Protective Effect of Membrane-Free Stem Cells against Lipopolysaccharide and Interferon-Gamma-Stimulated Inflammatory Responses in RAW 264.7 Macrophages
Abstract
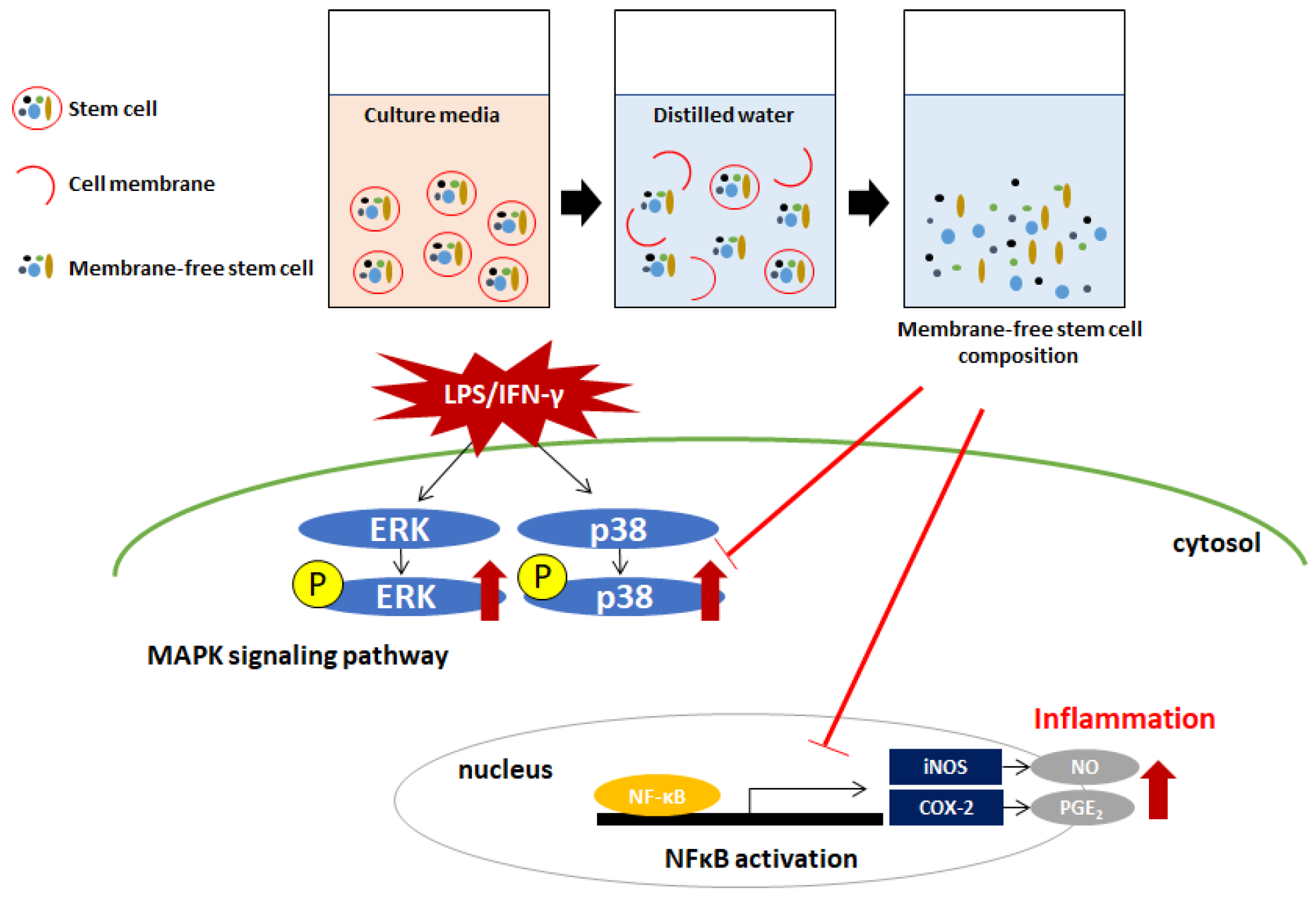
:1. Introduction
2. Results
2.1. Effect of MFSC-Ex on Cell Viability in LPS/IFN-γ-Stimulated RAW 264.7 Macrophage Cells
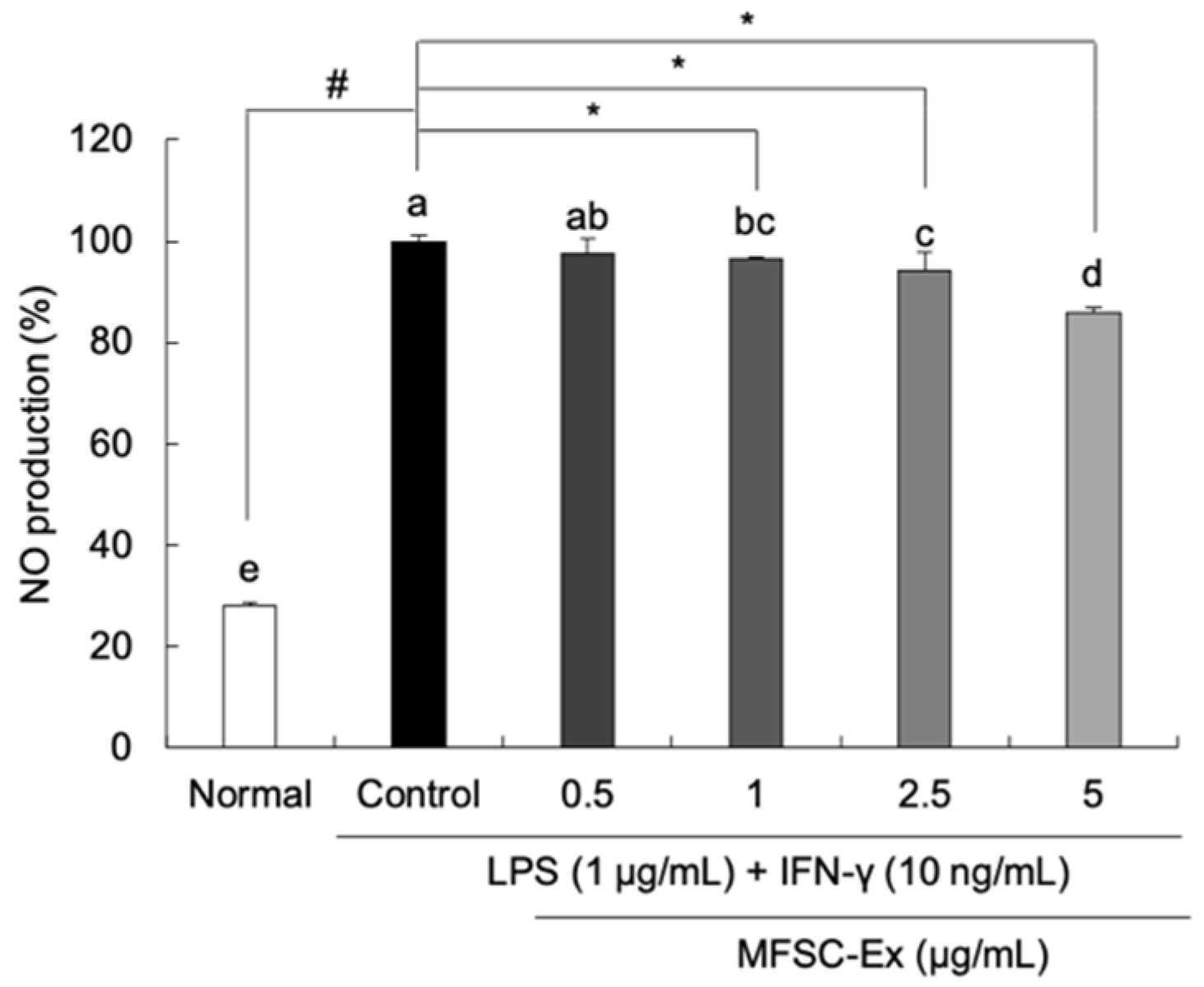
2.2. Inhibitory Effect of MFSC-Ex on NO Generation in LPS/IFN-γ-Stimulated RAW 264.7 Macrophage Cells
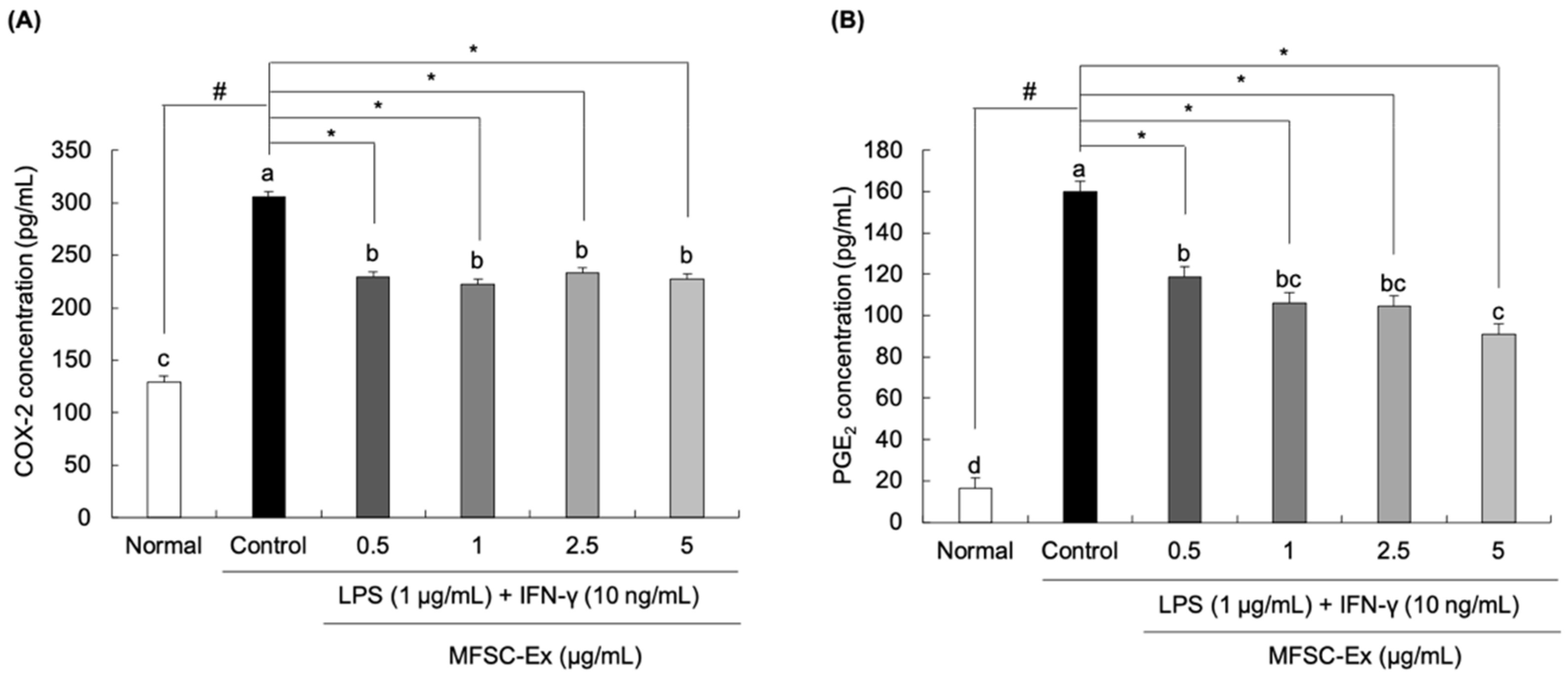
2.3. Inhibitory Effect of MFSC-Ex on COX-2 and PGE2 Generation in LPS/IFN-γ-Stimulated RAW 264.7 Macrophage Cells
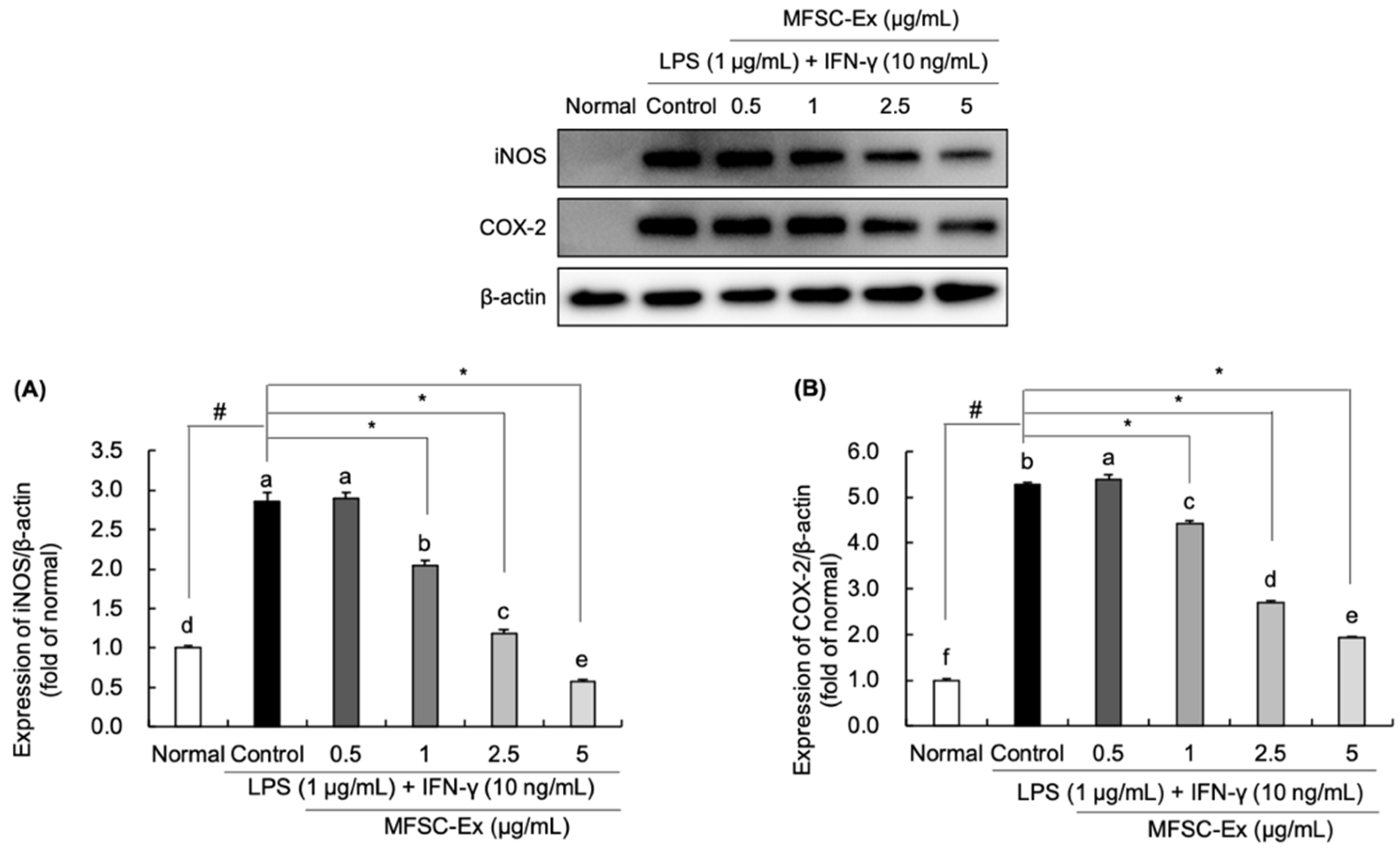
2.4. Regulatory Effect of MFSC-Ex on the Protein Levels of iNOS and COX-2 in LPS/IFN-γ-Stimulated RAW 264.7 Macrophage Cell
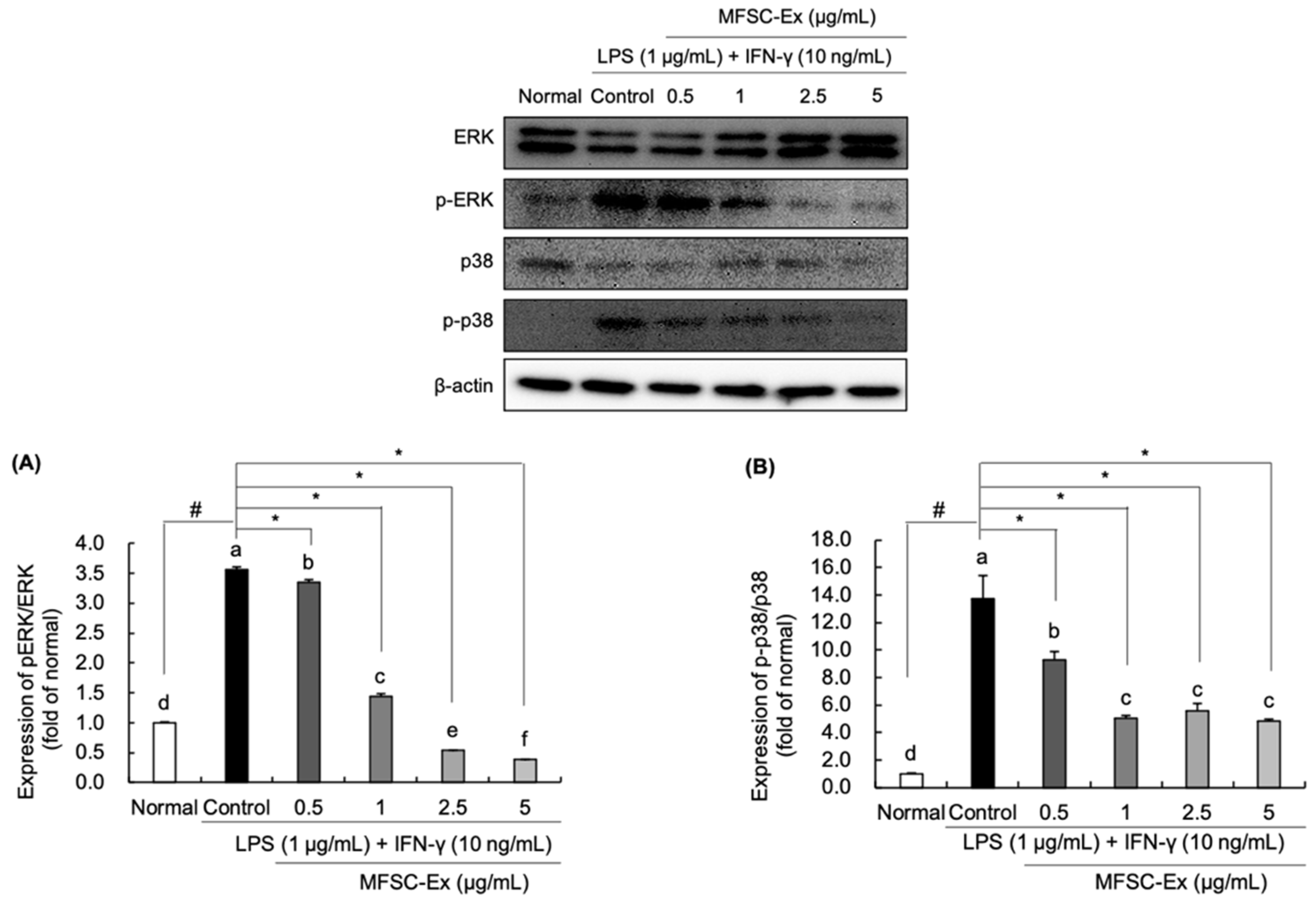
2.5. Regulatory Effect of MFSC-Ex on the Protein Levels of ERK and p-38 Phosphorylation in LPS/IFN-γ-Stimulated RAW 264.7 Macrophage Cell
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents
4.2. Preparation of MFSC-Ex
4.3. Cell Culture
4.4. Cell Viability
4.5. Measurement of NO Levels
4.6. Measurement of COX-2 and PGE2 Levels
4.7. Western Blotting
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Newton, K.; Dixit, V.M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef] [PubMed]
- MacMicking, J.; Xie, Q.W.; Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef]
