Effect of Morphological Characteristics and Biomineralization of 3D-Printed Gelatin/Hyaluronic Acid/Hydroxyapatite Composite Scaffolds on Bone Tissue Regeneration
Abstract
:1. Introduction
2. Results and Discussion
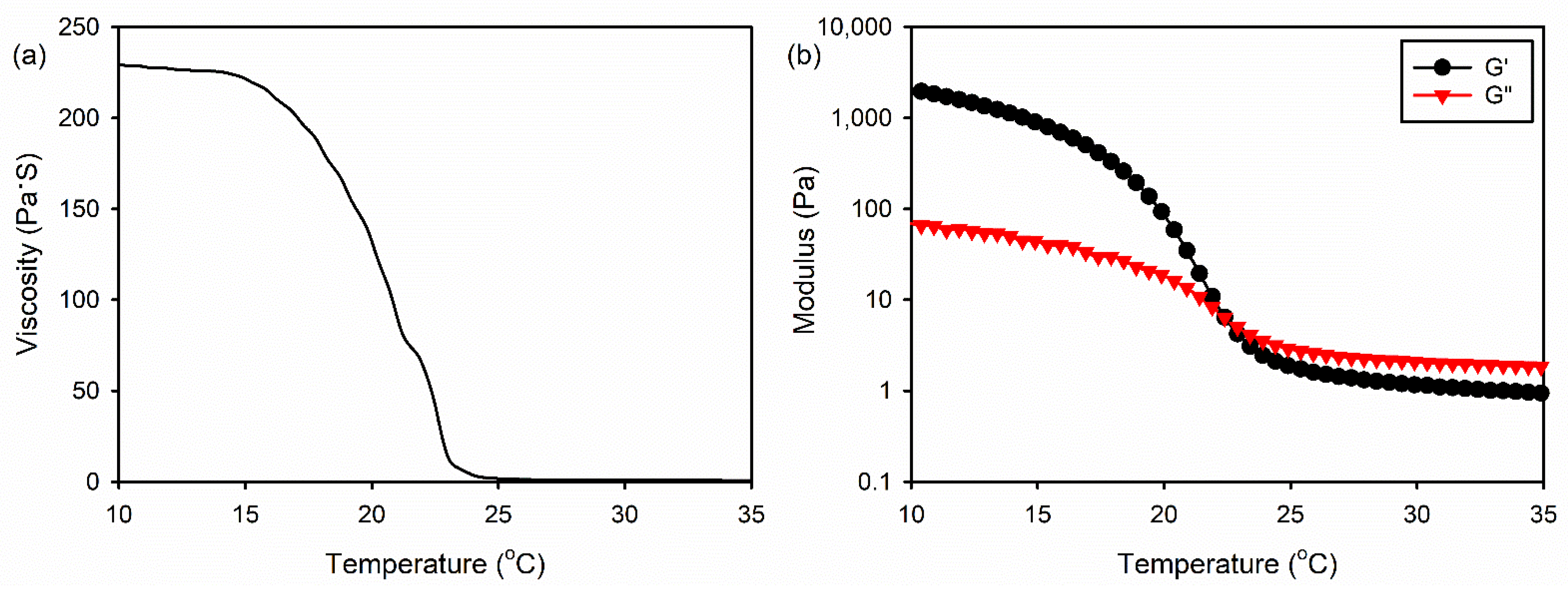
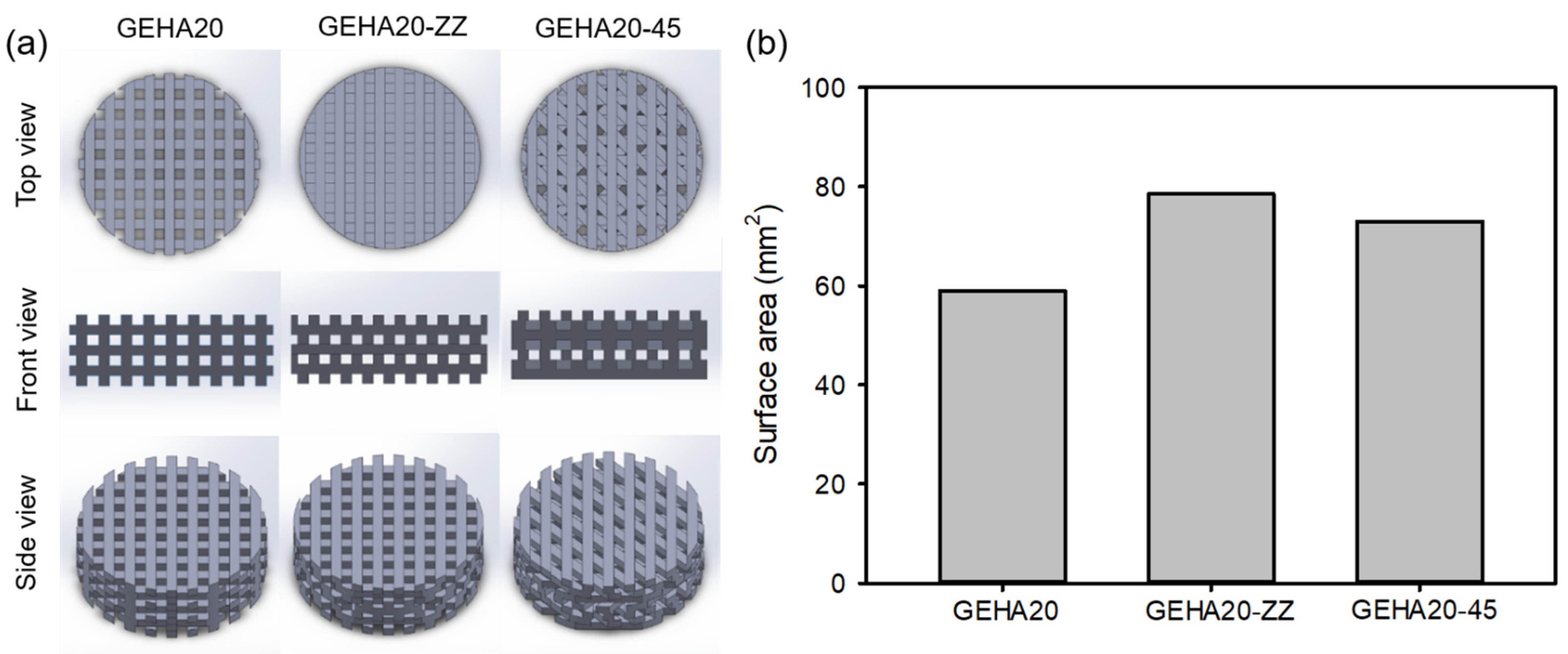
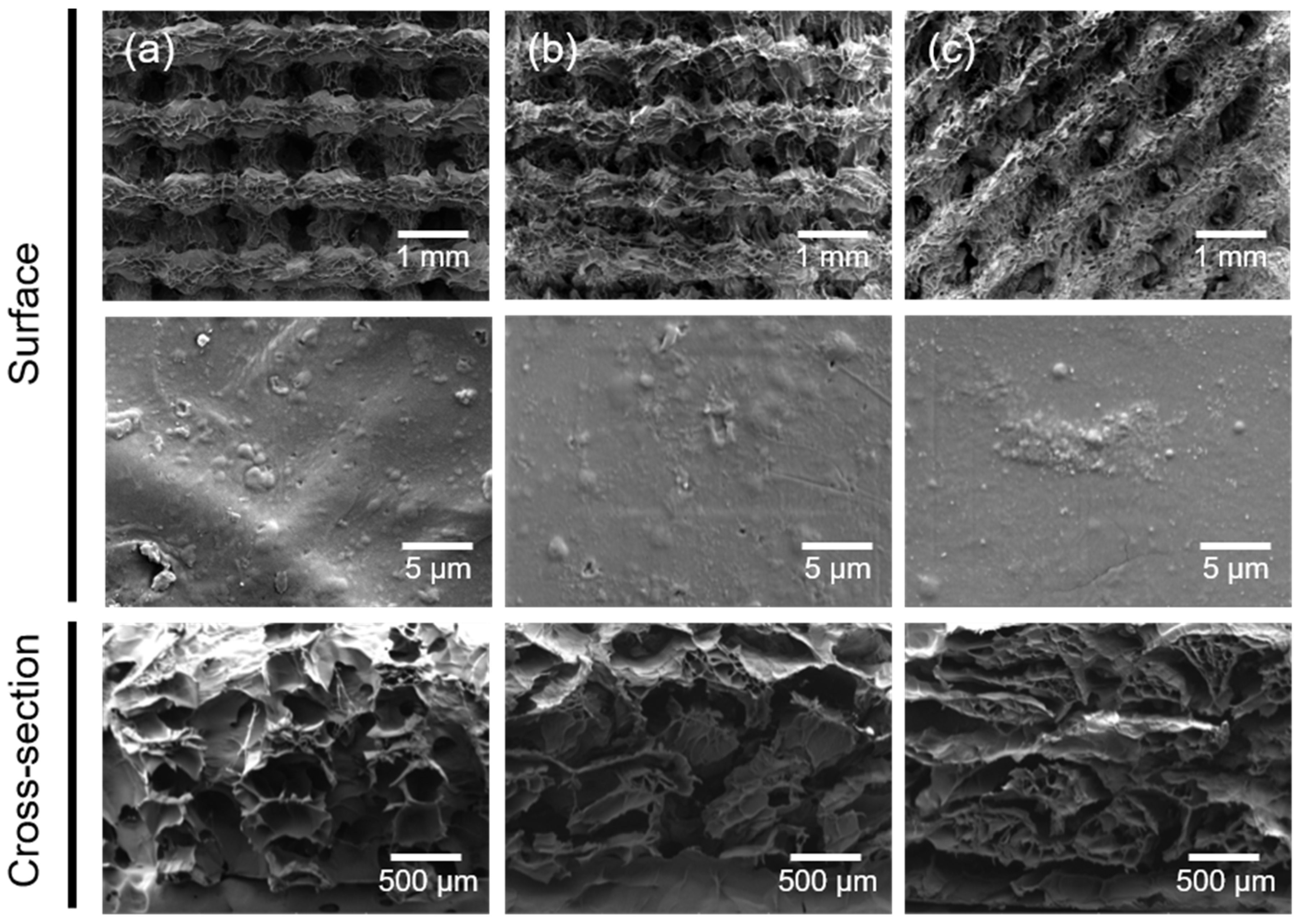
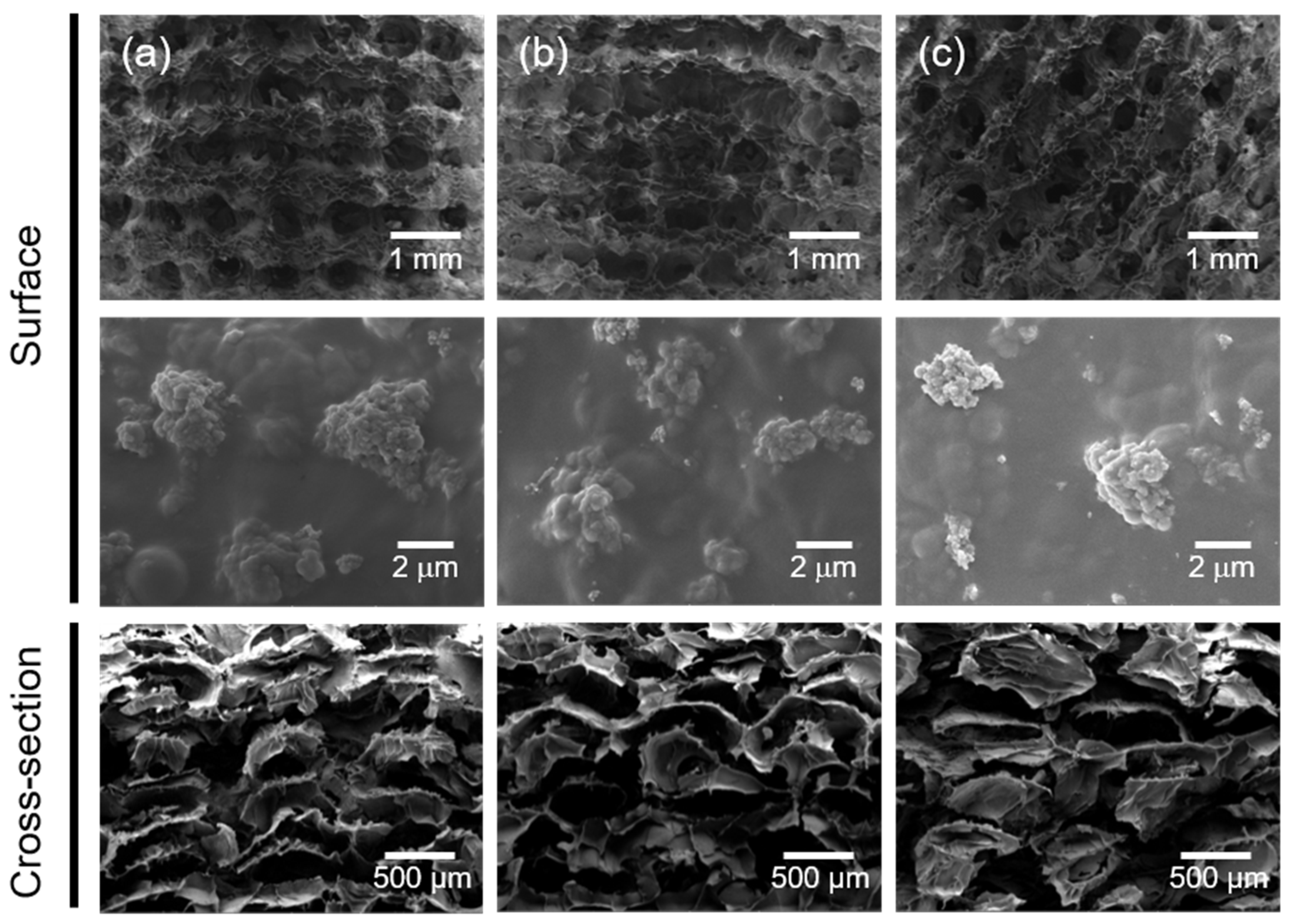
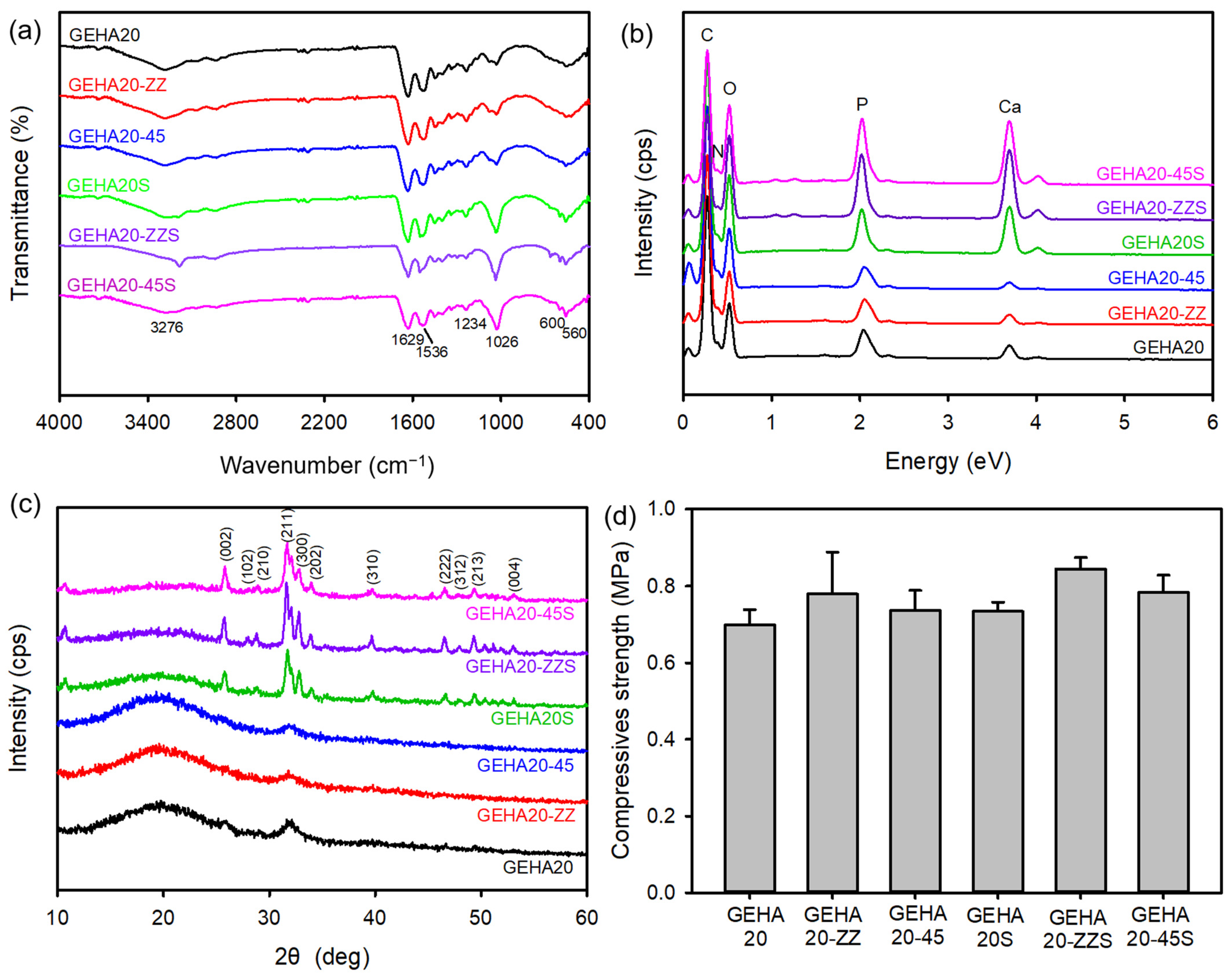
2.1. Fabrication and Characterization of the 3D Composite Scaffolds
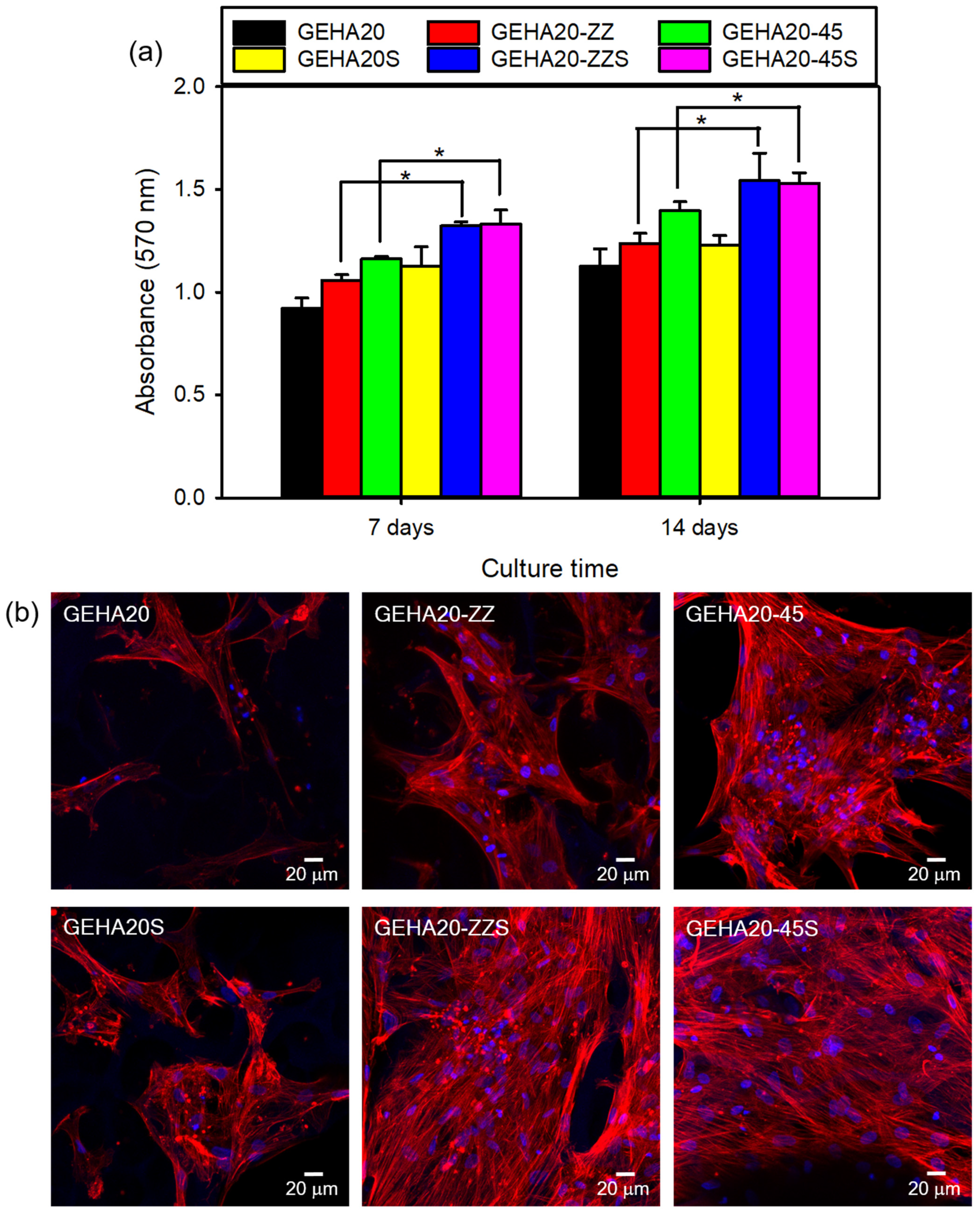
2.2. Cell Attachment and Proliferation on the 3D Composite Scaffolds
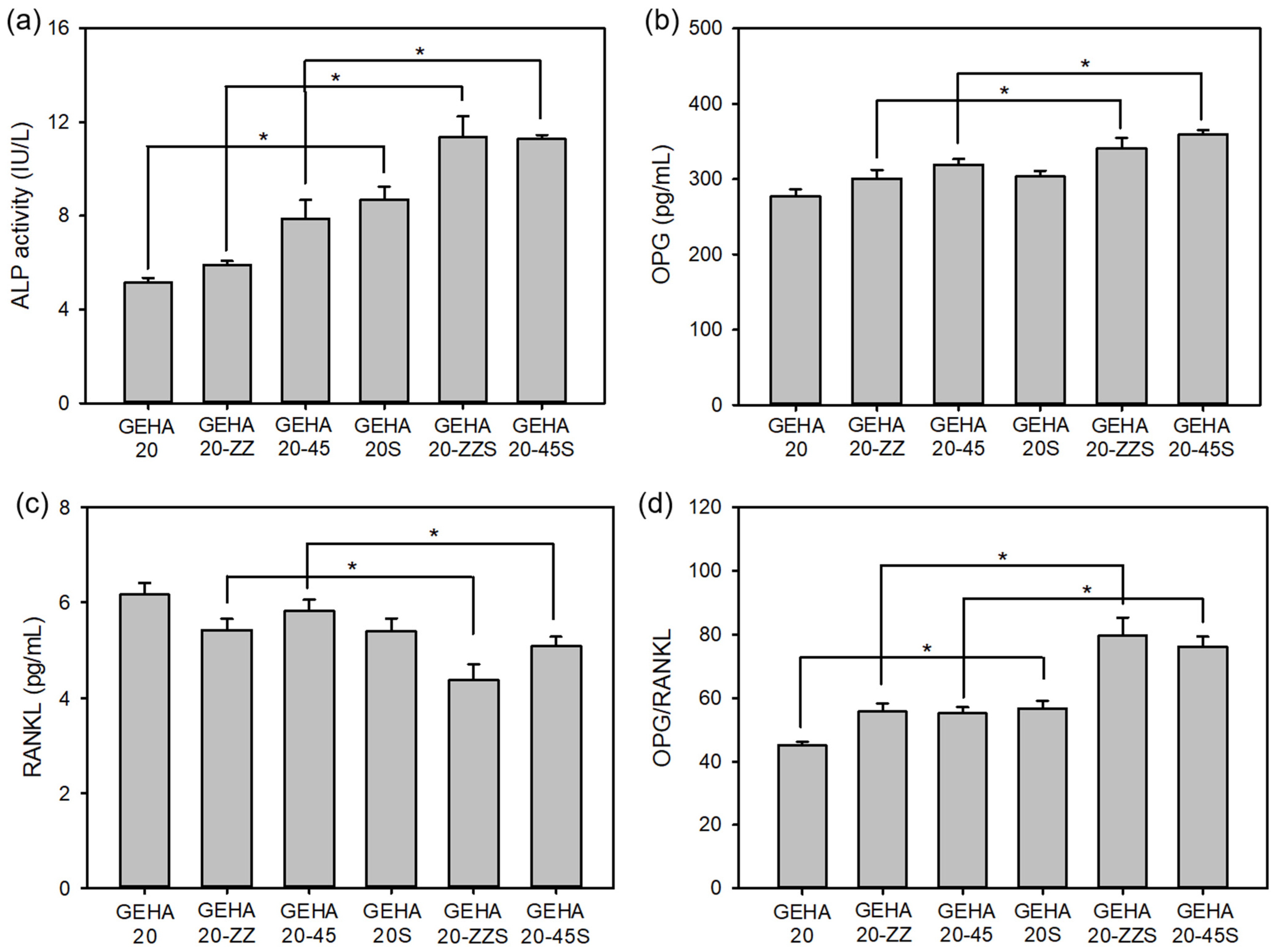
2.3. Cell Differentiation on the 3D Composite Scaffolds
3. Materials and Methods
3.1. Materials
3.2. Rheological Measurements of the Gelatin/HA Solution
3.3. Fabrication of the 3D Composite Scaffolds
3.4. Crosslinking and Biomineralization of the 3D Composite Scaffolds
3.5. Surface Morphology of the 3D Composite Scaffolds
3.6. Physicochemical and Mechanical Properties of the 3D Composite Scaffolds
3.7. Cell Attachment and Proliferation on the 3D Composite Scaffolds
3.8. ALP Activity
3.9. Expression of OPG and RANKL
3.10. qRT-PCR Analysis
