Thrombopoietin Contributes to Enhanced Platelet Activation in Patients with Type 1 Diabetes Mellitus
Abstract
1. Introduction
2. Results
2.1. Study Subjects
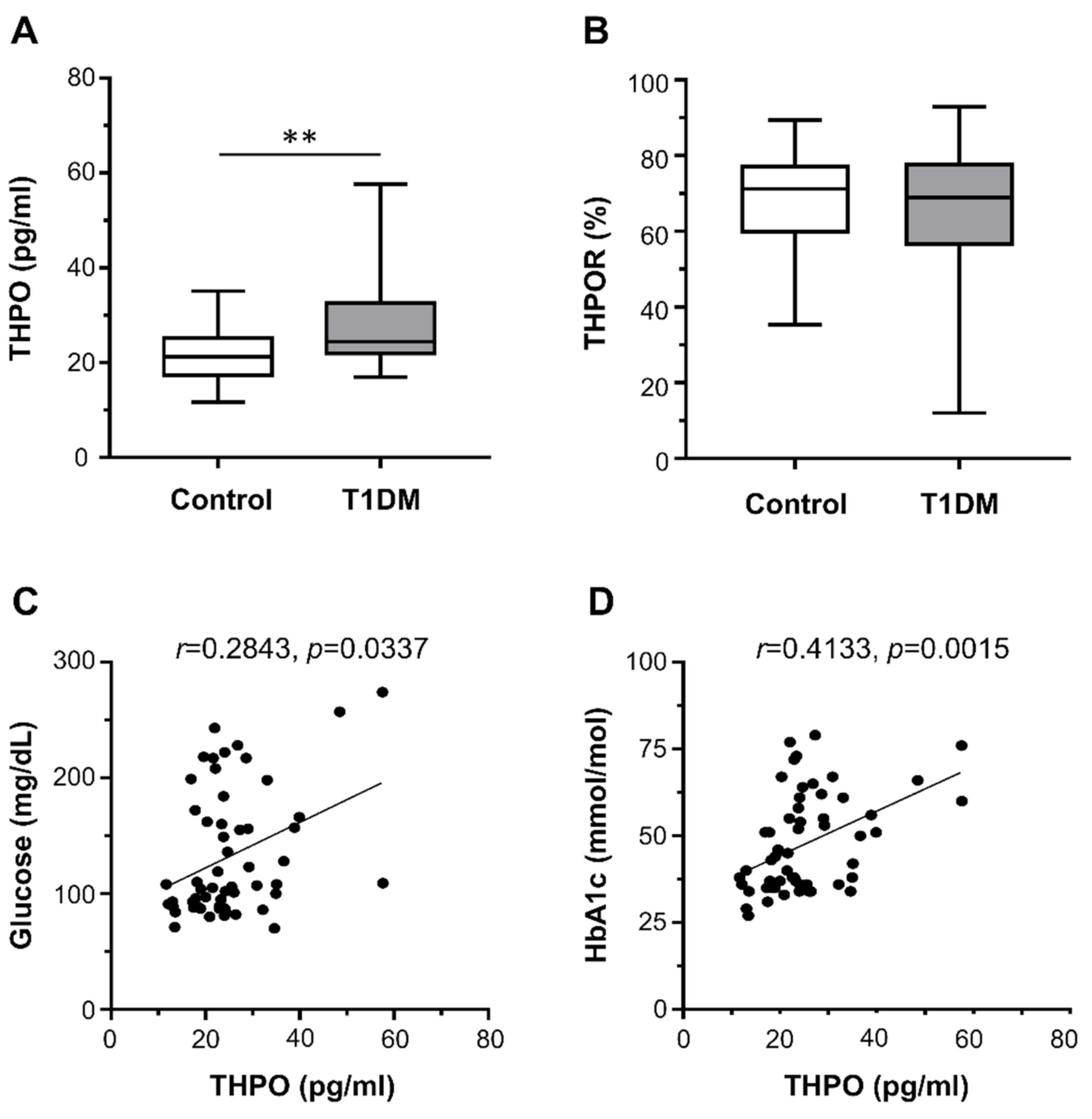
2.2. THPO Concentrations and Platelet THPOR Expression in Type 1 Diabetes Mellitus Patients
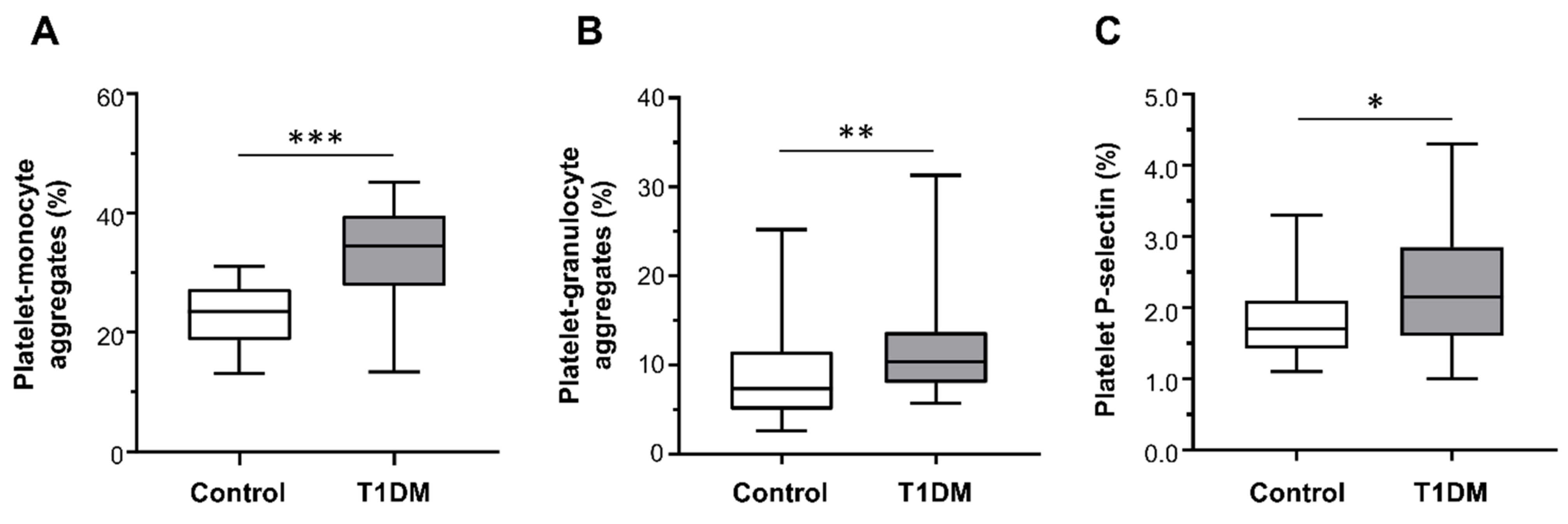
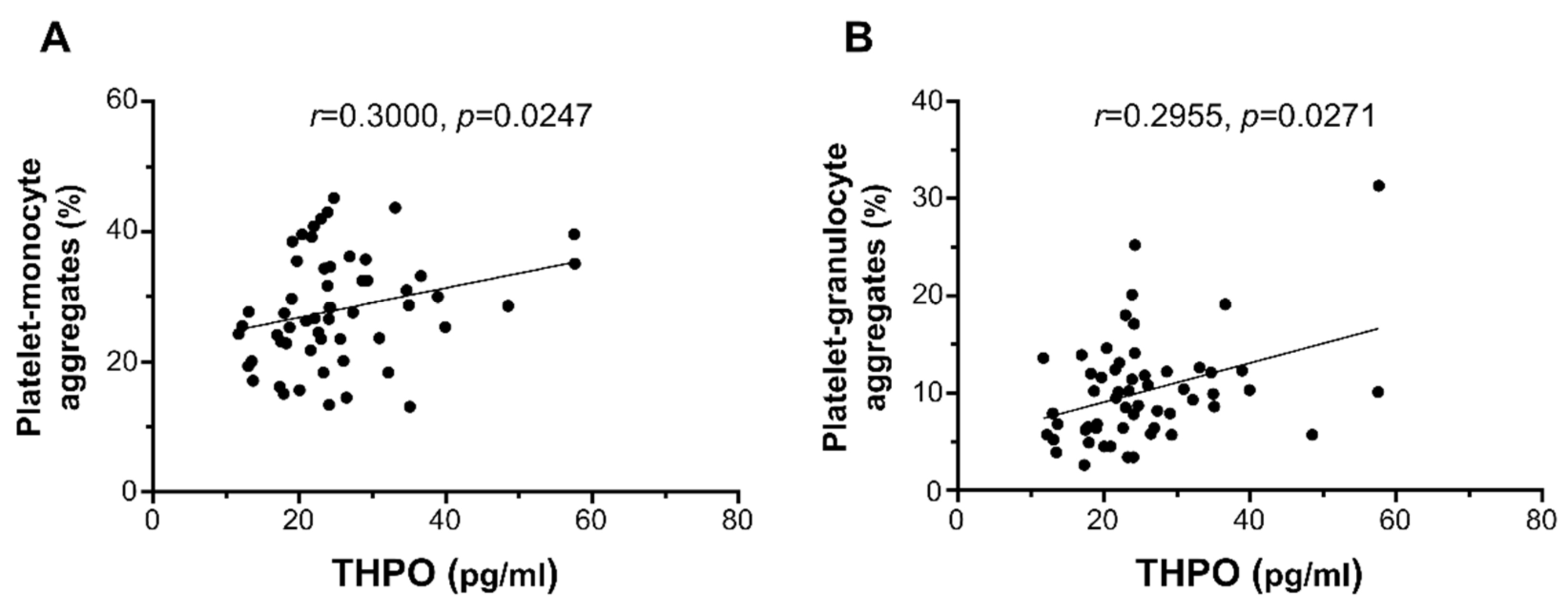
2.3. Ex Vivo Platelet Activation Studies
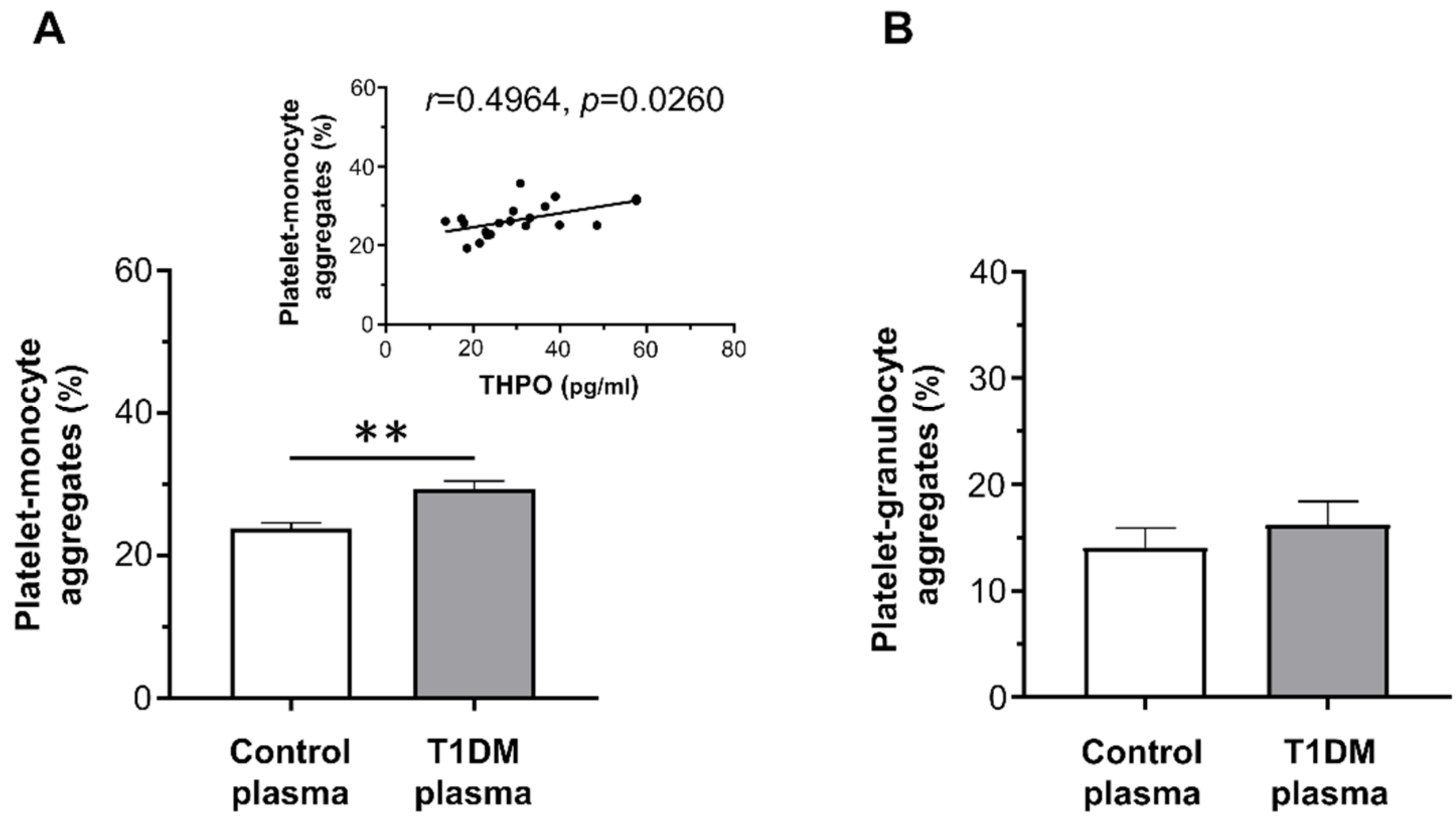
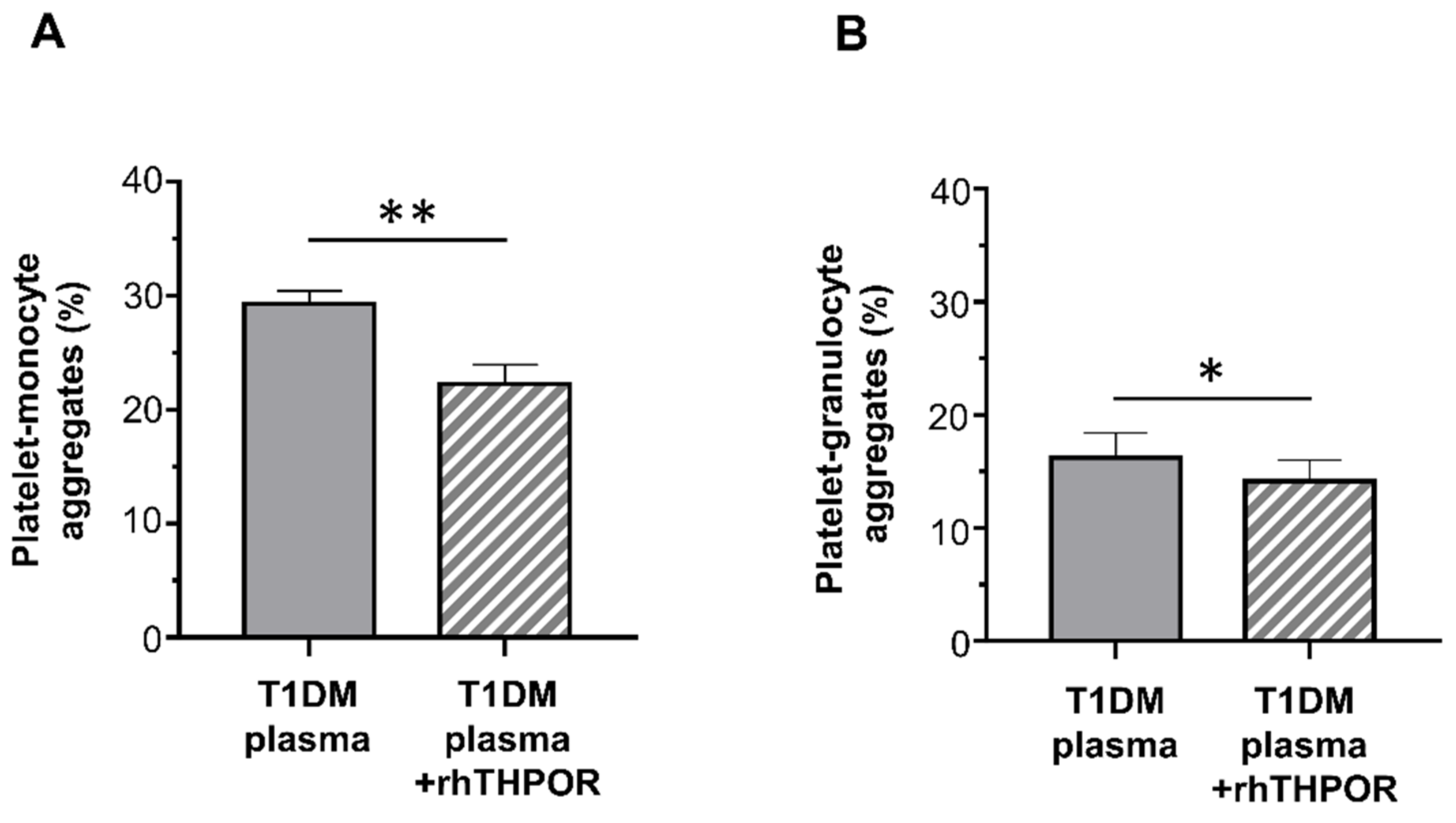
2.4. Effect of Plasma of Type 1 Diabetes Mellitus Patients on Platelet Activation In Vitro
3. Discussion
4. Materials and Methods
4.1. Patients and Healthy Controls
4.2. Blood Collection Protocol
4.3. Leucocyte–Platelet Adhesion and Platelet THPOR Expression Ex Vivo
4.4. Leucocyte–Platelet Adhesion In Vitro
4.5. Biochemical Analyses
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, M.; Tang, R.; Huang, F.; Zhong, T.; Chen, Y.; Li, X.; Zhou, Z. Cardiovascular disease in patients with type 1 diabetes: Early evaluation, risk factors and possible relation with cardiac autoimmunity. Diabetes Metab. Res. Rev. 2020. [Google Scholar] [CrossRef]
- Vergès, B. Cardiovascular disease in type 1 diabetes: A review of epidemiological data and underlying mechanisms. Diabetes Metab. 2020, 46, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Bratseth, V.; Margeirsdottir, H.D.; Chiva-Blanch, G.; Heier, M.; Solheim, S.; Arnesen, H.; Dahl-Jørgensen, K.; Seljeflot, I. Annexin V+ Microvesicles in Children and Adolescents with Type 1 Diabetes: A Prospective Cohort Study. J. Diabetes Res. 2020, 2020, 7216863. [Google Scholar] [CrossRef] [PubMed]
- Orchard, T.J.; Costacou, T. Cardiovascular complications of type 1 diabetes: Update on the renal link. Acta Diabetol. 2017, 54, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.N.; Bailey, R.; Wu, M.; Foster, N.C.; Pop-Busui, R.; Katz, M.; Crandall, J.; Bacha, F.; Nadeau, K.; Libman, I.; et al. Risk Factors for Cardiovascular Disease (CVD) in Adults with Type 1 Diabetes: Findings from Prospective Real-life T1D Exchange Registry. J. Clin. Endocrinol. Metab. 2020, 105. [Google Scholar] [CrossRef]
- Schneider, D.J. Factors contributing to increased platelet reactivity in people with diabetes. Diabetes Care 2009, 32, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Zahran, A.M.; El-Badawy, O.; Mohamad, I.L.; Tamer, D.M.; Abdel-Aziz, S.M.; Elsayh, K.I. Platelet Activation and Platelet-Leukocyte Aggregates in Type I Diabetes Mellitus. Clin. Appl. Thromb. Hemost. 2018, 24, 230S–239S. [Google Scholar] [CrossRef]
