F. Analysis of the human seminal plasma glycome reveals the presence of immunomodulatory carbohydrate functional groups. J. Proteome Res. 2009, 8, 4906–4915. [Google Scholar] [CrossRef]
- Clark, G.F.; Grassi, P.; Pang, P.C.; Panico, M.; Lafrenz, D.; Drobnis, E.Z.; Baldwin, M.R.; Morris, H.R.; Haslam, S.M.; Schedin-Weiss, S.; et al. Tumor biomarker glycoproteins in the seminal plasma of healthy human males are endogenous ligands for DC-SIGN. Mol. Cell. Proteom. 2012, 11, M111.008730. [Google Scholar] [CrossRef] [Green Version]
- Pang, P.C.; Chiu, P.C.; Lee, C.L.; Chang, L.Y.; Panico, M.; Morris, H.R.; Haslam, S.M.; Khoo, K.H.; Clark, G.F.; Yeung, W.S.; et al. Human sperm binding is mediated by the sialyl- Lewis(x) oligosaccharide on the zona pellucida. Science 2011, 23, 1761–1764. [Google Scholar] [CrossRef] [Green Version]
- Olejnik, B.; Kratz, E.M.; Zimmer, M.; Ferens-Sieczkowska, M. Glycoprotein fucosylation is increased in seminal plasma of subfertile men. Asian J. Androl. 2015, 17, 274–280. [Google Scholar]
- Kałuża, A.; Ferens-Sieczkowska, M.A.; Olejnik, B.; Kołodziejczyk, J.; Zimmer, M.; Kratz, E.M. The content of immunomodulatory glycoepitopes in seminal plasma glycoproteins of fertile and infertile men. Reprod. Fertil. Dev. 2019, 31, 579–589. [Google Scholar] [CrossRef]
- Kratz, E.M.; Faundez, R.; Katnik-Prastowska, I. Fucose and sialic acid expressions in human seminal fibronectin and α{1} -acid glycoprotein associated with leukocytospermia of infertile men. Dis. Markers 2011, 31, 317–325. [Google Scholar] [CrossRef]
- Sarrats, A.; Saldova, R.; Comet, J.; O’Donoghue, N.; de Llorens, R.; Rudd, P.M.; Peracaula, R. Glycan characterization of PSA 2-DE subforms from serum and seminal plasma. OMICS 2010, 14, 465–474. [Google Scholar] [CrossRef] [Green Version]
- Merlotti, A.; Dantas, E.; Remes Lenicov, F.; Ceballos, A.; Jancic, C.; Varese, A.; Rubione, J.; Stover, S.; Geffner, J.; Sabatté, J. Fucosylated clusterin in semen promotes the uptake of stress-damaged proteins by dendritic cells via DC-SIGN. Hum. Reprod. 2015, 30, 1545–1556. [Google Scholar] [CrossRef]
- Milutinović, B.; Goč, S.; Mitić, N.; Kosanović, M.; Janković, M. Surface glycans contribute to differences between seminal prostasomes from normozoospermic and oligozoospermic men. Ups. J. Med. Sci. 2019, 124, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Fu, C.; Zhao, H.; Wang, Y.; Cai, H.; Xiao, Y.; Zeng, Y.; Chen, H. Tumor-associated antigens: Tn antigen, sTn antigen, and T antigen. HLA 2016, 88, 275–286. [Google Scholar] [CrossRef]
- Cornelissen, L.A.; Van Vliet, S.J. A Bitter Sweet Symphony: Immune Responses to Altered O-glycan Epitopes in Cancer. Biomolecules 2016, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Itano, N. Implications of altered O-glycosylation in tumour immune evasion. J. Biochem. 2019, 165, 387–390. [Google Scholar] [CrossRef]
- Springer, G.F. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J. Mol. Med. 1997, 75, 594–602. [Google Scholar] [CrossRef]
- Springer, G.F. Tn epitope (N-acetyl-D-galactosamine-ct-Oserine/threonine) density in primary breast carcinoma: A functional predictor of aggressiveness. Mol. Immunol. 1989, 26, 1–5. [Google Scholar] [CrossRef]
- Springer, G.F. T and Tn, general carcinoma autoantigens. Science 1984, 224, 1198–1206. [Google Scholar] [CrossRef]
