Glucocorticoids, Stress and Delta-9 Tetrahydrocannabinol (THC) during Early Embryonic Development
Abstract
:1. Introduction
1.1. Trends in Cannabis Use
1.2. The Endocannabinoid System, Stress Signaling, and Glucocorticoids in Target Organs
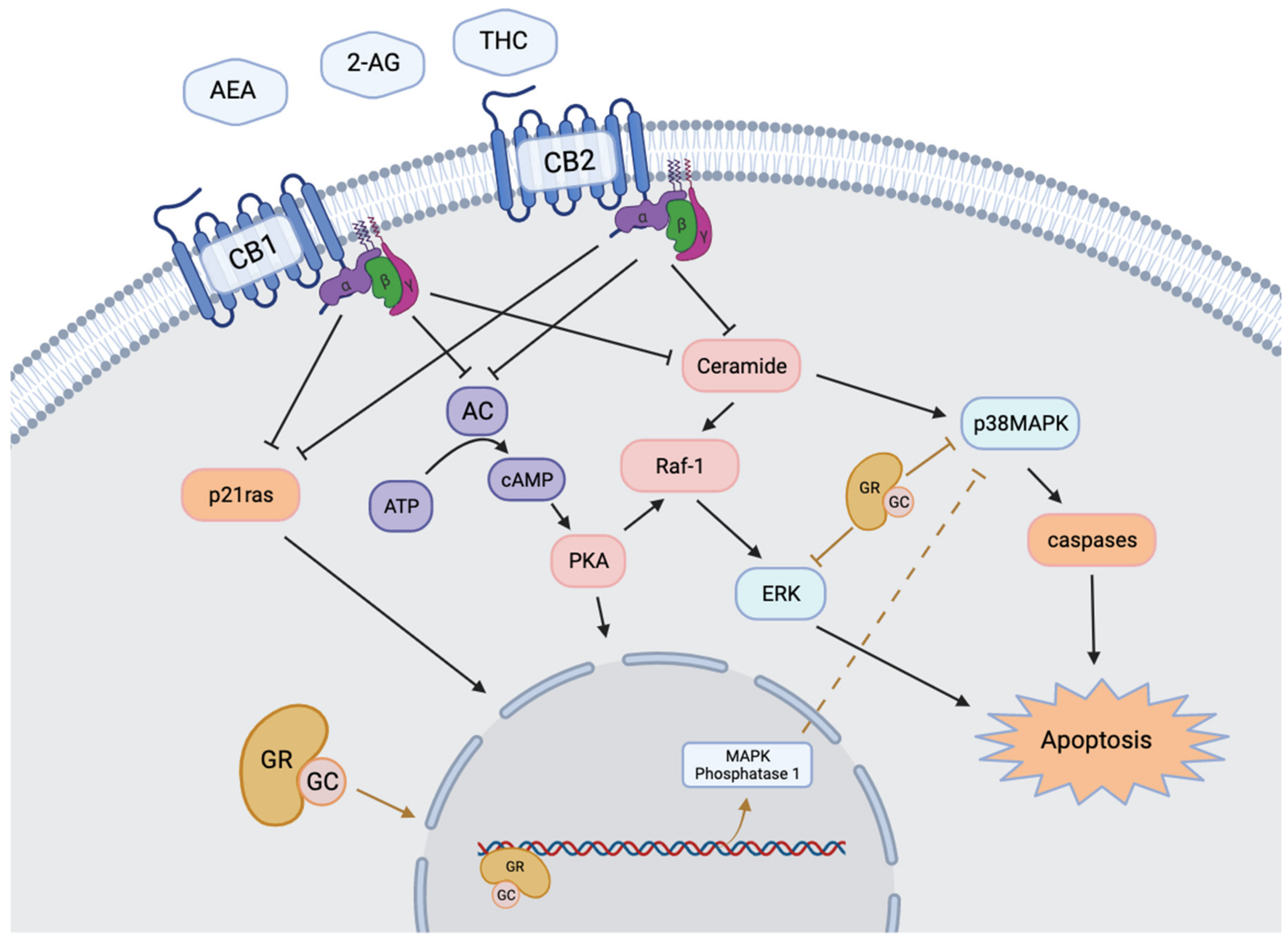
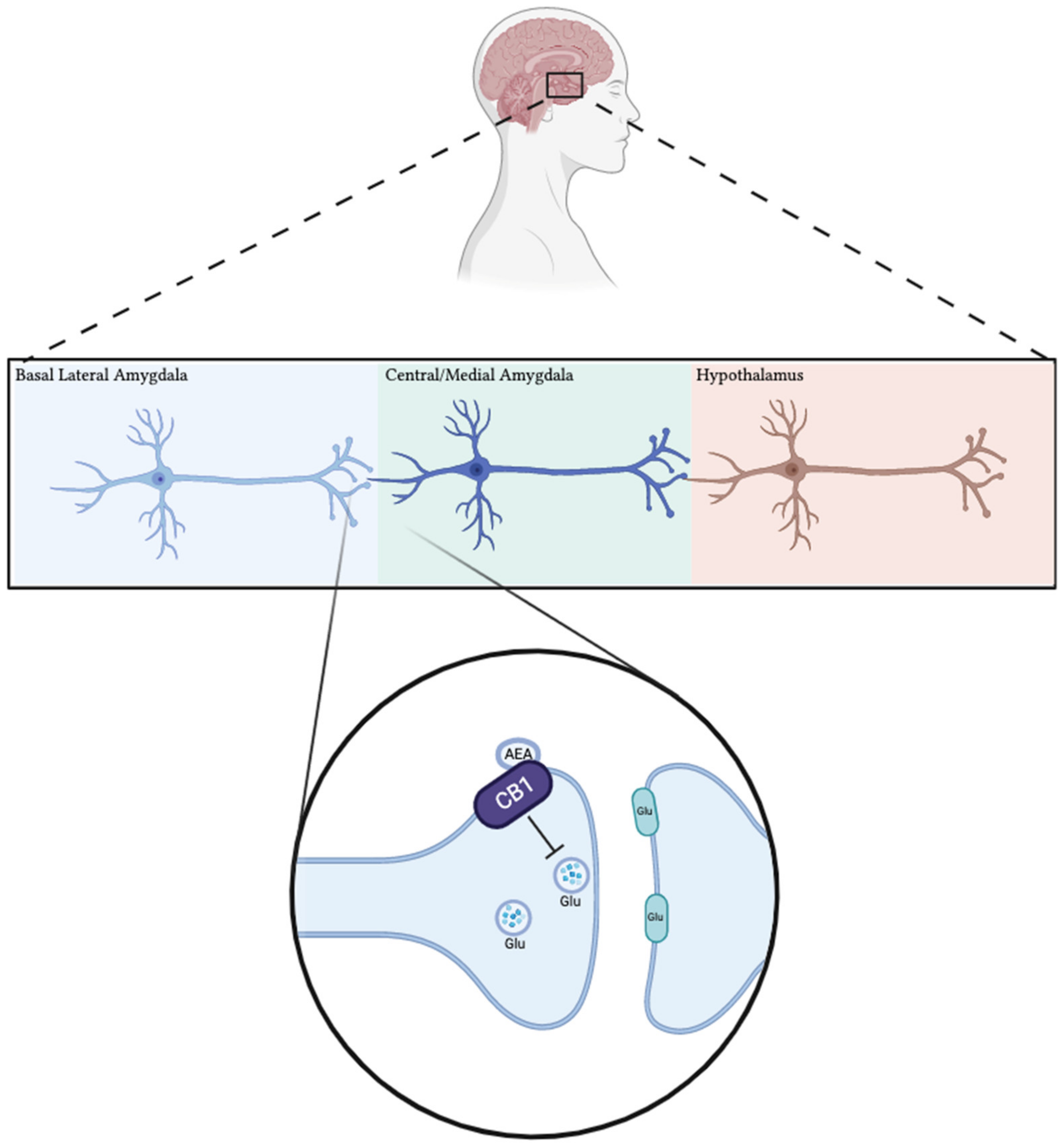
1.3. Animal Models
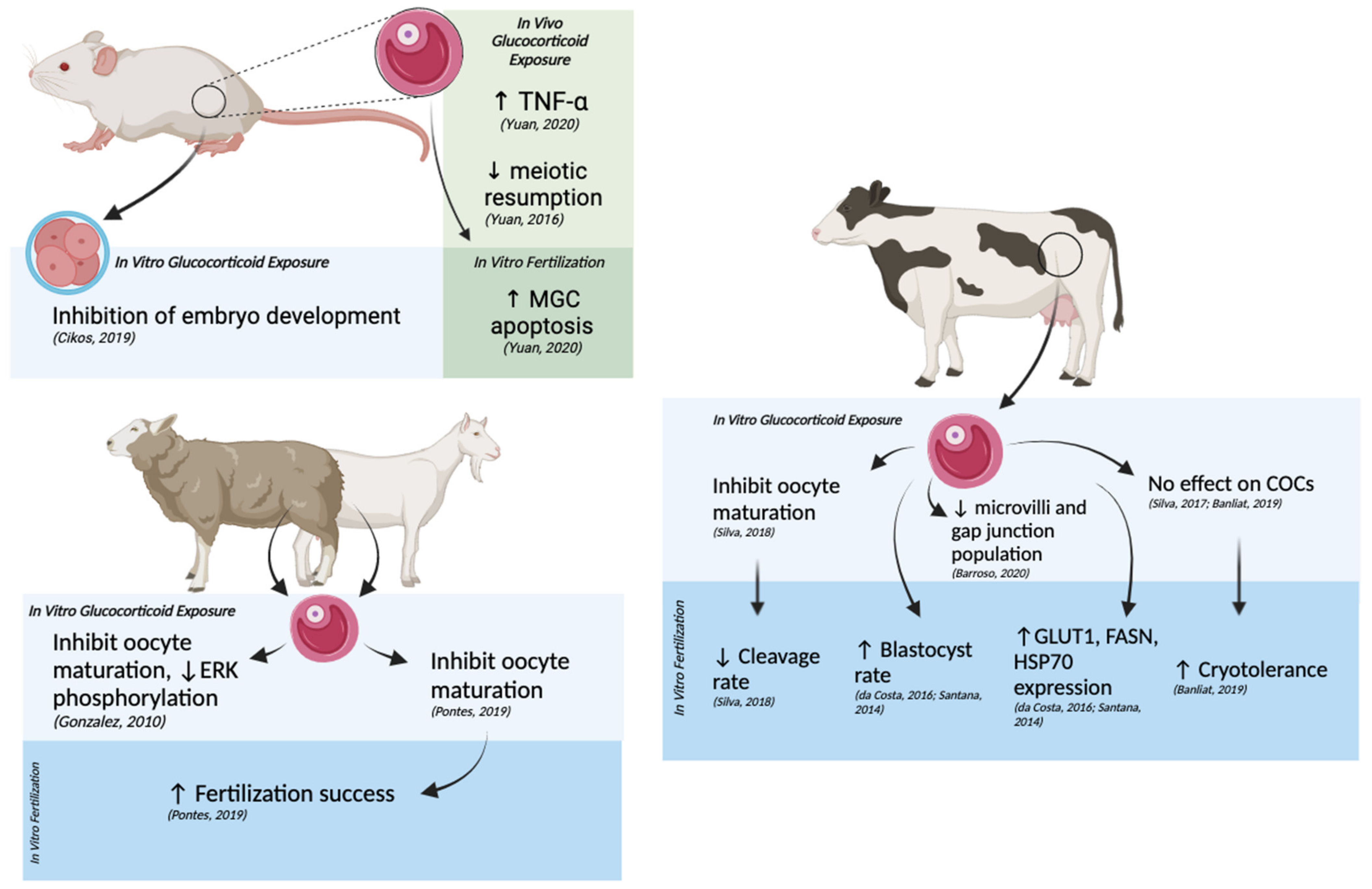
2. Impacts of Molecular Stress on Oogenesis and Early Embryonic Development
2.1. The Effects of Glucocorticoid Exposure on Oocyte Maturation and Early Embryonic Development
2.2. Effects of Glucocorticoids on Apoptotic Pathways and Cell Viability during Occyte Maturation and Early Embryo Development
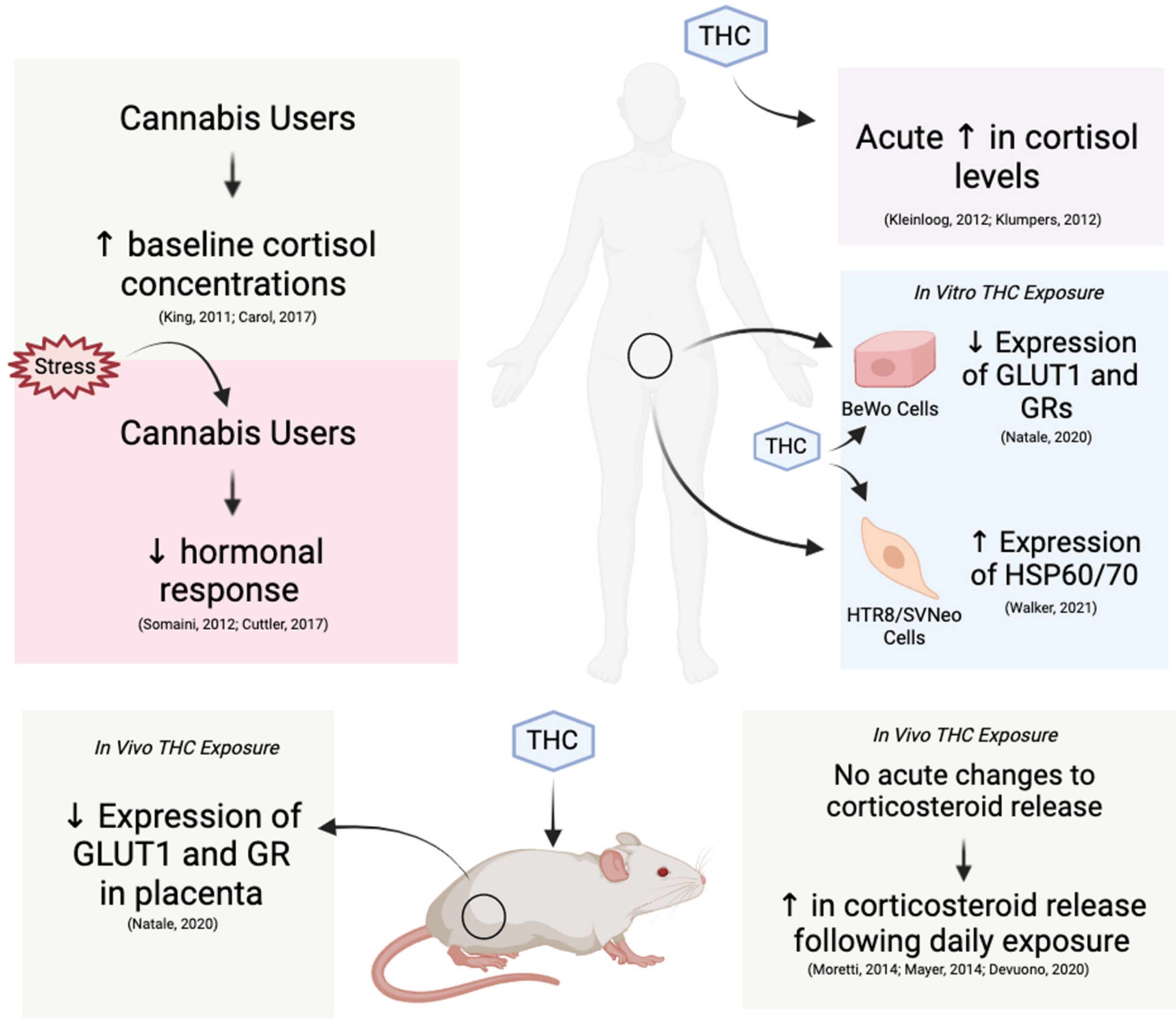
3. The Effects of THC on Molecular Stress Levels
3.1. Influence of THC on Physiological Glucocorticoid Levels and Sensitivity
3.2. Effects of THC on Glucocorticoid Receptors and Downstream Gene Expression
4. Conclusions: Potential Interactions between Glucocorticoids and THC at the Systemic, Cellular, and Molecular Levels
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ilnitsky, S.; van Uum, S. Marijuana and Fertility. Can. Med. Assoc. J. 2019, 191, E638. [Google Scholar] [CrossRef] [Green Version]
- National Institute on Drug Abuse. National Survey on Drug Use and Health: Trends in Prevalence of Various Drugs for Ages 12 or Older, Ages 12 to 17, Ages 18 to 25, and Ages 26 or Older; 2015–2017 (in Percent). 2018. Available online: https://www.samhsa.gov/data/data-we-collect/nsduh-national-survey-drug-use-and-health (accessed on 16 May 2021).