- Angiolillo, D.J. Antiplatelet therapy in diabetes: Efficacy and limitations of current treatment strategies and future directions. Diabetes Care 2009, 32, 531–540. [Google Scholar] [CrossRef]
- Rivas Rios, J.R.; Franchi, F.; Rollini, F.; Angiolillo, D.J. Diabetes and antiplatelet therapy: From bench to bedside. Cardiovasc. Diagn. Ther. 2018, 8, 594–609. [Google Scholar] [CrossRef]
- Kaushansky, K. Thrombopoietin: A tool for understanding thrombopoiesis. J. Thromb. Haemost. 2003, 1, 1587–1592. [Google Scholar] [CrossRef]
- Kuter, D.J.; Begley, C.G. Recombinant human thrombopoietin: Basic biology and evaluation of clinical studies. Blood 2002, 100, 3457–3469. [Google Scholar] [CrossRef] [PubMed]
- Montrucchio, G.; Brizzi, M.F.; Calosso, G.; Marengo, S.; Pegoraro, L.; Camussi, G. Effects of recombinant human megakaryocyte growth and development factor on platelet activation. Blood 1996, 87, 2762–2768. [Google Scholar] [CrossRef] [PubMed]
- Oda, A.; Miyakawa, Y.; Druker, B.J.; Ozaki, K.; Yabusaki, K.; Shirasawa, Y.; Handa, M.; Kato, T.; Miyazaki, H.; Shimosaka, A.; et al. Thrombopoietin primes human platelet aggregation induced by shear stress and by multiple agonists. Blood 1996, 87, 4664–4670. [Google Scholar] [CrossRef]
- Tibbles, H.E.; Navara, C.S.; Hupke, M.A.; Vassilev, A.O.; Uckun, F.M. Thrombopoietin induces p-selectin expression on platelets and subsequent platelet/leukocyte interactions. Biochem. Biophys. Res. Commun. 2002, 292, 987–991. [Google Scholar] [CrossRef]
- Lupia, E.; Bosco, O.; Bergerone, S.; Dondi, A.E.; Goffi, A.; Oliaro, E.; Cordero, M.; Del Sorbo, L.; Trevi, G.; Montrucchio, G. Thrombopoietin Contributes to Enhanced Platelet Activation in Patients With Unstable Angina. J. Am. Coll. Cardiol. 2006, 48, 2195–2203. [Google Scholar] [CrossRef]
- Lupia, E.; Bosco, O.; Mariano, F.; Dondi, A.E.; Goffi, A.; Spatola, T.; Cuccurullo, A.; Tizzani, P.; Brondino, G.; Stella, M.; et al. Elevated thrombopoietin in plasma of burned patients without and with sepsis enhances platelet activation. J. Thromb. Haemost. 2009, 7, 1000–1008. [Google Scholar] [CrossRef]
- Lupia, E.; Bosco, O.; Goffi, A.; Poletto, C.; Locatelli, S.; Spatola, T.; Cuccurullo, A.; Montrucchio, G. Thrombopoietin contributes to enhanced platelet activation in cigarette smokers. Atherosclerosis 2010, 210, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Keaney, J.F.; Loscalzo, J. Diabetes, oxidative stress, and platelet activation. Circulation 1999, 99, 189–191. [Google Scholar] [CrossRef]
