Targeting Intra-Pulmonary P53-Dependent Long Non-Coding RNA Expression as a Therapeutic Intervention for Systemic Lupus Erythematosus-Associated Diffuse Alveolar Hemorrhage
Abstract
:1. Introduction
2. Results
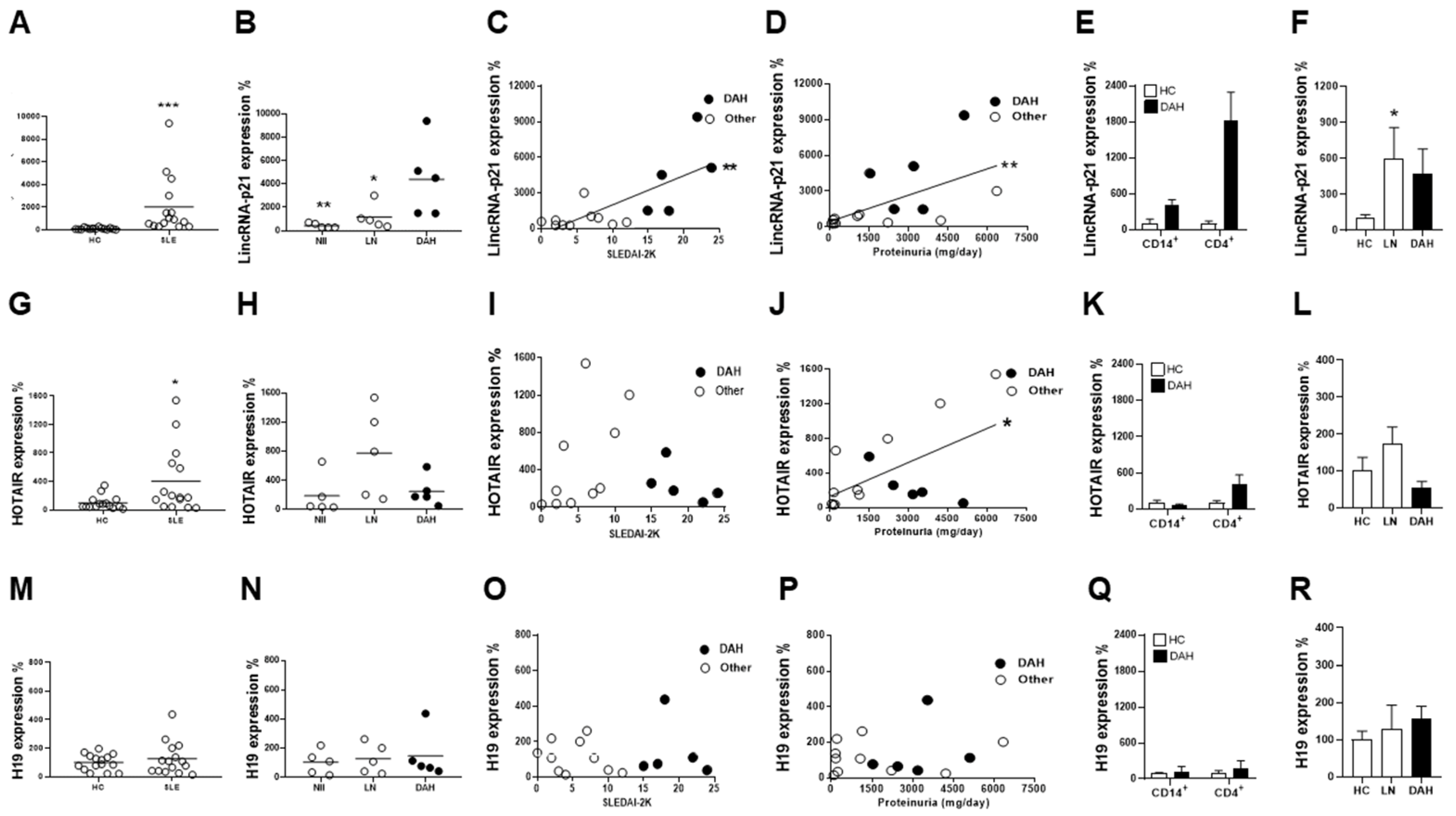
2.1. LncRNA Expression in Circulating MNCs and Urine Cells from SLE-Associated DAH Patients
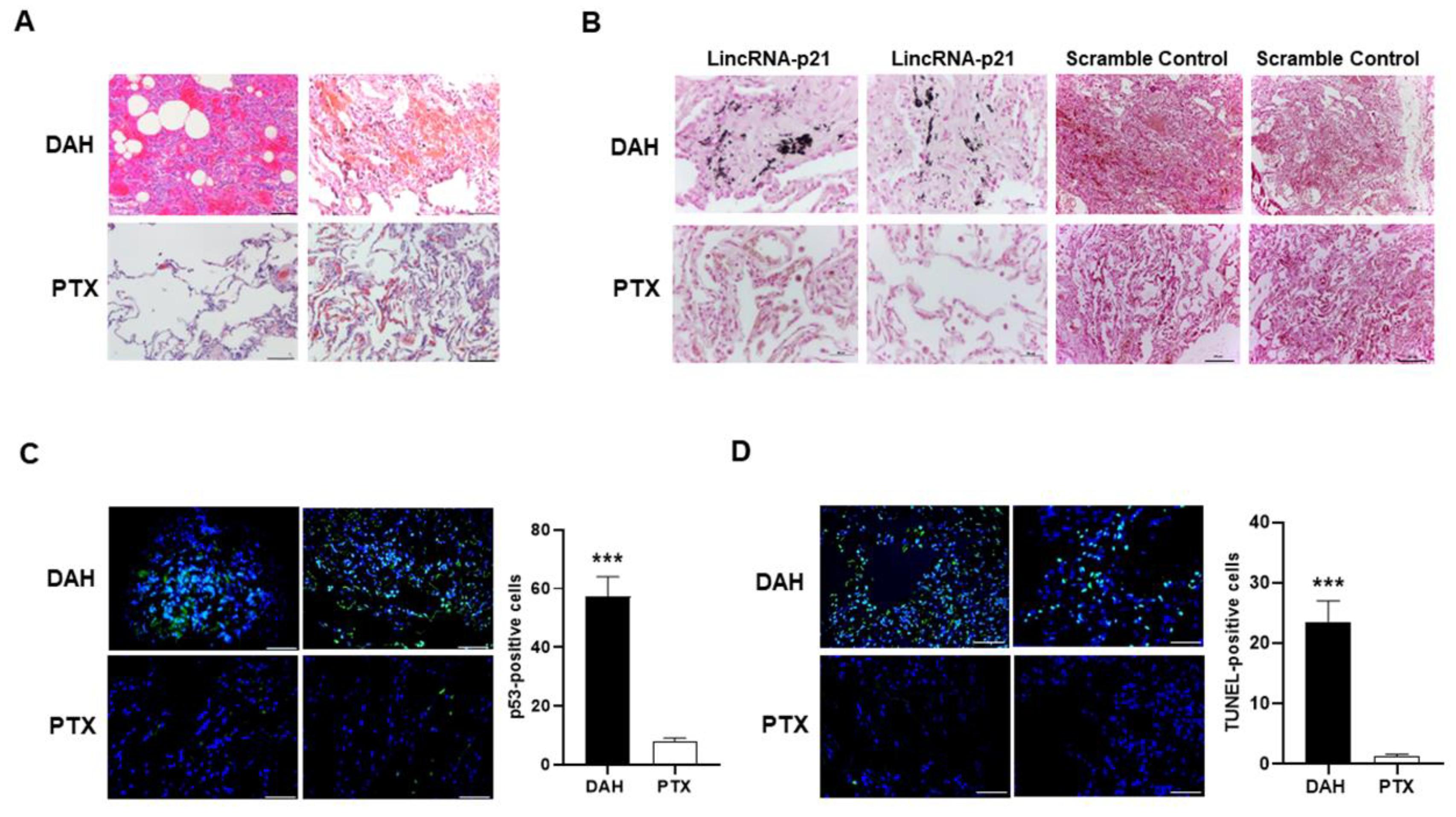
2.2. Increased Pulmonary p53 and LincRNA-p21 Expression and Cell Apoptosis in SLE-Associated DAH Patients
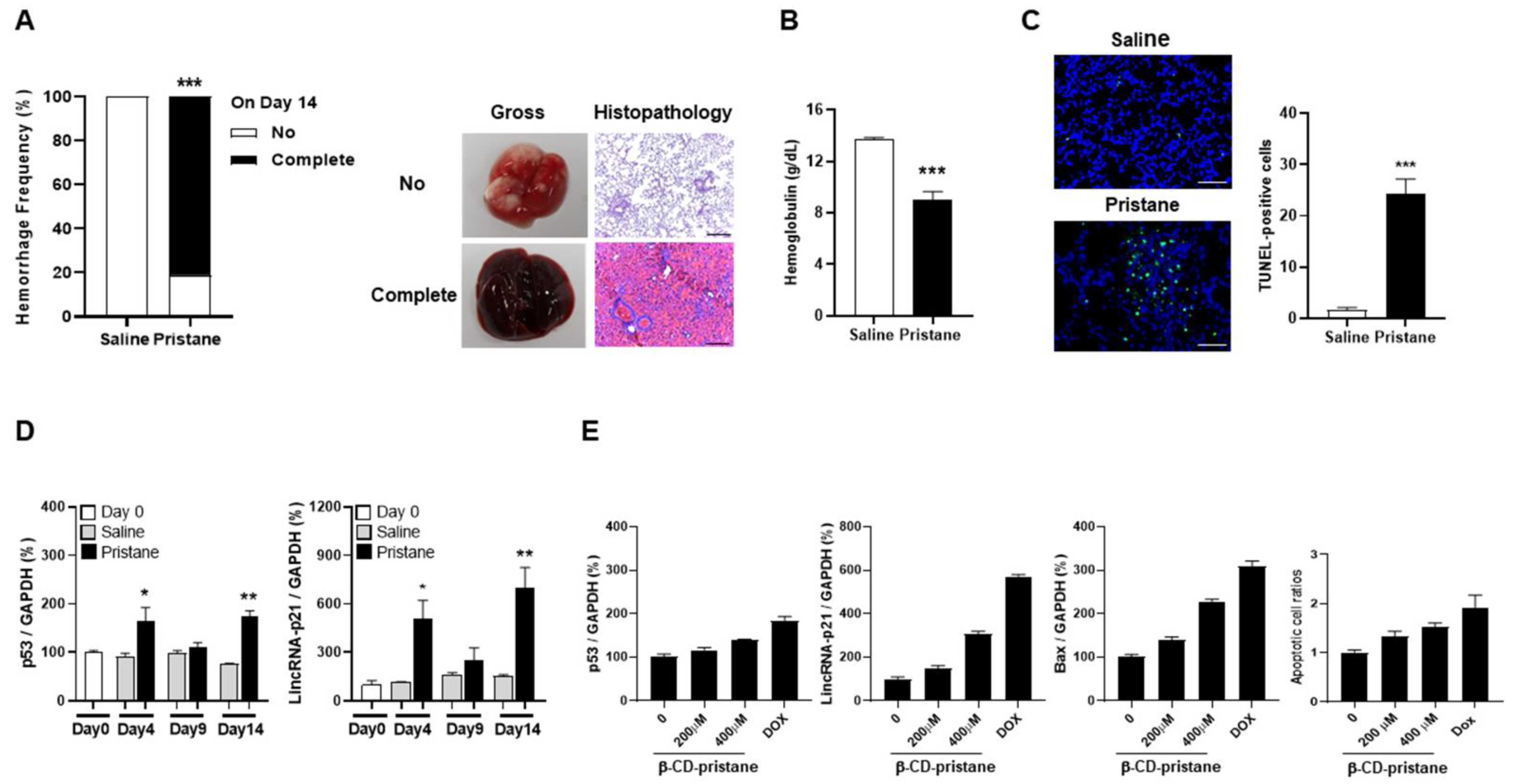
2.3. LincRNA-p21 Expression in Pristane-Induced Model of DAH and Hydrophilic Pristane-Stimulated Alveolar Epithelial Cells
2.4. Induction of CD4+ T Cell Apoptosis with Increased LincRNA-p21 Expression in Pristane-Injected Mice
2.5. LV-Based shRNA Targeting LincRNA-p21 Efficacy Screening
2.6. Intra-Pulmonary Delivery of sh-lincRNAp21 in Pristane-Induced Model of DAH
3. Discussion
4. Materials and Methods
4.1. SLE Patients and Age/Sex-Matched HC Subjects
4.2. Purification of Human and Mouse Cells
4.3. Pristane-Induced Model of DAH
4.4. Quantitative Real Time Polymerase Chain Reaction (qRT-PCR) Examination
4.5. Hydrophilic Pristane Preparation
4.6. LV-Based shRNA Targeting LincRNA-p21 Construction
4.7. Stable Transfectant Preparation for Screening LincRNA-p21 Knockdown Efficacy
4.8. In Vitro Hydrophylic Pristane Stimulation
4.9. LV-sh-lincRNAp21 IT Administration and Therapeutic Evaluation
4.10. Hemogram, RNP Antibody and Proteinuria Determination in Mice
4.11. Cell Apoptosis Measurement
4.12. Immunoblotting Assay
4.13. H&E, TUNEL and IF Histopathological Staining
4.14. ISH Assessment
4.15. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| β-CyD | β-cyclodextrin |
| DAH | Diffuse alveolar hemorrhage |
| DAPI | 4,6-diamidino-2-phenylindole |
| DIG | Digoxigenin |
| Dox | Doxorubicin |
| GN | Glomerulonephritis |
| Hb | Hemoglobulin |
| Hct | Hematocrit |
| IC | Immune complexes |
| IF | Immunofluorescence |
| Ig | Immunoglobulin |
| LncRNA | Long noncoding RNA |
| LN | Lupus nephritis |
| LV | Lentivirus |
| MiRNA | MicroRNA |
| MNC NCKU | Mononuclear cell National Cheng Kung University |
| PBS | Phosphate-buffered saline |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| RBC | Red blood cell |
| RT RA | Reverse transcription Rheumatoid arthritis |
| SEM ShRNA | Standard error of the mean Short hairpin RNA |
| SLE | Systemic lupus erythematosus |
| SLEDAI | SLE disease activity index |
| Tm | Temperature for primer melting |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP nick end labeling |
References
- Di Bartolomeo, S.; Alunno, A.; Carubbi, F. Respiratory manifestations in systemic lupus erythematosus. Pharmaceuticals 2021, 14, 276. [Google Scholar] [CrossRef] [PubMed]