- Statistics Canada. Table 13-10-0383-01- Prevalence of Cannabis Use in the Past Three Months, Self-Reported. 2021. Available online: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310038301 (accessed on 20 May 2021).
- El Sholy, M.; Foster, S.; Gon, C.; Chandra, S.; Church, J. Changes in Cannabis Potency over the Last Two Decades (1995–2014): Analysis of Current Data in the United States. Biol. Psychiatry 2016, 79, 813–819. [Google Scholar] [CrossRef] [Green Version]
- Ewing, A.C.; Schauer, G.L.; Grant-Lenzy, A.M.; Njai, R.; Coy, K.C.; Ko, J.Y. Current Marijuana Use among Women of Reproductive Age. Drug Alcohol Depend. 2020, 214, 108161. [Google Scholar] [CrossRef]
- Corsi, D.J.; Hsu, H.; Weiss, D.; Fell, D.B.; Walker, M. Trends and Correlates of Cannabis Use in Pregnancy: A Population-Based Study in Ontario, Canada from 2012 to 2017. Can. J. Public Health 2019, 110, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.Y.; Farr, S.; Tong, V.; Creanga, A.; Callaghan, W. Prevalence and Patterns of Marijuana Use among Pregnant and Nonpregnant Women of Reproductive Age. Am. J. Obstet. Gynecol. 2015, 213, 201. [Google Scholar] [CrossRef]
- Roberson, E.K.; Patrick, W.K.; Hurwitz, E.L. Marijuana Use and Maternal Experiences of Severe Nausea during Pregnancy in Hawai’i. Hawai’i J. Med. Public Health 2014, 73, 283–287. [Google Scholar]
- FDA (2004) MARINOL © (Dronabinol) Capsules, for Oral Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/018651s021lbl.pdf (accessed on 20 June 2021).
- Childs, E.; Lutz, J.A.; de Wit, H. Dose-Related Effects of Delta-9-THC on Emotional Responses to Acute Psychosocial Stress. Drug Alcohol Depend. 2017, 177, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, S.; Rapino, C.; Di Nisio, V.; Rossi, G.; Maccarrone, M. The (Endo)Cannabinoid Signaling in Female Reproduction: What Are the Latest Advances? Prog. Lipid Res. 2020, 77, 101019. [Google Scholar] [CrossRef]
- Da Silva, F.M. Effect of Cortisol on Bovine Oocytes Maturation and Further Embryonic Development after In Vitro Fertilization. Biomed. J. Sci. Tech. Res. 2018, 10, 8029–8034. [Google Scholar] [CrossRef]
- López-Cardona, A.P.; Sánchez-Calabuig, M.J.; Beltran-Breña, P.; Agirregoitia, N.; Rizos, D.; Agirregoitia, E.; Gutierrez-Adán, A. Exocannabinoids Effect on in Vitro Bovine Oocyte Maturation via Activation of AKT and ERK1/2. Reproduction 2016, 152, 603–612. [Google Scholar] [CrossRef] [Green Version]
- López-Cardona, A.P.; Pérez-Cerezales, S.; Fernández-González, R.; Laguna-Barraza, R.; Pericuesta, E.; Agirregoitia, N.; Gutiérrez-Adán, A.; Agirregoitia, E. CB1 Cannabinoid Receptor Drives Oocyte Maturation and Embryo Development via PI3K/Akt and MAPK Pathways. FASEB J. 2017, 31, 3372–3382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, R.; Ruiz-León, Y.; Gomendio, M.; Roldan, E.R.S. The Effect of Glucocorticoids on ERK-1/2 Phosphorylation during Maturation of Lamb Oocytes and Their Subsequent Fertilization and Cleavage Ability in Vitro. Reprod. Toxicol. 2010, 29, 198–205. [Google Scholar] [CrossRef]
- Simerman, A.A.; Hill, D.L.; Grogan, T.R.; Elashoff, D.; Clarke, N.J.; Goldstein, E.H.; Manrriquez, A.N.; Chazenbalk, G.D.; Dumesic, D.A. Intrafollicular Cortisol Levels Inversely Correlate with Cumulus Cell Lipid Content as a Possible Energy Source during Oocyte Meiotic Resumption in Women Undergoing Ovarian Stimulation for in Vitro Fertilization. Fertil. Steril. 2015, 103, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Pontes, J.T.; Maside, C.; Lima, L.F.; Magalhães-Padilha, D.M.; Padilha, R.T.; Matos, M.H.T.; Figueiredo, J.R.; Campello, C.C. Immunolocalization for Glucocorticoid Receptor and Effect of Cortisol on in Vitro Development of Preantral Follicles. Vet. Anim. Sci. 2019, 7, 100060. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.J.; Zhi-Bin, L.; Xin-Yue, Z.; Guang-Yi, S.; Guo-Liang, W.; Ying-Qi, Z.; Min, Z.; Jing-He, T. Glucocorticoids Impair Oocyte Competence and Trigger Apoptosis of Ovarian Cells via Activating the TNF-α System. Reproduction 2020, 160, 129–140. [Google Scholar] [CrossRef]
- El-Talatini, M.R.; Taylor, A.H.; Elson, J.C.; Brown, L.; Davidson, A.C.; Konje, J.C. Localisation and Function of the Endocannabinoid System in the Human Ovary. PLoS ONE 2009, 4, e4579. [Google Scholar] [CrossRef] [Green Version]
- Gong, S.; Sun, G.Y.; Zhang, M.; Yuan, H.J.; Zhu, S.; Jiao, G.Z.; Luo, M.J.; Tan, J.H. Mechanisms for the Species Difference between Mouse and Pig Oocytes in Their Sensitivity to Glucorticoids. Biol. Reprod. 2017, 96, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Tetsuka, M.; Tanakadate, M. Activation of Hsd11b1 in the Bovine Cumulus-Oocyte Complex during Ivm and Ivf. Endocr. Connect. 2019, 8, 1029–1039. [Google Scholar] [CrossRef] [Green Version]
- Tetsuka, M.; Takagi, R.; Ambo, N.; Myat, T.S.; Zempo, Y.; Onuma, A. Glucocorticoid Metabolism in the Bovine Cumulus-Oocyte Complex Matured In Vitro. Reproduction 2016, 151, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Misner, M.J.; Taborek, A.; Dufour, J.; Sharifi, L.; Khokhar, J.Y.; Favetta, L.A. Effects of Delta-9 Tetrahydrocannabinol (THC) on Oocyte Competence and Early Embryonic Development. Front. Toxicol. 2021, 3, 1–18. [Google Scholar] [CrossRef]
- Walker, O.L.S.; Gurm, H.; Sharma, R.; Verma, N.; May, L.L. Delta-9-Tetrahydrocannabinol Inhibits Invasion of HTR8/SVneo Human Extravillous Trophoblast Cells and Negatively Impacts Mitochondrial Function. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef]
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.H.; Di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid Receptors and Their Ligands: Beyond CB1 and CB2. Pharmacol. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef] [Green Version]
- Newsom, R.J.; Garcia, R.J.; Stafford, J.; Osterlund, C.; O’Neill, C.E.; Day, H.E.W.; Campeau, S. Remote CB1 Receptor Antagonist Administration Reveals Multiple Sites of Tonic and Phasic Endocannabinoid Neuroendocrine Regulation. Psychoneuroendocrinology 2020, 113, 104549. [Google Scholar] [CrossRef] [PubMed]
