Effect of Plasma Treatment on Titanium Surface on the Tissue Surrounding Implant Material
Abstract
:1. Introduction
2. Results
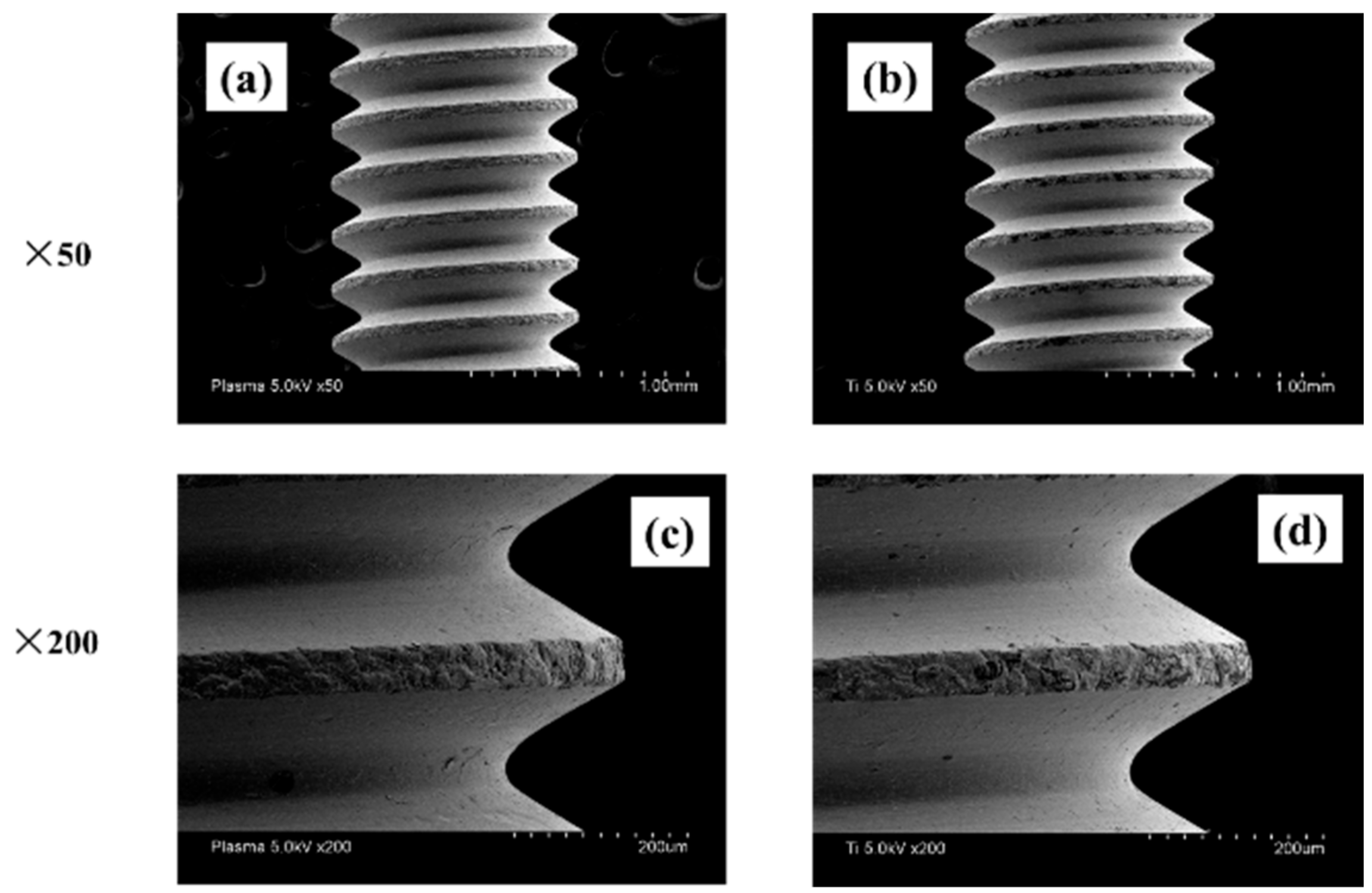
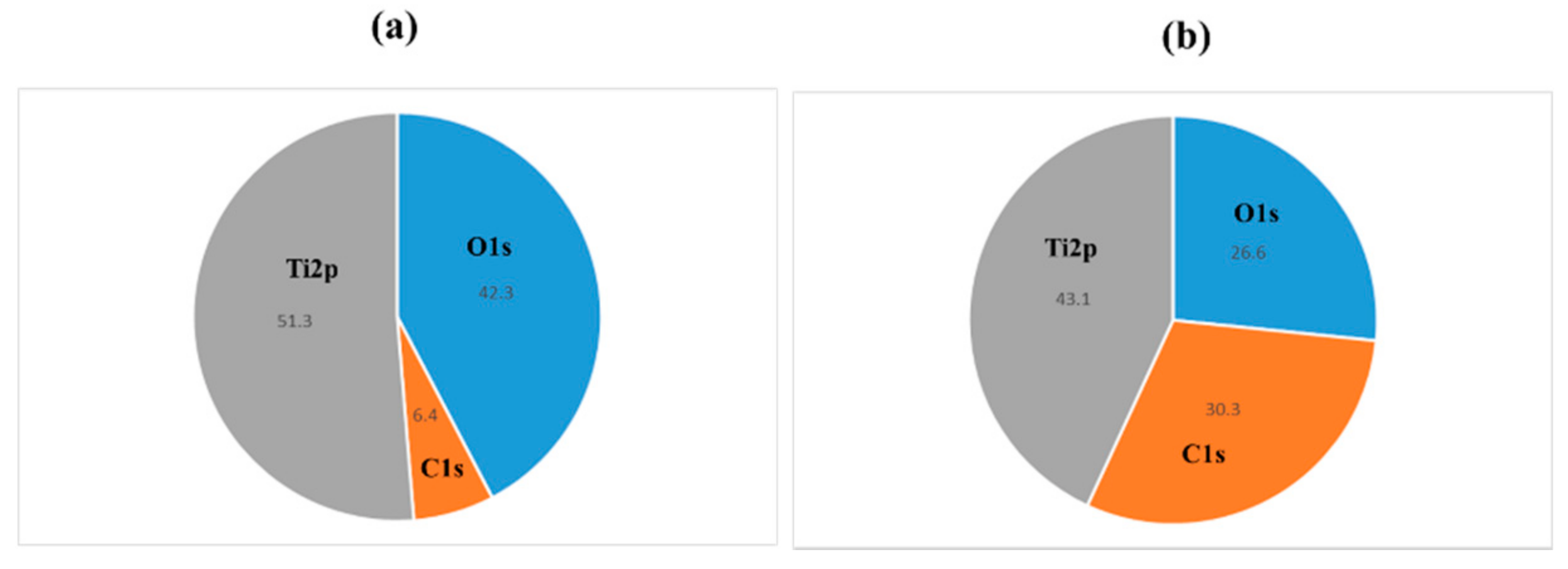
2.1. Sample Preparation
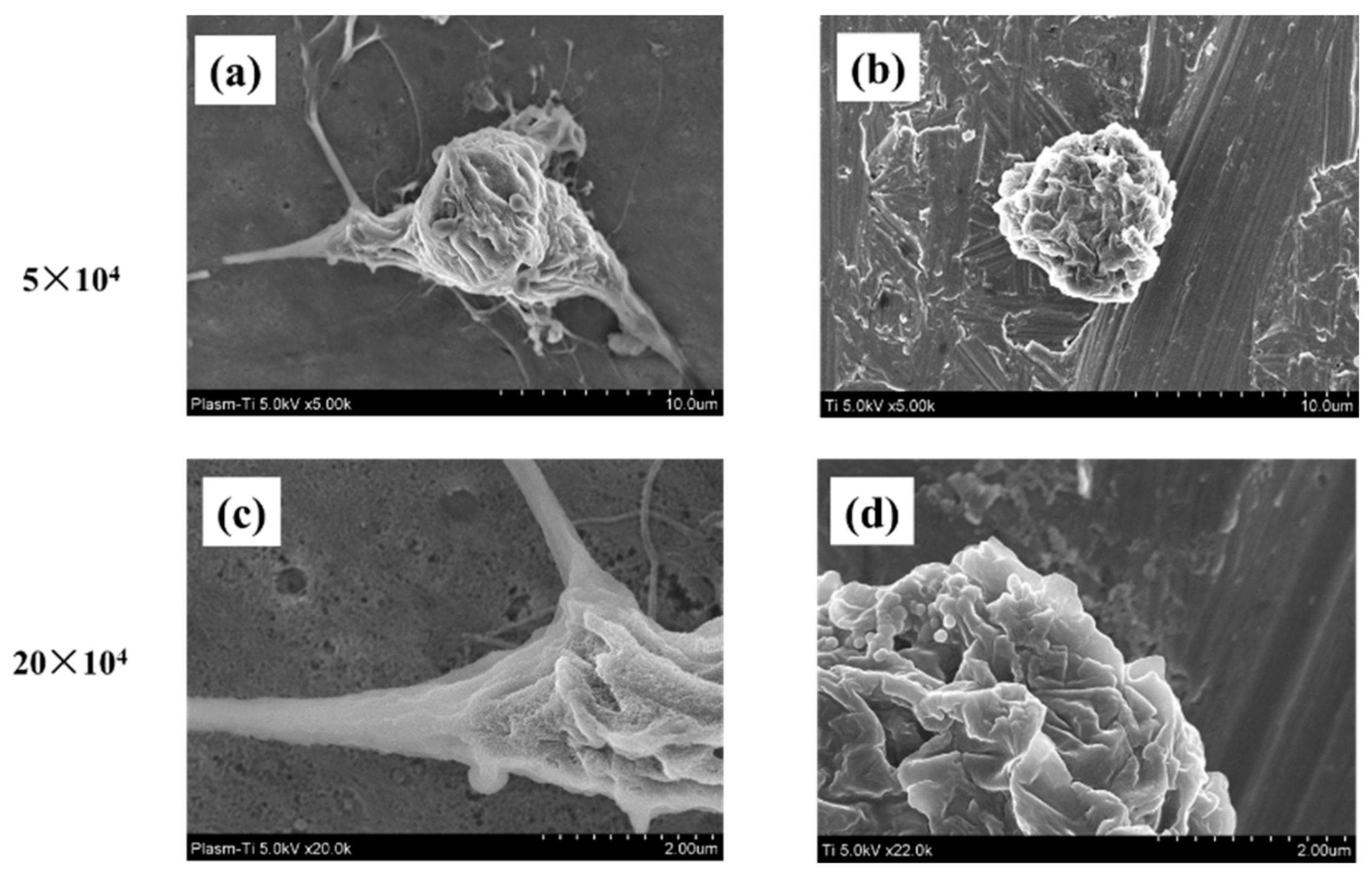
2.2. Cell Morphology
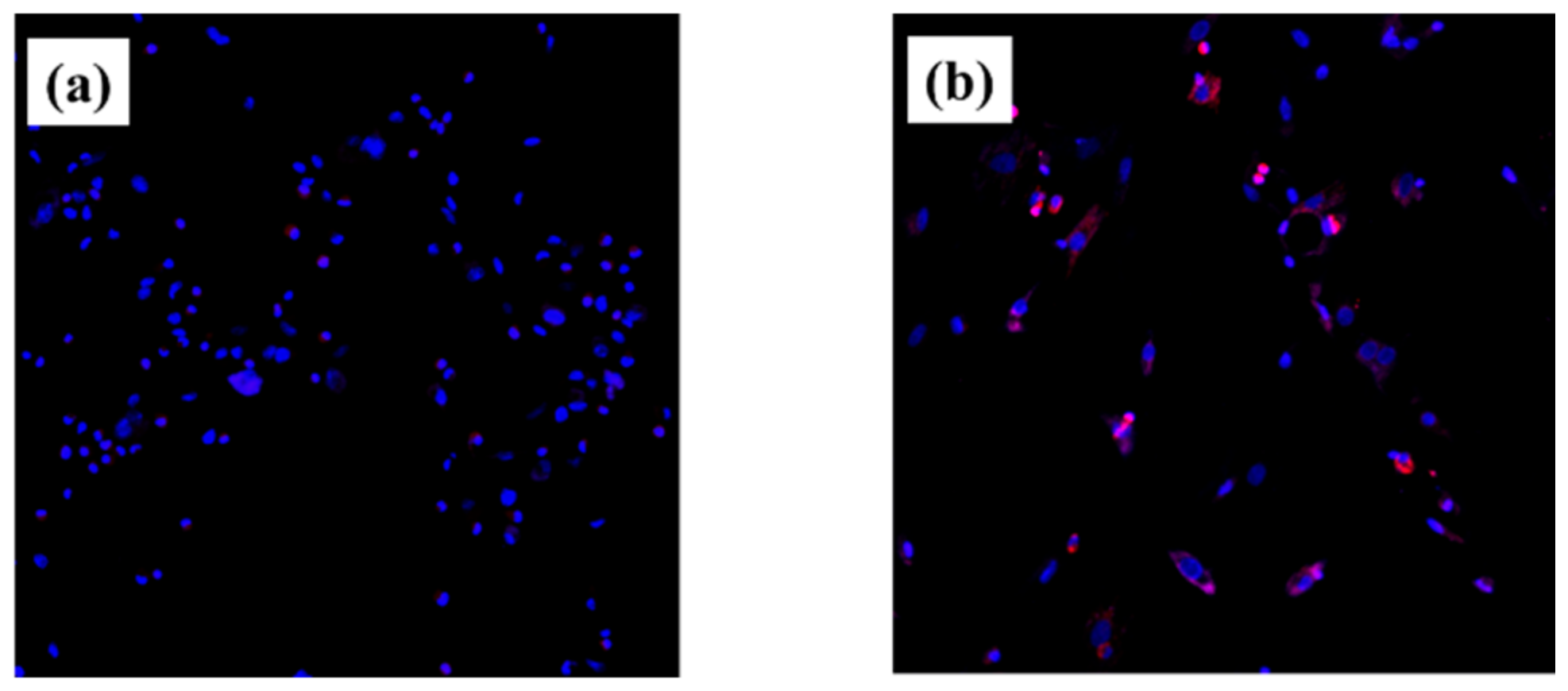
2.3. Intracellular ROS Level for RBMCs
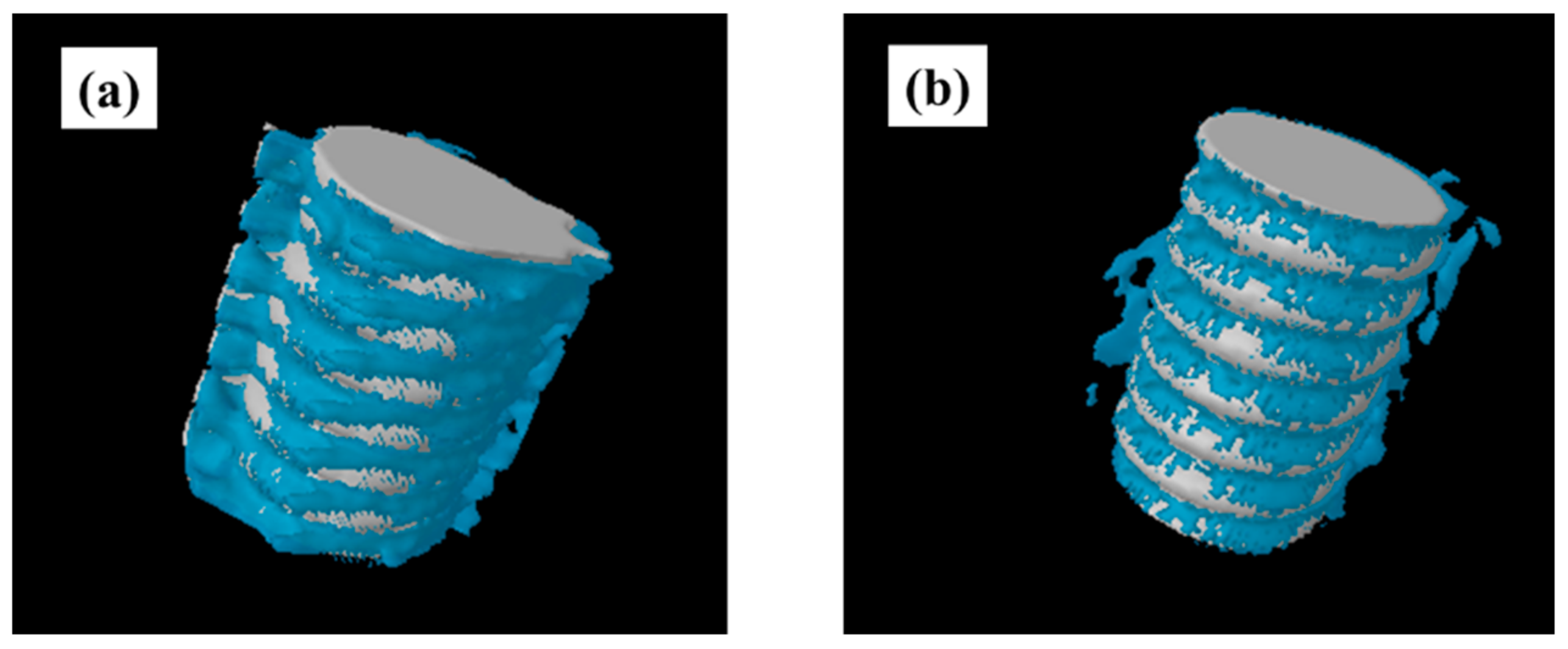
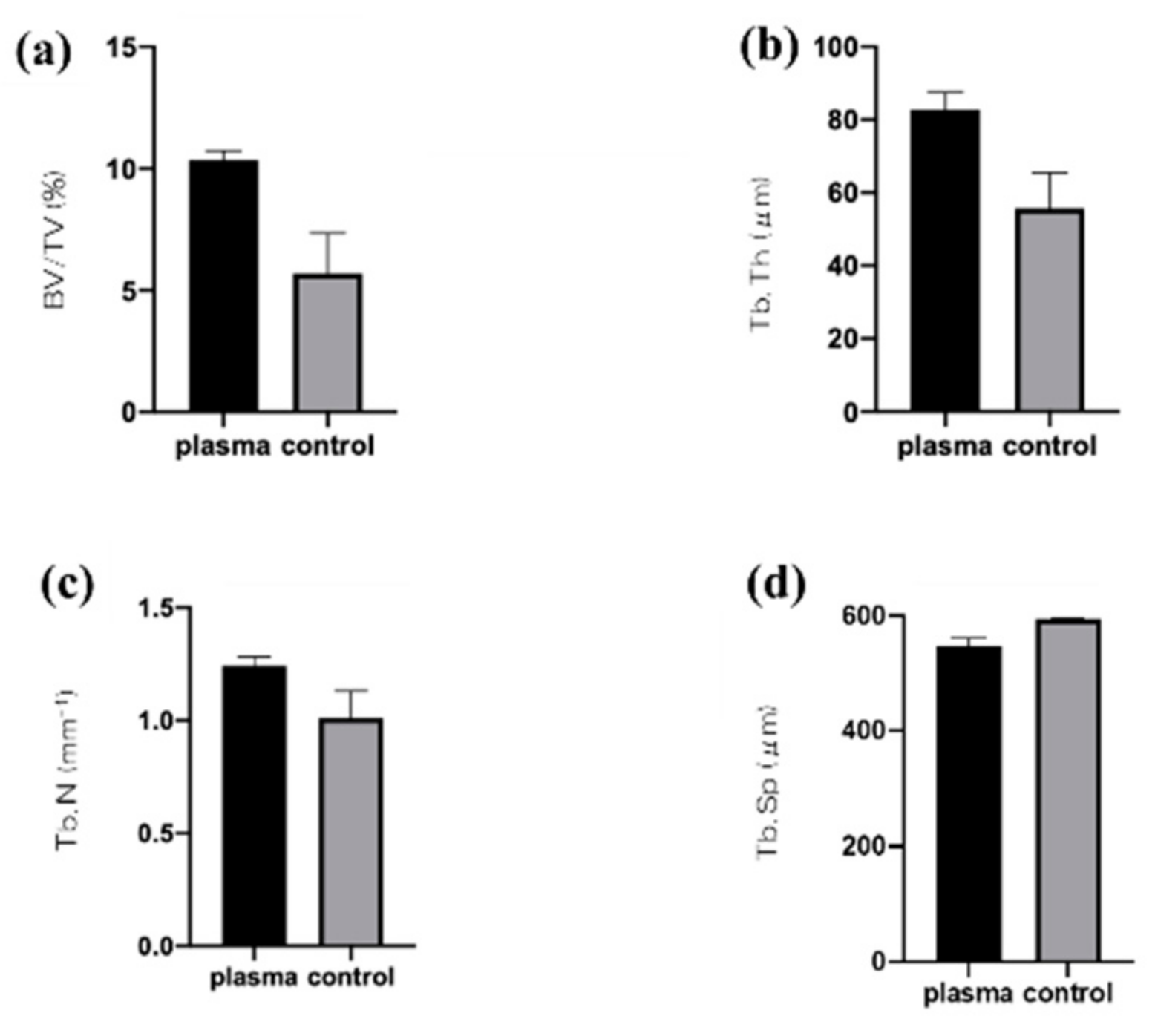
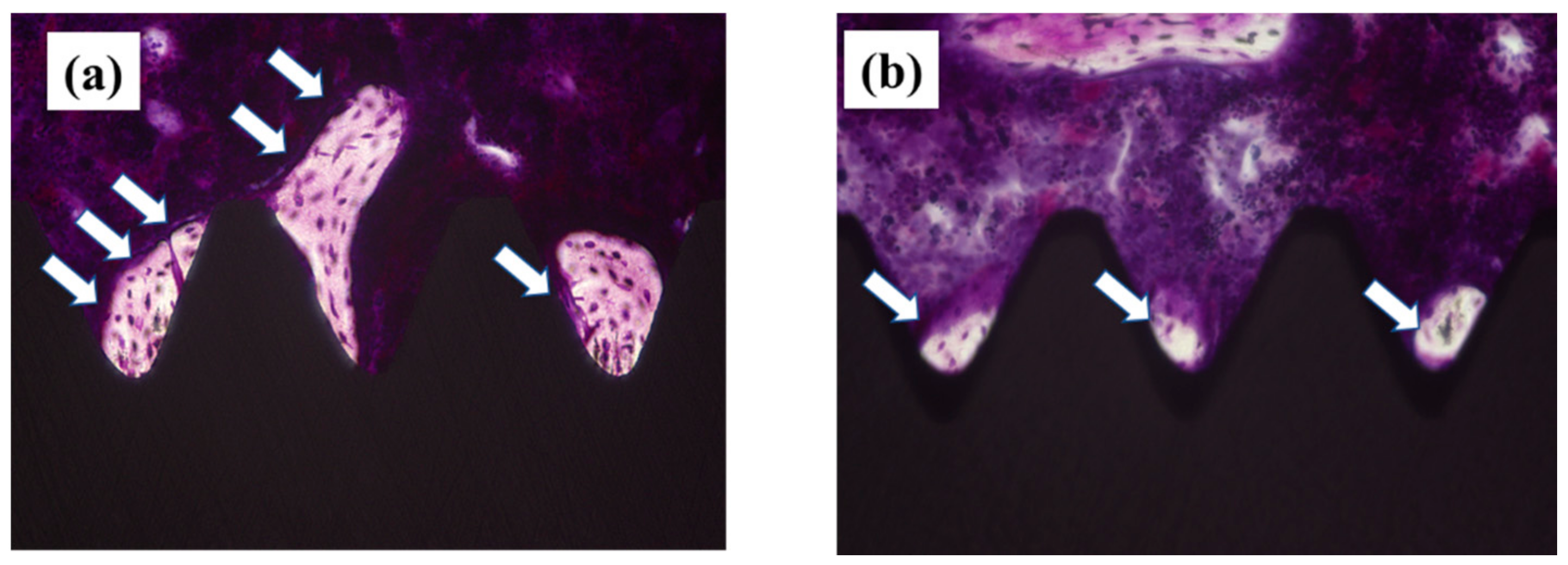
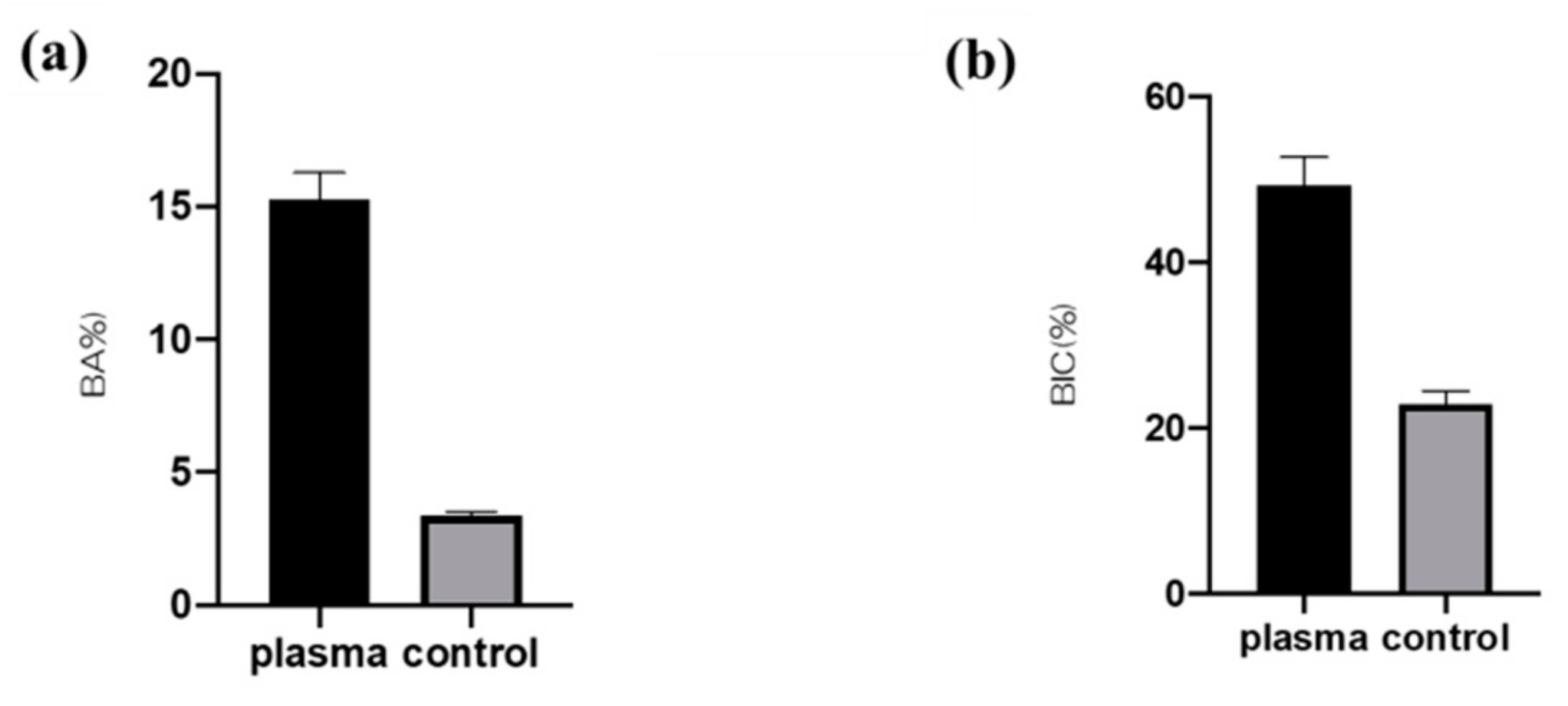
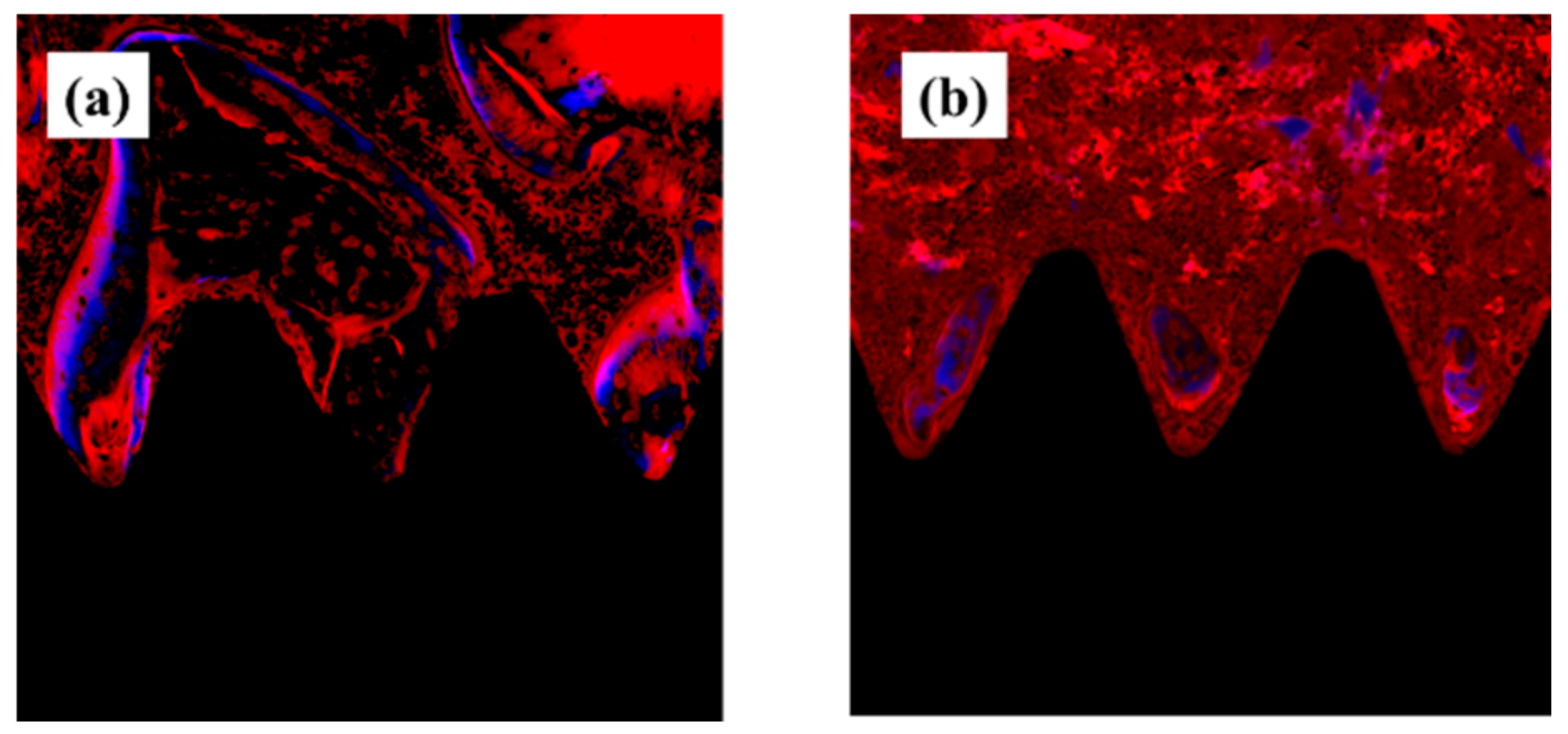
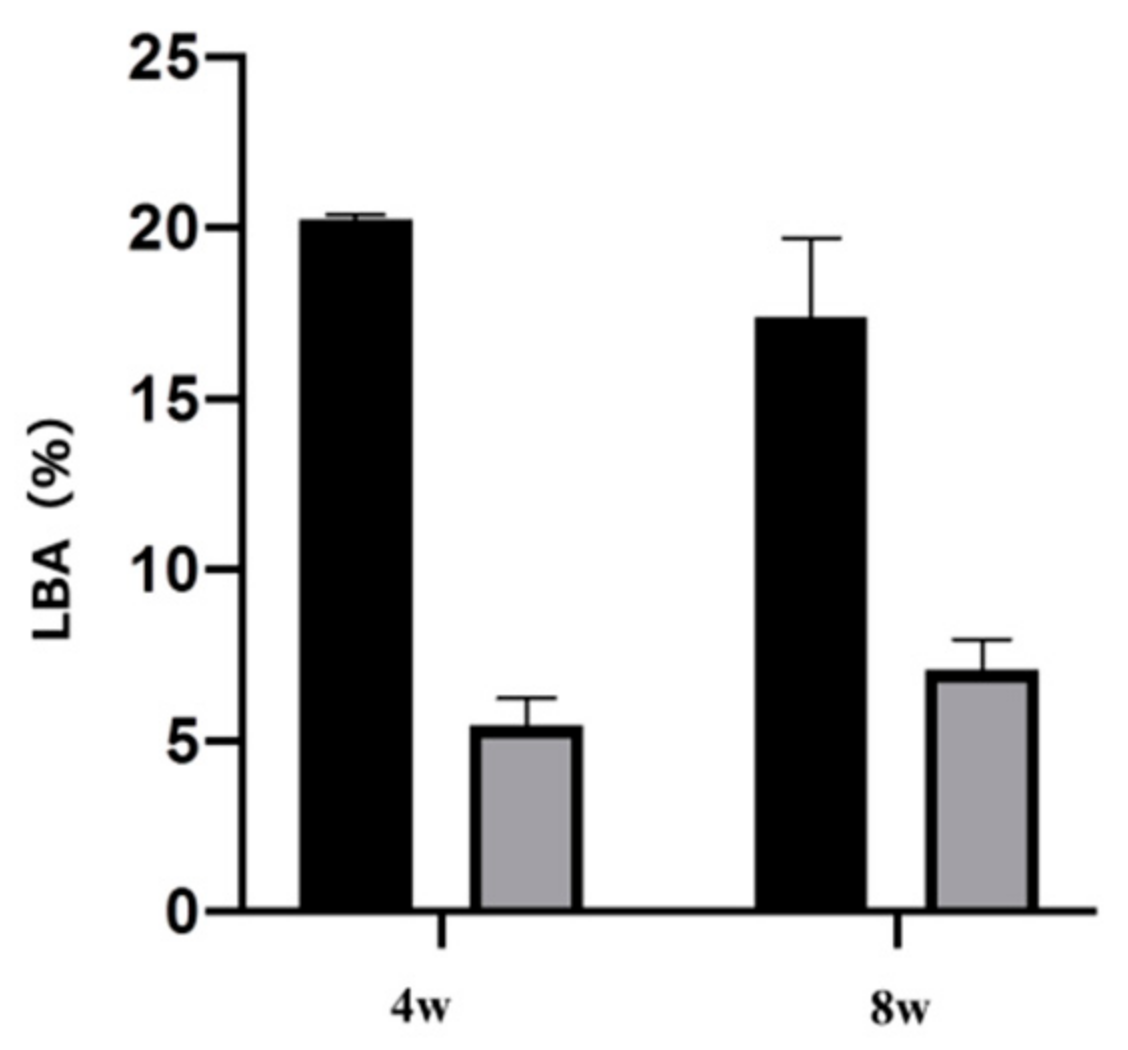
2.4. Plasma-Induced Bone Differentiation on Titanium Surfaces In Vivo
3. Discussion
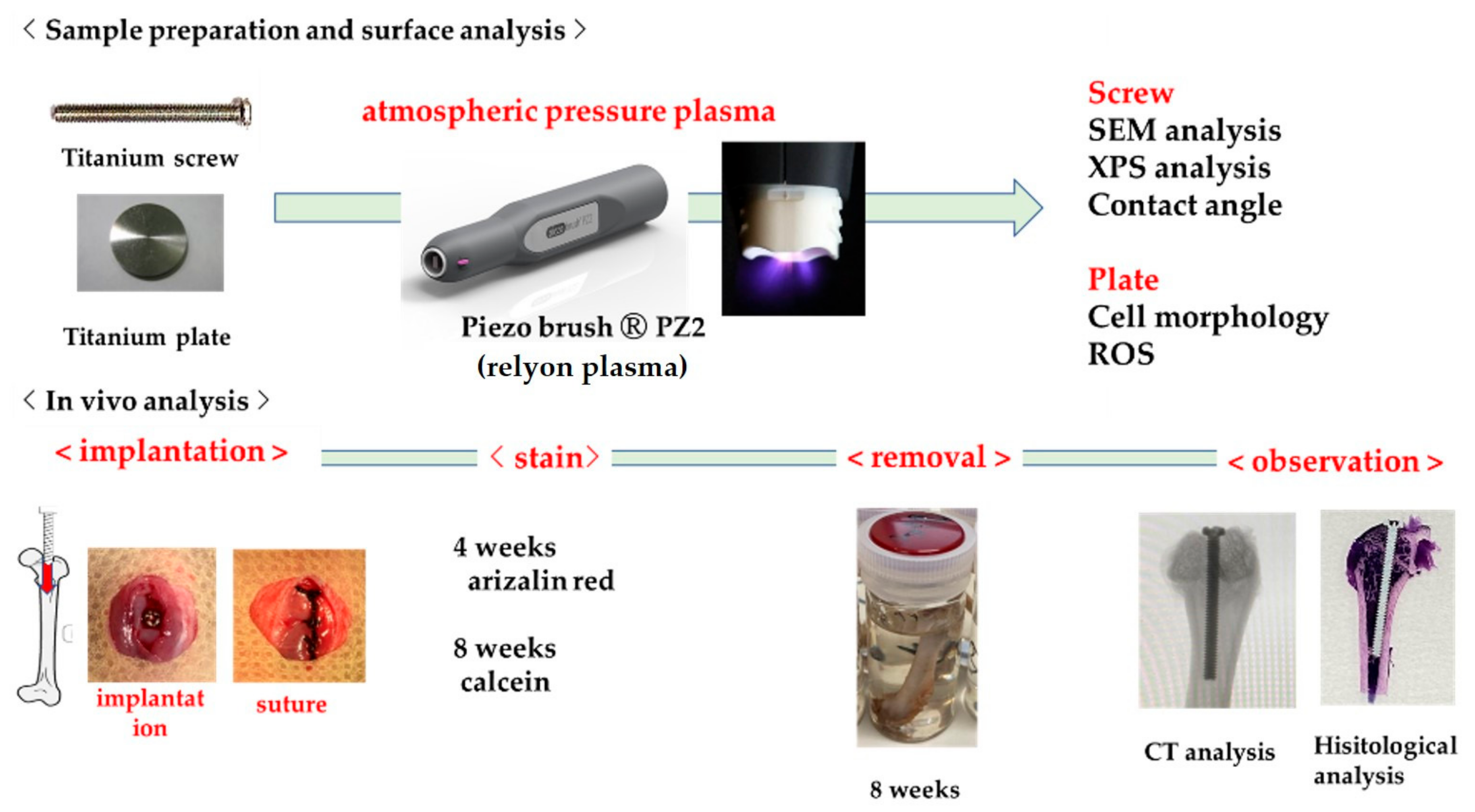
4. Materials and Methods
4.1. Sample Preparation
4.2. Cell Culture
4.3. Cell Morphology
4.4. Cell Intracellular ROS Level of RBMCs
4.5. Rat Distal Femur Model In Vivo
4.6. Sequential Fluorescence Labeling
4.7. Plasma-Induced Bone Differentiation on the TNS-Modified Titanium Surface In Vivo
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. Biomed. Res. Int. 2016. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Chen, S.; Tsoi, J.K.H.; Matinlinna, J.P. Binary Titanium Alloys as Dental Implant Materials—A Review. Regen. Biomater. 2017, 4, 315–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehrke, S.A.; Cavalcanti de Lima, J.H.; Rodriguez, F.; Calvo-Guirado, J.L.; Aramburú Júnior, J.; Pérez-Díaz, L.; Mazón, P.; Aragoneses, J.M.; De Aza, P.N. Microgrooves and Microrugosities in Titanium Implant Surfaces: An In Vitro and In Vivo Evaluation. Materials 2019, 12, 1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshinari, M.; Matsuzaka, K.; Inoue, T.; Oda, Y.; Shimono, M. Bio-functionalization of titanium surfaces for dental implants. Mater. Trans. 2002, 43, 2494–2501. [Google Scholar] [CrossRef] [Green Version]