- Osler, W. On the visceral manifestations of the erythema exudativum mutiforme. Am. J. Med. Sci. 1895, 110, 629–646. [Google Scholar] [CrossRef]
- Park, J.A. Treatment of diffuse alveolar hemorrhage: Controlling inflammation and obtaining rapid and effective hemostasis. Int. J. Mol. Sci. 2021, 22, 793. [Google Scholar] [CrossRef]
- Kwok, S.K.; Moon, S.J.; Ju, J.H.; Ju, J.H.; Park, K.S.; Kim, W.U.; Cho, C.S.; Kim, H.Y.; Park, S.H. Diffuse alveolar hemorrhage in systemic lupus erythematosus: Risk factors and clinical outcome: Results from affiliated hospitals of Catholic University of Korea. Lupus 2011, 20, 102–107. [Google Scholar] [CrossRef]
- Martínez-Martínez, M.U.; Abud-Mendoza, C. Predictors of mortality in diffuse alveolar hemorrhage associated with systemic lupus erythematosus. Lupus 2011, 20, 568–574. [Google Scholar] [CrossRef]
- Araujo, D.B.; Borba, E.F.; Silva, C.A.; Campos, L.M.; Pereira, R.M.; Bonfa, E.; Shinjo, S.K. Alveolar hemorrhage: Distinct features of juvenile and adult onset systemic lupus erythematosus. Lupus 2012, 21, 872–877. [Google Scholar] [CrossRef]
- Martinez-Martinez, M.U.; Sturbaum, A.K.; Alcocer-Varela, J.; Merayo-Chalico, J.; Gómez-Martin, D.; Gómez-Bañuelos, J.D.J.E.; Saavedra, M.; Enciso-Peláez, S.; Faugier-Fuentes, E.; Maldonado-Velázquez, R.; et al. Factors associated with mortality and infections in patients with systemic lupus erythematosus with diffuse alveolar hemorrhage. J. Rheumatol. 2014, 41, 1656–1661. [Google Scholar]
- Kazzaz, N.M.; Coit, P.; Lewis, E.E.; McCune, W.J.; Sawalha, A.H.; Knight, J.S. Systemic lupus erythematosus complicated by diffuse alveolar hemorrhage: Risk factors, therapy and survival. Lupus Sci. Med. 2015, 2, e000117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Choi, J.; Cho, S.K.; Choi, C.B.; Kim, T.H.; Jun, J.B.; Yoo, D.H.; Bae, S.C.; Sung, Y.K. Clinical characteristics and outcomes of diffuse alveolar hemorrhage in patients with systemic lupus erythematosus. Semin. Arthritis Rheum. 2017, 46, 782–787. [Google Scholar] [CrossRef]
- Wang, C.R.; Liu, M.F.; Weng, C.T.; Lin, W.C.; Li, W.T.; Tsai, H.W. Systemic lupus erythematosus-associated diffuse alveolar hemorrhage: A monocentric experience in Han Chinese patients. Scand. J. Rheumatol. 2018, 47, 392–399. [Google Scholar] [CrossRef]
- Blay, G.; Rodrigues, J.C.; Ferreira, J.C.O.; Leal, G.N.; Gormezano, N.W.; Novak, G.V.; Pereira, R.M.R.; Terreri, M.T.; Magalhães, C.S.; Molinari, B.C.; et al. Diffuse alveolar hemorrhage in childhood-onset systemic lupus erythematosus: A severe disease flare with serious outcome. Adv. Rheumatol. 2018, 58, 39. [Google Scholar] [CrossRef] [PubMed]
