Structure and Function of Piezophilic Hyperthermophilic Pyrococcus yayanosii pApase
Abstract
1. Introduction
2. Results
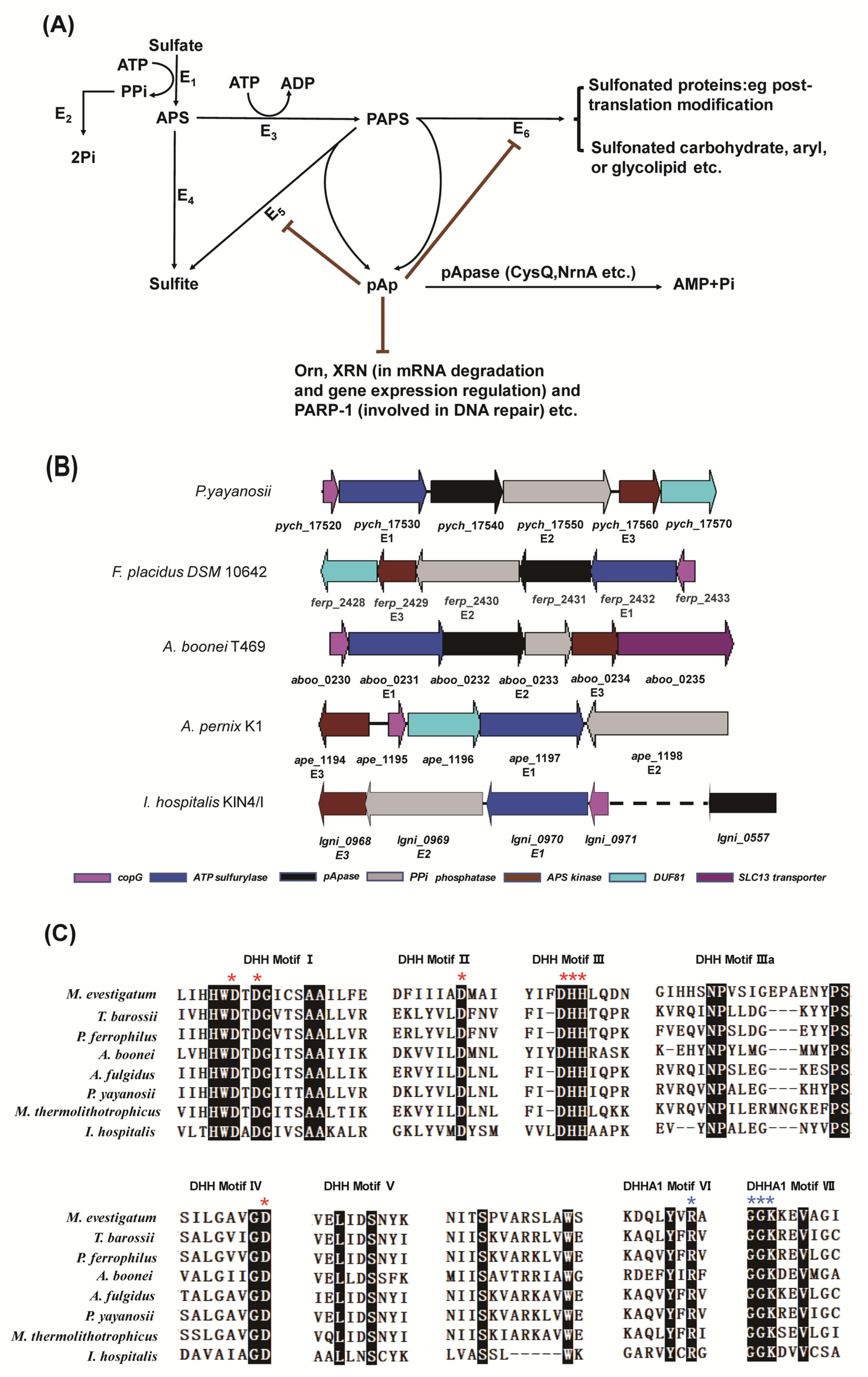
2.1. The Gene Cluster for Assimilatory Sulfate Reduction
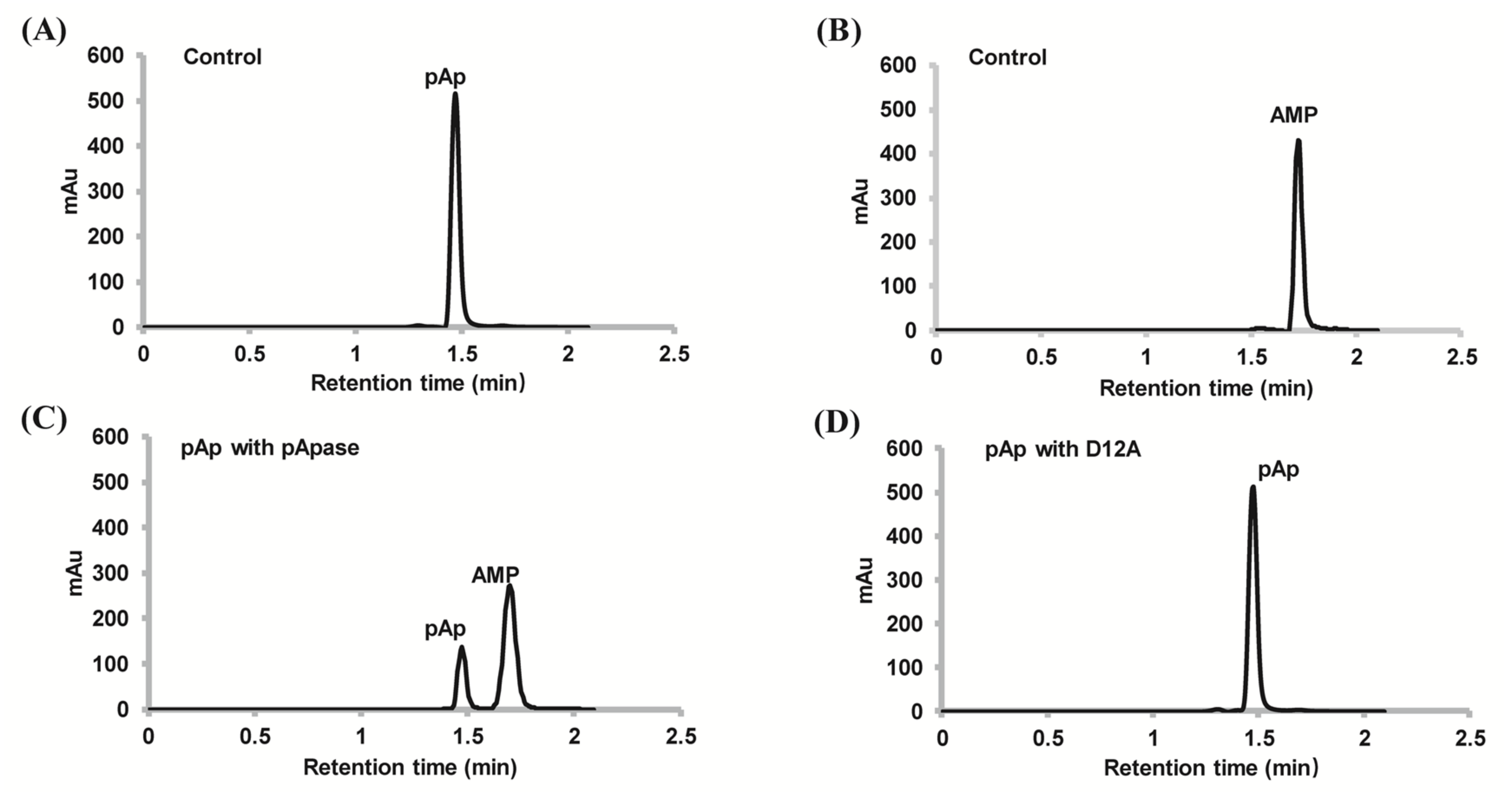
2.2. PyapApase Can Hydrolyze pAp In Vitro
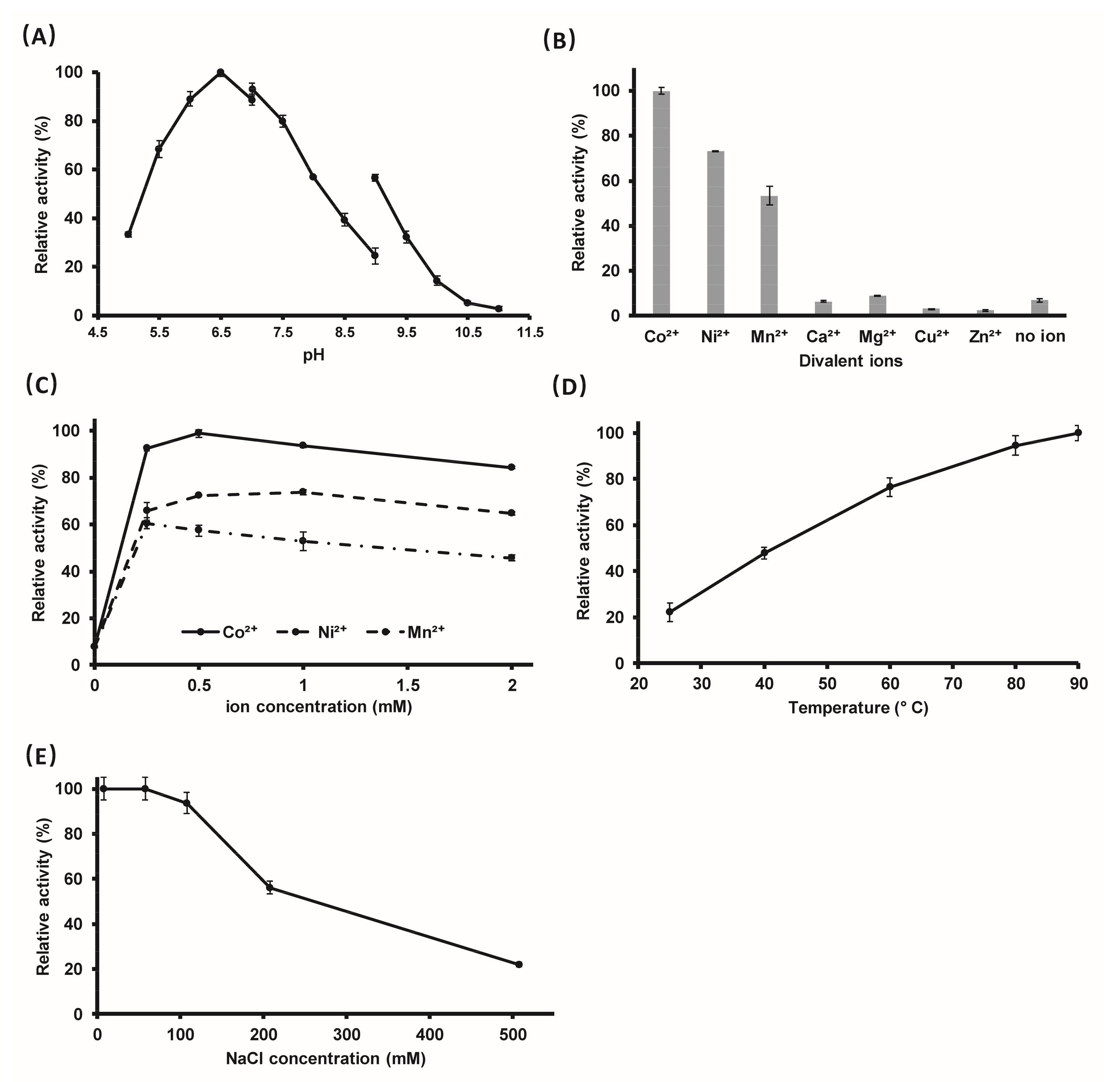
2.3. The Optimal Reaction Conditions
2.4. Substrate Specificity of PyapApase Is High
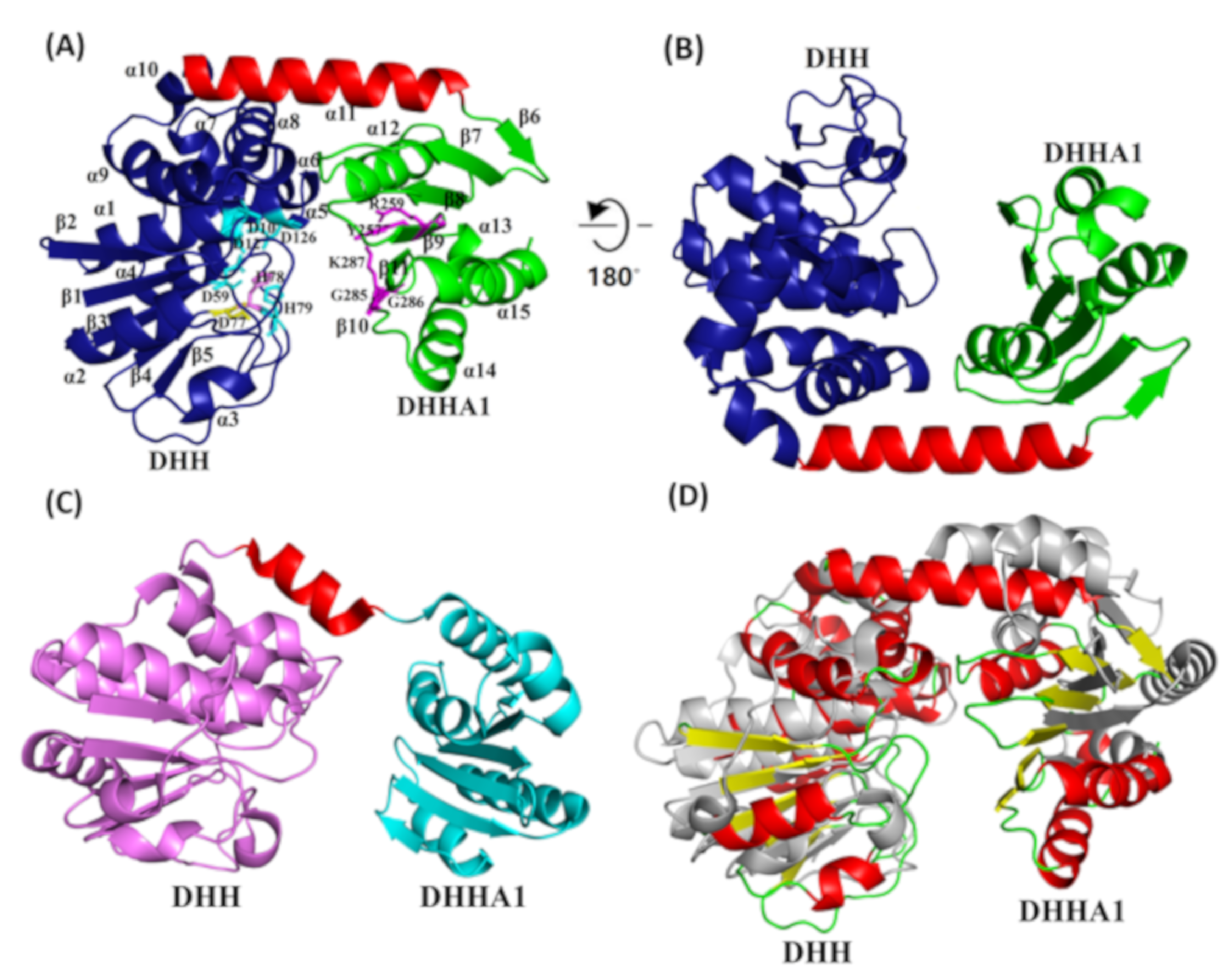
2.5. PyapApase Possesses a Similar Structure to Bacterial pApase
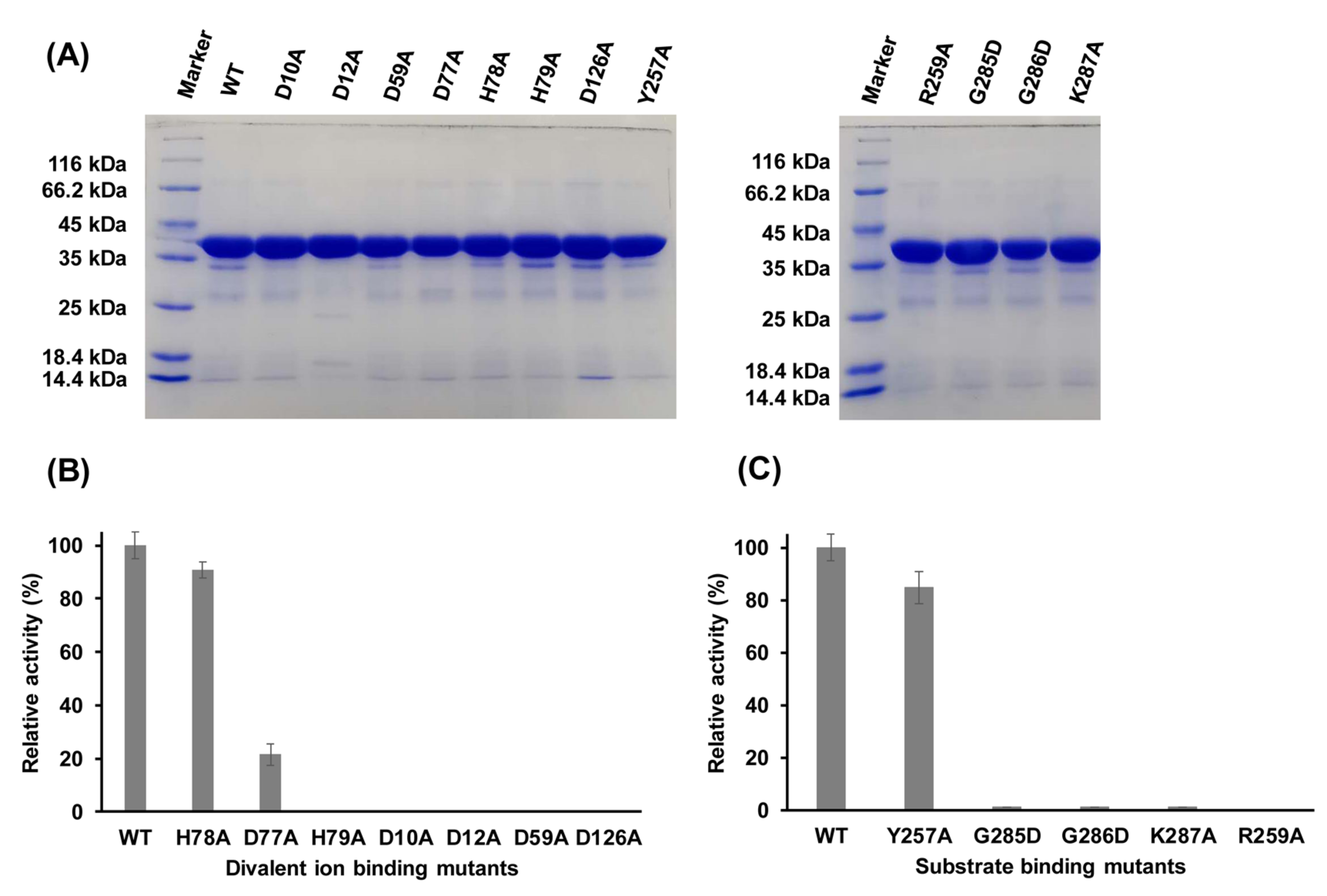
2.6. Functions of Key Amino Acid Residues
2.7. Enzyme Kinetics
2.8. Growth Phenotype of the pApase-Deleted Mutant
3. Discussion
3.1. PyapApase Converts pAp Efficiently
3.2. PyapApase Has High Substrate Specificity
3.3. The Function of PyapApase In Vivo
4. Materials and Methods
4.1. Materials
4.2. Construction of Phylogenetic Trees of pApases and Other Members of the DHH Superfamily
4.3. Preparation of Recombinant pApase and Its Mutants
4.4. Protein Expression and Purification
4.5. Optimization of the Reaction Conditions
4.6. Characterization of Enzymatic Activity on Dinucleotide Substrates
4.7. Analyzing Substrate and Product by HPLC
4.8. Measurement of Enzyme Activity toward ssDNA and ssRNA
4.9. Crystallization, Structure Determination, and Refinement
4.10. Enzyme Kinetics
4.11. Deletion of pApase in P. yayanosii A1
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Q.; Gao, Y.; Yang, A. Sulfur Homeostasis in Plants. Int. J. Mol. Sci. 2020, 21, 8926. [Google Scholar] [CrossRef]
- Linder, T. Assimilation of alternative sulfur sources in fungi. World J. Microbiol. Biotechnol. 2018, 34, 51. [Google Scholar] [CrossRef]
- Schelle, M.W.; Bertozzi, C.R. Sulfate Metabolism in Mycobacteria. ChemBioChem 2006, 7, 1516–1524. [Google Scholar] [CrossRef]