- Desai, P.R. Immunoreactive T and Tn antigens in malignancy: Role in carcinoma diagnosis, prognosis, and immunotherapy. Transfus. Med. Rev. 2000, 14, 312–325. [Google Scholar] [CrossRef]
- Loureiro, L.R.; Carrascal, M.A.; Barbas ARamalho, J.S.; Novo, C.; Delannoy, P.; Videira, P.A. Challenges in antibody development against Tn and sialyl-Tn antigens. Biomolecules 2015, 5, 1783–1809. [Google Scholar] [CrossRef] [Green Version]
- Baldus, S.E.; Zirbes, T.K.; Hanisch, F.G.; Kunze, D.; Shafizadeh, S.T.; Nolden, S.; Mönig, S.P.; Schneider, P.M.; Karsten, U.; Thiele, J.; et al. Thomsen-Friedenreich antigen presents as a prognostic factor in colorectal carcinoma: A clinicopathologic study of 264 patients. Cancer 2000, 88, 1536–1543. [Google Scholar] [CrossRef]
- Laack, E.; Nikbakht, H.; Peters, A.; Kugler, C.; Jasiewicz, Y.; Edler, L.; Hossfeld, D.K.; Schumacher, U. Lectin histochemistry of resected adenocarcinoma of the lung: Helix pomatia agglutinin binding is an independent prognostic factor. Am. J. Pathol. 2002, 160, 1001–1008. [Google Scholar] [CrossRef]
- Welinder, C.; Baldetorp, B.; Blixt, O.; Grabau, D.; Jansson, B. Primary breast cancer tumours contain high amounts of IgA1 immunoglobulin: An immunohistochemical analysis of a possible carrier of the tumour-associated Tn antigen. PLoS ONE 2013, 8, 87–89. [Google Scholar]
- Konno, A.; Hoshino, Y.; Terashima, S.; Motoki, R.; Kawaguchi, T. Carbohydrate expression profile of colorectal cancer cells is relevant to metastatic pattern and prognosis. Clin. Exp. Metastasis 2002, 19, 61–70. [Google Scholar] [CrossRef]
- Milutinovic, B.; Mitic, N.; Roncevic, J.G.S.; Jankovic, M. Glycome complexity of human seminal plasma high molecular mass components: Evaluation of the contribution of acid-soluble glycoproteins/mucins and extracellular vesicles. Arch. Biochem. Biophys. 2016, 609, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Takazawa, H.; Imai, S.; Morimoto, J.; Watanabe, T.; Kanno, M.; Igarashi, S. Expression of Vicia villosa agglutinin (VVA)-binding glycoprotein in primary breast cancer cells in relation to lymphatic metastasis: Is atypical MUC1 bearing Tn antigen a receptor of VVA? Breast. Cancer Res. Treat. 2006, 98, 31–43. [Google Scholar] [CrossRef]
- Wu, A.M. Polyvalent GalNAcalpha1-->Ser/Thr (Tn) and Galbeta1-->3GalNAcalpha1-->Ser/Thr (T alpha) as the most potent recognition factors involved in Maclura pomifera agglutinin-glycan interactions. J. Biomed. Sci. 2005, 12, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Eiselé, J.L.; Tello, D.; Osinaga, E.; Roseto, A.; Alzari, P.M. Crystallization and preliminary crystallographic analysis of a tetrameric isolectin from Vicia villosa, specific for the Tn antigen. J. Mol. Biol. 1993, 230, 670–672. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, S.; Takeishi, S.; Yamamoto, A.; Ikeda, K.; Matsunari, H.; Yamada, M.; Okabe, M.; Miyoshi, E.; Fukuzawa, M.; Nagashima, H. Survey of glycoantigens in cells from alpha1-3galactosyltransferase knockout pig using a lectin microarray. Xenotransplantation 2010, 17, 61–70. [Google Scholar] [CrossRef]
