Nanomaterials in Skin Regeneration and Rejuvenation
Abstract
1. Introduction
1.1. Aging
1.2. Wound Healing
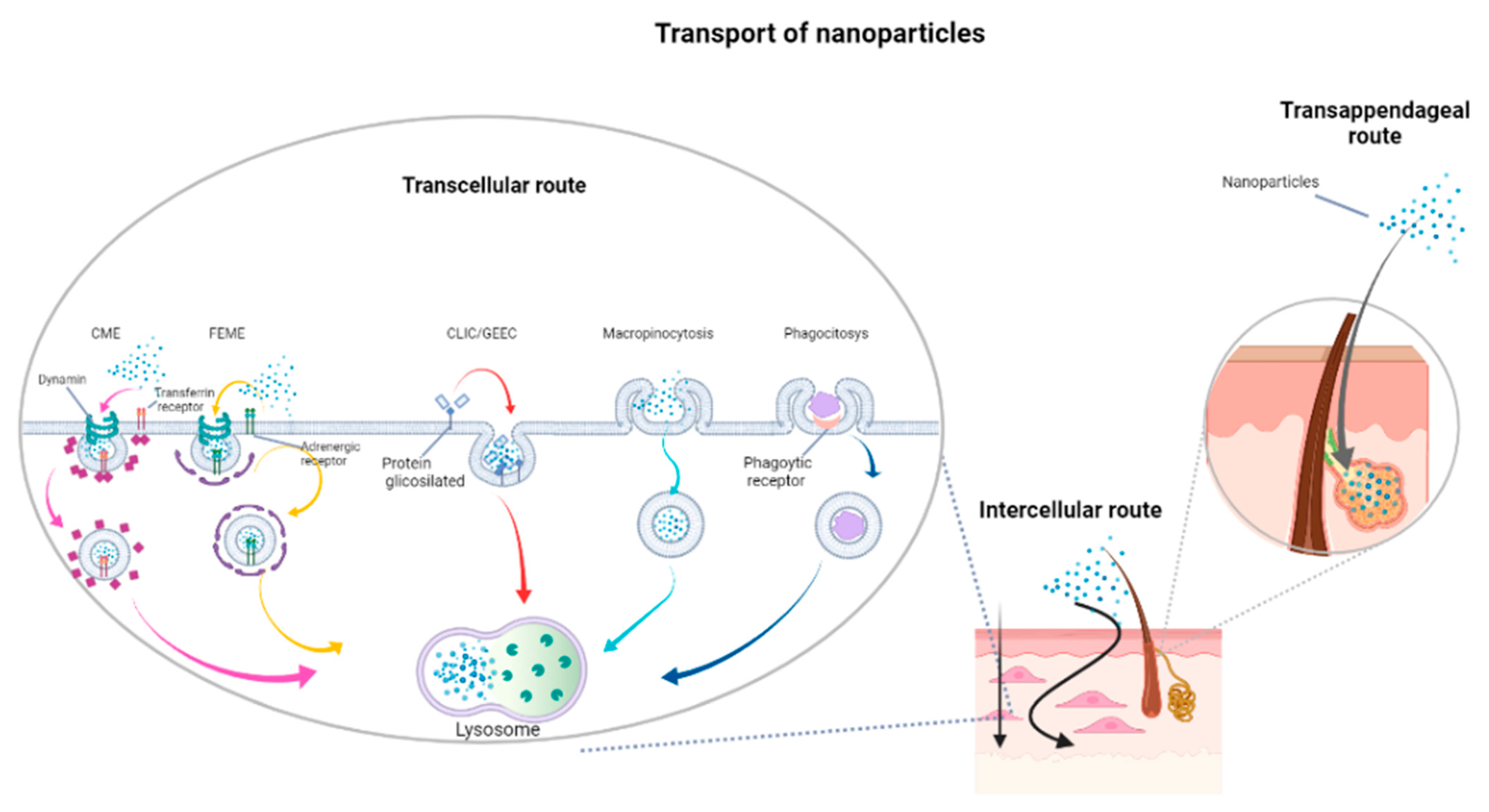
1.3. Skin Permeation by Topical Treatments
2. Tissue Regeneration and Rejuvenation Strategies
- (i)
- Direct stimulation of cell regrowth;
- (ii)
- Antibacterial activity;
- (iii)
- Drug delivery.
2.1. Nanohydrogels and Nanoparticle–Hydrogel Superstructures
2.1.1. Antibacterial Action
2.1.2. Antioxidant Properties
2.1.3. Drug Release
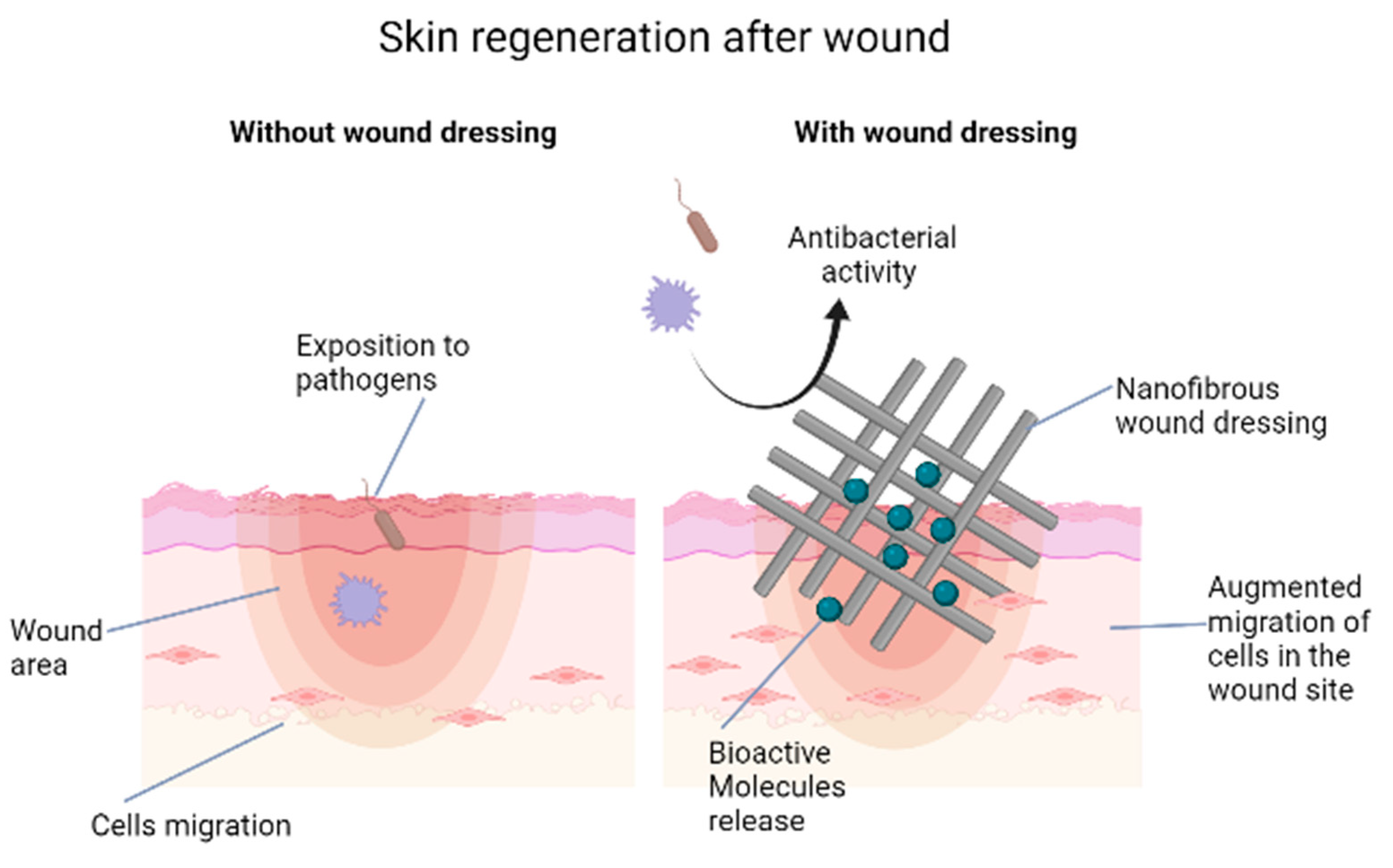
2.2. Nanofibers and Scaffolds
2.3. Antiscar Action
3. Silver Sulfadiazine Nanomaterials
4. Nanoformulations for Skin Care and Anti-Aging Products
5. Considerations on the Toxicity of Nanomaterials in Wound Dressings
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lu, C.; Fuchs, E. Sweat gland progenitors in development, homeostasis, and wound repair. Cold Spring Harb. Perspect. Med. 2014, 4, a015222. [Google Scholar] [CrossRef]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen 2009, 17, 763–771. [Google Scholar] [CrossRef]
- Kirkwood, T.B.; Melov, S. On the programmed/non-programmed nature of ageing within the life history. Curr. Biol. 2011, 21, R701–R707. [Google Scholar] [CrossRef] [PubMed]
- Brink, T.C.; Demetrius, L.; Lehrach, H.; Adjaye, J. Age-related transcriptional changes in gene expression in different organs of mice support the metabolic stability theory of aging. Biogerontology 2009, 10, 549–564. [Google Scholar] [CrossRef]
- Krutmann, J.; Morita, A.; Chung, J.H. Sun exposure: What molecular photodermatology tells us about its good and bad sides. J. Investig. Derm. 2012, 132, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Stern, M.M.; Bickenbach, J.R. Epidermal stem cells are resistant to cellular aging. Aging Cell 2007, 6, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; He, T.; Kang, S.; Voorhees, J.J.; Fisher, G.J. Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor-beta type II receptor/Smad signaling. Am. J. Pathol. 2004, 165, 741–751. [Google Scholar] [CrossRef]
- Mizukoshi, K.; Nakamura, T.; Oba, A. The relationship between dermal papillary structure and skin surface properties, color, and elasticity. Ski. Res. Technol. 2016, 22, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Kwon, S.H.; Choi, J.Y.; Na, J.I.; Huh, C.H.; Choi, H.R.; Park, K.C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef]
- Kehlet, S.N.; Willumsen, N.; Armbrecht, G.; Dietzel, R.; Brix, S.; Henriksen, K.; Karsdal, M.A. Age-related collagen turnover of the interstitial matrix and basement membrane: Implications of age- and sex-dependent remodeling of the extracellular matrix. PLoS ONE 2018, 13, e0194458. [Google Scholar] [CrossRef]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef]
- Yurchenco, P.D.; Schittny, J.C. Molecular architecture of basement membranes. FASEB J. 1990, 4, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Bellu, E.; Garroni, G.; Balzano, F.; Satta, R.; Montesu, M.A.; Kralovic, M.; Fedacko, J.; Cruciani, S.; Maioli, M. Isolating stem cells from skin: Designing a novel highly efficient non-enzymatic approach. Physiol. Res. 2019, 68, S385–S388. [Google Scholar] [CrossRef]
- Stadelmann, W.K.; Digenis, A.G.; Tobin, G.R. Physiology and healing dynamics of chronic cutaneous wounds. Am. J. Surg. 1998, 176, 26S–38S. [Google Scholar] [CrossRef]
- Ahmed, A.S.; Sheng, M.H.; Wasnik, S.; Baylink, D.J.; Lau, K.W. Effect of aging on stem cells. World J. Exp. Med. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, S.; Maioli, M.; Pigliaru, G.; Castagna, A.; Santaniello, S.; Basoli, V.; Fontani, V.; Ventura, C. Stem cell senescence. Effects of REAC technology on telomerase-independent and telomerase-dependent pathways. Sci. Rep. 2014, 4, 6373. [Google Scholar] [CrossRef]
- Boukamp, P. Non-melanoma skin cancer: What drives tumor development and progression? Carcinogenesis 2005, 26, 1657–1667. [Google Scholar] [CrossRef]
- Parrinello, S.; Coppe, J.P.; Krtolica, A.; Campisi, J. Stromal-epithelial interactions in aging and cancer: Senescent fibroblasts alter epithelial cell differentiation. J. Cell Sci. 2005, 118, 485–496. [Google Scholar] [CrossRef]
- Wang, Y.; Lauer, M.E.; Anand, S.; Mack, J.A.; Maytin, E.V. Hyaluronan synthase 2 protects skin fibroblasts against apoptosis induced by environmental stress. J. Biol. Chem. 2014, 289, 32253–32265. [Google Scholar] [CrossRef]
- Bellu, E.; Garroni, G.; Cruciani, S.; Balzano, F.; Serra, D.; Satta, R.; Montesu, M.A.; Fadda, A.; Mulas, M.; Sarais, G.; et al. Smart Nanofibers with Natural Extracts Prevent Senescence Patterning in a Dynamic Cell Culture Model of Human Skin. Cells 2020, 9, 2530. [Google Scholar] [CrossRef]
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of nanotechnology in cosmeceuticals: A review of recent advances. J. Pharm. 2018, 2018, 3420204. [Google Scholar] [CrossRef]