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aki, D.; Ulag, S.; Unal, S.; Sengor, M.; Ekren, N.; Lin, C.-C.; Yılmazer, H.; Ustundag, C.B.; Kalaskar, D.M.; Gunduz, O. 3D printing of PVA/hexagonal boron nitride/bacterial cellulose composite scaffolds for bone tissue engineering. Mater. Des. 2020, 196, 109094. [Google Scholar] [CrossRef]
- Simurda, T.; Kubisz, P.; Dobrotova, M.; Necas, L.; Stasko, J. Patient with congenital afibrinogenemia during revision total hip arthroplasty. Semin. Thromb. Hemost. 2016, 42, 689–692. [Google Scholar]
- Du, X.; Wei, D.; Huang, L.; Zhu, M.; Zhang, Y.; Zhu, Y. 3D printing of mesoporous bioactive glass/silk fibroin composite scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2019, 103, 109731. [Google Scholar] [CrossRef]
- Baptista, R.; Guedes, M. Morphological and mechanical characterization of 3D printed PLA scaffolds with controlled porosity for trabecular bone tissue replacement. Mater. Sci. Eng. C 2021, 118, 111528. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pathak, J.L.; Liang, D.; Zhong, N.; Guan, H.; Wan, M.; Miao, G.; Li, Z.; Ge, L. Fabrication and characterization of strontium-hydroxyapatite/silk fibroin biocomposite nanospheres for bone-tissue engineering. Int. J. Biol. Macromol. 2020, 142, 366–375. [Google Scholar] [CrossRef]