- Hillard, C.J.; Beatka, M.; Sarvaideo, J. Endocannabinoid Signaling and the Hypothalamic-Pituitary-Adrenal Axis. Compr. Physiol. 2017, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Cecconi, S.; Rossi, G.; Oddi, S.; di Nisio, V.; Maccarrone, M. Role of Major Endocannabinoid-Binding Receptors during Mouse Oocyte Maturation. Int. J. Mol. Sci. 2019, 20, 2866. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Xie, H.; Dey, S.K. Endocannabinoid Signaling Directs Periimplantation Events. Am. Assoc. Pharm. Sci. J. 2006, 8, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Totorikaguena, L.; Olabarrieta, E.; López-Cardona, A.P.; Agirregoitia, N.; Agirregoitia, E. Tetrahydrocannabinol Modulates in Vitro Maturation of Oocytes and Improves the Blastocyst Rates after in Vitro Fertilization. Cell. Physiol. Biochem. 2019, 53, 439–452. [Google Scholar] [CrossRef]
- Walker, O.L.S.; Holloway, A.C.; Raha, S. The Role of the Endocannabinoid System in Female Reproductive Tissues. J. Ovarian Res. 2019, 12, 1–10. [Google Scholar] [CrossRef]
- Joy, J.E.; Watson, S.J.; John, A. Marijuana and Medicine: Assessing the Science Base; The National Academic Press: Washington, DC, USA, 1999; p. 288. ISBN 978-0-309-13290-9. [Google Scholar]
- Bénard, G.; Massa, F.; Puente, N.; Lourenço, J.; Bellocchio, L.; Soria-Gómez, E.; Matias, I.; Delamarre, A.; Metna-Laurent, M.; Cannich, A.; et al. Mitochondrial CB 1 Receptors Regulate Neuronal Energy Metabolism. Nat. Neurosci. 2012, 15, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Walker, O.S.; Ragos, R.; Gurm, H.; Lapierre, M.; May, L.L.; Raha, S. Delta-9-Tetrahydrocannabinol Disrupts Mitochondrial Function and Attenuates Syncytialization in Human Placental BeWo Cells. Physiol. Rep. 2020, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lojpur, T.; Easton, Z.; Raez-Villanueva, S.; Laviolette, S.; Holloway, A.C.; Hardy, D.B. Δ9-Tetrahydrocannabinol Leads to Endoplasmic Reticulum Stress and Mitochondrial Dysfunction in Human BeWo Trophoblasts. Reprod. Toxicol. 2019, 87, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Bian, Y.; He, Q.; Yao, J.; Zhu, J.; Wu, J.; Wang, K.; Duan, T. Suppression of STAT3 Signaling by Δ 9 -Tetrahydrocannabinol (THC) Induces Trophoblast Dysfunction. Cell Physiol. Biochem. 2017, 42, 537–550. [Google Scholar] [CrossRef]
- Evanson, N.K.; Tasker, J.G.; Hill, M.N.; Hillard, C.J.; Herman, J.P. Fast Feedback Inhibition of the HPA Axis by Glucocorticoids Is Mediated by Endocannabinoid Signaling. Endocrinology 2010, 151, 4811–4819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeVuono, M.V.; la Caprara, O.; Sullivan, M.T.; Bath, A.; Petrie, G.N.; Limebeer, C.L.; Rock, E.M.; Hill, M.N.; Parker, L.A. Role of the Stress Response and the Endocannabinoid System in Δ9-Tetrahydrocannabinol (THC)-Induced Nausea. Psychopharmacology 2020, 237, 2187–2199. [Google Scholar] [CrossRef]
- Hill, M.N.; Tasker, J.G. Endocannabinoid Signaling, Glucocorticoid-Mediated Negative Feedback, and Regulation of the Hypothalamic-Pituitary-Adrenal Axis. Neuroscience 2012, 204, 5–16. [Google Scholar] [CrossRef] [Green Version]
- King, G.R.; Ernst, T.; Deng, W.; Stenger, A.; Gonzales, R.M.K.; Nakama, H.; Chang, L. Altered Brain Activation during Visuomotor Integration in Chronic Active Cannabis Users: Relationship to Cortisol Levels. J. Neurosci. 2011, 31, 17923–17931. [Google Scholar] [CrossRef] [Green Version]
- Ranganathan, M.; Braley, G.; Pittman, B.; Cooper, T.; Perry, E.; Krystal, J.; D’Souza, D.C. The Effects of Cannabinoids on Serum Cortisol and Prolactin in Humans. Psychopharmacology 2009, 203, 737–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cservenka, A.; Lahanas, S.; Dotson-Bossert, J. Marijuana Use and Hypothalamic Pituitary-Adrenal Axis Functioning in Humans. Front. Psychiatry 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeVuono, M.V.; Hrelja, K.M.; Sabaziotis, L.; Rajna, A.; Rock, E.M.; Limebeer, C.L.; Mutch, D.M.; Parker, L.A. Conditioned Gaping Produced by High Dose Δ9-Tetrahydracannabinol: Dysregulation of the Hypothalamic Endocannabinoid System. Neuropharmacology 2018, 141, 272–282. [Google Scholar] [CrossRef]
- Eldridge, J.C.; Murphy, L.L.; Landneid, P.W. Cannabinoids and the Hippocampal Glucocorticoid Receptor: Recent Findings and Possible Significance. Steroids 1991, 56, 226–231. [Google Scholar] [CrossRef]
- Paronis, C.A.; Nikas, S.P.; Shukla, V.G.; Makriyannis, A. Δ9-Tetrahydrocannabinol Acts as a Partial Agonist/Antagonist in Mice. Behav. Pharmacol. 2012, 23, 802–805. [Google Scholar] [CrossRef] [Green Version]
- Kearn, C.S.; Greenberg, M.J.; DiCamelli, R.; Kurzawa, K.; Hillard, C.J. Relationships between Ligand Affinities for the Cerebellar Cannabinoid Receptor CB1 and the Induction of GDP/GTP Exchange. J. Neurochem. 1999, 72, 2379–2387. [Google Scholar] [CrossRef] [Green Version]
- Tai, S.; Hyatt, W.S.; Gu, C.; Franks, L.N.; Vasiljevik, T.; Brents, L.K.; Prather, P.L.; Fantegrossi, W.E. Repeated Administration of Phytocannabinoid Δ9-THC or Synthetic Cannabinoids JWH-018 and JWH-073 Induces Tolerance to Hypothermia but Not Locomotor Suppression in Mice, and Reduces CB1 Receptor Expression and Function in a Brain Region-Specific Manner. Pharmacol. Res. 2015, 102, 22–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joëls, M.; Angela Sarabdjitsingh, R.; Karst, H. Unraveling the Time Domains of Corticosteroid Hormone Influences on Brain Activity: Rapid, Slow, and Chronic Modes. Pharmacol. Rev. 2012, 64, 901–938. [Google Scholar] [CrossRef] [PubMed]
- Natale, B.V.; Gustin, K.N.; Lee, K.; Holloway, A.C.; Laviolette, S.R.; Natale, D.R.C.; Hardy, D.B. Δ9-Tetrahydrocannabinol Exposure During Rat Pregnancy Leads To Symmetrical Fetal Growth Restriction and Labyrinth-Specific Vascular Defects in the Placenta. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ravisankar, S.; Brooks, K.E.; Murphy, M.J.; Redmayne, N.; Ryu, J.; Kinchen, J.M.; Chavez, S.L.; Hennebold, J.D. Metabolomics Analysis of Follicular Fluid Coupled with Oocyte Aspiration Reveals Importance of Glucocorticoids in Primate Periovulatory Follicle Competency. Sci. Rep. 2021, 11, 1–18. [Google Scholar] [CrossRef]