- Whitney, J.D. Overview: Acute and chronic wounds. Nurs. Clin. N. Am. 2005, 40, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Zare, M.R.; Khorram, M.; Barzegar, S.; Asadian, F.; Zareshahrabadi, Z.; Jamal Saharkhiz, M.; Ahadian, S.; Zomorodian, K. Antimicrobial core-shell electrospun nanofibers containing Ajwain essential oil for accelerating infected wound healing. Int. J. Pharm. 2021, 603, 120698. [Google Scholar] [CrossRef]
- Braund, R.; Hook, S.; Medlicott, N.J. The role of topical growth factors in chronic wounds. Curr. Drug Deliv. 2007, 4, 195–204. [Google Scholar] [CrossRef]
- Gainza, G.; Villullas, S.; Pedraz, J.L.; Hernandez, R.M.; Igartua, M. Advances in drug delivery systems (DDSs) to release growth factors for wound healing and skin regeneration. Nanomedicine 2015, 11, 1551–1573. [Google Scholar] [CrossRef] [PubMed]
- Addis, R.; Cruciani, S.; Santaniello, S.; Bellu, E.; Sarais, G.; Ventura, C.; Maioli, M.; Pintore, G. Fibroblast Proliferation and Migration in Wound Healing by Phytochemicals: Evidence for a Novel Synergic Outcome. Int. J. Med. Sci. 2020, 17, 1030–1042. [Google Scholar] [CrossRef]
- Oda, Y.; Bikle, D.D. Vitamin D and calcium signaling in epidermal stem cells and their regeneration. World J. Stem Cells 2020, 12, 604–611. [Google Scholar] [CrossRef]
- Kim, E.K.; Kim, H.O.; Park, Y.M.; Park, C.J.; Yu, D.S.; Lee, J.Y. Prevalence and risk factors of depression in geriatric patients with dermatological diseases. Ann. Derm. 2013, 25, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Bolzinger, M.-A.; Briançon, S.; Pelletier, J.; Chevalier, Y. Penetration of drugs through skin, a complex rate-controlling membrane. Curr. Opin. Colloid Interface Sci. 2012, 17, 156–165. [Google Scholar] [CrossRef]
- Trommer, H.; Neubert, R.H. Overcoming the stratum corneum: The modulation of skin penetration. A review. Ski. Pharm. Physiol. 2006, 19, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Batisse, D.; Bazin, R.; Baldeweck, T.; Querleux, B.; Leveque, J.L. Influence of age on the wrinkling capacities of skin. Ski. Res. Technol. 2002, 8, 148–154. [Google Scholar] [CrossRef]
- Rennick, J.J.; Johnston, A.P.R.; Parton, R.G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 2021, 16, 266–276. [Google Scholar] [CrossRef]
- Vitorino, C.; Almeida, J.; Goncalves, L.M.; Almeida, A.J.; Sousa, J.J.; Pais, A.A. Co-encapsulating nanostructured lipid carriers for transdermal application: From experimental design to the molecular detail. J. Control. Release 2013, 167, 301–314. [Google Scholar] [CrossRef]
- Naik, A.; Kalia, Y.N.; Guy, R.H. Transdermal drug delivery: Overcoming the skin’s barrier function. Pharm. Sci. Technol. Today 2000, 3, 318–326. [Google Scholar] [CrossRef]
- Kurian, S.J.; Miraj, S.S.; Benson, R.; Munisamy, M.; Saravu, K.; Rodrigues, G.S.; Rao, M. Vitamin D Supplementation in Diabetic Foot Ulcers: A Current Perspective. Curr. Diabetes Rev. 2021, 17, 512–521. [Google Scholar] [CrossRef]
- Cruciani, S.; Santaniello, S.; Garroni, G.; Fadda, A.; Balzano, F.; Bellu, E.; Sarais, G.; Fais, G.; Mulas, M.; Maioli, M. Myrtus Polyphenols, from Antioxidants to Anti-Inflammatory Molecules: Exploring a Network Involving Cytochromes P450 and Vitamin D. Molecules 2019, 24, 1515. [Google Scholar] [CrossRef]
- Barry, B.W. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur. J. Pharm. Sci. 2001, 14, 101–114. [Google Scholar] [CrossRef]
- Benson, H.A. Transdermal drug delivery: Penetration enhancement techniques. Curr. Drug Deliv. 2005, 2, 23–33. [Google Scholar] [CrossRef]
- Landsiedel, R.; Ma-Hock, L.; Van Ravenzwaay, B.; Schulz, M.; Wiench, K.; Champ, S.; Schulte, S.; Wohlleben, W.; Oesch, F. Gene toxicity studies on titanium dioxide and zinc oxide nanomaterials used for UV-protection in cosmetic formulations. Nanotoxicology 2010, 4, 364–381. [Google Scholar] [CrossRef]
- Nardini, M.; Perteghella, S.; Mastracci, L.; Grillo, F.; Marrubini, G.; Bari, E.; Formica, M.; Gentili, C.; Cancedda, R.; Torre, M.L.; et al. Growth Factors Delivery System for Skin Regeneration: An Advanced Wound Dressing. Pharmaceutics 2020, 12, 120. [Google Scholar] [CrossRef]
- Fathi-Azarbayjani, A.; Qun, L.; Chan, Y.W.; Chan, S.Y. Novel vitamin and gold-loaded nanofiber facial mask for topical delivery. AAPS PharmSciTech 2010, 11, 1164–1170. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle uptake: The phagocyte problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef]
- Chou, L.Y.; Ming, K.; Chan, W.C. Strategies for the intracellular delivery of nanoparticles. Chem. Soc. Rev. 2011, 40, 233–245. [Google Scholar] [CrossRef]
- Li, K.; Li, D.; Li, C.-H.; Zhuang, P.; Dai, C.; Hu, X.; Wang, D.; Liu, Y.; Mei, X.; Rotello, V.M. Efficient in vivo wound healing using noble metal nanoclusters. Nanoscale 2021, 13, 6531–6537. [Google Scholar] [CrossRef]