- Wang, C.; Huang, W.; Zhou, Y.; He, L.; He, Z.; Chen, Z.; He, X.; Tian, S.; Liao, J.; Lu, B.; et al. 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 2020, 5, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Yazdanian, M.; Tabesh, H.; Houshmand, B.; Tebyanian, H.; Soufdoost, R.S.; Tahmasebi, E.; Karami, A.; Ghullame, S. Fabrication and properties of βTCP/Zeolite/Gelatin scaffold as developed scaffold in bone regeneration: In vitro and in vivo studies. Biocybern. Biomed. Eng. 2020, 40, 1626–1637. [Google Scholar] [CrossRef]
- He, J.; Hu, X.; Cao, J.; Zhang, Y.; Xiao, J.; Peng, L.; Chen, D.; Xiong, C.; Zhang, L. Chitosan-coated hydroxyapatite and drug-loaded polytrimethylene carbonate/polylactic acid scaffold for enhancing bone regeneration. Carbohydr. Polym. 2021, 253, 117198. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Wu, M.; Yan, F.; Xie, Y.; Liu, Z.; Huang, H.; Yang, Z.; Yao, S.; Cai, L. A radial 3D polycaprolactone nanofiber scaffold modified by biomineralization and silk fibroin coating promote bone regeneration in vivo. Int. J. Biol. Macromol. 2021, 172, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Fisch, P.; Molnar, M.; Eggert, S.; Binelli, M.; Maniura-Weber, K.; Zenobi-Wong, M. Development and through characterization of the processing steps of an ink for 3D printing for bone tissue engineering. Mater. Sci. Eng. C 2020, 108, 110510. [Google Scholar] [CrossRef] [PubMed]