- Patron, N.J.; Durnford, D.G.; Kopriva, S. Sulfate assimilation in eukaryotes: Fusions, relocations and lateral transfers. BMC Evol. Biol. 2008, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.; Hiraoka, N.; Akama, T.O.; Fukuda, M.N. Carbohydrate-modifying Sulfotransferases: Structure, Function, and Pathophysiology. J. Biol. Chem. 2001, 276, 47747–47750. [Google Scholar] [CrossRef] [PubMed]
- Burkart, M.D.; Wong, C.H. A Continuous Assay for the Spectrophotometric Analysis of Sulfotransferases Using Aryl Sulfotransferase IV. Anal. Biochem. 1999, 274, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, J.W.; Bertozzi, C.R. Tyrosine sulfation: A modulator of extracellular protein–protein interactions. Chem. Biol. 2000, 7, R57–R61. [Google Scholar] [CrossRef]
- Han, S.W.; Lee, S.W.; Bahar, O.; Schwessinger, B.; Robinson, M.R.; Shaw, J.B.; Madsen, J.A.; Brodbelt, J.S.; Ronald, P.C. Tyrosine sulfation in a Gram-negative bacterium. Nat. Commun. 2012, 3, 1153. [Google Scholar] [CrossRef][Green Version]
- Klaassen, C.D.; Boles, J.W. Sulfation and sulfotransferases 5: The importance of 3′-phosphoadenosine 5′-phosphosulfate (PAPS) in the regulation of sulfation. FASEB J. 1997, 11, 404–418. [Google Scholar] [CrossRef]
- Mechold, U.; Ogryzko, V.; Ngo, S.; Danchin, A. Oligoribonuclease is a common downstream target of lithium-induced pAp accumulation in Escherichia coli and human cells. Nucleic Acids Res. 2006, 34, 2364–2373. [Google Scholar] [CrossRef]
- Estavillo, G.M.; Crisp, P.A.; Pornsiriwong, W.; Wirtz, M.; Collinge, D.; Carrie, C.; Giraud, E.; Whelan, J.; David, P.; Javot, H.; et al. Evidence for a SAL1-PAP chloroplast retrograde pathway that functions in drought and high light signaling in Arabidopsis. Plant Cell 2011, 23, 3992–4012. [Google Scholar] [CrossRef]