- Tetsuka, M.; Nishimoto, H.; Miyamoto, A.; Okuda, K.; Hamano, S. Gene Expression of 11β-HSD and Glucocorticoid Receptor in the Bovine (Bos Taurus) Follicle during Follicular Maturation and Atresia: The Role of Follicular Stimulating Hormone. J. Reprod. Dev. 2010, 56, 616–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Čikoš, Š.; Babeïová, J.; Špirková, A.; Burkuš, J.; Kovaříková, V.; Šefčíková, Z.; Fabian, D.; Koppel, J. Glucocorticoid Receptor Isoforms and Effects of Glucocorticoids in Ovulated Mouse Oocytes and Preimplantation Embryos. Biol. Reprod. 2019, 100, 351–364. [Google Scholar] [CrossRef]
- Oakley, R.H.; Cidlowski, J.A. The Biology of the Glucocorticoid Receptor: New Signaling Mechanisms in Health and Disease. J. Allergy Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef] [Green Version]
- Da Costa, N.N.; Brito, K.N.L.; Santana, P.d.P.B.; da Cordeiro, M.S.; Silva, T.V.G.; Santos, A.X.; do Ramos, P.C.; Santos, S.D.; King, W.A.; Miranda, M.S.; et al. Effect of Cortisol on Bovine Oocyte Maturation and Embryo Development in Vitro. Theriogenology 2016, 85, 323–329. [Google Scholar] [CrossRef]
- Misner, M. The Effects of Delta-9 Tetrahydrocannabinol (THC) on Oocyte Competence and Early Embryonic Development. Master’s Thesis, University of Guelph, Guelph, ON, Canada, 2020. [Google Scholar]
- Li, Q.N.; Li, L.; Hou, G.; Wang, Z.B.; Hou, Y.; Liu, Z.H.; Schatten, H.; Sun, Q.Y. Glucocorticoid Exposure Affects Female Fertility by Exerting Its Effect on the Uterus but Not on the Oocyte: Lessons from a Hypercortisolism Mouse Model. Hum. Reprod. 2018, 33, 2285–2294. [Google Scholar] [CrossRef]
- Costa, M.A.; Fonseca, B.M.; Mendes, A.; Braga, J.; Teixeira, N.A.; Correia-Da-Silva, G. The Endocannabinoid 2-Arachidonoylglycerol Dysregulates the Synthesis of Proteins by the Human Syncytiotrophoblast. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2016, 1861, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Qu, Y.; Li, J.; Cui, L.; Wang, Y.; Lin, J.; Wang, H. Cortisol Inhibits NF-ΚB and MAPK Pathways in LPS Activated Bovine Endometrial Epithelial Cells. Int. Immunopharmacol. 2018, 56, 71–77. [Google Scholar] [CrossRef]
- Charles Eldridge, J.; Landfield, P.W. Cannabinoid Interactions with Glucocorticoid Receptors in Rat Hippocampus. Brain Res. 1990, 534, 135–141. [Google Scholar] [CrossRef]
- Yuan, H.J.; Han, X.; He, N.; Wang, G.L.; Gong, S.; Lin, J.; Gao, M.; Tan, J.H. Glucocorticoids Impair Oocyte Developmental Potential by Triggering Apoptosis of Ovarian Cells via Activating the Fas System. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Intawicha, P.; Tsai, L.-K.; Yen, S.-Y.; Lo, N.-W.; Ju, J.-C. Nucleus, Cytoskeleton, and Mitogen-Activated Protein Kinase P38 Dynamics during In Vitro Maturation of Porcine Oocytes. Animals 2019, 9, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Domenico, E.; Todaro, F.; Rossi, G.; Dolci, S.; Geremia, R.; Rossi, P.; Grimaldi, P. Overactive Type 2 Cannabinoid Receptor Induces Meiosis in Fetal Gonads and Impairs Ovarian Reserve. Cell Death Dis. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Beker van Woudenberg, A.; Gröllers-Mulderij, M.; Snel, C.; Jeurissen, N.; Stierum, R.; Wolterbeek, A. The Bovine Oocyte in Vitro Maturation Model: A Potential Tool for Reproductive Toxicology Screening. Reprod. Toxicol. 2012, 34, 251–260. [Google Scholar] [CrossRef]
- Luciano, A.M.; Franciosi, F.; Lodde, V.; Corbani, D.; Lazzari, G.; Crotti, G.; Galli, C.; Pellizzer, C.; Bremer, S.; Weimer, M.; et al. Transferability and Inter-Laboratory Variability Assessment of the in Vitro Bovine Oocyte Maturation (IVM) Test within ReProTect. Reprod. Toxicol. 2010, 30, 81–88. [Google Scholar] [CrossRef]
- Santos, R.R.; Schoevers, E.J.; Roelen, B.A.J. Usefulness of Bovine and Porcine IVM/IVF Models for Reproductive Toxicology. Reprod. Biol. Endocrinol. 2014, 12, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Menezo, Y.J.; Francois, H. Mouse and Bovine Models for Human IVF. Reprod. Biomed. Online 2002, 4, 170–175. [Google Scholar] [CrossRef]
- Sylvestre, E.L.; Robert, C.; Pennetier, S.; Labrecque, R.; Gilbert, I.; Dufort, I.; Léveillé, M.C.; Sirard, M.A. Evolutionary Conservation of the Oocyte Transcriptome among Vertebrates and Its Implications for Understanding Human Reproductive Function. Mol. Hum. Reprod. 2013, 19, 369–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Z.; Sun, J.; Dong, H.; Luo, O.; Zheng, X.; Obergfell, C.; Tang, Y.; Bi, J.; O’Neill, R.; Ruan, Y.; et al. Transcriptional Profiles of Bovine in Vivo Pre-Implantation Development. BMC Genom. 2014, 15, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagavandoss, P.; Grimshaw, S. Temporal and Spatial Distribution of the Cannabinoid Receptors (CB 1, CB2) and Fatty Acid Amide Hydroxylase in the Rat Ovary. Anat. Rec. 2010, 293, 1425–1432. [Google Scholar] [CrossRef]
- Paria, B.C.; Das, S.K.; Dey, S.K. The Preimplantation Mouse Embryo Is a Target for Cannabinoid Ligand-Receptor Signaling. Proc. Natl. Acad. Sci. USA 1995, 92, 9460–9464. [Google Scholar] [CrossRef] [Green Version]
- Ernst, E.H.; Grøndahl, M.L.; Grund, S.; Hardy, K.; Heuck, A.; Sunde, L.; Franks, S.; Andersen, C.Y.; Villesen, P.; Lykke-Hartmann, K. Dormancy and Activation of Human Oocytes from Primordial and Primary Follicles: Molecular Clues to Oocyte Regulation. Hum. Reprod. 2017, 32, 1684–1700. [Google Scholar] [CrossRef] [PubMed]
- Jamnongjit, M.; Hammes, S.R. Oocyte Maturation: The Coming of Age of a Germ Cell. Semin. Reprod. Med. 2005, 23, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Hoshino, Y. Updating the Markers for Oocyte Quality Evaluation: Intracellular Temperature as a New Index. Reprod. Med. Biol. 2018, 17, 434–441. [Google Scholar] [CrossRef] [Green Version]
- Hill, M.A. Embryology Oocyte Development. Available online: https://embryology.med.unsw.edu.au/embryology/index.php/Oocyte_Development (accessed on 21 June 2021).