- Dohan Ehrenfest, D.M.; Coelho, P.G.; Kang, B.-S.; Sul, Y.-T.; Albrektsson, T. Classification of Osseointegrated Implant Surfaces: Materials, Chemistry and Topography. Trends Biotechnol. 2010, 28, 198–206. [Google Scholar] [CrossRef]
- An, N.; Rausch-fan, X.; Wieland, M.; Matejka, M.; Andrukhov, O.; Schedle, A. Initial attachiment, subsequent cell proliferation/viability and gene expression of epithelial cells related to attachment and wound healing in response to different titanium surfaces. Dent. Mater. 2012, 28, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Zareidoost, A.; Yousefpour, M.; Ghaseme, B.; Amanzadeh, A. The relationship of surface roughness and cell response of chemical surface modification of titanium. J. Mater. Sci. Mater. Med. 2012, 23, 1479–1488. [Google Scholar] [CrossRef]
- Huang, H.-H.; Ho, C.-T.; Lee, T.-H.; Lee, T.-L.; Liao, K.-K.; Chen, F.-L. Effect of surface roughness of ground titanium on initial cell adhesion. Biomol. Eng. 2004, 21, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Räisänen, L.; Könönen, M.; Juhanoja, J.; Varpavaara, P.; Hautaniemi, J.; Kivilahti, J.; Hormia, M. Expression of cell adhe sion complexes in epithelial cells seeded on biomaterial surfaces. J. Biomed. Mater. Res. 2000, 49, 79–87. [Google Scholar] [CrossRef]
- Lauer, G.; Wiedmann-Al-Ahmad, M.; Otten, J.E.; Hübner, U.; Schmelzeisen, R.; Schilli, W. The titanium surface texture effects adherence and growth of human gingival keratinocytes and human maxillar osteoblast-like cells in vitro. Biomaterials 2001, 22, 2799–2809. [Google Scholar] [CrossRef]