- Hu, H.; Li, N.; Yngen, M.; Ostenson, C.G.; Wallén, N.H.; Hjemdahl, P. Enhanced leukocyte-platelet cross-talk in Type 1 diabetes mellitus: Relationship to microangiopathy. J. Thromb. Haemost. 2004, 2, 58–64. [Google Scholar] [CrossRef]
- Véricel, E.; Januel, C.; Carreras, M.; Moulin, P.; Lagarde, M. Diabetic patients without vascular complications display enhanced basal platelet activation and decreased antioxidant status. Diabetes 2004, 53, 1046–1051. [Google Scholar] [CrossRef]
- Wisinski, J.A.; Kimple, M.E. Platelet Dysfunction in Type 1 Diabetes: Stressing the Thromboxanes. Diabetes 2016, 65, 349–351. [Google Scholar] [CrossRef][Green Version]
- Harding, S.A.; Sommerfield, A.J.; Sarma, J.; Twomey, P.J.; Newby, D.E.; Frier, B.M.; Fox, K.A. Increased CD40 ligand and platelet-monocyte aggregates in patients with type 1 diabetes mellitus. Atherosclerosis 2004, 176, 321–325. [Google Scholar] [CrossRef]
- Freedman, J.E.; Loscalzo, J. Platelet-monocyte aggregates: Bridging thrombosis and inflammation. Circulation 2002, 105, 2130–2132. [Google Scholar] [CrossRef]
- Lupia, E.; Goffi, A.; Bosco, O.; Montrucchio, G. Thrombopoietin as biomarker and mediator of cardiovascular damage in critical diseases. Mediators Inflamm. 2012, 2012, 390892. [Google Scholar] [CrossRef] [PubMed]
- Lupia, E.; Spatola, T.; Cuccurullo, A.; Bosco, O.; Mariano, F.; Pucci, A.; Ramella, R.; Alloatti, G.; Montrucchio, G. Thrombopoietin modulates cardiac contractility in vitro and contributes to myocardial depressing activity of septic shock serum. Basic Res. Cardiol. 2010, 105, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Wolber, E.M.; Jelkmann, W. Interleukin-6 increases thrombopoietin production in human hepatoma cells HepG2 and Hep3B. J. Interferon Cytokine Res. 2000, 20, 499–506. [Google Scholar] [CrossRef]
- Wolber, E.M.; Haase, B.; Jelkmann, W. Thrombopoietin production in human hepatic cell cultures (HepG2) is resistant to IFN-alpha, IFN-beta, and IFN-gamma treatment. J. Interferon Cytokine Res. 2002, 22, 1185–1189. [Google Scholar] [CrossRef]
- Coban, E.; Bostan, F.; Ozdogan, M. The mean platelet volume in subjects with impaired fasting glucose. Platelets 2006, 17, 67–69. [Google Scholar] [CrossRef]
- Santilli, F.; Formoso, G.; Sbraccia, P.; Averna, M.; Miccoli, R.; Di Fulvio, P.; Ganci, A.; Pulizzi, N.; Lattanzio, S.; Ciabattoni, G.; et al. Postprandial hyperglycemia is a determinant of platelet activation in early type 2 diabetes mellitus. J. Thromb. Haemost. 2010, 8, 828–837. [Google Scholar] [CrossRef]