- Sha, Q.Q.; Dai, X.X.; Dang, Y.; Tang, F.; Liu, J.; Zhang, Y.L.; Fan, H.Y. A MAPK Cascade Couples Maternal MRNA Translation and Degradation to Meiotic Cell Cycle Progression in Mouse Oocytes. Development 2017, 144, 452–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niakan, K.K.; Han, J.; Pedersen, R.A.; Simon, C.; Pera, R.A.R. Human Pre-Implantation Embryo Development. Development 2012, 139, 829–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krupinski, P.; Chickarmane, V.; Peterson, C. Simulating the Mammalian Blastocyst—Molecular and Mechanical Interactions Pattern the Embryo. PLoS Comput. Biol. 2011, 7, e1001128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.Q.; Denegre, J.M.; Wigglesworth, K.; Pendola, F.L.; O’Brien, M.J.; Eppig, J.J. Oocyte-Dependent Activation of Mitogen-Activated Protein Kinase (ERK1/2) in Cumulus Cells Is Required for the Maturation of the Mouse Oocyte-Cumulus Cell Complex. Dev. Biol. 2003, 263, 126–138. [Google Scholar] [CrossRef] [Green Version]
- Robker, R.L.; Hennebold, J.D.; Russell, D.L. Coordination of Ovulation and Oocyte Maturation: A Good Egg at the Right Time. Endocrinology 2018, 159, 3209–3218. [Google Scholar] [CrossRef]
- Santana, P.P.B.; Carvalho, C.M.F.; da Costa, N.N.; Silva, T.V.G.; Ramos, P.C.A.; Cordeiro, M.S.; Santos, S.S.D.; Khayat, A.S.; Ohashi, O.M.; Miranda, M.S. Effect of Dexamethasone on Development of Invitro-Produced Bovine Embryos. Theriogenology 2014, 82, 10–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banliat, C.; Dubuisson, F.; Corbin, E.; Beurois, J.; Tomas, D.; Le Bourhis, D.; Salvetti, P.; Labas, V.; Mermillod, P.; Saint-Dizier, M. Intraoviductal Concentrations of Steroid Hormones during In Vitro Culture Changed Phospholipid Profiles and Cryotolerance of Bovine Embryos. Mol. Reprod. Dev. 2019, 86, 661–672. [Google Scholar] [CrossRef]
- Arena, R.; Bisogno, S.; Gasior, Ł.; Rudnicka, J.; Bernhardt, L.; Haaf, T.; Zacchini, F.; Bochenek, M.; Fic, K.; Bik, E.; et al. Lipid Droplets in Mammalian Eggs Are Utilized during Embryonic Diapause. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Leese, H.J. Quiet Please, Do Not Disturb: A Hypothesis of Embryo Metabolism and Viability. BioEssays 2002, 24, 845–849. [Google Scholar] [CrossRef]
- Hułas-Stasiak, M.; Dobrowolski, P.; Pawlikowska-Pawlęga, B.; Tomaszewska, E.; Muszyński, S. The Effects of Dexamethasone Administered during Pregnancy on the Postpartum Spiny Mouse Ovary. PLoS ONE 2017, 12, e0183528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.R.; Hong, K.; Choi, S.H.; Park, C.; Park, J.K.; Lee, J.; Bang, J.; Seol, D.W.; Lee, J.E.; Lee, D.R. Anti-Apoptotic Regulation Contributes to the Successful Nuclear Reprogramming Using Cryopreserved Oocytes. Stem Cell Rep. 2019, 12, 545–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, A.W.B.; Ribeiro, R.P.; Menezes, V.G.; Barberino, R.S.; Passos, J.R.S.; Dau, A.M.P.; Costa, J.J.N.; Melo, L.R.F.; Bezerra, F.T.G.; Donato, M.A.M.; et al. Expression of TNF-α System Members in Bovine Ovarian Follicles and the Effects of TNF-α or Dexamethasone on Preantral Follicle Survival, Development and Ultrastructure in Vitro. Anim. Reprod. Sci. 2017, 182, 56–68. [Google Scholar] [CrossRef]
- Barroso, P.A.A.; Paulino, L.R.F.M.; Silva, B.R.; Vasconcelos, G.L.; Gomes, D.S.; Lima Neto, M.F.; Silva, A.W.B.; Souza, A.L.P.; Donato, M.A.M.; Peixoto, C.A.; et al. Effects of Dexamethasone on Growth, Viability and Ultrastructure of Bovine Secondary Follicles Cultured in Vitro. Zygote 2020, 28, 504–510. [Google Scholar] [CrossRef]
- Williams, D.M. Clinical Pharmacology of Corticosteroids. Respir. Care 2018, 63, 655–670. [Google Scholar] [CrossRef] [Green Version]
- Vining, R.F.; McGinley, R.A.; Maksvytis, J.J.; Ho, K.Y. Salivary Cortisol: A Better Measure of Adrenal Cortical Function than Serum Cortisol. Ann. Clin. Biochem. 1983, 20, 329–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ash, H.; Smith, T.E.; Knight, S.; Buchanan-Smith, H.M. Measuring Physiological Stress in the Common Marmoset (Callithrix Jacchus): Validation of a Salivary Cortisol Collection and Assay Technique. Physiol. Behav. 2018, 185, 14–22. [Google Scholar] [CrossRef]