- Fang, X.; Zhao, G.; Zhang, S.; Li, Y.; Gu, H.; Li, Y.; Zhao, Q.; Qi, Y. Chloroplast-to-Nucleus Signaling Regulates MicroRNA Biogenesis in Arabidopsis. Dev. Cell 2019, 48, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Neuwald, A.F.; Krishnan, B.R.; Brikun, I.; Kulakauskas, S.; Suziedelis, K.; Tomcsanyi, T.; Leyh, T.S.; Berg, D.E. cysQ, a gene needed for cysteine synthesis in Escherichia coli K-12 only during aerobic growth. J. Bacteriol. 1992, 174, 415–425. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peng, Z.; Verma, D.P. A Rice HAL2-like Gene Encodes a Ca2+-sensitive 3′(2′),5′-Diphosphonucleoside 3′(2′)-Phosphohydrolase and Complements Yeast met22 and Escherichia coli cysQ Mutations. J. Biol. Chem. 1995, 270, 29105–29110. [Google Scholar] [CrossRef]
- Vaupotič, T.; Gunde Cimerman, N.; Plemenitaš, A. Novel 3′-phosphoadenosine-5′-phosphatases from extremely halotolerant Hortaea werneckii reveal insight into molecular determinants of salt tolerance of black yeasts. Fungal Genet. Biol. 2007, 44, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Mechold, U.; Fang, G.; Ngo, S.; Ogryzko, V.; Danchin, A. YtqI from Bacillus subtilis has both oligoribonuclease and pAp-phosphatase activity. Nucleic Acids Res. 2007, 35, 4552–4561. [Google Scholar] [CrossRef]
- Wakamatsu, T.; Kim, K.; Uemura, Y.; Nakagawa, N.; Kuramitsu, S.; Masui, R. Role of RecJ-like protein with 5′-3′ exonuclease activity in oligo(deoxy)nucleotide degradation. J. Biol. Chem. 2011, 286, 2807–2816. [Google Scholar] [CrossRef]
- Cummings, J.A.; Vetting, M.; Ghodge, S.V.; Xu, C.; Hillerich, B.; Seidel, R.D.; Almo, S.C.; Raushel, F.M. Prospecting for unannotated enzymes: Discovery of a 3′,5′-nucleotide bisphosphate phosphatase within the amidohydrolase superfamily. Biochemistry 2014, 53, 591–600. [Google Scholar] [CrossRef]
- Srivastav, R.; Sharma, R.; Tandon, S.; Tandon, C. Role of DHH superfamily proteins in nucleic acids metabolism and stress tolerance in prokaryotes and eukaryotes. Int. J. Biol. Macromol. 2019, 127, 66–75. [Google Scholar] [CrossRef]