- McDaniel, M.L.; Kwon, G.; Hill, J.R.; Marchall, C.A.; Corbett, J.A. Cytokines and nitric oxide in islet inflammation and diabetes. Exp. Biol. Med. 1996, 211, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Alexander, C.; Rietschel, E.T. Bacterial lipopolysaccharides and innate immunity. J. Endotoxin Res. 2001, 7, 167–202. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S. NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef]
- Yoon, W.J.; Moon, J.; Song, G.; Lee, Y.; Han, M.; Lee, J.; Ihm, B.; Lee, W.; Lee, N.; Hyun, C. Artemisia fukudo essential oil attenuates LPS-induced inflammation by suppressing NF-κB and MAPK activation in RAW 264.7 macrophages. Food Chem. Toxicol. 2010, 48, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Chi, G.; Wei, M.; Xie, X.; Soromou, L.W.; Liu, F.; Zhao, S. Suppression of MAPK and NF-κB pathways by limonene contributes to attenuation of lipopolysaccharide-Induced inflammatory responses in acute lung injury. Inflammation 2013, 36, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.A.; Kim, S.Y.; Ye, B.R.; Kim, J.; Ko, S.C.; Lee, W.W.; Kim, K.N.; Choi, I.W.; Jung, W.K.; Heo, S.J. Anti-inflammatory effect of Apo-9′-fucoxanthinone via inhibition of MAPKs and NF-κB signaling pathway in LPS-stimulated RAW 264.7 macrophages and zebrafish model. Int. Immunopharmacol. 2018, 59, 339–346. [Google Scholar] [CrossRef]