- Korb, O.; Möller, H.M.; Exner, T.E. NMR-guided molecular docking of a protein-peptide complex based on ant colony optimization. Chem. Med. Chem. 2010, 5, 1001–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geijtenbeek, T.; Gringhuis, S. Signalling through C-type lectin receptors: Shaping immune responses. Nat. Rev. Immunol. 2009, 9, 465–479. [Google Scholar] [CrossRef]
- Dambuza, I.M.; Brown, G.D. C-type lectins in immunity: Recent developments. Curr. Opin. Immunol. 2015, 32, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Brown, G.D.; Willment, J.A.; Whitehead, L. C-type lectins in immunity and homeostasis. Nat. Rev. Immunol. 2018, 18, 374–389. [Google Scholar] [CrossRef] [PubMed]
- van de Wall, S.; Santegoets, K.C.M.; van Houtum, E.J.H.; Büll, C.; Adema, G.J. Sialoglycans and Siglecs Can Shape the Tumor Immune Microenvironment. Trends Immunol. 2020, 41, 274–285. [Google Scholar] [CrossRef]
- Bärenwaldt, A.; Läubli, H. The sialoglycan-Siglec glyco-immune checkpoint—A target for improving innate and adaptive anti-cancer immunity. Expert Opin. Ther. Targets 2019, 23, 839–853. [Google Scholar] [CrossRef]
- Läubli, H.; Varki, A. Sialic acid-binding immunoglobulin-like lectins (Siglecs) detect self-associated molecular patterns to regulate immune responses. Cell Mol. Life Sci. 2020, 77, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Kaltner, H.; Toegel, S.; Caballero, G.G.; Manning, J.C.; Ledeen, R.W.; Gabius, H.J. Galectins: Their network and roles in immunity/tumor growth control. Histochem. Cell Biol. 2017, 147, 239–256. [Google Scholar] [CrossRef]
- van Kooyk, Y.; Geijtenbeek, T.B. DC-SIGN: Escape mechanism for pathogens. Nat. Rev. Immunol. 2003, 3, 697–709. [Google Scholar] [CrossRef]
- Borsig, L. Selectins in cancer immunity. Glycobiology 2018, 28, 648–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinchmann, M.F.; Patel, D.M.; Iversen, M.H. The Role of Galectins as Modulators of Metabolism and Inflammation. Mediat. Inflamm 2018, 49, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Stanczak, M.A.; Siddiqui, S.S.; Trefny, M.P.; Thommen, D.S.; Boligan, K.F.; von Gunten, S.; Tzankov, A.; Tietze, L.; Lardinois, D.; Heinzelmann-Schwarz, V.; et al. Self-associated molecular patterns mediate cancer immune evasion by engaging Siglecs on T cells. J. Clin. Investig. 2018, 128, 4912–4923. [Google Scholar] [CrossRef] [Green Version]
- Chou, F.C.; Chen, H.Y.; Kuo, C.C.; Sytwu, H.K. Role of Galectins in Tumors and in Clinical Immunotherapy. Int. J. Mol. Sci. 2018, 19, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morante-Palacios, O.; Fondelli, F.; Ballestar, E.; Martínez-Cáceres, E.M. Tolerogenic Dendritic Cells in Autoimmunity and Inflammatory Diseases. Trends. Immunol. 2021, 42, 59–75. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.; Pearce, E.J. Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 2016, 213, 15–23. [Google Scholar] [CrossRef]
- Steinman, R.M.; Hawiger, D.; Nussenzweig, M.C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 2003, 21, 685–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remes Lenicov, F.; Rodriguez Rodrigues, C.; Sabatté, J.; Cabrini, M.; Jancic, C.; Ostrowski, M.; Merlotti, A.; Gonzalez, H.; Alonso, A.; Pasqualini, R.A.; et al. Semen promotes the differentiation of tolerogenic dendritic cells. J. Immunol. 2012, 189, 4777–4786. [Google Scholar] [CrossRef] [Green Version]