- Quintana, J.H.; Aragón, C.C.; Santos, V.A.; de Las Salas, A.; Tafúr, R.A.; Aguirre-Valencia, D.; Cañas, C.A.; Tobón, G.J. Diffuse Alveolar Hemorrhage: A cohort of patients with systemic lupus erythematosus. J. Clin. Rheumatol. 2020, 26 (Suppl. 2), S153–S157. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, C.; Zhao, J.; Wang, Q.; Xu, D.; Zhang, S.; Shen, M.; Hou, Y.; Tian, X.; Li, M.; et al. Systemic lupus erythematosus-associated diffuse alveolar hemorrhage: A single-center, matched case-control study in China. Lupus 2020, 29, 795–803. [Google Scholar] [CrossRef]
- Mistry, P.; Kaplan, M.J. Cell death in the pathogenesis of systemic lupus erythematosus and lupus nephritis. Clin. Immunol. 2017, 185, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Calvani, N.; Caricchio, R.; Tucci, M.; Sobel, E.S.; Silvestris, F.; Tartaglia, P.; Richards, H.B. Induction of apoptosis by the hydrocarbon oil pristane: Implications for pristane-induced lupus. J. Immunol. 2005, 175, 4777–4782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Adhoubi, N.K.; Bystrom, J. Systemic lupus erythematosus and diffuse alveolar hemorrhage, etiology and novel treatment strategies. Lupus 2020, 29, 355–363. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, L.; Cao, H.; Gu, Y.; Zhou, P.; Shi, M.; Li, G.; Jiao, X.; Li, N.; Li, X.; et al. Erythropoietin protects against diffuse alveolar hemorrhage in mice by regulating macrophage polarization through the EPOR/JAK2/STAT3 axis. J. Immunol. 2021, 206, 1752–1764. [Google Scholar] [CrossRef]
- Jarrot, P.A.; Tellier, E.; Plantureux, L.; Crescence, L.; Robert, S.; Chareyre, C.; Daniel, L.; Secq, V.; Garcia, S.; Dignat-George, F.; et al. Neutrophil extracellular traps are associated with the pathogenesis of diffuse alveolar hemorrhage in murine lupus. J. Autoimmun. 2019, 100, 120–130. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Zhu, W.; Dong, J.; Cheng, Y.; Yin, Z.; Shen, F. Mechanisms and functions of long non-coding RNAs at multiple regulatory levels. Int. J. Mol. Sci. 2019, 20, 5573. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.N.; Mao, Y.M.; Liu, L.N.; Li, X.M.; Wang, D.G.; Pan, H.F. Emerging role of lncRNAs in systemic lupus erythematosus. Biomed. Pharmacother. 2018, 106, 584–592. [Google Scholar] [CrossRef]
- Taheri, M.; Eghtedarian, R.; Dinger, M.E.; Ghafouri-Fard, S. Exploring the role of non-coding RNAs in the pathophysiology of systemic lupus erythematosus. Biomolecules 2020, 10, 937. [Google Scholar] [CrossRef] [PubMed]
- Miret, C.; Molina, R.; Filella, X.; García-Carrasco, M.; Claver, G.; Ingelmo, M.; Ballesta, A.; Font, J. Relationship of p53 with other oncogenes, cytokines and systemic lupus erythematosus activity. Tumor Biol. 2003, 24, 185–188. [Google Scholar] [CrossRef]