- Lo, S.; Fauzi, M.B. Current Update of Collagen Nanomaterials—Fabrication, Characterisation and Its Applications: A Review. Pharmaceutics 2021, 13, 316. [Google Scholar] [CrossRef]
- Ovais, M.; Ahmad, I.; Khalil, A.T.; Mukherjee, S.; Javed, R.; Ayaz, M.; Raza, A.; Shinwari, Z.K. Wound healing applications of biogenic colloidal silver and gold nanoparticles: Recent trends and future prospects. Appl. Microbiol. Biotechnol. 2018, 102, 4305–4318. [Google Scholar] [CrossRef]
- Neema, S.; Chatterjee, M. Nano-silver dressing in toxic epidermal necrolysis. Indian J. Dermatol. Venereol. Leprol. 2017, 83. [Google Scholar] [CrossRef]
- Ribeiro, F.M.; de Oliveira, M.M.; Singh, S.; Sakthivel, T.S.; Neal, C.J.; Seal, S.; Ueda-Nakamura, T.; Lautenschlager, S.d.O.S.; Nakamura, C.V. Ceria Nanoparticles decrease UVA-induced fibroblast death through cell redox regulation leading to cell survival, migration and proliferation. Front. Bioeng. Biotechnol. 2020, 8, 577557. [Google Scholar] [CrossRef]
- Alizadeh, S.; Seyedalipour, B.; Shafieyan, S.; Kheime, A.; Mohammadi, P.; Aghdami, N. Copper nanoparticles promote rapid wound healing in acute full thickness defect via acceleration of skin cell migration, proliferation, and neovascularization. Biochem. Biophys. Res. Commun. 2019, 517, 684–690. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Nurchi, V.M.; Zoroddu, M.A. Medical uses of silver: History, myths, and scientific evidence. J. Med. Chem. 2019, 62, 5923–5943. [Google Scholar] [CrossRef]
- Akram, M.; Hussain, R. Nanohydrogels: History, development, and applications in drug delivery. In Nanocellulose and Nanohydrogel Matrices: Biotechnological and Biomedical Applications; Wiley Online Library: Hoboken, NJ, USA, 2017; pp. 297–330. [Google Scholar]
- Paiva-Santos, A.C.; Herdade, A.M.; Guerra, C.; Peixoto, D.; Pereira-Silva, M.; Zeinali, M.; Mascarenhas-Melo, F.; Paranhos, A.; Veiga, F. Plant-mediated green synthesis of metal-based nanoparticles for dermopharmaceutical and cosmetic applications. Int. J. Pharm. 2021, 597, 120311. [Google Scholar] [CrossRef]
- Kong, Y.; Hou, Z.; Zhou, L.; Zhang, P.; Ouyang, Y.; Wang, P.; Chen, Y.; Luo, X. Injectable Self-Healing Hydrogels Containing CuS Nanoparticles with Abilities of Hemostasis, Antibacterial activity, and Promoting Wound Healing. ACS Biomater. Sci. Eng. 2021, 7, 335–349. [Google Scholar] [CrossRef]
- Manatunga, D.; Godakanda, V.; Herath, H.; de Silva, R.M.; Yeh, C.-Y.; Chen, J.-Y.; Akshitha de Silva, A.; Rajapaksha, S.; Nilmini, R.; Nalin de Silva, K. Nanofibrous cosmetic face mask for transdermal delivery of nano gold: Synthesis, characterization, release and zebra fish employed toxicity studies. R. Soc. Open Sci. 2020, 7, 201266. [Google Scholar] [CrossRef]
- Jiménez-Pérez, Z.E.; Singh, P.; Kim, Y.-J.; Mathiyalagan, R.; Kim, D.-H.; Lee, M.H.; Yang, D.C. Applications of Panax ginseng leaves-mediated gold nanoparticles in cosmetics relation to antioxidant, moisture retention, and whitening effect on B16BL6 cells. J. Ginseng Res. 2018, 42, 327–333. [Google Scholar] [CrossRef]
- Taufikurohmah, T.; Sanjaya, I.G.M.; Syahrani, A. Nanogold synthesis using matrix mono glyceryl stearate as antiaging compounds in modern cosmetics. J. Mater. Sci. Eng. A 2011, 1, 857. [Google Scholar]
- Arafa, M.G.; El-Kased, R.F.; Elmazar, M. Thermoresponsive gels containing gold nanoparticles as smart antibacterial and wound healing agents. Sci. Rep. 2018, 8, 13674. [Google Scholar] [CrossRef]
- Stefan, L.M.; Iosageanu, A.; Ilie, D.; Stanciuc, A.M.; Matei, C.; Berger, D.; Craciunescu, O. Extracellular matrix biomimetic polymeric membranes enriched with silver nanoparticles for wound healing. Biomed. Mater. 2021, 16, 035010. [Google Scholar] [CrossRef]
- Bundjaja, V.; Santoso, S.P.; Angkawijaya, A.E.; Yuliana, M.; Soetaredjo, F.E.; Ismadji, S.; Ayucitra, A.; Gunarto, C.; Ju, Y.-H.; Ho, M.-H. Fabrication of cellulose carbamate hydrogel-dressing with rarasaponin surfactant for enhancing adsorption of silver nanoparticles and antibacterial activity. Mater. Sci. Eng. C 2021, 118, 111542. [Google Scholar] [CrossRef]
- Amer, S.; Attia, N.; Nouh, S.; El-Kammar, M.; Korittum, A.; Abu-Ahmed, H. Fabrication of sliver nanoparticles/polyvinyl alcohol/gelatin ternary nanofiber mats for wound healing application. J. Biomater. Appl. 2020, 35, 287–298. [Google Scholar] [CrossRef]
- Rahman, M.A.; Islam, M.S.; Haque, P.; Khan, M.N.; Takafuji, M.; Begum, M.; Chowdhury, G.W.; Khan, M.; Rahman, M.M. Calcium ion mediated rapid wound healing by nano-ZnO doped calcium phosphate-chitosan-alginate biocomposites. Materialia 2020, 13, 100839. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, F.; Hou, Z.; Chen, Y.; Luo, X. Injectable self-healing CuS nanoparticle complex hydrogels with antibacterial, anti-cancer, and wound healing properties. Chem. Eng. J. 2021, 409, 128224. [Google Scholar] [CrossRef]