- Wakamatsu, T.; Kitamura, Y.; Kotera, Y.; Nakagawa, N.; Kuramitsu, S.; Masui, R. Structure of RecJ exonuclease defines its specificity for single-stranded DNA. J. Biol. Chem. 2010, 285, 9762–9769. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chang, C.C.; Song, D.; Jiang, C.; Song, Y.; Wang, C.F.; Deng, W.; Zou, Y.J.; Chen, H.F.; Xiao, X.; et al. The trimeric Hef-associated nuclease HAN is a 3′→5′ exonuclease and is probably involved in DNA repair. Nucleic Acids Res. 2018, 46, 9027–9043. [Google Scholar] [CrossRef] [PubMed]
- Schmier, B.J.; Nelersa, C.M.; Malhotra, A. Structural Basis for the Bidirectional Activity of Bacillus nanoRNase NrnA. Sci. Rep. 2017, 7, 11085. [Google Scholar] [CrossRef] [PubMed]
- Postic, G.; Danchin, A.; Mechold, U. Characterization of NrnA homologs from Mycobacterium tuberculosis and Mycoplasma pneumoniae. RNA 2012, 18, 155–165. [Google Scholar] [CrossRef]
- Yang, J.; Bai, Y.; Zhang, Y.; Gabrielle, V.D.; Jin, L.; Bai, G. Deletion of the cyclic di-AMP phosphodiesterase gene (cnpB) in Mycobacterium tuberculosis leads to reduced virulence in a mouse model of infection. Mol. Microbiol. 2014, 93, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Quintero, F.J.; Garciadeblás, B.; Rodríguez-Navarro, A. The SAL1 gene of Arabidopsis, encoding an enzyme with 3′(2′),5′-bisphosphate nucleotidase and inositol polyphosphate 1-phosphatase activities, increases salt tolerance in yeast. Plant Cell 1996, 8, 529–537. [Google Scholar]
- Zhang, J.Y.; Zou, J.; Bao, Q.; Chen, W.L.; Wang, L.; Yang, H.; Zhang, C.C. A lithium-sensitive and sodium-tolerant 3′-phosphoadenosine-5′-phosphatase encoded by halA from the cyanobacterium Arthrospira platensis is closely related to its counterparts from yeasts and plants. Appl. Environ. Microbiol. 2006, 72, 245–251. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, Y.J.; Lee, J.H.; Kang, S.G. Identification and characterization of inorganic pyrophosphatase and PAP phosphatase from Thermococcus onnurineus NA1. J. Bacteriol. 2009, 191, 3415–3519. [Google Scholar] [CrossRef]