- Ligon, S.D.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D printing and customized additive manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Wang, L.; Song, P.; Pei, X.; Sun, H.; Wu, L.; Zhou, C.; Wang, K.; Fan, Y.; Zhang, X. 3D printed bone tissue regenerative PLA/HA scaffolds with comprehensive performance optimizations. Mater. Des. 2021, 201, 109490. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Y.; Qin, X.; Wa, Q. 3D printing of alginate/gelatin scaffolds with homogenous nano apatite coating for bone tissue engineering. Mater. Des. 2018, 146, 12–19. [Google Scholar] [CrossRef]
- Maji, S.; Agarwal, T.; Das, J.; Maiti, T.K. Development of gelatin/carboxymethyl chitosan/nano-hydroxyapatite composite 3D macroporous scaffold for bone tissue engineering applications. Carbohydr. Polym. 2018, 189, 115–125. [Google Scholar] [CrossRef]
- Wu, M.; Wu, P.; Xiao, L.; Zhao, Y.; Yan, F.; Liu, X.; Xie, Y.; Zhang, C. Biomimetic mineralization of novel hydroxyethyl cellulose/soy protein isolate scaffolds promote bone regeneration in vitro and in vivo. Int. J. Biol. Macromol. 2020, 162, 1627–1641. [Google Scholar] [CrossRef]