- Baharloo, B.; Textor, M.; Brunette, D.M. Substratum roughness alters the growth, area, and focal adhesions of epithelial cells, and their proximity to titanium surfaces. J. Biomed. Mater. Res. Part A 2005, 74, 12–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wieland, M.; Textor, M.; Spencer, N.D.; Brunette, D.M. Wavelength-dependent roughness: A quantitative approach to characterizing the topography of rough titanium suefaces. Int. J. Oral Maxillofac. Implants 2001, 16, 163–181. [Google Scholar]
- Boyan, B.D.; Bonewald, L.F.; Paschalis, E.P.; Lohmann, C.H.; Rosser, J.; Cochran, D.L.; Dean, D.D.; Schwartz, Z.; Boskey, A.L. Osteoblast-mediated mineral deposition in culture is dependent on surface microtopography. Calcif. Tissue Int. 2002, 71, 519–529. [Google Scholar] [CrossRef]
- Poh, C.K.; Shi, Z.; Lim, T.Y.; Neoh, K.G.; Wang, W. The effect of VEGF functionalization of titanium on endothelial cells in vitro. Biomaterialsl 2010, 31, 1578–1585. [Google Scholar] [CrossRef]
- Jayaraman, M.; Meyer, U.; Bühner, M.; Joos, U.; Wiesmann, H.P. Influence of titanium surfaces on attachment of osteoblast-like cells in vitro. Biomaterials 2004, 25, 625–631. [Google Scholar] [CrossRef]
- Komasa, S.; Taguchi, Y.; Nishida, H.; Tanaka, M.; Kawazoe, T. Bioactivity of nanostructure on titanium surface modified by chemical processing at room temperature. J. Prosthodont. Res. 2012, 56, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Komasa, S.; Taguchi, Y.; Sekino, T.; Okazaki, J. Osteogenic activity of titanium surface with nanonetwork structures. Int. J. Nanomed. 2014, 9, 1741–1755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujino, T.; Taguchi, Y.; Komasa, S.; Sekino, T.; Tanaka, M. Cell differentiation on nanoscale feature of a titanium surface: Effects of deposition tme in NaOH solution. J. Hard Tissue Boil. 2014, 23, 63–70. [Google Scholar] [CrossRef]
- Nakano, Y.; Komasa, S.; Taguchi, Y.; Sekino, T.; Okazaki, J. Rat endothelial cell attachment, behavior and gene expression on NaOH-treated titanium surfaces. J. Oral Tissue Eng. 2013, 11, 189–200. [Google Scholar]