- Ahmed, K.B.A.; Anbazhagan, V. Synthesis of copper sulfide nanoparticles and evaluation of in vitro antibacterial activity and in vivo therapeutic effect in bacteria-infected zebrafish. RSC Adv. 2017, 7, 36644–36652. [Google Scholar] [CrossRef]
- Haghniaz, R.; Rabbani, A.; Vajhadin, F.; Khan, T.; Kousar, R.; Khan, A.R.; Montazerian, H.; Iqbal, J.; Libanori, A.; Kim, H.J.; et al. Anti-bacterial and wound healing-promoting effects of zinc ferrite nanoparticles. J. Nanobiotechnol. 2021, 19, 38. [Google Scholar] [CrossRef]
- Patel, K.K.; Surekha, D.B.; Tripathi, M.; Anjum, M.M.; Muthu, M.; Tilak, R.; Agrawal, A.K.; Singh, S. Antibiofilm potential of silver sulfadiazine-loaded nanoparticle formulations: A study on the effect of DNase-I on microbial biofilm and wound healing activity. Mol. Pharm. 2019, 16, 3916–3925. [Google Scholar] [CrossRef]
- Singh, S.K.; Dhyani, A.; Juyal, D. Hydrogel: Preparation, characterization and applications. Pharma Innov. 2017, 6, 25. [Google Scholar]
- Asadi, N.; Pazoki-Toroudi, H.; Del Bakhshayesh, A.R.; Akbarzadeh, A.; Davaran, S.; Annabi, N. Multifunctional hydrogels for wound healing: Special focus on biomacromolecular based hydrogels. Int. J. Biol. Macromol. 2021, 170, 728–750. [Google Scholar] [CrossRef]
- Jiang, Y.; Krishnan, N.; Heo, J.; Fang, R.H.; Zhang, L. Nanoparticle–hydrogel superstructures for biomedical applications. J. Control. Release 2020, 324, 505–521. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Qiu, L.; Wang, C.; Lan, M.; Guo, Q.; Du, X.; Zhou, S.; Cui, P.; Hong, T.; Jiang, P.; Wang, J. Antibacterial Photodynamic Gold Nanoparticles for Skin Infection. ACS Appl. Bio Mater. 2021, 4, 3124–3132. [Google Scholar] [CrossRef]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef]
- Li, S.; Dong, S.; Xu, W.; Tu, S.; Yan, L.; Zhao, C.; Ding, J.; Chen, X. Antibacterial Hydrogels. Adv. Sci. 2018, 5, 1700527. [Google Scholar] [CrossRef]
- Zhao, X.; Li, P.; Guo, B.; Ma, P.X. Antibacterial and conductive injectable hydrogels based on quaternized chitosan-graft-polyaniline/oxidized dextran for tissue engineering. Acta Biomater. 2015, 26, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Atefyekta, S.; Blomstrand, E.; Rajasekharan, A.K.; Svensson, S.; Trobos, M.; Hong, J.; Webster, T.J.; Thomsen, P.; Andersson, M. Antimicrobial Peptide-Functionalized Mesoporous Hydrogels. ACS Biomater. Sci. Eng. 2021, 7, 1693–1702. [Google Scholar] [CrossRef]
- Azoulay, Z.; Aibinder, P.; Gancz, A.; Moran-Gilad, J.; Navon-Venezia, S.; Rapaport, H. Assembly of cationic and amphiphilic beta-sheet FKF tripeptide confers antibacterial activity. Acta Biomater. 2021, 125, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Li, Q.; Fang, Z.; Jin, M.; Zeng, Q.; Huang, G.; Jia, Y.G.; Wang, L.; Chen, Y. Conductive and antimicrobial macroporous nanocomposite hydrogels generated from air-in-water Pickering emulsions for neural stem cell differentiation and skin wound healing. Biomater. Sci. 2020, 8, 6957–6968. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919. [Google Scholar]
- Sadidi, H.; Hooshmand, S.; Ahmadabadi, A.; Javad Hosseini, S.; Baino, F.; Vatanpour, M.; Kargozar, S. Cerium Oxide Nanoparticles (Nanoceria): Hopes in Soft Tissue Engineering. Molecules 2020, 25, 4559. [Google Scholar] [CrossRef]
- Yu, R.; Yang, Y.; He, J.; Li, M.; Guo, B. Novel supramolecular self-healing silk fibroin-based hydrogel via host–guest interaction as wound dressing to enhance wound healing. Chem. Eng. J. 2021, 417, 128278. [Google Scholar] [CrossRef]
- Contardi, M.; Kossyvaki, D.; Picone, P.; Summa, M.; Guo, X.; Heredia-Guerrero, J.A.; Giacomazza, D.; Carzino, R.; Goldoni, L.; Scoponi, G. Electrospun Polyvinylpyrrolidone (PVP) hydrogels containing hydroxycinnamic acid derivatives as potential wound dressings. Chem. Eng. J. 2021, 409, 128144. [Google Scholar] [CrossRef]
- Ahmadian, Z.; Correia, A.; Hasany, M.; Figueiredo, P.; Dobakhti, F.; Eskandari, M.R.; Hosseini, S.H.; Abiri, R.; Khorshid, S.; Hirvonen, J.; et al. A Hydrogen-Bonded Extracellular Matrix-Mimicking Bactericidal Hydrogel with Radical Scavenging and Hemostatic Function for pH-Responsive Wound Healing Acceleration. Adv. Healthc. Mater. 2021, 10, e2001122. [Google Scholar] [CrossRef]
- Silva, V.C.; Silva, A.M.; Basílio, J.A.; Xavier, J.A.; do Nascimento, T.G.; Naal, R.M.; Del Lama, M.P.; Leonelo, L.A.; Mergulhão, N.L.; Maranhão, F.C. New Insights for Red Propolis of Alagoas—Chemical Constituents, Topical Membrane Formulations and Their Physicochemical and Biological Properties. Molecules 2020, 25, 5811. [Google Scholar] [CrossRef] [PubMed]