- Deng, Y.J.; Feng, L.; Zhou, H.; Xiao, X.; Wang, F.P.; Liu, X.P. NanoRNase from Aeropyrum pernix shows nuclease activity on ssDNA and ssRNA. DNA Repair 2018, 65, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Spiegelberg, B.D.; Xiong, J.P.; Smith, J.J.; Gu, R.F.; York, J.D. Cloning and characterization of a mammalian lithium-sensitive bisphosphate 3′-nucleotidase inhibited by inositol 1,4-bisphosphate. J. Biol. Chem. 1999, 274, 13619–13628. [Google Scholar] [CrossRef]
- Li, X.; Fu, L.; Li, Z.; Ma, X.; Xiao, X.; Xu, J. Genetic tools for the piezophilic hyperthermophilic archaeon Pyrococcus yayanosii. Extremophiles 2015, 19, 59–67. [Google Scholar] [CrossRef]
- He, Q.; Wang, F.; Liu, S.; Zhu, D.; Cong, H.; Gao, F.; Li, B.; Wang, H.; Lin, Z.; Liao, J.; et al. Structural and Biochemical Insight into the Mechanism of Rv2837c from Mycobacterium tuberculosis as a c-di-NMP Phosphodiesterase. J. Biol. Chem. 2016, 291, 3668–3681. [Google Scholar] [CrossRef] [PubMed]
- Weinitschke, S.; Denger, K.; Cook, A.M.; Smits, T.H. The DUF81 protein TauE in Cupriavidus necator H16, a sulfite exporter in the metabolism of C2 sulfonates. Microbiology 2007, 153, 3055–3060. [Google Scholar] [CrossRef] [PubMed]
- Chodur, D.M.; Coulter, P.; Isaacs, J.; Pu, M.; Fernandez, N.; Waters, C.M.; Rowe-Magnus, D.A. Environmental Calcium Initiates a Feed-Forward Signaling Circuit That Regulates Biofilm Formation and Rugosity in Vibrio vulnificus. MBio 2018, 9, e01377-18. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.; Motta, S.; Mauri, P.; Landini, P. Sulfate assimilation pathway intermediate phosphoadenosine 5’-phosphosulfate acts as a signal molecule affecting production of curli fibres in Escherichia coli. Microbiology 2014, 160, 1832–1844. [Google Scholar] [CrossRef] [PubMed]