- Li, Y.; He, S.; Tang, J.; Ding, N.; Chu, X.; Cheng, L.; Ding, X.; Liang, T.; Feng, S.; Rahman, S.U.; et al. Andrographolide inhibits inflammatory cytokines secretion in LPS-stimulated RAW 264.7 cells through suppression of NF-κB/MAPK signaling pathway. Evid. Based Complement. Altern. Med. 2017, 2017, 8248142. [Google Scholar]
- Kim, H.J.; Lee, H.S.; Chong, Y.H.; Kang, J.L. p38 Mitogen-activated protein kinase up-regulates LPS-induced NF kappa B activation in the development of lung injury and RAW 264.7 macrophages. Toxicology 2006, 225, 36–47. [Google Scholar] [CrossRef]
- Kim, E.K.; Choi, E.J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta. 2010, 1802, 396–405. [Google Scholar] [CrossRef] [Green Version]
- Frese, L.; Dijkman, P.E.; Hoerstrup, S.P. Adipose tissue-derived stem cells in regenerative medicine. Transfus. Med. Hemother. 2016, 43, 268–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camussi, G.; Deregibus, M.C.; Cantaluppi, V. Role of stem-cell-derived microvesicles in the paracrine action of stem cells. Biochem. Soc. Trans. 2013, 41, 283–287. [Google Scholar] [CrossRef]
- Lai, R.C.; Chen, T.S.; Lim, S.K. Mesenchymal stem cell exosome: A novel stem cell-based therapy for cardiovascular disease. Regen. Med. 2011, 6, 481–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saralamma, V.V.G.; Vetrivel, P.; Kim, S.M.; Ha, S.E.; Lee, H.J.; Lee, S.J.; Kim, Y.S.; Pak, J.E.; Lee, H.J.; Heo, J.D.; et al. Proteome profiling of membrane-free stem cell components by nano-LS/MS analysis and its anti-inflammatory activity. Evid.-Based Complement. Altern. Med. 2019, 2019, 4683272. [Google Scholar]