- Rahbar, M.H.; Rahbar, M.R.; Mardanpour, N.; Mardanpour, S. The potential diagnostic utility of coexpression of Ki-67 and P53 in the renal biopsy in pediatric lupus nephritis. Int. J. Nephrol. Renovasc. Dis. 2018, 11, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Zhang, A.; Xu, M.; Mo, Y.Y. Role of the lncRNA-p53 regulatory network in cancer. J. Mol. Cell Biol. 2014, 6, 181–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, H.; Han, B.; Zhang, Y.; Shen, L.; Huang, R. Non-coding RNAs and autophagy. Adv. Exp. Med. Biol. 2019, 1206, 199–220. [Google Scholar] [PubMed]
- Matouk, I.J.; Mezan, S.; Mizrahi, A.; Ohana, P.; Abu-Lail, R.; Fellig, Y.; Degroot, N.; Galun, E.; Hochberg, A. The oncofetal H19 RNA connection: Hypoxia, p53 and cancer. Biochim. Biophys. Acta 2010, 1803, 443–451. [Google Scholar] [CrossRef] [Green Version]
- Zhai, N.; Xia, Y.; Yin, R.; Liu, J.; Gao, F. A negative regulation loop of long noncoding RNA HOTAIR and p53 in non-small-cell lung cancer. OncoTargets Ther. 2016, 9, 5713–5720. [Google Scholar]
- Amirinejad, R.; Rezaei, M.; Shirvani-Farsani, Z. An update on long intergenic noncoding RNA p21: A regulatory molecule with various significant functions in cancer. Cell Biosci. 2020, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Reeves, W.H.; Lee, P.Y.; Weinstein, J.S.; Satoh, M.; Lu, L. Induction of autoimmunity by pristane and other naturally occurring hydrocarbons. Trends Immunol. 2009, 30, 455–464. [Google Scholar] [CrossRef] [Green Version]
- Mokrzycki, M.H.; Balogun, R.A. Therapeutic apheresis: A review of complications and recommendations for prevention and management. J. Clin. Apher. 2011, 26, 243–248. [Google Scholar] [CrossRef]
- Ednalino, C.; Yip, J.; Carsons, S.E. Systematic review of diffuse alveolar hemorrhage in systemic lupus erythematosus: Focus on outcome and therapy. J. Clin. Rheumatol. 2015, 21, 305–310. [Google Scholar] [CrossRef]
- Heslet, L.; Nielsen, J.D.; Nepper-Christensen, S. Local pulmonary administration of factor VIIa (rFVIIa) in diffuse alveolar hemorrhage (DAH)—A review of a new treatment paradigm. Biologics 2012, 6, 37–46. [Google Scholar] [PubMed] [Green Version]
- Esper, R.C.; Estrada, I.E.; de la Torre León, T.; Gutiérrez, A.O.; López, J.A. Treatment of diffuse alveolar hemorrhage secondary to lupus erythematosus with recombinant activated factor VII administered with a jet nebulizer. J. Intensive Care 2014, 2, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alabed, I.B. Treatment of diffuse alveolar hemorrhage in systemic lupus erythematosus patient with local pulmonary administration of factor VIIa (rFVIIa): A case report. Medicine 2014, 93, e72. [Google Scholar] [CrossRef] [PubMed]