- Ditta, L.A.; Rao, E.; Provenzano, F.; Sanchez, J.L.; Santonocito, R.; Passantino, R.; Costa, M.A.; Sabatino, M.A.; Dispenza, C.; Giacomazza, D.; et al. Agarose/kappa-carrageenan-based hydrogel film enriched with natural plant extracts for the treatment of cutaneous wounds. Int. J. Biol. Macromol. 2020, 164, 2818–2830. [Google Scholar] [CrossRef]
- Back, P.I.; Balestrin, L.A.; Fachel, F.N.S.; Nemitz, M.C.; Falkembach, M.; Soares, G.; Marques, M.D.S.; Silveira, T.; Dal Pra, M.; Horn, A.P.; et al. Hydrogels containing soybean isoflavone aglycones-rich fraction-loaded nanoemulsions for wound healing treatment—In vitro and in vivo studies. Colloids Surf. B Biointerfaces 2020, 196, 111301. [Google Scholar] [CrossRef] [PubMed]
- Sami, D.G.; Abdellatif, A.; Azzazy, H.M.E. Turmeric/oregano formulations for treatment of diabetic ulcer wounds. Drug Dev. Ind. Pharm. 2020, 46, 1613–1621. [Google Scholar] [CrossRef]
- Zhang, W.; Qi, X.; Zhao, Y.; Liu, Y.; Xu, L.; Song, X.; Xiao, C.; Yuan, X.; Zhang, J.; Hou, M. Study of injectable Blueberry anthocyanins-loaded hydrogel for promoting full-thickness wound healing. Int. J. Pharm. 2020, 586, 119543. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hoshi, R.; Chen, S.; Yi, J.; Duan, C.; Galiano, R.D.; Zhang, H.F.; Ameer, G.A. Sustained release of stromal cell derived factor-1 from an antioxidant thermoresponsive hydrogel enhances dermal wound healing in diabetes. J. Control. Release 2016, 238, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Abella, L.; Ruiz, V.; Pérez-San Vicente, A.; Grande, H.-J.; Loinaz, I.; Dupin, D. Reactive oxygen species (ROS)-responsive biocompatible polyethylene glycol nanocomposite hydrogels with different graphene derivatives. J. Mater. Sci. 2021, 56, 10041–10052. [Google Scholar] [CrossRef]
- Gallelli, G.; Cione, E.; Serra, R.; Leo, A.; Citraro, R.; Matricardi, P.; Di Meo, C.; Bisceglia, F.; Caroleo, M.C.; Basile, S. Nano-hydrogel embedded with quercetin and oleic acid as a new formulation in the treatment of diabetic foot ulcer: A pilot study. Int. Wound J. 2020, 17, 485–490. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, Y.; Lee, J.; Hua, J.; Li, S.; Panchamukhi, A.; Yue, J.; Gou, X.; Xia, Z.; Zhu, L.; et al. A pulsatile release platform based on photo-induced imine-crosslinking hydrogel promotes scarless wound healing. Nat. Commun. 2021, 12, 1670. [Google Scholar] [CrossRef]
- Gugerell, A.; Gouya-Lechner, G.; Hofbauer, H.; Laggner, M.; Trautinger, F.; Almer, G.; Peterbauer-Scherb, A.; Seibold, M.; Hoetzenecker, W.; Dreschl, C.; et al. Safety and clinical efficacy of the secretome of stressed peripheral blood mononuclear cells in patients with diabetic foot ulcer-study protocol of the randomized, placebo-controlled, double-blind, multicenter, international phase II clinical trial MARSYAS II. Trials 2021, 22, 10. [Google Scholar] [CrossRef]
- Hu, C.; Long, L.; Cao, J.; Zhang, S.; Wang, Y. Dual-crosslinked mussel-inspired smart hydrogels with enhanced antibacterial and angiogenic properties for chronic infected diabetic wound treatment via pH-responsive quick cargo release. Chem. Eng. J. 2021, 411, 128564. [Google Scholar] [CrossRef]
- Li, H.; Yin, D.; Li, W.; Tang, Q.; Zou, L.; Peng, Q. Polydopamine-based nanomaterials and their potentials in advanced drug delivery and therapy. Colloids Surf. B 2020, 199, 111502. [Google Scholar] [CrossRef]
- Cao, M.; Li, J.; Tang, J.; Chen, C.; Zhao, Y. Gold Nanomaterials in Consumer Cosmetics Nanoproducts: Analyses, Characterization, and Dermal Safety Assessment. Small 2016, 12, 5488–5496. [Google Scholar] [CrossRef] [PubMed]
- Ben Haddada, M.; Gerometta, E.; Chawech, R.; Sorres, J.; Bialecki, A.; Pesnel, S.; Spadavecchia, J.; Morel, A.-L. Assessment of antioxidant and dermoprotective activities of gold nanoparticles as safe cosmetic ingredient. Colloids Surf. B 2020, 189, 110855. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Rong, J.; Nie, G.; Qiao, J.; Wang, H.; Wu, D.; Su, Z.; Niu, Z.; Huang, Y. Enhanced orientation of PEO polymer chains induced by nanoclays in electrospun PEO/clay composite nanofibers. Colloid. Polym. Sci. 2013, 291, 1541–1546. [Google Scholar] [CrossRef]
- Righi, T.M.; Almeida, R.S.; d’Ávila, M.A. Electrospinning of Gelatin/PEO Blends: Influence of Process Parameters in the Nanofiber Properties. Macromol. Symp. 2012, 319, 230–234. [Google Scholar] [CrossRef]
- Panzavolta, S.; Gioffrè, M.; Focarete, M.L.; Gualandi, C.; Foroni, L.; Bigi, A. Electrospun gelatin nanofibers: Optimization of genipin cross-linking to preserve fiber morphology after exposure to water. Acta Biomater. 2011, 7, 1702–1709. [Google Scholar] [CrossRef] [PubMed]
- Farokhzad, O.C.; Langer, R. Impact of nanotechnology on drug delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef]
- Sylvester, M.A.; Amini, F.; Tan, C.K. Electrospun nanofibers in wound healing. Mater. Today Proc. 2020, 29, 1–6. [Google Scholar] [CrossRef]
- Sahana, T.; Rekha, P. Biopolymers: Applications in wound healing and skin tissue engineering. Mol. Biol. Rep. 2018, 45, 2857–2867. [Google Scholar] [CrossRef]