- Lian, H.; Zhang, L.; Meng, Z. Biomimetic hydroxyapatite/gelatin composites for bone tissue regeneration: Fabrication, characterization, and osteogenic differentiation in vitro. Mater. Des. 2018, 156, 381–388. [Google Scholar] [CrossRef]
- Domingos, M.; Chiellini, F.; Gloria, A.; Ambrosio, L.; Bartolo, P.; Chiellini, E. Effect of process parameters on the morphological and mechanical properties of 3D bioextruded poly(ε-caprolactone) scaffolds. Rapid Prototyp. J. 2012, 18, 56–67. [Google Scholar] [CrossRef]
- He, Y.; Yang, F.F.; Zhao, H.M.; Xia, B.; Fu, J.Z. Research on the printability of hydrogels in 3D bioprinting. Sci. Rep. 2016, 6, 29977. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.J.; Park, S.J.; Gu, B.K.; Kim, Y.-J.; Chung, S.; Kim, C.-H. Effect of the pore size in a 3D printed gelatin scaffold on fibroblast proliferation. J. Ind. Eng. Chem. 2018, 67, 388–395. [Google Scholar] [CrossRef]
- Lee, K.Y.; Bouhadir, K.H.; Mooney, D.J. Degradation behavior of covalently cross-linked poly(aldehyde guluronate) hydrogels. Macromolecules 2000, 33, 97–101. [Google Scholar] [CrossRef]
- Xia, J.; Yuan, Y.; Wu, H.; Huang, Y.; Weitz, D.A. Decoupling the effects of nanopore size and surface roughness on the attachment, spreading and differentiation of bone marrow-derived stem cells. Biomaterials 2020, 248, 120014. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Yu, X.; Jiang, X.; Brody, H.D.; Rowe, D.W.; Wei, M. Fabrication and characterization of biomimetic collagen-apatite scaffolds with tunable structures for bone tissue engineering. Acta Biomater. 2013, 9, 7308–7319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fayyazbakhsh, F.; Solati-Hashjin, M.; Keshtkar, A.; Shokrgozar, M.A.; Dehghan, M.M.; Larijani, B. Novel layered double hydroxides-hydroxyapatite/gelatin bone tissue engineering scaffolds: Fabrication, characterization, and in vivo study. Mater. Sci. Eng. C 2017, 76, 701–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabha, R.D.; Nair, B.P.; Ditzel, N.; Kjems, J.; Nair, P.D.; Kassem, M. Strontium functionalized scaffold for bone tissue engineering. Mater. Sci. Eng. C 2019, 94, 509–515. [Google Scholar] [CrossRef]
- Huh, J.T.; Lee, J.U.; Kim, W.J.; Yeo, M.; Kim, G.H. Preparation and characterization of gelatin/α-TCP/SF biocomposite scaffold for bone tissue regeneration. Int. J. Biol. Macromol. 2018, 110, 488–496. [Google Scholar] [CrossRef]
- Kim, D.; Lee, J.U.; Kim, G.H. Biomimetic gelatin/HA biocomposites with effective elastic properties and 3D-structural flexibility using a 3D-printing process. Addit. Manuf. 2020, 36, 101616. [Google Scholar] [CrossRef]