- Michoud, G.; Jebbar, M. High hydrostatic pressure adaptive strategies in an obligate piezophile Pyrococcus yayanosii. Sci. Rep. 2016, 6, 27289. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, J.; Luo, Y.; Fu, Y.; Su, J.; He, J. Highly efficient enzymatic preparation of c-di-AMP using the diadenylate cyclase DisA from Bacillus thuringiensis. Enzym. Microb. Tech. 2013, 52, 319–324. [Google Scholar] [CrossRef]
- Winn, M.D.; Ballard, C.C.; Cowtan, K.D.; Dodson, E.J.; Emsley, P.; Evans, P.R.; Keegan, R.M.; Krissinel, E.B.; Leslie, A.G.; McCoy, A.; et al. Overview of the CCP4 suite and current developments. Acta Cryst. D 2011, 67, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Kabsch, W. XDS. Acta Cryst. D 2010, 66, 125–132. [Google Scholar] [CrossRef]
- Bricogne, G.; Vonrhein, C.; Flensburg, C.; Schiltz, M.; Paciorek, W. Generation, representation and flow of phase information in structure determination: Recent developments in and around SHARP 2.0. Acta Cryst. D 2003, 59, 2023–2030. [Google Scholar] [CrossRef]
- Adams, P.D.; Afonine, P.V.; Bunkóczi, G.; Chen, V.B.; Davis, I.W.; Echols, N.; Headd, J.J.; Hung, L.W.; Kapral, G.J.; Grosse-Kunstleve, R.W.; et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Cryst. D 2010, 66, 213–221. [Google Scholar] [CrossRef]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Cryst. D 2010, 66, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.B.; Arendall Iii, W.B.; Headd, J.J.; Keedy, D.A.; Immormino, R.M.; Kapral, G.J.; Murray, L.W.; Richardson, J.S.; Richardson, D.C. MolProbity: All-atom structure validation for macromolecular crystallography. Acta Cryst. D 2010, 66, 12–21. [Google Scholar] [CrossRef]