- Hara, Y.; Komasa, S.; Yoshimine, S.; Nisizaki, H.; Okazaki, J. Effect of Nano modified titanium surface on adsorption of rat periodontal ligament cells. J. Osaka Dent. Univ. 2018, 52, 37–44. [Google Scholar]
- Terada, C.; Komasa, S.; Kusumoto, T.; Kawazoe, T.; Okazaki, J. Effect of amelogenin coating of a nano-modified titanium surface on bioactivity. Int. J. Mol. Sci. 2018, 19, 1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Komasa, S.; Mashimo, C.; Sekino, T.; Okazaki, J. Effect of ultraviolet treatment on bacterial attachment and osteogenic activity to alkali-treated titanium with nanonetwork structures. Int. J. Nanomed. 2017, 12, 4633–4646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishizaki, M.; Komasa, S.; Taguchi, Y.; Nishizaki, H.; Okazaki, J. Bioactivity of NANOZR induced by alkali treatment. Int. J. Mol. Sci. 2017, 18, 780. [Google Scholar] [CrossRef] [Green Version]
- Komasa, S.; Nishizaki, M.; Zhang, H.; Takao, S.; Yin, D.; Terada, C.; Kobayashi, Y.; Kusumoto, T.; Yoshimine, S.; Nishizaki, H.; et al. Osseointegration of alkali-modified NANOZR implants: An in vivo study. Int. J. Mol. Sci. 2019, 20, 842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komasa, S.; Nisizaki, M.; Kusumoto, T.; Terada, C.; Derong, Y.; Kawamoto, A.; Yamamoto, S.; Yoshimine, S.; Nisizaki, H.; Shimizu, H.; et al. Osteogenesis-related gene expression on alkalimodified NANOZR and titanium surfaces with nanonetwork structures. J. Bio-Integr. 2017, 7, 87–94. [Google Scholar]
- Stevens, N.; Priest, C.I.; Sedev, R.; Ralston, J. Wettability of photoresponsive titanium dioxide surfaces. Langmuir 2003, 19, 3272–3275. [Google Scholar] [CrossRef]