- Sritharan, S.; Kannan, T.P.; Norazmi, M.N.; Nurul, A.A. The synergistic effects of IL-6/IL-17A promote osteogenic differentiation by improving OPG/RANKL ratio and adhesion of MC3T3-E1 cells on hydroxyapatite. J. Craniomaxillofac. Surg. 2018, 46, 1361–1367. [Google Scholar] [CrossRef]
- Kapasa, E.R.; Ginnoudius, P.V.; Jia, X.; Hatton, P.V.; Yang, X.B. The effects of RANKL/OPG balance on remodeling implant complications. J. Funct. Biomater. 2017, 8, 42. [Google Scholar] [CrossRef]
- Mahato, A.; Sandy, Z.; Bysakh, S.; Hupa, L.; Das, I.; Bhattacharjee, P.; Kundu, B.; De, G.; Nandi, S.K.; Vallittu, P.; et al. Development of nano-porous hydroxyapatite coated e-glass for potential bone-tissue engineering application: An in vitro approach. Mater. Sci. Eng. C 2020, 111, 110764. [Google Scholar] [CrossRef] [PubMed]


| Gene | Primer Sequence (5′-3′) | |
|---|---|---|
| Forward | Reverse | |
| COL1 | TCTAGACATGTTCAGCTTTGTGGAC | TCTGTACGCAGGTGATTGGTG |
| RUNX2 | CACTGGCGCTGCAACAAGA | CATTCCGGAGCTCAGCAGAATAA |
| OPN | TCACCAGTCTGATGAGTCTCACCATTC | TAGCATCAGGGTACTGGATGTCAGGTC |
