Characterization of an Atypical Trypanosoma brucei Hsp70 Demonstrates Its Cytosolic-Nuclear Localization and Modulation by Quercetin and Methylene Blue
Abstract
:1. Introduction
2. Results
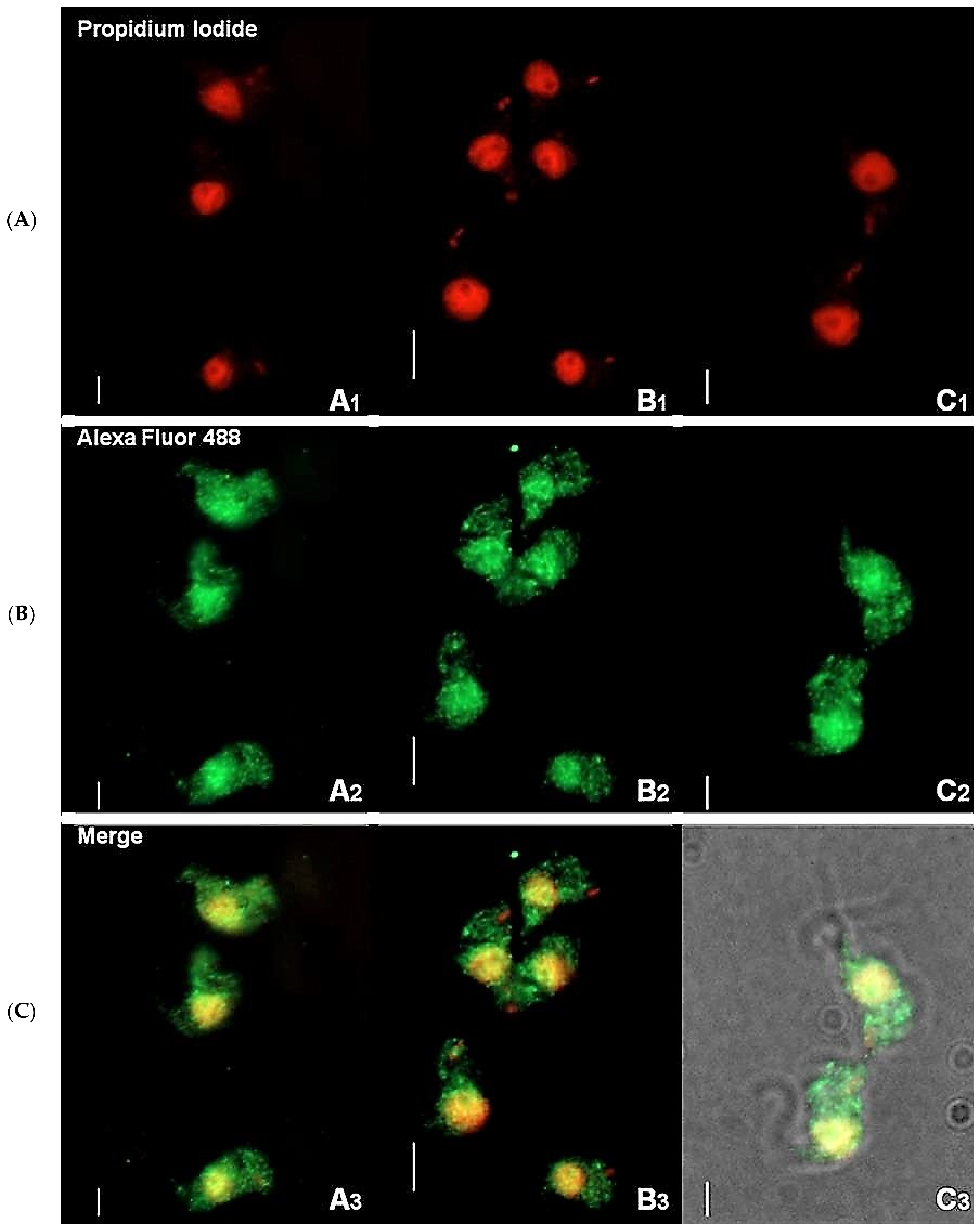
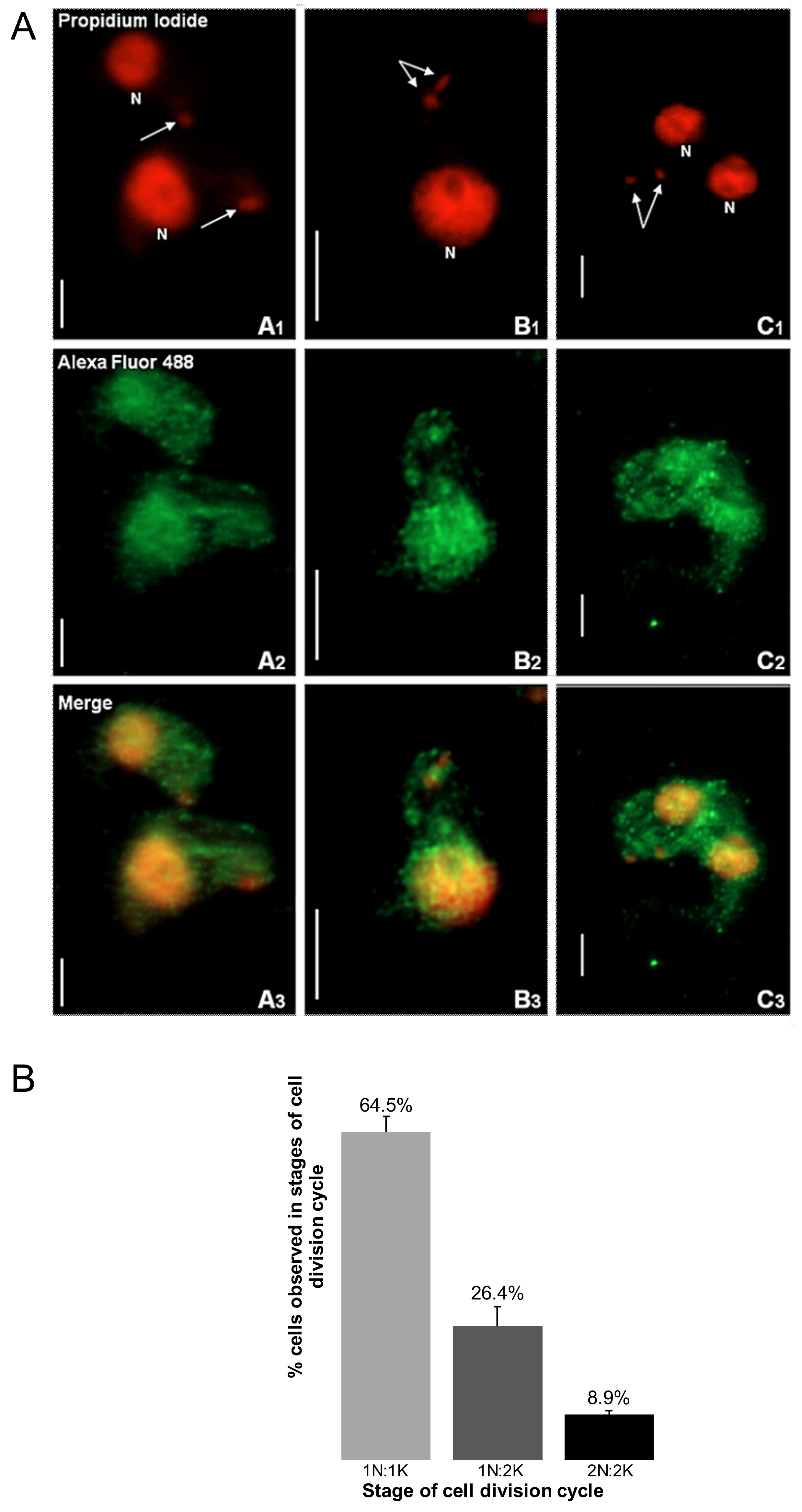