- He, M.T.; Kim, J.H.; Kim, Y.S.; Park, H.S.; Cho, E.J. Protective effects of membrane-free stem cell extract from H2O2-induced inflammation responses in human periodontal ligament fibroblasts. J. Korea Acad.-Ind. Coop. Soc. 2019, 20, 95–103. [Google Scholar]
- Kim, M.J.; Kim, J.H.; Park, H.S.; Kim, Y.S.; Cho, E.J. Protective effect of membrane-free stem cell extract against oxidative stress in LLC-PK1 cells. J. Korea Acad. Ind. Coop. Soc. 2019, 20, 303–312. [Google Scholar]
- Park, H.S.; Pang, Q.Q.; Kim, Y.S.; Kim, J.H.; Cho, E.J. Neuroprotective effect of membrane-free stem cell extract against amyloid beta 25-35-indcued neurotoxicity in SH-SY5Y cells. Appl. Sci. 2021, 11, 2219. [Google Scholar] [CrossRef]
- Medzhitov, R.; Janeway, C.A., Jr. Innate immunity: The virtues of a nonclonal system of recognition. Cell 1997, 91, 295–298. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.S.; Shin, J.S.; Jang, D.S.; Lee, K.T. Cnidilide, an alkylphthalide isolated from the roots of Cnidium officinale, suppresses LPS-induced NO, PGE2, IL-1β, IL-6 and TNF-α production by AP-1 and NF-κB inactivation in RAW 264.7 macrophages. Int. Immunopharmacol. 