- Firestein, G.S.; Nguyen, K.; Aupperle, K.R.; Yeo, M.; Boyle, D.L.; Zvaifler, N.J. Apoptosis in rheumatoid arthritis: p53 overexpression in rheumatoid arthritis synovium. Am. J. Pathol. 1996, 149, 2143–2151. [Google Scholar]
- Spurlock, C.F., 3rd; Tossberg, J.T.; Matlock, B.K.; Olsen, N.J.; Aune, T.M. Methotrexate inhibits NF-κB activity via long intergenic (noncoding) RNA-p21 induction. Arthritis Rheumatol. 2014, 66, 2947–2957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.R.; Chen, S.Y.; Chou, Y.C.; Wu, C.L.; Lo, Y.T.; Shiau, A.L. Experimental rheumatoid joint ameliorated by CRISPR interference targeting long non-coding RNA H19 through Wnt signaling inactivation. Arthritis Rheumatol. 2019, 71, 1770. [Google Scholar]
- Rapicavoli, N.A.; Qu, K.; Zhang, J.; Mikhail, M.; Laberge, R.M.; Chang, H.Y. A mammalian pseudogene lncRNA at the interface of inflammation and anti-inflammatory therapeutics. Elife 2013, 2, e00762. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Hsieh, S.C.; Lu, C.S.; Wu, T.H.; Liao, H.T.; Wu, C.H.; Li, K.J.; Kuo, Y.M.; Lee, H.T.; Shen, C.Y.; et al. Cross-talk between mitochondrial dysfunction-provoked oxidative stress and aberrant noncoding RNA expression in the pathogenesis and pathophysiology of SLE. Int. J. Mol. Sci. 2019, 20, 5183. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Liu, H.; Tang, Y.; Wei, Y.; Wei, W.; Zhang, L.; Chen, J. The development and controversy of competitive endogenous RNA hypothesis in non-coding genes. Mol. Cell Biochem. 2021, 476, 109–123. [Google Scholar] [CrossRef]
- Ala, U. Competing endogenous RNAs, non-coding RNAs and diseases: An intertwined story. Cells 2020, 9, 1574. [Google Scholar] [CrossRef]
- Freitas, E.C.; de Oliveira, M.S.; Monticielo, O.A. Pristane-induced lupus: Considerations on this experimental model. Clin. Rheumatol. 2017, 36, 2403–2414. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, Y.; Meng, Y.; Xiao, C.; Liu, Z.; Brohawn, P.; Higgs, B.W.; Jallal, B.; Jia, Q.; Qu, B.; et al. In Vivo Therapeutic success of microRNA-155 antagomir in a mouse model of lupus alveolar hemorrhage. Arthritis Rheumatol. 2016, 68, 953–964. [Google Scholar] [CrossRef]
- Smith, S.; Wu, P.W.; Seo, J.J.; Fernando, T.; Jin, M.; Contreras, J.; Montano, E.N.; Gabhann, J.N.; Cunningham, K.; Widaa, A.; et al. IL-16/miR-125a axis controls neutrophil recruitment in pristane-induced lung inflammation. JCI Insight 2018, 3, e120798. [Google Scholar] [CrossRef] [Green Version]
- Dong, G.; Yang, Y.; Li, X.; Yao, X.; Zhu, Y.; Zhang, H.; Wang, H.; Ma, Q.; Zhang, J.; Shi, H.; et al. Granulocytic myeloid-derived suppressor cells contribute to IFN-I signaling activation of B cells and disease progression through the lncRNA NEAT1-BAFF axis in systemic lupus erythematosus. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165554. [Google Scholar] [CrossRef]