- Guthikonda, S.; Lev, E.I.; Patel, R.; DeLao, T.; Bergeron, A.L.; Dong, J.F.; Kleiman, N.S. Reticulated platelets and uninhibited COX-1 and COX-2 decrease the antiplatelet effects of aspirin. J. Thromb. Haemost. 2007, 5, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Guthikonda, S.; Alviar, C.L.; Vaduganathan, M.; Arikan, M.; Tellez, A.; DeLao, T.; Granada, J.F.; Dong, J.F.; Kleiman, N.S.; Lev, E.I. Role of reticulated platelets and platelet size heterogeneity on platelet activity after dual antiplatelet therapy with aspirin and clopidogrel in patients with stable coronary artery disease. J. Am. Coll. Cardiol. 2008, 52, 743–749. [Google Scholar] [CrossRef]
- Folman, C.C.; Linthorst, G.E.; van Mourik, J.; van Willigen, G.; de Jonge, E.; Levi, M.; de Haas, M.; von dem Borne, A.E. Platelets release thrombopoietin (Tpo) upon activation: Another regulatory loop in thrombocytopoiesis? Thromb. Haemost. 2000, 83, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Brizzi, M.F.; Battaglia, E.; Montrucchio, G.; Dentelli, P.; Del Sorbo, L.; Garbarino, G.; Pegoraro, L.; Camussi, G. Thrombopoietin stimulates endothelial cell motility and neoangiogenesis by a platelet-activating factor-dependent mechanism. Circ. Res. 1999, 84, 785–796. [Google Scholar] [CrossRef]
- Brizzi, M.F.; Battaglia, E.; Rosso, A.; Strippoli, P.; Montrucchio, G.; Camussi, G.; Pegoraro, L. Regulation of polymorphonuclear cell activation by thrombopoietin. J. Clin. Investig. 1997, 99, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, P.; Basili, S.; Falco, A.; Davì, G. Platelet activation in type 2 diabetes mellitus. J. Thromb. Haemost. 2004, 2, 1282–1291. [Google Scholar] [CrossRef]
- Rollini, F.; Franchi, F.; Muñiz-Lozano, A.; Angiolillo, D.J. Platelet function profiles in patients with diabetes mellitus. J. Cardiovasc. Transl. Res. 2013, 6, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Santilli, F.; Simeone, P.; Liani, R.; Davì, G. Platelets and diabetes mellitus. Prostaglandins Other Lipid Mediat. 2015, 120, 28–39. [Google Scholar] [CrossRef]
- Russo, I.; Penna, C.; Musso, T.; Popara, J.; Alloatti, G.; Cavalot, F.; Pagliaro, P. Platelets, diabetes and myocardial ischemia/reperfusion injury. Cardiovasc. Diabetol. 2017, 16, 71. [Google Scholar] [CrossRef]
- Carrizzo, A.; Izzo, C.; Oliveti, M.; Alfano, A.; Virtuoso, N.; Capunzo, M.; Di Pietro, P.; Calabrese, M.; de Simone, E.; Sciarretta, S.; et al. The Main Determinants of Diabetes Mellitus Vascular Complications: Endothelial Dysfunction and Platelet Hyperaggregation. Int. J. Mol. Sci. 2018, 19, 2968. [Google Scholar] [CrossRef]