2016, 40, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Tamai, R.; Sugawara, S.; Takeuchi, O.; Akira, S.; Takada, H. Synergistic effects of lipopolysaccharide and interferon-gamma in inducing interleukin-8 production in human monocytic THP-1 cells is accompanied by up-regulation of CD14, Toll-like receptor 4, MD-2 and MyD88 expression. J. Endotoxin. Res. 2003, 9, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Cao, J.; Fang, L.; Zhao, H.; Liu, Z.; Ran, J.; Zheng, X.; Li, X.; Zhou, Y.; Ge, D.; et al. Geniposide suppresses LPS-induced nitric oxide, PGE2 and inflammatory cytokine by downregulating NF-kappaB, MAPK and AP-1 signaling pathways in macrophages. Int. Immunopharmacol. 2014, 20, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Gomez, P.F.; Pillinger, M.H.; Attur, M.; Marjanovic, N.; Dave, M.; Park, J.; Bingham, C.O.; Al-Mussawir, H.; Abramson, S.B. Resolution of inflammation: Prostaglandin E2 dissociates nuclear trafficking of individual NF-kappa B subunits (p65, p50) in stimulated rheumatoid synovial fibroblasts. J. Immunol. 2005, 175, 6924–6930. [Google Scholar] [CrossRef] [Green Version]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Al-Ghadban, S.; Bunnell, B.A. Adipose tissue-derived stem cells: Immunomodulatory effects and therapeutic potential. Physiology 2020, 35, 125–133. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, S.M.; Moon, Y.G.; Jung, Y.S.; Lee, J.H.; Saralamma, V.V.G.; Kim, Y.S.; Pak, J.E.; Lee, H.J.; Kim, G.S.; et al. Membrane-free stem cell components inhibit