2.1. Immunofluorescence Suggests That TbHsp70.c Localizes in Both the Cytosol and Nucleus
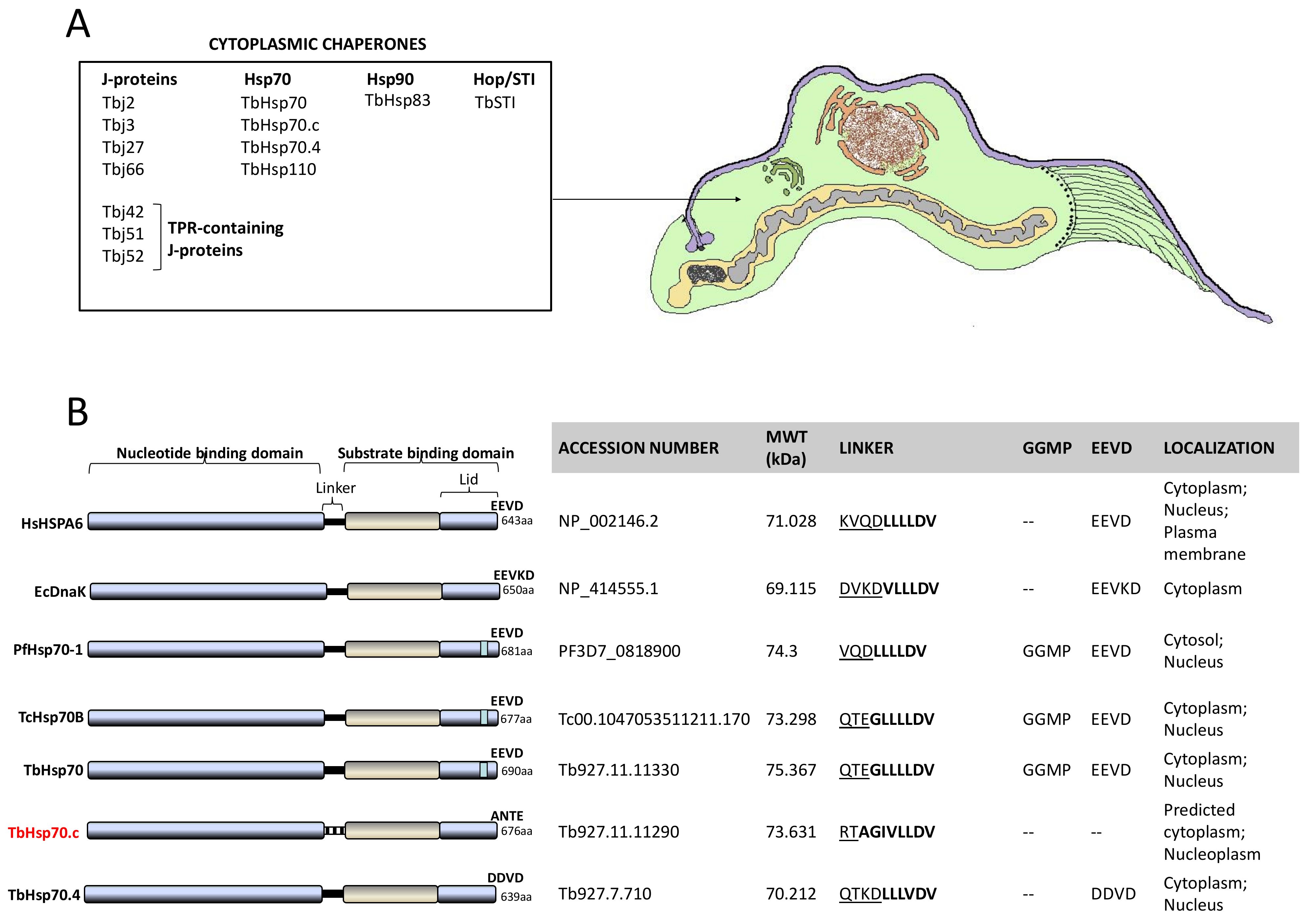
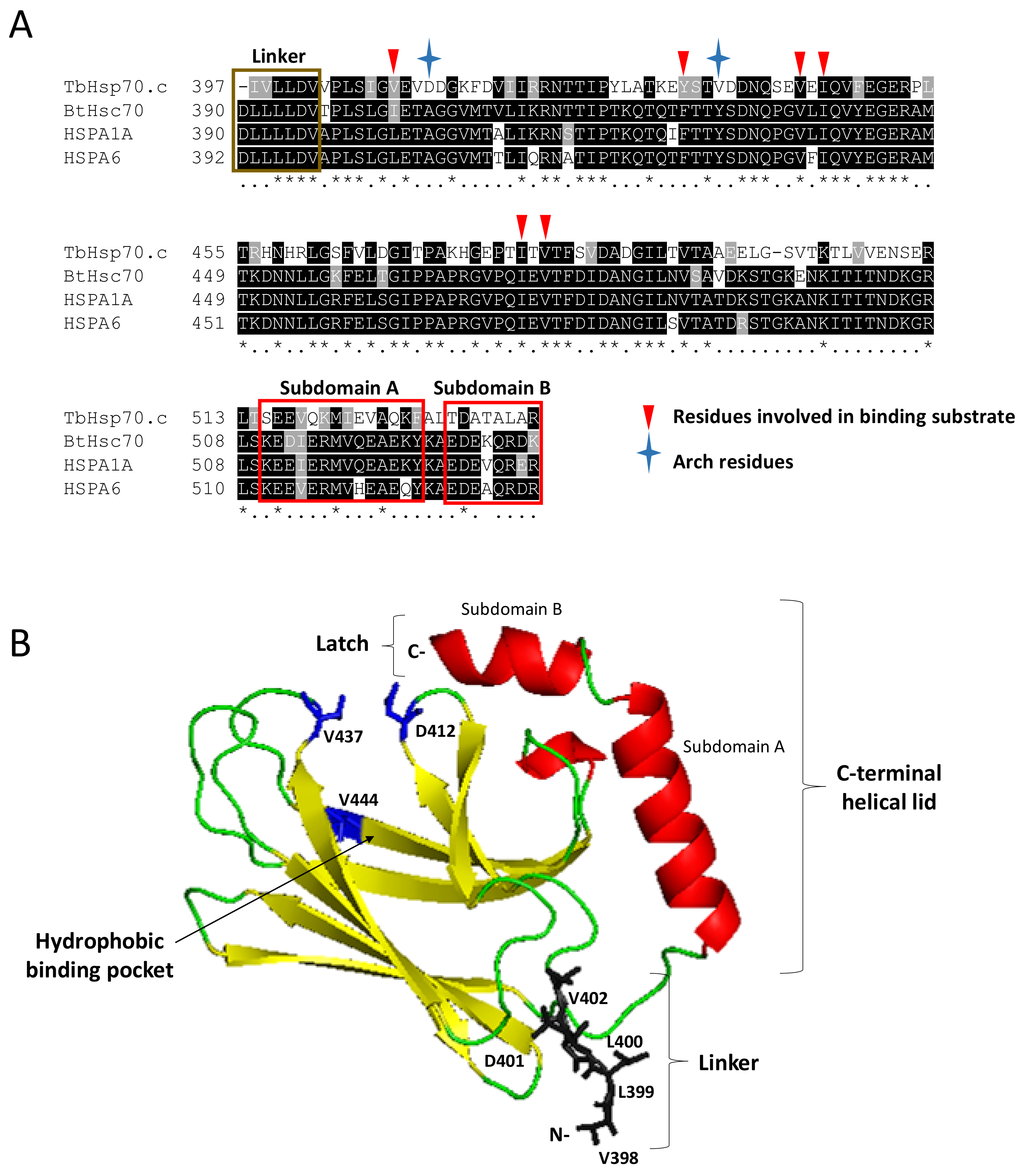
2.2. TbHsp70.c Possesses Unique Residues in the Hydrophobic