- Adawiyah, J.H.; Zainab, N.J.; Imad, H.M.; Al-Hussaini, I.H. Review on: Titanium dioxide applications. Energy Procedia 2019, 157, 17–29. [Google Scholar]
- Ohler, B.; Langel, W. Molecular Dynamics Simulations on the Interface between Titanium Dioxide and Water Droplets: A New Model for the Contact Angle. J. Phys. Chem. C 2009, 113, 10189–10197. [Google Scholar] [CrossRef]
- Rupp, F.; Scheideler, L.; Olshanska, N.; de Wild, M.; Wielden, M.; Geis-Gerstorfer, J. Enhancing surface free energy and hydrophilicity through chemical modification of micro structured titanium implant surfaces. J. Biomed. Mater. Res. 2006, 76, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Schwartz, Z.; Wieland, M.; Rupp, F.; Geis-Gerstorfer, J.; Cochran, D.L.; Boyan, B.D. High surface energy enhances cell response to titanium substrate microstructure. J. Biomed. Mater. Res. Part A 2005, 74, 49–58. [Google Scholar] [CrossRef]
- Aita, H.; Hori, N.; Takeuchi, M.; Suzuki, T.; Yamada, M.; Anpo, M.; Ogawa, T. The effect of ultraviolet functionalization of titanium on integration with bone. Biomaterials 2009, 30, 1015–1025. [Google Scholar] [CrossRef]
- Aita, H.; Att, W.; Ueno, T.; Yamada, M.; Hori, N.; Iwasa, F.; Tsukimura, N.; Ogawa, T. Ultraviolet light-mediated photofunctionalization of titanium to promote human mesenchymal stem cell migration, attachment, proliferation and differentiation. Acta Biomater. 2009, 5, 3247–3257. [Google Scholar] [CrossRef]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied plasma medicine. Plasma Process Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]
- Schröder, K.; Finke, B.; Polak, M.; Lüthen, F.; Nebe, B.; Rychly, J.; Bader, R.; Lukowski, G.; Walschus, U.; Schlosser, M.; et al. Gas-discharge plasma-assisted functionalization of titanium implant surfaces. Mater. Sci. Forum 2010, 638–642, 700–705. [Google Scholar] [CrossRef] [Green Version]