- Robertson, S.A.; Care, A.S.; Moldenhauer, L.M. Regulatory T cells in embryo implantation and the immune response to pregnancy. J. Clin. Investig. 2018, 128, 4224–4235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itzkowitz, S.H.; Yuan, M.; Montgomery, C.K.; Kjeldsen, T.; Takahashi, H.K.; Bigbee, W.L.; Kim, Y.S. Expression of Tn, sialosyl-Tn, and T antigens in human colon cancer. Cancer Res. 1989, 49, 197–204. [Google Scholar]
- Cervoni, G.E.; Cheng, J.J.; Stackhouse, K.A.; Heimburg-Molinaro, J.; Cummings, R.D. O-glycan recognition and function in mice and human cancers. Biochem. J. 2020, 477, 1541–1564. [Google Scholar] [CrossRef]
- Moreira, I.B.; Pinto, F.; Gomes, C.; Campos, D.; Reis, C.A. Impact of Truncated O-glycans in gastric-cancer-associated CD44v9 detection. Cells 2020, 9, 264. [Google Scholar] [CrossRef] [Green Version]
- Park, G.W.; Lee, J.W.; Lee, H.K.; Shin, J.H.; Kim, J.Y.; Yoo, J.S. Classification of mucin-type O-glycopeptides using higher-energy collisional dissociation in mass spectrometry. Anal. Chem. 2020, 92, 9772–9781. [Google Scholar] [CrossRef]
- Yang, W.; Ao, M.; Song, A.; Xu, Y.; Sokoll, L.; Zhang, H. Mass spectrometric mapping of glycoproteins modified by Tn-antigen using solid-phase capture and enzymatic release. Anal. Chem. 2020, 92, 9230–9238. [Google Scholar] [CrossRef]
- Zizzari, I.G.; Martufi, P.; Battisti, F.; Rahimi, H.; Caponnetto, S.; Bellati, F.; Nuti, M.; Rughetti, A.; Napoletano, C. The Macrophage Galactose-Type C-Type Lectin (MGL) modulates regulatory T cell functions. PLoS ONE 2015, 10, e0132617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zizzari, I.G.; Napoletano, C.; Battisti, F.; Rahimi, H.; Caponnetto, S.; Pierelli, L.; Nuti, M.; Rughetti, A. MGL receptor and immunity: When the ligand can make the difference. J. Immunol. Res. 2015, 2015, 450695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napoletano, C.; Steentoff, C.; Battisti, F.; Ye, Z.; Rahimi, H.; Zizzari, I.G.; Dionisi, M.; Cerbelli, B.; Tomao, F.; French, D.; et al. Investigating patterns of immune interaction in ovarian cancer: Probing the O-glycoproteome by the Macrophage Galactose-Like C-type Lectin (MGL). Cancers 2020, 12, 2841. [Google Scholar] [CrossRef]
- Pirro, M.; Mohammed, Y.; van Vliet, S.J.; Rombouts, Y.; Sciacca, A.; de Ru, A.H.; Janssen, G.M.C.; Tjokrodirijo, R.T.N.; Wuhrer, M.; van Veelen, P.A.; et al. N-glycoproteins have a major role in MGL binding to colorectal cancer cell lines: Associations with overall proteome diversity. Int. J. Mol. Sci. 2020, 21, 5522. [Google Scholar] [CrossRef]
- Pirro, M.; Rombouts, Y.; Stella, A.; Neyrolles, O.; Burlet-Schiltz, O.; van Vliet, S.J.; de Ru, A.H.; Mohammed, Y.; Wuhrer, M.; van Veelen, P.A.; et al. Characterization of Macrophage Galactose-type Lectin (MGL) ligands in colorectal cancer cell lines. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129513. [Google Scholar] [CrossRef]