| GAPDH | AGATCATCAGCAATGCAATGCCTCC | ATGGCATGGACTGTGGTCAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-W.; Han, Y.-S.; Lee, H.-M.; Kim, J.-K.; Kim, Y.-J. Effect of Morphological Characteristics and Biomineralization of 3D-Printed Gelatin/Hyaluronic Acid/Hydroxyapatite Composite Scaffolds on Bone Tissue Regeneration. Int. J. Mol. Sci. 2021, 22, 6794. https://doi.org/10.3390/ijms22136794
Kim J-W, Han Y-S, Lee H-M, Kim J-K, Kim Y-J. Effect of Morphological Characteristics and Biomineralization of 3D-Printed Gelatin/Hyaluronic Acid/Hydroxyapatite Composite Scaffolds on Bone Tissue Regeneration. International Journal of Molecular Sciences. 2021; 22(13):6794. https://doi.org/10.3390/ijms22136794
Chicago/Turabian StyleKim, Jae-Woo, Yoon-Soo Han, Hyun-Mee Lee, Jin-Kyung Kim, and Young-Jin Kim. 2021. "Effect of Morphological Characteristics and Biomineralization of 3D-Printed Gelatin/Hyaluronic Acid/Hydroxyapatite Composite Scaffolds on Bone Tissue Regeneration" International Journal of Molecular Sciences 22, no. 13: 6794. https://doi.org/10.3390/ijms22136794
APA StyleKim, J.-W., Han, Y.-S., Lee, H.-M., Kim, J.-K., & Kim, Y.-J. (2021). Effect of Morphological Characteristics and Biomineralization of 3D-Printed Gelatin/Hyaluronic Acid/Hydroxyapatite Composite Scaffolds on Bone Tissue Regeneration. International Journal of Molecular Sciences, 22(13), 6794. https://doi.org/10.3390/ijms22136794