- Cao, H.Y.; Li, D.; Wang, Y.P.; Lu, H.X.; Sun, J.; Li, H.B. The protection of NF-κB inhibition on kidney injury of systemic lupus erythematosus mice may be correlated with lncRNA TUG1. Kaohsiung J. Med. Sci. 2020, 36, 354–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Titov, A.; Morel, L. An update on lupus animal models. Curr. Opin. Rheumatol. 2017, 29, 434–441. [Google Scholar] [CrossRef]
- Barker, T.T.; Lee, P.Y.; Kelly-Scumpia, K.M.; Weinstein, J.S.; Nacionales, D.C.; Kumagai, Y.; Akira, S.; Croker, B.P.; Sobel, E.S.; Reeves, W.H.; et al. Pathogenic role of B cells in the development of diffuse alveolar hemorrhage induced by pristane. Lab. Investig. 2011, 91, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, V.R.; Grande, J.P.; Luthra, H.S.; David, C.S. Characterization of haemorrhagic pulmonary capillaritis: Another manifestation of pristane-induced lupus. Rheumatology 2007, 46, 1405–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, H.; Han, S.; Lee, P.Y.; Khaybullin, R.; Shumyak, S.; Lu, L.; Chatha, A.; Afaneh, A.; Zhang, Y.; Xie, C.; et al. Pathogenesis of diffuse alveolar hemorrhage in murine lupus. Arthritis Rheumatol. 2017, 69, 1280–1293. [Google Scholar] [CrossRef] [Green Version]
- Cavalcante, G.C.; Schaan, A.P.; Cabral, G.F.; Santana-da-Silva, M.N.; Pinto, P.; Vidal, A.F.; Ribeiro-Dos-Santos, Â. A cell’s fate: An overview of the molecular biology and genetics of apoptosis. Int. J. Mol. Sci. 2019, 20, 4133. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.T.; Chen, S.Y.; Wang, C.R.; Liu, M.F.; Lin, C.C.; Jou, I.M.; Shiau, A.L.; Wu, C.L. Amelioration of collagen-induced arthritis in mice by lentivirus-mediated silencing of microRNA-223. Arthritis Rheum. 2012, 64, 3240–3245. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.S.; Chen, S.Y.; Wu, C.L.; Chong, H.E.; Ding, Y.C.; Shiau, A.L.; Wang, C.R. Amelioration of experimental autoimmune arthritis through targeting synovial fibroblasts by the intra-articular delivery of microRNA-140-3p and -5p. Arthritis Rheumatol. 2016, 68, 370–381. [Google Scholar] [CrossRef] [Green Version]
- Milone, M.C.; O’Doherty, U. Clinical use of lentiviral vectors. Leukemia 2018, 32, 1529–1541. [Google Scholar] [CrossRef]
- Hochberg, M.C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997, 40, 1725. [Google Scholar] [CrossRef] [PubMed]