- van Vliet, S.J.; Vuist, I.M.; Lenos, K.; Tefsen, B.; Kalay, H.; García-Vallejo, J.J.; van Kooyk, Y. Human T cell activation results in extracellular signal-regulated kinase (ERK)-calcineurin-dependent exposure of Tn antigen on the cell surface and binding of the macrophage galactose-type lectin (MGL). J. Biol. Chem. 2013, 288, 27519–27532. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Xu, Z.; Wang, D.; Ogata, C.M.; Palczewski, K.; Lee, X.; Young, N.M. Characterization of the secondary binding sites of Maclura pomifera agglutinin by glycan array and crystallographic analyses. Glycobiology 2010, 20, 1643–1653. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.M. Carbohydrate structural units in glycoproteins and polysaccharides as important ligands for Gal and GalNAc reactive lectins. J. Biomed. Sci. 2003, 10, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Gilboa-Garber, N.; Lerrer, B.; Lesman-Movshovich, E.; Dgani, O. Differential staining of Western blots of human secreted glycoproteins from serum, milk, saliva, and seminal fluid using lectins displaying diverse sugar specificities. Electrophoresis 2005, 26, 4396–4401. [Google Scholar] [CrossRef] [PubMed]
- Kratz, E.M.; Kałuża, A.; Zimmer, M.; Ferens-Sieczkowska, M. The analysis of sialylation, N-glycan branching, and expression of O-glycans in seminal plasma of infertile men. Dis. Markers. 2015, 2015, 941871. [Google Scholar]
- Kołodziejczyk, J.; Blixt, O.; Olejnik, B.; Zimmer, M.; Ferens-Sieczkowska, M. Application of lectin microarrays for the analysis of seminal plasma glycome. Andrologia 2018, 50, e13018. [Google Scholar] [CrossRef]
- Drabovich, A.P.; Saraon, P.; Jarvi, K.; Diamandis, E.P. Seminal plasma as a diagnostic fluid for male reproductive system disorders. Nat. Rev. Urol. 2014, 11, 278–288. [Google Scholar] [CrossRef]
- Buckett, W.M.; Luckas, M.J.; Gazvani, M.R.; Aird, I.A.; Lewis-Jones, D.I. Seminal plasma lactoferrin concentrations in normal and abnormal semen samples. J. Androl. 1997, 18, 302–304. [Google Scholar]
- Autiero, M.; Sansone, G.; Abrescia, P. Relative ratios of lactoferrin, albumin, and acid phosphatase seminal levels as sperm quality markers in fertile and infertile men. J. Androl. 1991, 12, 191–200. [Google Scholar]
- Chen, H.D.; Zhou, X.; Yu, G.; Zhao, Y.L.; Ren, Y.; Zhou, Y.D.; Li, Q.; Zhang, X.L. Knockdown of Core 1 Beta 1, 3-galactosyltransferase Prolongs Skin Allograft Survival with Induction of Galectin-1 Secretion and Suppression of CD8+T Cells. J. Clin. Immunol. 2012, 32, 820–836. [Google Scholar] [CrossRef] [PubMed]
- Wiegandt, A.; Behnken, H.N.; Meyer, B. Unusual N-type glycosylation of salivary prolactin-inducible protein (PIP): Multiple LewisY epitopes generate highly-fucosylated glycan structures. Glycoconj. J. 2018, 35, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Block, A.S.; Saraswati, S.; Lichti, C.F.; Mahadevan, M.; Diekman, A.B. Co-purification of Mac-2 binding protein with galectin-3 and association with prostasomes in human semen. Prostate 2011, 71, 711–721. [Google Scholar] [CrossRef] [Green Version]
- Kovak, M.R.; Saraswati, S.; Goddard, S.D.; Diekman, A.B. Proteomic identification of galectin-3 binding ligands and characterization of galectin-3 proteolytic cleavage in human prostasomes. Andrology 2013, 1, 682–691. [Google Scholar] [CrossRef]
- Hassan, M.I.; Waheed, A.; Yadav, S.; Singh, T.P.; Ahmad, F. Prolactin inducible protein in cancer, fertility and immunoregulation: Structure, function and its clinical implications. Cell Mol. Life Sci. 2009, 66, 447–459. [Google Scholar] [CrossRef]
- Umadat, V.; Ihedioha, O.; Shiu, R.; Uzonna, J.; Myal, Y. The prolactin-inducible-protein (PIP): A regulatory molecule in adaptive and innate immunity. Open J. Immunol. 2013, 3, 210–217. [Google Scholar] [CrossRef] [Green Version]