- Barale, C.; Cavalot, F.; Frascaroli, C.; Bonomo, K.; Morotti, A.; Guerrasio, A.; Russo, I. Association between High On-Aspirin Platelet Reactivity and Reduced Superoxide Dismutase Activity in Patients Affected by Type 2 Diabetes Mellitus or Primary Hypercholesterolemia. Int. J. Mol. Sci. 2020, 21, 4983. [Google Scholar] [CrossRef]
- Anfossi, G.; Mularoni, E.M.; Burzacca, S.; Ponziani, M.C.; Massucco, P.; Mattiello, L.; Cavalot, F.; Trovati, M. Platelet resistance to nitrates in obesity and obese NIDDM, and normal platelet sensitivity to both insulin and nitrates in lean NIDDM. Diabetes Care 1998, 21, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Santilli, F.; Lapenna, D.; La Barba, S.; Davì, G. Oxidative stress-related mechanisms affecting response to aspirin in diabetes mellitus. Free Radic Biol. Med. 2015, 80, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Calverley, D.C.; Hacker, M.R.; Loda, K.A.; Brass, E.; Buchanan, T.A.; Tsao-Wei, D.D.; Groshen, S. Increased platelet Fc receptor expression as a potential contributing cause of platelet hypersensitivity to collagen in diabetes mellitus. Br. J. Haematol. 2003, 121, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.S.; Blann, A.D.; Lip, G.Y. Soluble CD40 ligand, soluble P-selectin, interleukin-6, and tissue factor in diabetes mellitus: Relationships to cardiovascular disease and risk factor intervention. Circulation 2004, 109, 2524–2528. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alzahrani, S.H.; Ajjan, R.A. Coagulation and fibrinolysis in diabetes. Diab. Vasc. Dis. Res. 2010, 7, 260–273. [Google Scholar] [CrossRef]
- Kearney, K.; Tomlinson, D.; Smith, K.; Ajjan, R. Hypofibrinolysis in diabetes: A therapeutic target for the reduction of cardiovascular risk. Cardiovasc. Diabetol. 2017, 16, 34. [Google Scholar] [CrossRef]
- Ajjan, R.A.; Gamlen, T.; Standeven, K.F.; Mughal, S.; Hess, K.; Smith, K.A.; Dunn, E.J.; Anwar, M.M.; Rabbani, N.; Thornalley, P.J.; et al. Diabetes is associated with posttranslational modifications in plasminogen resulting in reduced plasmin generation and enzyme-specific activity. Blood 2013, 122, 134–142. [Google Scholar] [CrossRef]
- Sumaya, W.; Wallentin, L.; James, S.K.; Siegbahn, A.; Gabrysch, K.; Bertilsson, M.; Himmelmann, A.; Ajjan, R.A.; Storey, R.F. Fibrin clot properties independently predict adverse clinical outcome following acute coronary syndrome: A PLATO substudy. Eur. Heart J. 2018, 39, 1078–1085. [Google Scholar] [CrossRef]