interleukin-1α-stimulated inflammation and cartilage degradation in vitro and in vivo: A rat model of osteoarthritis. Int. J. Mol. Sci. 2019, 20, 4869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Vincent, S.R. Nitric oxide neurons and neurotransmission. Prog. Neurobiol. 2010, 90, 246–255. [Google Scholar] [CrossRef]
- Muntané, J.; De la Mata, M. Nitric oxide and cancer. World J. Hepatol. 2010, 2, 337–344. [Google Scholar] [CrossRef]
- Nagy, G.; Koncz, A.; Telarico, T.; Fernandez, D.; Ersek, B.; Buzas, E.; Perl, A. Central role of nitric oxide in the pathogenesis of rheumatoid arthritis and systemic lupus erythematosus. Arthritis Res. Ther. 2010, 12, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuste, J.E.; Tarragon, E.; Campuzano, C.M.; Ros-Bernal, F. Implications of glial nitric oxide in neurodegenerative diseases. Front. Cell. Neurosci. 2015, 9, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giroux, M.; Descoteaux, A. Cyclooxygenase-2 expression in macrophages: Modulation by protein kinase C-alpha. J. Immunol. 2000, 165, 3985–3991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinz, B.; Brune, K. Cyclooxygenase-2-10 years later. J. Pharmacol. Exp. Ther. 2002, 300, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, D.J.; Yun, J.C.; Jung, M.H.; Yeo, H.D.; Kim, H.J.; Kim, D.W.; Yang, J.I.; Lee, G.W.; Jeong, S.H.; et al. Human adipose tissue-derived mesenchymal stem cells protect kidneys from cisplatin nephrotoxicity in rats. Am. J. Physiol. Ren. Physiol. 2012, 302, F1141–F1150. [Google Scholar] [CrossRef] [Green Version]