- Yoneyama, T.; Tobisawa, Y.; Kaneko, T.; Kaya, T.; Hatakeyama, S.; Mori, K.; Sutoh Yoneyama, M.; Okubo, T.; Mitsuzuka, K.; Duivenvoorden, W.; et al. Clinical significance of the LacdiNAc- glycosylated prostate-specific antigen assay for prostate cancer detection. Cancer. Sci. 2019, 110, 2573–2589. [Google Scholar] [CrossRef] [Green Version]
- Kaya, T.; Kaneko, T.; Kojima, S.; Nakamura, Y.; Ide, Y.; Ishida, K.; Suda, Y.; Yamashita, K. High-sensitivity immunoassay with surface plasmon field-enhanced fluorescence spectroscopy using a plastic sensor chip: Application to quantitative analysis of total prostate-specific antigen and GalNAcβ1-4GlcNAc-linked prostate-specific antigen for prostate cancer diagnosis. Anal. Chem. 2015, 87, 1797–1803. [Google Scholar] [PubMed]
- Inoue, T.; Kaneko, T.; Muramatsu, S.; Kimura, H.; Yoshino, T.; Goto, T.; Sawada, A.; Akamatsu, S.; Kobayashi, T.; Yamasaki, T.; et al. LacdiNAc-Glycosylated Prostate-specific Antigen Density is a Potential Biomarker of Prostate Cancer. Clin. Genitourin. Cancer. 2020, 18, e28–e36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haga, Y.; Uemura, M.; Baba, S.; Inamura, K.; Takeuchi, K.; Nonomura, N.; Ueda, K. Identification of multisialylated LacdiNAc structures as highly prostate cancer specific glycan signatures on PSA. Anal. Chem. 2019, 91, 2247–2254. [Google Scholar] [CrossRef]
- Haji-Ghassemi, O.; Gilbert, M.; Spence, J.; Schur, M.J.; Parker, M.J.; Jenkins, M.L.; Burke, J.E.; van Faassen, H.; Young, N.M.; Evans, S.V. Molecular Basis for Recognition of the Cancer Glycobiomarker, LacdiNAc (GalNAc[β1→4]GlcNAc), by Wisteria floribunda Agglutinin. J. Biol. Chem. 2016, 291, 24085–24095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olejnik, B.; Jarząb, A.; Kratz, E.M.; Zimmer, M.; Gamian, A.; Ferens-Sieczkowska, M. Terminal mannose residues in seminal plasma glycoproteins of infertile men compared to fertile donors. Int. J. Mol. Sci. 2015, 16, 14933–14950. [Google Scholar] [CrossRef] [Green Version]
- Malm, J.; Hellman, J.; Magnusson, H.; Laurell, C.-B.; Lilja, H. Isolation and Characterization of the Major Gel Proteins in Human Semen, Semenogen I and Semenogen II. Eur. J. Biochem. 1996, 238, 48–53. [Google Scholar] [CrossRef]
- Amaya, S.; Sasaki, M.; Watanabe, Y.; Tsui, W.M.; Tsuneyama, K.; Harada, K.; Nakanuma, Y. Expression of MUC1 and MUC2 and carbohydrate antigen Tn change during malignant transformation of biliary papillomatosis. Histopathology 2001, 38, 550–560. [Google Scholar] [CrossRef]
- Santos-Silva, F.; Fonseca, A.; Caffrey, T.; Carvalho, F.; Mesquita, P.; Reis, C.; Almeida, R.; David, L.; Hollingsworth, M.A. Thomsen-Friedenreich antigen expression in gastric carcinomas is associated with MUC1 mucin VNTR polymorphism. Glycobiology 2005, 15, 511–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hockl, P.F.; Wolosiuk, A.; Pérez-Sáez, J.M.; Bordoni, A.V.; Croci, D.O.; Toum-Terrones, Y.; Soler-Illia, G.J.; Rabinovich, G.A. Glyco-nano-oncology: Novel therapeutic opportunities by combining small and sweet. Pharm. Res. 2016, 109, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- GeneTools, version 4.03; Image Analysis Software; Syngene: Cambridge, UK, 2013.