- Herman, S.; Kny, A.; Schorn, C.; Pfatschbacher, J.; Niederreiter, B.; Herrmann, M.; Holmdahl, R.; Steiner, G.; Hoffmann, M.H. Cell death and cytokine production induced by autoimmunogenic hydrocarbon oils. Autoimmunity 2012, 45, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Janz, S.; Shacter, E. A new method for delivering alkanes to mammalian cells: Preparation and preliminary characterization of an inclusion complex between beta-cyclodextrin and pristane (2,6,10,14-tetramethylpentadecane). Toxicology 1991, 69, 301–315. [Google Scholar] [CrossRef]
- Zufferey, R.; Donello, J.E.; Trono, D.; Hope, T.J. Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. J. Virol. 1999, 73, 2886–2892. [Google Scholar] [CrossRef] [Green Version]
- Moffat, J.; Grueneberg, D.A.; Yang, X.; Kim, S.Y.; Kloepfer, A.M.; Hinkle, G.; Piqani, B.; Eisenhaure, T.M.; Luo, B.; Grenier, J.K.; et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell 2006, 124, 1283–1298. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.Y.; Shiau, A.L.; Li, Y.T.; Lin, C.C.; Jou, I.M.; Liu, M.F.; Wu, C.L.; Wang, C.R. Transcription factor snail regulates tumor necrosis factor α-mediated synovial fibroblast activation in the rheumatoid joint. Arthritis Rheumatol. 2015, 67, 39–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.Y.; Shieh, C.C.; Yu, C.K.; Lei, H.Y. Allergen-induced bronchial inflammation is associated with decreased levels of surfactant proteins A and D in a murine model of asthma. Clin. Exp. Allergy 2001, 31, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Elshikha, A.S.; Abboud, G.; van der Meijden-Erkelens, L.; Lu, Y.; Chen, M.J.; Yuan, Y.; Ponjee, G.; Zeumer, L.; Satoh, M.; Morel, L.; et al. Alpha-1-Antitrypsin Ameliorates Pristane Induced Diffuse Alveolar Hemorrhage in Mice. J. Clin. Med. 2019, 8, 1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.R.; Shiau, A.L.; Chen, S.Y.; Lin, L.L.; Tai, M.H.; Shieh, G.S.; Lin, P.R.; Yo, Y.T.; Lee, C.H.; Kuo, S.M.; et al. Amelioration of collagen-induced arthritis in rats by adenovirus-mediated PTEN gene transfer. Arthritis Rheum. 2008, 58, 1650–1656. [Google Scholar] [CrossRef] [PubMed]



| No. | Source | Case No. | Incidence | # Age/Sex | CYC | MP | TP | IVIG | RTX | MR | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2011 ROK | 21 | 1.4% | 30/F 91% | 38% | NR | 67% | 38% | Nil | 62% | 4 |
| 2 | 2011 Mexico | 22 | 9.0% | 25/F 76% | 59% | NR | 0% | 5% | 9% | 68% | 5 |
| 3 | 2012 Brazil | 28 | 1.6% | 23/F 82% | 57% | 100% | 18% | 25% | Nil | 39% | 6 |
| 4 | 2014 Mexico | 50 | NR | 23/F 98% | 49% | NR | 0% | 8% | 6% | 48% | 7 |
| 5 | 2015 United States | 22 | 2.2% | 37/NR | 41% | 45% | 18% | 14% | 14% | 0% @ | 8 |
| 6 | 2017 ROK | 24 | 2.9% | 30/F 100% | 17% | NR | 33% | 50% | Nil | 29% | 9 |
| 7 | 2018 Taiwan | 17 | 2.0% | 38/F 88% | 35% | 71% | 43% | Nil | 24% | 35% | 10 |
| 8 | 2018 Brazil | 19 | 2.2% | 13/F 74% | 47% | 95% | 0% | Nil | Nil | 47% | 11 |