- Mir, M.; Ali, M.N.; Barakullah, A.; Gulzar, A.; Arshad, M.; Fatima, S.; Asad, M. Synthetic polymeric biomaterials for wound healing: A review. Prog. Biomater. 2018, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Andreu, V.; Mendoza, G.; Arruebo, M.; Irusta, S. Smart dressings based on nanostructured fibers containing natural origin antimicrobial, anti-inflammatory, and regenerative compounds. Materials 2015, 8, 5154–5193. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, L.; Wang, J.; Jin, M.; Tang, Q.; Chen, Z.; Cheng, Y.; Yang, R.; Zhao, G. Electrospun nanofibers promote wound healing: Theories, techniques, and perspectives. J. Mater. Chem. B 2021, 9, 3106–3130. [Google Scholar] [CrossRef]
- Lanno, G.-M.; Ramos, C.; Preem, L.; Putrinš, M.; Laidmäe, I.; Tenson, T.; Kogermann, K. Antibacterial Porous Electrospun Fibers as Skin Scaffolds for Wound Healing Applications. ACS Omega 2020, 5, 30011–30022. [Google Scholar] [CrossRef]
- Coelho, D.S.; Veleirinho, B.; Alberti, T.; Maestri, A.; Yunes, R.; Dias, P.F.; Maraschin, M. Electrospinning technology: Designing nanofibers toward wound healing application. In Nanomaterials: Toxicity, Human Health and Environment; BoD—Books on Demand: Norderstedt, Germany, 2018; pp. 1–19. [Google Scholar]
- Beznoska, J.; Uhlik, J.; Kestlerova, A.; Kralovic, M.; Divin, R.; Fedacko, J.; Benes, J.; Benes, M.; Vocetkova, K.; Sovkova, V.; et al. PVA and PCL nanofibers are suitable for tissue covering and regeneration. Physiol. Res. 2019, 68, S501–S508. [Google Scholar] [CrossRef]
- Vocetkova, K.; Sovkova, V.; Buzgo, M.; Lukasova, V.; Divin, R.; Rampichova, M.; Blazek, P.; Zikmund, T.; Kaiser, J.; Karpisek, Z.; et al. A Simple Drug Delivery System for Platelet-Derived Bioactive Molecules, to Improve Melanocyte Stimulation in Vitiligo Treatment. Nanomaterials 2020, 10, 1801. [Google Scholar] [CrossRef]
- Vocetkova, K.; Buzgo, M.; Sovkova, V.; Bezdekova, D.; Kneppo, P.; Amler, E. Nanofibrous polycaprolactone scaffolds with adhered platelets stimulate proliferation of skin cells. Cell Prolif. 2016, 49, 568–578. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, S.; Gao, Y.; Zhai, Y. Electrospun nanofibers as a wound dressing for treating diabetic foot ulcer. Asian J. Pharm. Sci. 2019, 14, 130–143. [Google Scholar] [CrossRef]
- Hivechi, A.; Milan, P.B.; Modabberi, K.; Amoupour, M.; Ebrahimzadeh, K.; Gholipour, A.R.; Sedighi, F.; Amini, N.; Bahrami, S.H.; Rezapour, A.; et al. Synthesis and Characterization of Exopolysaccharide Encapsulated PCL/Gelatin Skin Substitute for Full-Thickness Wound Regeneration. Polymers 2021, 13, 854. [Google Scholar] [CrossRef]
- Zhu, C.; Cao, R.; Zhang, Y.; Chen, R. Metallic Ions Encapsulated in Electrospun Nanofiber for Antibacterial and Angiogenesis Function to Promote Wound Repair. Front. Cell Dev. Biol. 2021, 9, 660571. [Google Scholar] [CrossRef]
- Ahmed, M.; Zayed, M.; El-Dek, S.; Hady, M.A.; El Sherbiny, D.H.; Uskoković, V. Nanofibrous ε-polycaprolactone scaffolds containing Ag-doped magnetite nanoparticles: Physicochemical characterization and biological testing for wound dressing applications in vitro and in vivo. Bioact. Mater. 2021, 6, 2070–2088. [Google Scholar] [CrossRef] [PubMed]
- Mirmajidi, T.; Chogan, F.; Rezayan, A.H.; Sharifi, A.M. In vitro and in vivo evaluation of a nanofiber wound dressing loaded with melatonin. Int. J. Pharm. 2021, 596, 120213. [Google Scholar] [CrossRef]
- Dankova, J.; Buzgo, M.; Vejpravova, J.; Kubickova, S.; Sovkova, V.; Vyslouzilova, L.; Mantlikova, A.; Necas, A.; Amler, E. Highly efficient mesenchymal stem cell proliferation on poly-epsilon-caprolactone nanofibers with embedded magnetic nanoparticles. Int. J. Nanomed. 2015, 10, 7307–7317. [Google Scholar] [CrossRef]
- Graça, M.F.P.; de Melo-Diogo, D.; Correia, I.J.; Moreira, A.F. Electrospun Asymmetric Membranes as Promising Wound Dressings: A Review. Pharmaceutics 2021, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Xu, Z.; Ikegami, Y.; Yoshida, K.; Sakai, Y.; Joshi, A.; Kaur, T.; Nakao, Y.; Yamashita, Y.-I.; Baba, H. Exploiting synergistic effect of externally loaded bFGF and endogenous growth factors for accelerated wound healing using heparin functionalized PCL/gelatin co-spun nanofibrous patches. Chem. Eng. J. 2021, 404, 126518. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, A.; Dey, A.D.; Behl, T.; Chadha, S. Stem cells and growth factors-based delivery approaches for chronic wound repair and regeneration: A promise to heal from within. Life Sci. 2021, 268, 118932. [Google Scholar] [CrossRef]
- Başaran, D.D.A.; Gündüz, U.; Tezcaner, A.; Keskin, D. Topical delivery of heparin from PLGA nanoparticles entrapped in nanofibers of sericin/gelatin scaffolds for wound healing. Int. J. Pharm. 2021, 597, 120207. [Google Scholar] [CrossRef]