- Merril, C.R.; Dunau, M.L.; Goldman, D. A rapid sensitive silver stain for polypeptides in polyacrylamide gels. Anal. Biochem. 1981, 110, 201–207. [Google Scholar] [CrossRef]
- Shevchenko, A.; Tomas, H.; Havlis, J.; Olsen, J.V.; Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006, 1, 2856–2860. [Google Scholar] [CrossRef] [PubMed]
- Steentoft, C.; Vakhrushev, S.Y.; Joshi, H.J.; Kong, Y.; Vester-Christensen, M.B.; Schjoldager, K.T.; Lavrsen, K.; Dabelsteen, S.; Pedersen, N.B.; Marcos-Silva, L.; et al. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 2013, 32, 1478–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Statistica, version 1.3.0; Software for Statistical Analysis StatSoft; Polska: Kraków, Poland, 2015.

| Fraction | Protein | Score | Matches | Molar Mass [Da] |
|---|---|---|---|---|
| 135 kDa | Mucin 6 | 12,688 | 271 | 263,159 |
| Aminopeptidase N | 8732 | 204 | 109,870 | |
| Serum albumin | 5456 | 111 | 71,317 | |
| Fibronectin | 5109 | 111 | 266,052 | |
| Lactotransferrin | 4699 | 96 | 80,014 | |
| Dipeptidyl peptidase 4 | 3143 | 75 | 88,907 | |
| Laminin subunit beta 2 | 2410 | 56 | 202,982 | |
| Beta mannosidase | 1174 | 31 | 101,800 | |
| 83 kDa | Lactotranferrin | 43,323 | 872 | 83,000 |
| Fibronectin | 14,382 | 309 | 266,052 | |
| Serum albumin | 4267 | 108 | 71,317 | |
| Serotransferrin | 2896 | 78 | 79,294 | |
| Mucin-6 | 2692 | 62 | 263,159 | |
| Polymeric immunoglobulin receptor | 1972 | 37 | 84,429 | |
| Alpha 2 macroglubulin | 1049 | 32 | 164,613 | |
| 70 kDa | Serum albumin | 38,789 | 946 | 71,317 |
| Galectin 3 binding protein | 2960 | 59 | 66,202 | |
| Lactotransferrin | 1668 | 44 | 80,014 | |
| Fibronectin | 1061 | 41 | 266,052 | |
| 53 kDa | Prostatic acid phosphatase | 12,624 | 443 | 44,880 |
| Alpha 1 antitrypsin | 3616 | 139 | 46,878 | |
| Fibronectin | 1528 | 46 | 266,052 | |
| Lactotransferrin | 1390 | 42 | 80,014 | |
| 32 kDa | Fibronectin | 9291 | 221 | 266,052 |
| Prostate specific antigen | 6171 | 222 | 29,293 | |
| Annexin | 4182 | 80 | 35,971 | |
| Clusterin | 2358 | 66 | 53,031 | |
| Prostatic acid phosphatase | 1381 | 35 | 44,880 | |
| Prosaposin | 1263 | 42 | 59,899 | |
| Serum albumin | 1098 | 42 | 71,317 | |
| Carboxypeptidase E | 1059 | 34 | 53,516 | |
| Cysteine rich secretory protein 1 | 1026 | 19 | 29,432 | |
| Purine nucleoside phosphorylase | 1010 | 23 | 32,325 | |
| 15 kDa | Prolactin inducible protein | 37,959 | 916 | 16,847 |
| Peptidyl prolyl cis trans isomerase A | 1145 | 33 | 18,229 | |
| Prostatic acid phosphatase | 1084 | 37 | 44,880 | |
| <15 kDa | Prolactin inducible protein precursor | 1823 | 39 | 16,847 |