- Hommes, D.; Peppelenbosch, M.; Van Deventer, S. Mitogen activated protein (MAP) kinase signal transduction pathways and novel anti-inflammatory targets. Gut 2003, 52, 144–151. [Google Scholar] [CrossRef] [Green Version]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy-from molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta-Proteins Proteom. 2005, 1754, 253–262. [Google Scholar] [CrossRef]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef] [Green Version]
- Herlaar, E.; Brown, Z. p38 MAPK signalling cascades in inflammatory disease. Mol. Med. Today 1999, 5, 439–447. [Google Scholar] [CrossRef]
- Chun, J.; Choi, R.J.; Khan, S.; Lee, D.S.; Kim, Y.C.; Nam, Y.J.; Lee, D.U.; Kim, Y.S. Alantolactone suppresses inducible nitric oxide synthase and cyclooxygenase-2 expression by down-regulating NF-κB, MAPK and AP-1 via the MyD88 signaling pathway in LPS-activated RAW 264.7 cells. Int. Immunopharmacol. 2012, 14, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Xagorari, A.; Roussos, C.; Papapetropoulos, A. Inhibition of LPS-stimulated pathways in macrophages by the flavonoid luteolin. Br. J. Pharmacol. 2002, 136, 1058–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Schmidt, H.H.; Warner, T.D.; Nakane, M.; Förstermann, U.; Murad, F. Regulation and subcellular location of nitrogen oxide synthases in RAW264.7 macrophages. Mol. Pharmacol. 