- Yngen, M.; Ostenson, C.G.; Li, N.; Hjemdahl, P.; Wallén, N.H. Acute hyperglycemia increases soluble P-selectin in male patients with mild diabetes mellitus. Blood Coagul. Fibrinolysis 2001, 12, 109–116. [Google Scholar] [CrossRef]
- Vaidyula, V.R.; Rao, A.K.; Mozzoli, M.; Homko, C.; Cheung, P.; Boden, G. Effects of hyperglycemia and hyperinsulinemia on circulating tissue factor procoagulant activity and platelet CD40 ligand. Diabetes 2006, 55, 202–208. [Google Scholar] [CrossRef]
- Vaidyula, V.R.; Boden, G.; Rao, A.K. Platelet and monocyte activation by hyperglycemia and hyperinsulinemia in healthy subjects. Platelets 2006, 17, 577–585. [Google Scholar] [CrossRef]
- Yngen, M.; Norhammar, A.; Hjemdahl, P.; Wallén, N.H. Effects of improved metabolic control on platelet reactivity in patients with type 2 diabetes mellitus following coronary angioplasty. Diab. Vasc. Dis. Res. 2006, 3, 52–56. [Google Scholar] [CrossRef]
- Undas, A.; Wiek, I.; Stêpien, E.; Zmudka, K.; Tracz, W. Hyperglycemia is associated with enhanced thrombin formation, platelet activation, and fibrin clot resistance to lysis in patients with acute coronary syndrome. Diabetes Care 2008, 31, 1590–1595. [Google Scholar] [CrossRef]
- Winocour, P.D.; Watala, C.; Perry, D.W.; Kinlough-Rathbone, R.L. Decreased platelet membrane fluidity due to glycation or acetylation of membrane proteins. Thromb. Haemost. 1992, 68, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Watala, C.; Golański, J.; Boncler, M.A.; Pietrucha, T.; Gwoździński, K. Membrane lipid fluidity of blood platelets: A common denominator that underlies the opposing actions of various agents that affect platelet activation in whole blood. Platelets 1998, 9, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Keating, F.K.; Sobel, B.E.; Schneider, D.J. Effects of increased concentrations of glucose on platelet reactivity in healthy subjects and in patients with and without diabetes mellitus. Am. J. Cardiol 2003, 92, 1362–1365. [Google Scholar] [CrossRef] [PubMed]
- Assert, R.; Scherk, G.; Bumbure, A.; Pirags, V.; Schatz, H.; Pfeiffer, A.F. Regulation of protein kinase C by short term hyperglycaemia in human platelets in vivo and in vitro. Diabetologia 2001, 44, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Massucco, P.; Mattiello, L.; Russo, I.; Traversa, M.; Doronzo, G.; Anfossi, G.; Trovati, M. High glucose rapidly activates the nitric oxide/cyclic nucleotide pathway in human platelets via an osmotic mechanism. Thromb. Haemost. 2005, 93, 517–526. [Google Scholar] [CrossRef]