| Semenogelin 1 preproprotein | 1134 | 27 | 52,157 | |
| Prolactin induced protein | 1090 | 23 | 9232 | |
| Semenogelin 2 precursor | 1023 | 21 | 65,519 |
| VVL | |||
|---|---|---|---|
| Protein | Score | Matches | Molar mass (Da) |
| Semenogelin-2 | 2997 | 135 | 65,497 |
| Prolactin-inducible protein | 1482 | 39 | 16,792 |
| Semenogelin-1 | 1278 | 49 | 52,146 |
| Lactotransferrin | 504 | 16 | 79,650 |
| Prostate-specific antigen | 86 | 2 | 29,183 |
| Fibronectin | 67 | 2 | 27,5047 |
| MPL | |||
| Protein | Score | Matches | Molar mass (Da) |
| Prolactin-inducible protein | 9346 | 158 | 16,792 |
| Semenogelin-2 | 6473 | 159 | 65,497 |
| Semenogelin-1 | 3895 | 80 | 52,146 |
| Fibronectin | 1471 | 28 | 275,047 |
| Lactotransferrin | 1440 | 29 | 79,650 |
| Prostate-specific antigen | 809 | 21 | 29,183 |
| Aminopeptidase N | 758 | 16 | 109,793 |
| Prostatic acid phosphatase | 539 | 11 | 44,813 |
| Clusterin | 242 | 6 | 52,921 |
| Zinc-alpha-2-glycoprotein | 179 | 3 | 34,421 |
| Mucin-6 | 117 | 2 | 261,946 |
| Glycodelin | 97 | 2 | 29,244 |
| Group | Age (Years) | ||
|---|---|---|---|
| Mean ± SD | Median | Mode | |
| F | 34 ± 2.73 | 33 | 32 |
| N | 33 ± 3.69 | 32 | 32 |
| O | 35 ± 6.13 | 33 | 33 |
| A | 34 ± 4.72 | 32.5 | 32 |
| OA | 32 ± 3.15 | 31 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczykutowicz, J.; Tkaczuk-Włach, J.; Ferens-Sieczkowska, M. Glycoproteins Presenting Galactose and N-Acetylgalactosamine in Human Seminal Plasma as Potential Players Involved in Immune Modulation in the Fertilization Process. Int. J. Mol. Sci. 2021, 22, 7331. https://doi.org/10.3390/ijms22147331
Szczykutowicz J, Tkaczuk-Włach J, Ferens-Sieczkowska M. Glycoproteins Presenting Galactose and N-Acetylgalactosamine in Human Seminal Plasma as Potential Players Involved in Immune Modulation in the Fertilization Process. International Journal of Molecular Sciences. 2021; 22(14):7331. https://doi.org/10.3390/ijms22147331
Chicago/Turabian StyleSzczykutowicz, Justyna, Joanna Tkaczuk-Włach, and Mirosława Ferens-Sieczkowska. 2021. "Glycoproteins Presenting Galactose and N-Acetylgalactosamine in Human Seminal Plasma as Potential Players Involved in Immune Modulation in the Fertilization Process" International Journal of Molecular Sciences 22, no. 14: 7331. https://doi.org/10.3390/ijms22147331
APA StyleSzczykutowicz, J., Tkaczuk-Włach, J., & Ferens-Sieczkowska, M. (2021). Glycoproteins Presenting Galactose and N-Acetylgalactosamine in Human Seminal Plasma as Potential Players Involved in Immune Modulation in the Fertilization Process. International Journal of Molecular Sciences, 22(14), 7331. https://doi.org/10.3390/ijms22147331