- Coelho, P.G.; Giro, G.; Teixeira, H.S.; Marin, C.; Witek, L.; Thompson, V.P.; Tovar, N.; Silva, N.R.F.A. Argon-based atmospheric pressure plasma enhances early bone response to rough titanium surfaces. Biomaterials 2012, 100A, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- Yeung, K.W.K.; Chan, R.Y.L.; Lam, K.O.; Wu, S.L.; Liu, X.M.; Chung, C.Y.; Chu, P.K.; Lu, W.W.; Chan, D.; Luk, K.D.K.; et al. In vitro and in vivo characterization of novel plasma treated nickel titanium shape memory alloy for orthopedic implantation. Surf. Coat. Technol. 2007, 202, 1247–1251. [Google Scholar] [CrossRef]
- Matsumoto, T.; Tashiro, Y.; Komasa, S.; Miyake, A.; Komasa, Y.; Okazaki, J. Effect of modification on adsorption behavior f cell and protein on titanium surface by using quartz crystal microbalance system. Materials 2021, 14, 97. [Google Scholar] [CrossRef]
- Perrin, D.; Szmukler, M.S.; Echikou, C.; Pointaire, P.; Bernard, J.P. Bone response to alteration of surface topography and surface composition of sandblasted and acid etched (SLA) implants. Clin Oral Implants Res 2002, 13, 465–469. [Google Scholar] [CrossRef]
- Foest, R.; Kindel, E.; Ohl, A.; Stieber, M.; Weltmann, K.-D. Non-thermal atmospheric pressure discharges for surface modification. Plasma Phys. Control. Fusion 2005, 47, B525–B536. [Google Scholar] [CrossRef]
- Duske, K.; Koban, I.; Kindel, E.; Schröder, K.; Nebe, B.; Holtfreter, B.; Jablonowski, L.; Weltmann, K.D.; Kocher, T. Atmospheric plasma enhances wettability and cell spreading on dental implant metals. J. Clin. Periodontol. 2012, 39, 400–407. [Google Scholar] [CrossRef]
- Ujino, D.; Nisizaki, H.; Higuchi, S.; Komasa, S.; Okazaki, J. Effect of plasma treatment of titanium surface on bioactivity. Appl. Sci. 2019, 9, 2257. [Google Scholar] [CrossRef] [Green Version]