- Bergandi, L.; Cordero, M.; Anselmino, M.; Ferraro, G.; Ravera, L.; Dalmasso, P.; Moiraghi, C.; Trevi, G.P.; Ghigo, D.; Bosia, A.; et al. Altered nitric oxide/cGMP platelet signaling pathway in platelets from patients with acute coronary syndromes. Clin. Res. Cardiol. 2010, 99, 557–564. [Google Scholar] [CrossRef][Green Version]
- Vivas, D.; García-Rubira, J.C.; Bernardo, E.; Angiolillo, D.J.; Martín, P.; Calle-Pascual, A.; Núñez-Gil, I.; Macaya, C.; Fernández-Ortiz, A. Effects of intensive glucose control on platelet reactivity in patients with acute coronary syndromes. Results of the CHIPS Study (“Control de Hiperglucemia y Actividad Plaquetaria en Pacientes con Sindrome Coronario Agudo”). Heart 2011, 97, 803–809. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Malmberg, K. Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group. BMJ 1997, 314, 1512–1515. [Google Scholar] [CrossRef]
- Harker, L.A.; Marzec, U.M.; Hunt, P.; Kelly, A.B.; Tomer, A.; Cheung, E.; Hanson, S.R.; Stead, R.B. Dose-response effects of pegylated human megakaryocyte growth and development factor on platelet production and function in nonhuman primates. Blood 1996, 88, 511–521. [Google Scholar] [CrossRef]
- Zaccardi, F.; Rizzi, A.; Petrucci, G.; Ciaffardini, F.; Tanese, L.; Pagliaccia, F.; Cavalca, V.; Ciminello, A.; Habib, A.; Squellerio, I.; et al. In Vivo Platelet Activation and Aspirin Responsiveness in Type 1 Diabetes. Diabetes 2016, 65, 503–509. [Google Scholar] [CrossRef]
- Colwell, J.A. Is aspirin effective in diabetic patients? Yes. J. Thromb. Haemost. 2005, 3, 2612–2614. [Google Scholar] [CrossRef] [PubMed]
- Nicolucci, A.; de Berardis, G.; Sacco, M.; Tognoni, G. AHA/ADA vs. ESC/EASD recommendations on aspirin as a primary prevention strategy in people with diabetes: How the same data generate divergent conclusions. Eur. Heart J. 2007, 28, 1925–1927. [Google Scholar] [CrossRef][Green Version]
- Mehta, S.S.; Silver, R.J.; Aaronson, A.; Abrahamson, M.; Goldfine, A.B. Comparison of aspirin resistance in type 1 versus type 2 diabetes mellitus. Am. J. Cardiol. 2006, 97, 567–570. [Google Scholar] [CrossRef]
- Fitzgerald, R.; Pirmohamed, M. Aspirin resistance: Effect of clinical, biochemical and genetic factors. Pharmacol. Ther. 2011, 130, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.H.; Abdelmoneim, A.S.; Omran, D.; Featherstone, T.R. Prevalence of high on-treatment platelet reactivity in diabetic patients treated with aspirin. Am. J. Med. 2014, 127, 95.e1–95.e9. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Controls (n = 28) | T1DM (n = 28) |