1992, 41, 615–624. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.T.; Park, H.S.; Kim, Y.S.; Lee, A.Y.; Cho, E.J. Protective Effect of Membrane-Free Stem Cells against Lipopolysaccharide and Interferon-Gamma-Stimulated Inflammatory Responses in RAW 264.7 Macrophages. Int. J. Mol. Sci. 2021, 22, 6894. https://doi.org/10.3390/ijms22136894
He MT, Park HS, Kim YS, Lee AY, Cho EJ. Protective Effect of Membrane-Free Stem Cells against Lipopolysaccharide and Interferon-Gamma-Stimulated Inflammatory Responses in RAW 264.7 Macrophages. International Journal of Molecular Sciences. 2021; 22(13):6894. https://doi.org/10.3390/ijms22136894
Chicago/Turabian StyleHe, Mei Tong, Hye Sook Park, Young Sil Kim, Ah Young Lee, and Eun Ju Cho. 2021. "Protective Effect of Membrane-Free Stem Cells against Lipopolysaccharide and Interferon-Gamma-Stimulated Inflammatory Responses in RAW 264.7 Macrophages" International Journal of Molecular Sciences 22, no. 13: 6894. https://doi.org/10.3390/ijms22136894
APA StyleHe, M. T., Park, H. S., Kim, Y. S., Lee, A. Y., & Cho, E. J. (2021). Protective Effect of Membrane-Free Stem Cells against Lipopolysaccharide and Interferon-Gamma-Stimulated Inflammatory Responses in RAW 264.7 Macrophages. International Journal of Molecular Sciences, 22(13), 6894. https://doi.org/10.3390/ijms22136894