- Ronaldson, A.; Carvalho, L.A.; Kostich, K.; Lazzarino, A.I.; Urbanova, L.; Steptoe, A. The Effects of Six-Day SSRI Administration on Diurnal Cortisol Secretion in Healthy Volunteers. Psychopharmacology 2018, 235, 3415–3422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moretti, S.; Castelli, M.; Franchi, S.; Raggi, M.A.; Mercolini, L.; Protti, M.; Somaini, L.; Panerai, A.E.; Sacerdote, P. Δ9-Tetrahydrocannabinol-Induced Anti-Inflammatory Responses in Adolescent Mice Switch to Proinflammatory in Adulthood. J. Leukoc. Biol. 2014, 96, 523–534. [Google Scholar] [CrossRef]
- Mayer, T.A.; Matar, M.A.; Kaplan, Z.; Zohar, J.; Cohen, H. Blunting of the HPA-Axis Underlies the Lack of Preventive Efficacy of Early Post-Stressor Single-Dose Delta-9-Tetrahydrocannabinol (THC). Pharmacol. Biochem. Behav. 2014, 122, 307–318. [Google Scholar] [CrossRef]
- Cuttler, C.; Spradlin, A.; Nusbaum, A.T.; Whitney, P.; Hinson, J.M.; McLaughlin, R.J. Blunted Stress Reactivity in Chronic Cannabis Users. Psychopharmacology 2017, 234, 2299–2309. [Google Scholar] [CrossRef] [PubMed]
- Gougeon, A. Human Ovarian Follicular Development: From Activation of Resting Follicles to Preovulatory Maturation. Ann. D’endocrinologie 2010, 71, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Kleinloog, D.; Liem-Moolenaar, M.; Jacobs, G.; Klaassen, E.; de Kam, M.; Hijman, R.; van Gerven, J. Does Olanzapine Inhibit the Psychomimetic Effects of Δ9- Tetrahydrocannabinol? J. Psychopharmacol. 2012, 26, 1307–1316. [Google Scholar] [CrossRef]
- Klumpers, L.E.; Cole, D.M.; Khalili-Mahani, N.; Soeter, R.P.; te Beek, E.T.; Rombouts, S.A.R.B.; van Gerven, J.M.A. Manipulating Brain Connectivity with Δ9-Tetrahydrocannabinol: A Pharmacological Resting State FMRI Study. NeuroImage 2012, 63, 1701–1711. [Google Scholar] [CrossRef]
- Carol, E.E.; Spencer, R.L.; Mittal, V.A. The Relationship between Cannabis Use and Cortisol Levels in Youth at Ultra High-Risk for Psychosis. Psychoneuroendocrinology 2017, 83, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Somaini, L.; Manfredini, M.; Amore, M.; Zaimovic, A.; Raggi, M.A.; Leonardi, C.; Gerra, M.L.; Donnini, C.; Gerra, G. Psychobiological Responses to Unpleasant Emotions in Cannabis Users. Eur. Arch. Psychiatry Clin. Neurosci. 2012, 262, 47–57. [Google Scholar] [CrossRef]
- Pasch, L.A.; Holley, S.R.; Bleil, M.E.; Shehab, D.; Katz, P.P.; Adler, N.E. Addressing the Needs of Fertility Treatment Patients and Their Partners: Are They Informed of and Do They Receive Mental Health Services? Fertil. Steril. 2016, 106, 209–215.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, S.; Janicki-Deverts, D.; Doyle, W.J.; Miller, G.E.; Frank, E.; Rabin, B.S.; Turner, R.B. Chronic Stress, Glucocorticoid Receptor Resistance, Inflammation, and Disease Risk. Proc. Natl. Acad. Sci. USA 2012, 109, 5995–5999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, M.A. Embryology Trophoblast. Available online: https://embryology.med.unsw.edu.au/embryology/index.php/Trophoblast (accessed on 21 June 2021).
- Kurashova, N.A.; Madaeva, I.M.; Kolesnikova, L.I. Expression of HSP70 Heat-Shock Proteins under Oxidative Stress. Adv. Gerontol. 2020, 10, 20–25. [Google Scholar] [CrossRef]
- Pockley, A.G.; Shepherd, J.; Corton, J.M. Detection of Heat Shock Protein 70 (Hsp70) and Anti-Hsp70 Antibodies in the Serum of Normal Individuals. Immunol. Investig. 1998, 27, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Nixon, B.; Bromfield, E.G.; Dun, M.D.; Redgrove, K.A.; McLaughlin, E.A.; Aitken, R.J. The Role of the Molecular Chaperone Heat Shock Protein A2 (HSPA2) in Regulating Human Sperm-Egg Recognition. Asian J. Androl. 2015, 17, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Torres-Osorio, V.; Urrego, R.; Echeverri-Zuluaga, J.J.; López-Herrera, A. Oxidative Stress and Antioxidant Use during in Vitro Mammal Embryo Production. Review. Rev. Mex. Cienc. Pecu. 2019, 10, 433–459. [Google Scholar] [CrossRef]
- Vandaele, L.; Thys, M.; Bijttebier, J.; van Langendonckt, A.; Donnay, I.; Maes, D.; Meyer, E.; van Soom, A. Short-Term Exposure to Hydrogen Peroxide during Oocyte Maturation Improves Bovine Embryo Development. Reproduction 2010, 139, 505–511. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Lin, L.; Schmidt, M.; Bøgh, I.B.; Kragh, P.M.; Sørensen, C.B.; Li, J.; Purup, S.; Pribenszky, C.; Molnár, M.; et al. High Hydrostatic Pressure Treatment of Porcine Oocytes before Handmade Cloning Improves Developmental Competence and Cryosurvival. Cloning Stem Cells 2008, 10, 325–330. [Google Scholar] [CrossRef]