|---|---|---|
| Age (years), median (IQR) | 26.00 (23.00–36.00) | 28.00 (22.25–36.75) |
| Gender (n), female/male | 10/18 | 10/18 |
| WBC (109/L), median (IQR) | 5.38 (4.73–5.63) | 6.15 (4.64–7.27) |
| Monocytes (109/L), mean ± SE | 0.37 ± 0.02 | 0.40 ± 0.04 |
| Granulocytes (109/L), median (IQR) | 2.94 (2.56–3.39) | 3.17 (2.07–4.78) |
| Platelets (109/L), mean ± SE | 231.60 ± 7.75 | 235.80 ± 14.58 |
| MPV (fL), mean ± SE | 10.42 ± 0.15 | 10.74 ± 0.20 |
| PDW (fL), mean ± SE | 12.52 ± 0.32 | 12.98 ± 0.37 |
| Total cholesterol (mg/dL), mean ± SE | 186.00 ± 5.88 | 175.40 ± 5.18 |
| HDL cholesterol (mg/dL), mean ± SE | 56.75 ± 2.72 | 51.36 ± 2.30 |
| LDL cholesterol (mg/dL), mean ± SE | 112.60 ± 6.10 | 111.40 ± 4.40 |
| Triglycerides (mg/dL), median (IQR) | 67.50 (57.25–81.50) | 58.00 (48.00–78.00) |
| BMI (Kg/m2), mean ± SE | 21.90 ± 0.59 | 21.57 ± 0.39 |
| Diabetes duration (years), mean ± SE | n.a. | 13.31 ± 1.55 |
| Fasting glucose (mg/dL), median (IQR) | 90.00 (85.25–100.80) | 164.00 (130.00–217.00) *** |
| HbA1c (mmol/mol), median (IQR) | 36.00 (34.00–38.00) | 59.00 (51.25–66.75) *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosco, O.; Vizio, B.; Gruden, G.; Schiavello, M.; Lorenzati, B.; Cavallo-Perin, P.; Russo, I.; Montrucchio, G.; Lupia, E. Thrombopoietin Contributes to Enhanced Platelet Activation in Patients with Type 1 Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 7032. https://doi.org/10.3390/ijms22137032
Bosco O, Vizio B, Gruden G, Schiavello M, Lorenzati B, Cavallo-Perin P, Russo I, Montrucchio G, Lupia E. Thrombopoietin Contributes to Enhanced Platelet Activation in Patients with Type 1 Diabetes Mellitus. International Journal of Molecular Sciences. 2021; 22(13):7032. https://doi.org/10.3390/ijms22137032
Chicago/Turabian StyleBosco, Ornella, Barbara Vizio, Gabriella Gruden, Martina Schiavello, Bartolomeo Lorenzati, Paolo Cavallo-Perin, Isabella Russo, Giuseppe Montrucchio, and Enrico Lupia. 2021. "Thrombopoietin Contributes to Enhanced Platelet Activation in Patients with Type 1 Diabetes Mellitus" International Journal of Molecular Sciences 22, no. 13: 7032. https://doi.org/10.3390/ijms22137032
APA StyleBosco, O., Vizio, B., Gruden, G., Schiavello, M., Lorenzati, B., Cavallo-Perin, P., Russo, I., Montrucchio, G., & Lupia, E. (2021). Thrombopoietin Contributes to Enhanced Platelet Activation in Patients with Type 1 Diabetes Mellitus. International Journal of Molecular Sciences, 22(13), 7032. https://doi.org/10.3390/ijms22137032