- Marshall, C.D.; Hu, M.S.; Leavitt, T.; Barnes, L.A.; Lorenz, H.P.; Longaker, M.T. Cutaneous scarring: Basic science, current treatments, and future directions. Adv. Wound Care 2018, 7, 29–45. [Google Scholar] [CrossRef]
- Mulholland, E.J. Electrospun biomaterials in the treatment and prevention of scars in skin wound healing. Front. Bioeng. Biotechnol. 2020, 8, 481. [Google Scholar] [CrossRef]
- Basar, A.; Castro, S.; Torres-Giner, S.; Lagaron, J.; Sasmazel, H.T. Novel poly (ε-caprolactone)/gelatin wound dressings prepared by emulsion electrospinning with controlled release capacity of Ketoprofen anti-inflammatory drug. Mater. Sci. Eng. C 2017, 81, 459–468. [Google Scholar] [CrossRef]
- Atiyeh, B.S.; Amm, C.A.; El Musa, K.A. Improved scar quality following primary and secondary healing of cutaneous wounds. Aesthetic Plast. Surg. 2003, 27, 411–417. [Google Scholar] [CrossRef]
- Woo, H.; Joo, O.; Min, J.; Mi, B.; Jung, H.; Ri, Y.; Chae, M.; Hyeon, S.; Ren, J.; Seok, C. Wound healing effect of electrospun silk fibroin nanomatrix in burn-model. Int. J. Biol. Macromol. 2016, 85, 29–39. [Google Scholar]
- Hadjizadeh, A.; Ghasemkhah, F.; Ghasemzaie, N. Polymeric scaffold based gene delivery strategies to improve angiogenesis in tissue engineering: A review. Polym. Rev. 2017, 57, 505–556. [Google Scholar] [CrossRef]
- Venkataraman, M.; Nagarsenker, M. Silver sulfadiazine nanosystems for burn therapy. AAPS PharmSciTech 2013, 14, 254–264. [Google Scholar] [CrossRef][Green Version]
- Kurowska, A.; Ghate, V.; Kodoth, A.; Shah, A.; Shah, A.; Vishalakshi, B.; Prakash, B.; Lewis, S.A. Non-Propellant Foams of Green Nano-Silver and Sulfadiazine: Development and In Vivo Evaluation for Burn Wounds. Pharm. Res. 2019, 36, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Alipour, R.; Khorshidi, A.; Shojaei, A.F.; Mashayekhi, F.; Moghaddam, M.J.M. Silver Sulfadiazine-loaded PVA/CMC Nanofibers for the Treatment of Wounds Caused by Excision. Fibers Polym. 2019, 20, 2461–2469. [Google Scholar] [CrossRef]
- Ahmed, M.E.; Khalaf, Z.Z.; Ghafil, J.A.; Al-Awadi, A.Q. Effects of Silver Nanoparticles on Biofilms of Streptococcus Spps. Exec. Ed. 2018, 9, 1216. [Google Scholar] [CrossRef]
- Pérez-Díaz, M.A.; Silva-Bermudez, P.; Jiménez-López, B.; Martínez-López, V.; Melgarejo-Ramírez, Y.; Brena-Molina, A.; Ibarra, C.; Baeza, I.; Martínez-Pardo, M.E.; Reyes-Frías, M.L. Silver-pig skin nanocomposites and mesenchymal stem cells: Suitable antibiofilm cellular dressings for wound healing. J. Nanobiotechnol. 2018, 16, 1–16. [Google Scholar] [CrossRef]
- El-Deeb, N.M.; Abo-Eleneen, M.A.; Al-Madboly, L.A.; Sharaf, M.M.; Othman, S.S.; Ibrahim, O.M.; Mubarak, M.S. Biogenically Synthesized Polysaccharides-Capped Silver Nanoparticles: Immunomodulatory and Antibacterial Potentialities Against Resistant Pseudomonas aeruginosa. Front. Bioeng. Biotechnol. 2020, 8, 643. [Google Scholar] [CrossRef]
- Ahumada, M.; Lazurko, C.; Khatoon, Z.; Goel, K.; Sedlakova, V.; Cimenci, C.E.; Zhang, L.; Mah, T.-F.; Franco, W.; Suuronen, E.J. Multifunctional Nano and Collagen-Based Therapeutic Materials for Skin Repair. ACS Biomater. Sci. Eng. 2020, 6, 1124–1134. [Google Scholar]
- Krutmann, J.; Schalka, S.; Watson, R.E.B.; Wei, L.; Morita, A. Daily photoprotection to prevent photoaging. Photodermatol. Photoimmunol. Photomed. 2021. [Google Scholar] [CrossRef]
- Neale, R.; Khan, S.; Lucas, R.; Waterhouse, M.; Whiteman, D.; Olsen, C. The effect of sunscreen on vitamin D: A review. Br. J. Dermatol. 2019, 181, 907–915. [Google Scholar] [CrossRef]
- Bikle, D. Do sunscreens block vitamin D production? A critical review by an international panel of experts. Br. J. Dermatol. 2019, 181, 884. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Fernandes, A.R.; Martins-Gomes, C.; Coutinho, T.E.; Durazzo, A.; Lucarini, M.; Souto, S.B.; Silva, A.M.; Santini, A. Nanomaterials for skin delivery of cosmeceuticals and pharmaceuticals. Appl. Sci. 2020, 10, 1594. [Google Scholar] [CrossRef]
- Dhapte-Pawar, V.; Kadam, S.; Saptarsi, S.; Kenjale, P.P. Nanocosmeceuticals: Facets and aspects. Future Sci. OA 2020, 6, FSO613. [Google Scholar] [CrossRef]
- Cao, M.; Li, B.; Guo, M.; Liu, Y.; Zhang, L.; Wang, Y.; Hu, B.; Li, J.; Sutherland, D.S.; Wang, L. In vivo percutaneous permeation of gold nanomaterials in consumer cosmetics: Implication in dermal safety assessment of consumer nanoproducts. Nanotoxicology 2020, 15, 131–144. [Google Scholar] [CrossRef]
- Beamer, C.A. Toxicity of Nanomaterials to the Host and the Environment. In Mucosal Delivery of Drugs and Biologics in Nanoparticles; Springer: Berlin/Heidelberg, Germany, 2020; pp. 233–245. [Google Scholar]
- Sengupta, J.; Ghosh, S.; Datta, P.; Gomes, A.; Gomes, A. Physiologically important metal nanoparticles and their toxicity. J. Nanosci. Nanotechnol. 2014, 14, 990–1006. [Google Scholar] [CrossRef]