- Richani, D.; Gilchrist, R.B. The Epidermal Growth Factor Network: Role in Oocyte Growth, Maturation and Developmental Competence. Hum. Reprod. Update 2018, 24, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Xiao, H.; Pi, J.; Zhang, H.; Pan, A.; Pu, Y.; Liang, Z.; Shen, J.; Du, J. EGFR Promotes the Proliferation of Quail Follicular Granulosa Cells through the MAPK/Extracellular Signal-Regulated Kinase (ERK) Signaling Pathway. Cell Cycle 2019, 18, 2742–2756. [Google Scholar] [CrossRef] [PubMed]
- Yitzhak, R.; Judith, E.; Nava, D. Sustained Activity of the EGF Receptor Is an Absolute Requisite for LH-Induced Oocyte Maturation and Cumulus Expansion. Mol. Endocrinol. 2010, 24, 402–411. [Google Scholar] [CrossRef] [Green Version]
- Cagnol, S.; Chambard, J.C. ERK and Cell Death: Mechanisms of ERK-Induced Cell Death—Apoptosis, Autophagy and Senescence. FEBS J. 2010, 277, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Tsoupras, A.; Zabetakis, I.; Demopoulos, C.A. Forty Years since the Structural Elucidation of Platelet-Activating Factor (PAF): Historical, Current, and Future Research Perspectives. Molecules 2019, 24, 4414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesalam, A.; Lee, K.L.; Khan, I.; Chowdhury, M.M.R.; Zhang, S.; Song, S.H.; Joo, M.D.; Lee, J.H.; Jin, J.I.; Kong, I.K. A Combination of Bovine Serum Albumin with Insulin-Transferrin-Sodium Selenite and/or Epidermal Growth Factor as Alternatives to Fetal Bovine Serum in Culture Medium Improves Bovine Embryo Quality and Trophoblast Invasion by Induction of Matrix Metallopro. Reprod. Fertil. Dev. 2019, 31, 333–346. [Google Scholar] [CrossRef]
- Herrick, J.R.; Greene-Ermisch, A.F.; Schoolcraft, W.B.; Krisher, R.L. Exogenous Growth Factors Do Not Affect the Development of Individually Cultured Murine Embryos. J. Assist. Reprod. Genet. 2018, 35, 523–531. [Google Scholar] [CrossRef]
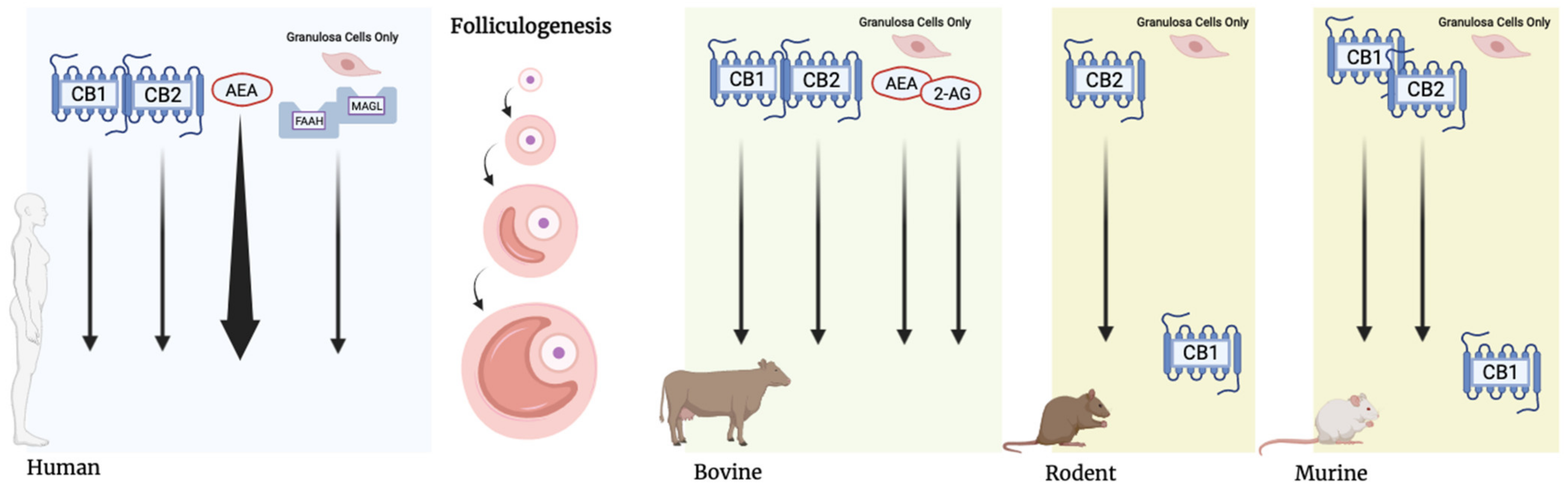
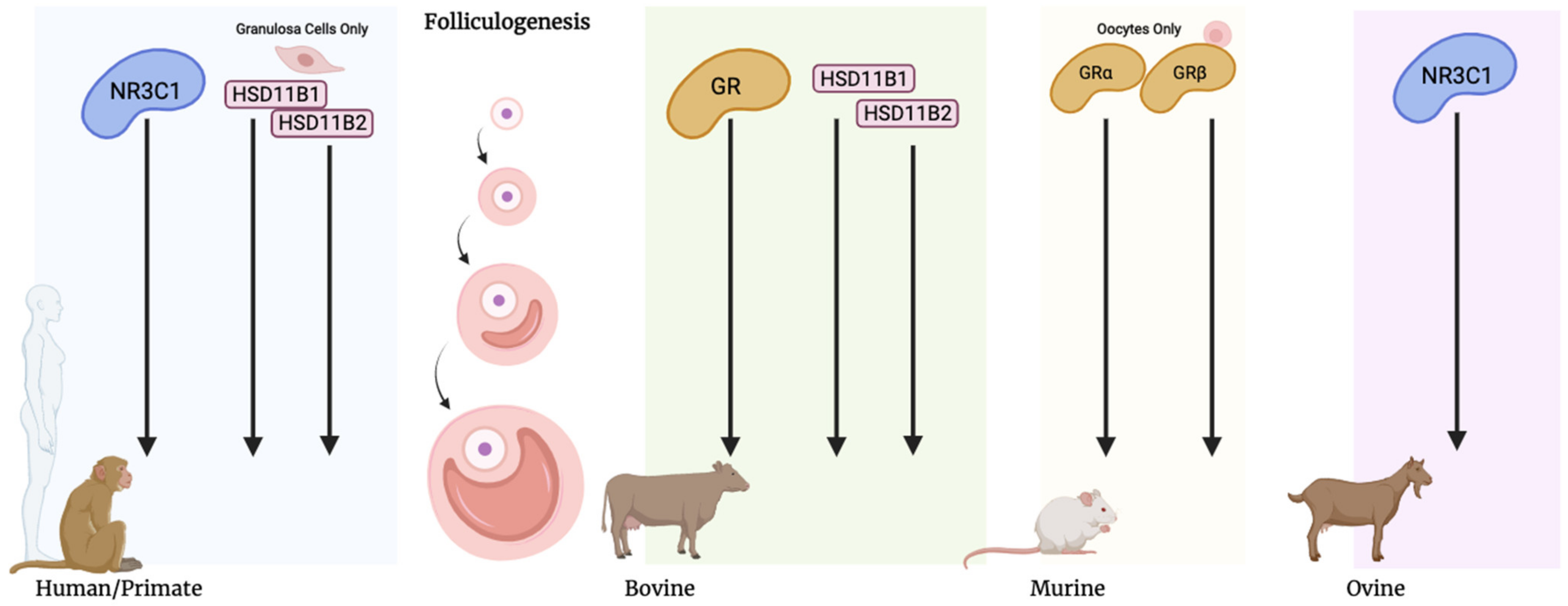
| System | Molecule | Function |
|---|---|---|
| Endocannabinoid System (ECS) | AEA (N-arachidonylethanolamine, anandamide) | Endocannabinoid |
| 2-AG (2-arachidonylglycerol) | Endocannabinoid | |
| CB1 | Cannabinoid receptor | |
| CB2 | Cannabinoid receptor | |
| FAAH (Fatty acid amide hydrolase) | Metabolize AEA into arachidonic acid and ethanolamine | |
| MAGL (Monoacylglycerol lipase) | Metabolize 2-AG into arachidonic acid and glycerol | |
| Stress Signaling | Cortisol | Endogenous stress hormone |
| Corticosterone | Endogenous stress hormone (murine only) | |
| Glucocorticoid Receptor | Cortisol receptor | |
| HSD11B1 (11β-Hydroxysteroid Dehydrogenase Type 1, cortisone reductase) | Convert biologically inactive cortisone into active cortisol | |
| HSD11B2 (11β-Hydroxysteroid Dehydrogenase Type 2) | Convert biologically active cortisol into inactive cortisone |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuzma-Hunt, A.G.; Truong, V.B.; Favetta, L.A. Glucocorticoids, Stress and Delta-9 Tetrahydrocannabinol (THC) during Early Embryonic Development. Int. J. Mol. Sci. 2021, 22, 7289. https://doi.org/10.3390/ijms22147289
Kuzma-Hunt AG, Truong VB, Favetta LA. Glucocorticoids, Stress and Delta-9 Tetrahydrocannabinol (THC) during Early Embryonic Development. International Journal of Molecular Sciences. 2021; 22(14):7289. https://doi.org/10.3390/ijms22147289
Chicago/Turabian StyleKuzma-Hunt, Alexander G., Vivien B. Truong, and Laura A. Favetta. 2021. "Glucocorticoids, Stress and Delta-9 Tetrahydrocannabinol (THC) during Early Embryonic Development" International Journal of Molecular Sciences 22, no. 14: 7289. https://doi.org/10.3390/ijms22147289
APA StyleKuzma-Hunt, A. G., Truong, V. B., & Favetta, L. A. (2021). Glucocorticoids, Stress and Delta-9 Tetrahydrocannabinol (THC) during Early Embryonic Development. International Journal of Molecular Sciences, 22(14), 7289. https://doi.org/10.3390/ijms22147289