| 9 | 2020 Colombia | 17 | NR | 28/F 65% | 77% | 100% | 100% | Nil | 6% | 29% | 12 |
| 10 | 2020 China | 94 | 2.0% | 29/F 87% | 76% | 79% | 38% | 71% | Nil | 38% | 13 |
| Patient Group | Nil | LN | DAH | p Values |
|---|---|---|---|---|
| Demographicfeature | ||||
| Patients number | 5 | 5 | 5 | |
| Age (year) | 35.4 ± 7.3 | 34.6 ± 9.3 | 36.0 ± 11.1 | NS |
| Female | 4 (80%) | 4 (80%) | 4 (80%) | NS |
| Clinical profile | ||||
| Disease period (years) | 5.4 ± 3.4 | 5.2 ± 4.3 | 4.6 ± 3.7 | NS |
| Activity score (SLEDAI-2K) | 2.2 ± 1.5 | 8.6 ± 2.4 | 19.2 ± 3.7 | * p < 0.01 |
| Involved organ number | 0.6 ± 0.5 | 2.8± 0.4 | 3.8 ± 0.4 | * p < 0.01 |
| Therapeutic modality | ||||
| Corticosteroid | 5 (100%) | 5 (100%) | 5 (100%) | NS |
| Cyclophosphamide | 0 (0%) | 4 (80%) | 4 (80%) | * p < 0.05 |
| Mycophenolate mofetil | 0 (0%) | 3 (60%) | 2 (40%) | NS |
| Azathioprine | 2 (40%) | 2 (40%) | 3 (60%) | NS |
| Rituximab | 0 (0%) | 1 (20%) | 2 (40%) | NS |
| Plasmapheresis | 0 (0%) | 0 (0%) | 1 (20%) | NS |
| Mechanic ventilator | 0 (0%) | 0 (0%) | 5 (100%) | #p < 0.01 |
| ECMO | 0 (0%) | 0 (0%) | 1 (20%) | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-C.; Chou, Y.-C.; Hsieh, Y.-T.; Kuo, P.-Y.; Yang, M.-L.; Chong, H.-E.; Wu, C.-L.; Shiau, A.-L.; Wang, C.-R. Targeting Intra-Pulmonary P53-Dependent Long Non-Coding RNA Expression as a Therapeutic Intervention for Systemic Lupus Erythematosus-Associated Diffuse Alveolar Hemorrhage. Int. J. Mol. Sci. 2021, 22, 6948. https://doi.org/10.3390/ijms22136948
Chen Y-C, Chou Y-C, Hsieh Y-T, Kuo P-Y, Yang M-L, Chong H-E, Wu C-L, Shiau A-L, Wang C-R. Targeting Intra-Pulmonary P53-Dependent Long Non-Coding RNA Expression as a Therapeutic Intervention for Systemic Lupus Erythematosus-Associated Diffuse Alveolar Hemorrhage. International Journal of Molecular Sciences. 2021; 22(13):6948. https://doi.org/10.3390/ijms22136948
Chicago/Turabian StyleChen, Yi-Cheng, Yu-Chi Chou, Yu-Tung Hsieh, Pin-Yu Kuo, Mei-Lin Yang, Hao-Earn Chong, Chao-Liang Wu, Ai-Li Shiau, and Chrong-Reen Wang. 2021. "Targeting Intra-Pulmonary P53-Dependent Long Non-Coding RNA Expression as a Therapeutic Intervention for Systemic Lupus Erythematosus-Associated Diffuse Alveolar Hemorrhage" International Journal of Molecular Sciences 22, no. 13: 6948. https://doi.org/10.3390/ijms22136948
APA StyleChen, Y.-C., Chou, Y.-C., Hsieh, Y.-T., Kuo, P.-Y., Yang, M.-L., Chong, H.-E., Wu, C.-L., Shiau, A.-L., & Wang, C.-R. (2021). Targeting Intra-Pulmonary P53-Dependent Long Non-Coding RNA Expression as a Therapeutic Intervention for Systemic Lupus Erythematosus-Associated Diffuse Alveolar Hemorrhage. International Journal of Molecular Sciences, 22(13), 6948. https://doi.org/10.3390/ijms22136948