- He, Y.; Zhang, W.; Guo, T.; Zhang, G.; Qin, W.; Zhang, L.; Wang, C.; Zhu, W.; Yang, M.; Hu, X. Drug nanoclusters formed in confined nano-cages of CD-MOF: Dramatic enhancement of solubility and bioavailability of azilsartan. Acta Pharm. Sin. B 2019, 9, 97–106. [Google Scholar] [CrossRef]
- Carnovale, C.; Bryant, G.; Shukla, R.; Bansal, V. Identifying trends in gold nanoparticle toxicity and uptake: Size, shape, capping ligand, and biological corona. ACS Omega 2019, 4, 242–256. [Google Scholar] [CrossRef]
- Ilić, K.; Hartl, S.; Galić, E.; Tetyczka, C.; Pem, B.; Barbir, R.; Milić, M.; Vrček, I.V.; Roblegg, E.; Pavičić, I. Interaction of Differently Coated Silver Nanoparticles with Skin and Oral Mucosal Cells. J. Pharm. Sci. 2021, 110, 2250–2261. [Google Scholar] [CrossRef] [PubMed]
- Guilger-Casagrande, M.; Germano-Costa, T.; Bilesky-José, N.; Pasquoto-Stigliani, T.; Carvalho, L.; Fraceto, L.F.; de Lima, R. Influence of the capping of biogenic silver nanoparticles on their toxicity and mechanism of action towards Sclerotinia sclerotiorum. J. Nanobiotechnol. 2021, 19, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Bengalli, R.; Colantuoni, A.; Perelshtein, I.; Gedanken, A.; Collini, M.; Mantecca, P.; Fiandra, L. In vitro skin toxicity of CuO and ZnO nanoparticles: Application in the safety assessment of antimicrobial coated textiles. NanoImpact 2021, 21, 100282. [Google Scholar] [CrossRef]
- Hashempour, S.; Ghanbarzadeh, S.; Maibach, H.I.; Ghorbani, M.; Hamishehkar, H. Skin toxicity of topically applied nanoparticles. Ther. Deliv. 2019, 10, 383–396. [Google Scholar] [CrossRef]
| Representative Image of Nanomaterial | Name | Materials Used in Skin Regeneration |
|---|---|---|
 | Nanocrystal | Silver, gold, carbon, polymers, etc. |
 | Nanoparticle | Silver, gold, copper, zinc oxide, copper oxide, sulfide, etc. |
 | Hydrogel | Polysaccharides, hyaluronic acid, chitosan, polyvinyl alcohol, sodium alginate, cyclodextrin, polyacrylic acid, polyvinyl pyrrolidone, polyvinyl acetate, collagen, pectin, chitin, etc. |
 | Nanofiber | Polycaprolactone, polyethylene glycol, polylactic acid, polyvinyl pyrrolidone, etc. |
| Nanoparticle | Description | Function/Use |
|---|---|---|
| Gold and silver nanoclusters | Size between 1.1 and 1.6 nm | Skin repair in rat models in vivo [45]. Enhance cell proliferation in vitro and full thickness wound healing [50]. |
| AuNPs | Biosynthesised AuNPs are highly biocompatible and have less side effects [53] | Reduction of inflammation, promotion of granulation tissue formation [46]. Antimicrobial activity [54]. Skin rejuvenation properties [55] including ability to reduce wrinkles [56] improve skin brightening, promote skin healing, have a cleansing effect, reduce inflammation and ROS damage, slow down collagen depletion [57] and elastin degradation [58]. |
| AgNPs | Enhance keratocyte and fibroblast proliferation, suppress the innate immune system increasing wound healing rate and decrease the scarring process rate [47]. Antimicrobial activity [59,60,61]. | |
| Nanoceria | Spherical cerium oxide nanoparticles, 3–5 nm | In low doses are able to counteract the effects of UVA-induced photodamage, favouring cell viability, migration, and proliferation [49]. |
| Copper nanoparticles (CuNPs and CuS) | 20, 40 and 80 nm, all spherical in shape | Promotion of size- and dose-dependent endothelial cell migration and proliferation, accelerate full-thickness skin wounds healing. Increased collagen 1A1 expression in vitro and increased formation of new blood vessels in rat models [51]. Antimicrobial activity [54,62,63,64]. |
| Zinc ferrite (ZnFe2O4) | Antimicrobial activity via multiple mechanisms [65]. | |
| Silver sulfadiazine | Antimicrobial activity in particular against biofilms [66]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellu, E.; Medici, S.; Coradduzza, D.; Cruciani, S.; Amler, E.; Maioli, M. Nanomaterials in Skin Regeneration and Rejuvenation. Int. J. Mol. Sci. 2021, 22, 7095. https://doi.org/10.3390/ijms22137095
Bellu E, Medici S, Coradduzza D, Cruciani S, Amler E, Maioli M. Nanomaterials in Skin Regeneration and Rejuvenation. International Journal of Molecular Sciences. 2021; 22(13):7095. https://doi.org/10.3390/ijms22137095
Chicago/Turabian StyleBellu, Emanuela, Serenella Medici, Donatella Coradduzza, Sara Cruciani, Evzen Amler, and Margherita Maioli. 2021. "Nanomaterials in Skin Regeneration and Rejuvenation" International Journal of Molecular Sciences 22, no. 13: 7095. https://doi.org/10.3390/ijms22137095
APA StyleBellu, E., Medici, S., Coradduzza, D., Cruciani, S., Amler, E., & Maioli, M. (2021). Nanomaterials in Skin Regeneration and Rejuvenation. International Journal of Molecular Sciences, 22(13), 7095. https://doi.org/10.3390/ijms22137095