- Takao, S.; Komasa, S.; Agariguchi, A.; Kusumoto, T.; Pezzotti, G.; Okazaki, J. Effects of plasma treatment on the bioactivity of alkali-treated ceria-stabilised zirconia/alumina nanocomposite (NANOZR). Int. J. Mol. Sci. 2020, 21, 7476. [Google Scholar] [CrossRef]
- Zeng, Y.; Komasa, S.; Nishida, H.; Agariguchi, A.; Sekino, T.; Okazaki, J. Enhanced osseointegration and bio-decontamination of nanostructured titanium, based on non-thermal atmospheric pressure plasma. Int. J. Mol. Sci. 2020, 21, 3533. [Google Scholar] [CrossRef]
- Toffoli, A.; Parisi, L.; Tatti, R.; Lorenzi, A.; Verucchi, R.; Manfredi, E.; Lumetti, S.; Macaluso, G.M. Thermal-induced hydrophilicity enhancement of titanium dental implant surfaces. J. Oral Sci. 2020, 62, 217–221. [Google Scholar] [CrossRef] [Green Version]
- Guastaldi, F.P.S.; Yoo, D.; Marin, C.; Jimbo, R.; Tavor, N.; Zanetta-Barbosa, D.; Chelho, P.G. Plasma treatment maintains surface energy of the implant surface and enhances osseointegration. Int. J. Biomater. 2013. [Google Scholar] [CrossRef]
- Tallarico, M.; Baldini, N.; Gatti, F.; Martinolli, M.; Xhanari, E.; Meloni, S.M.; Gabriele, C.; Immacolata, L.A. Role of new hydrophilic surfaces on early success rate and implantstability: 1-year post-loading results of a multicenter, split-mouth, randomized controlled trial. Eur. J. Dent. 2021, 15, 001–007. [Google Scholar] [CrossRef]
- Park, J.-W.; Jang, J.-H.; Lee, C.S.; Hanawa, T. Osteoconductivity of hydrophilic microstructured titanium implants with phosphate ion chemistry. Acta Biomater. 2009, 5, 2311–2321. [Google Scholar] [CrossRef]