- Zeng, X.; Birrien, J.L.; Fouquet, Y.; Cherkashov, G.; Jebbar, M.; Querellou, J.; Oger, P.; Cambon-Bonavita, M.A.; Xiao, X.; Prieur, D. Pyrococcus CH1, an obligate piezophilic hyperthermophile: Extending the upper pressure-temperature limits for life. ISME J. 2009, 3, 873–876. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Li, Z.; Chen, R.; Ma, X.; Xiao, X.; Xu, J. Induction of a toxin-antitoxin gene cassette under high hydrostatic pressure enables markerless gene disruption in the hyperthermophilic archaeon Pyrococcus yayanosii. Appl. Environ. Microbiol. 2019, 85, e02662-18. [Google Scholar] [CrossRef]
- Matsumi, R.; Manabe, K.; Fukui, T.; Atomi, H.; Imanaka, T. Disruption of a sugar transporter gene cluster in a hyperthermophilic archaeon using a host-marker system based on antibiotic resistance. J. Bacteriol. 2007, 189, 2683–2691. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, X.; Xiao, X.; Xu, J. An integrative genomic island affects the adaptations of the piezophilic hyperthermophilic archaeon Pyrococcus yayanosii to high temperature and high hydrostatic pressure. Front. Microbiol. 2016, 7, 1927. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Se-WT | DHH77–79AAA | H79A |
|---|---|---|---|
| Data collection | |||
| Wavelength (Å) | 0.9792 | 0.9792 | 0.9792 |
| Space group | P21 | P1211 | P1211 |
| Cell dimensions | |||
| a, b, c (Å) | 58.6, 67.1, 60.3 | 61.5, 149.4, 75.9 | 59.26, 72.3, 76.21 |
| β (°)b | 112.4 | 90, 99.3, 90 | 90, 101.73, 90 |
| Resolution (Å) | 50–2.10 (2.14–2.10) | 34.45–2.69 (2.76–2.69) | 29–2.05 (2.13–2.05) |
| No. reflections | 37,590 | 37,365 | 38,863 |
| Rmergeb (%) a | 8.5 (70.4) | 9 (157.4) | 13.2 (124.5) |
| Mean I/σ (I) a | 23.5 (10.2) | 18.2 (1.9) | 14.1 (3.7) |
| Completeness (%) a | 100 (100) | 99.46 (99.6) | 98.36 (96.98) |
| Redundancy a | 7.5 (3.1) | 6.8 (5.7) | 6.7 (6.0) |
| Refinement | |||
| Rwork/Rfree (%) c | 17.37/21.00 | 22.63/27.38 | 19.32/22.96 |