- Basu, S.; Michaëlsson, K.; Olofsson, H.; Johansson, S.; Melhus, H. Association between oxidative stress and bone mineral density. Biochem. Biophys. Res. Commun. 2001, 288, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xu, L.; Zhang, T.; Ren, G.; Yang, Z. Oxidative stress and apoptosis induced by nanosized titanium dioxide in PC12 cells. Toxicology 2010, 267, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Jeong, W.-S.; Seo, S.-J.; Kim, H.-W.; Kim, K.-N.; Choi, E.-H.; Kim, K.-M. Non-thermal atmospheric pressure plasma functionalized dental implant for enhancement of bacterial resistance and osseointegration. Dent. Mater. 2017, 33, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Leon-Ramos, J.-R.; Diosdado-Cano, J.-M.; López-Santos, C.; Barranco, A.; Torres-Lagares, D.; Serrera-Figallo, M.-A. Influence of titanium oxide pillar array nanometric structure and ultraviolet irradiation on the properties of the surface of dental implants—A pilot study. Nanomaterials 2019, 9, 1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsujita, H.; Nishizaki, H.; Miyake, A.; Takao, S.; Komasa, S. Effect of Plasma Treatment on Titanium Surface on the Tissue Surrounding Implant Material. Int. J. Mol. Sci. 2021, 22, 6931. https://doi.org/10.3390/ijms22136931
Tsujita H, Nishizaki H, Miyake A, Takao S, Komasa S. Effect of Plasma Treatment on Titanium Surface on the Tissue Surrounding Implant Material. International Journal of Molecular Sciences. 2021; 22(13):6931. https://doi.org/10.3390/ijms22136931
Chicago/Turabian StyleTsujita, Hitomi, Hiroshi Nishizaki, Akiko Miyake, Seiji Takao, and Satoshi Komasa. 2021. "Effect of Plasma Treatment on Titanium Surface on the Tissue Surrounding Implant Material" International Journal of Molecular Sciences 22, no. 13: 6931. https://doi.org/10.3390/ijms22136931
APA StyleTsujita, H., Nishizaki, H., Miyake, A., Takao, S., & Komasa, S. (2021). Effect of Plasma Treatment on Titanium Surface on the Tissue Surrounding Implant Material. International Journal of Molecular Sciences, 22(13), 6931. https://doi.org/10.3390/ijms22136931