| No. atoms | |||
| Protein | 3702 | 9832 | 4928 |
| Water | 178 | 15 | 241 |
| Ligand | 2 | / | / |
| R.M.S. Deviation | |||
| Bond lengths (Å) | 0.002 | 0.007 | 0.022 |
| Bond angles (°) | 0.473 | 1.15 | 1.08 |
| Ramachandran plot (%) | |||
| Favored | 98.29 | 94.57 | 96.58 |
| Allowed | 1.28 | 5.03 | 2.93 |
| Outliers | 0.43 | 0.41 | 0.49 |
| Protein | Km | kcat | kcat/Km |
|---|---|---|---|
| WT | 272 ± 12.6 μM | 1259 ± 94.1 s−1 | 4.63 × 103 M−1s−1 |
| D12A | 790 ± 47.7 μM | 1.7 ± 0.3 s−1 | 2.16 × 103 M−1s−1 |
| R259A | 6000 ± 753.4 μM | 61.57 ± 7.1 s−1 | 1.03 × 103 M−1s−1 |
| G285D | 4500 ± 607.7 μM | 71.04 ± 9.6 s−1 | 1.58 × 103 M−1s−1 |
| K287A | 720 ± 33.1 μM | 17.10 ± 2.1 s−1 | 2.38 × 104 M−1s−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Z.; Wang, W.; Li, X.; Zhou, H.; Yi, G.; Wang, Q.; Yu, F.; Xiao, X.; Liu, X. Structure and Function of Piezophilic Hyperthermophilic Pyrococcus yayanosii pApase. Int. J. Mol. Sci. 2021, 22, 7159. https://doi.org/10.3390/ijms22137159
Jin Z, Wang W, Li X, Zhou H, Yi G, Wang Q, Yu F, Xiao X, Liu X. Structure and Function of Piezophilic Hyperthermophilic Pyrococcus yayanosii pApase. International Journal of Molecular Sciences. 2021; 22(13):7159. https://doi.org/10.3390/ijms22137159
Chicago/Turabian StyleJin, Zheng, Weiwei Wang, Xuegong Li, Huan Zhou, Gangshun Yi, Qisheng Wang, Feng Yu, Xiang Xiao, and Xipeng Liu. 2021. "Structure and Function of Piezophilic Hyperthermophilic Pyrococcus yayanosii pApase" International Journal of Molecular Sciences 22, no. 13: 7159. https://doi.org/10.3390/ijms22137159
APA StyleJin, Z., Wang, W., Li, X., Zhou, H., Yi, G., Wang, Q., Yu, F., Xiao, X., & Liu, X. (2021). Structure and Function of Piezophilic Hyperthermophilic Pyrococcus yayanosii pApase. International Journal of Molecular Sciences, 22(13), 7159. https://doi.org/10.3390/ijms22137159