Pocket, and Lacks Some Key Motifs
2.3. Homology Modelling of the TbHsp70.c Substrate Binding Domain Highlights a Unique Hydrophobic Pocket
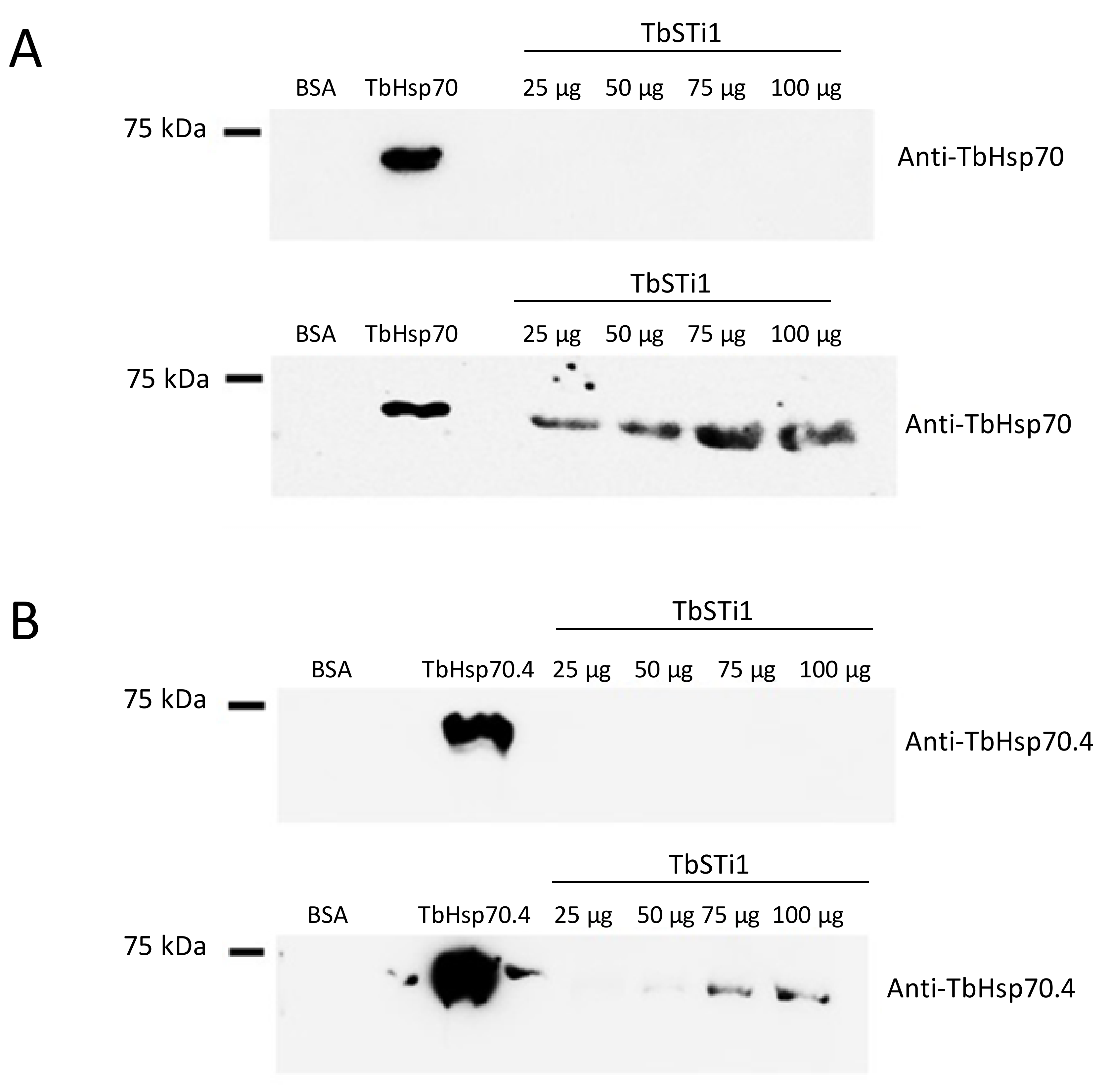
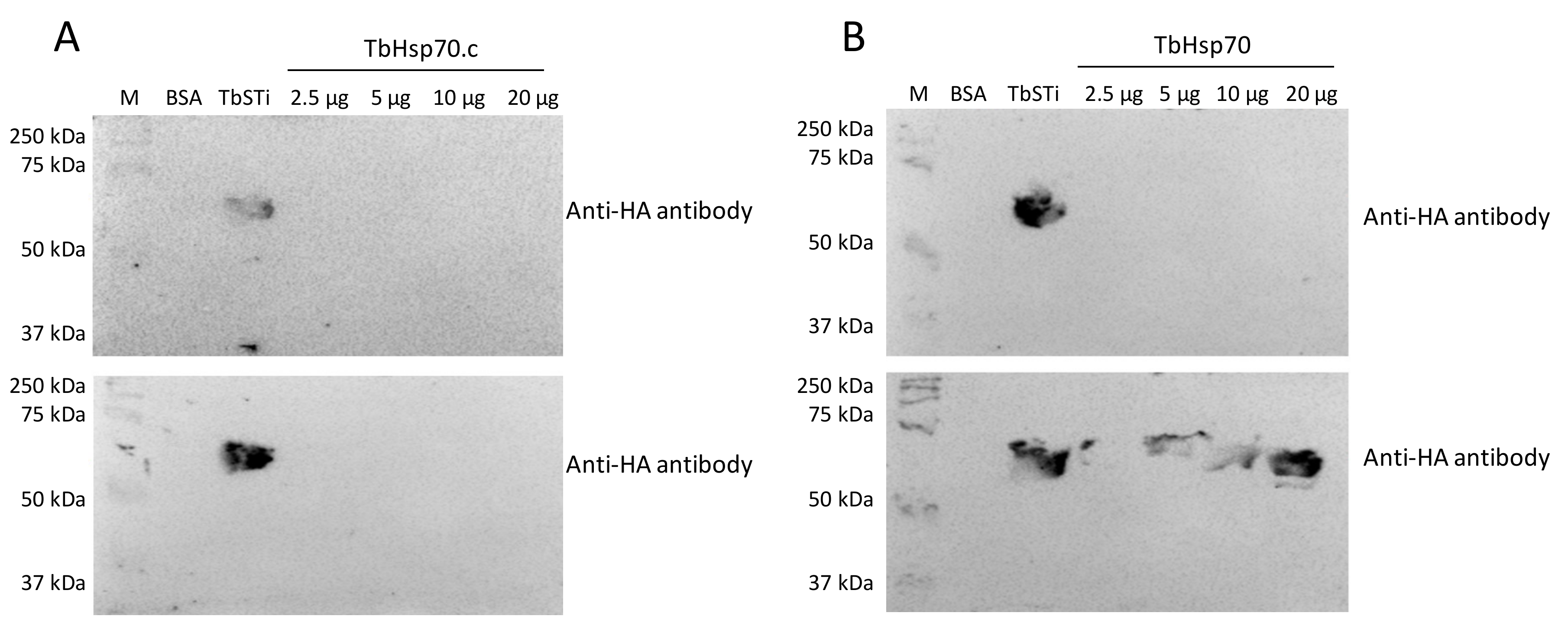
2.4. TbHsp70C Does Not Bind TbSTi1
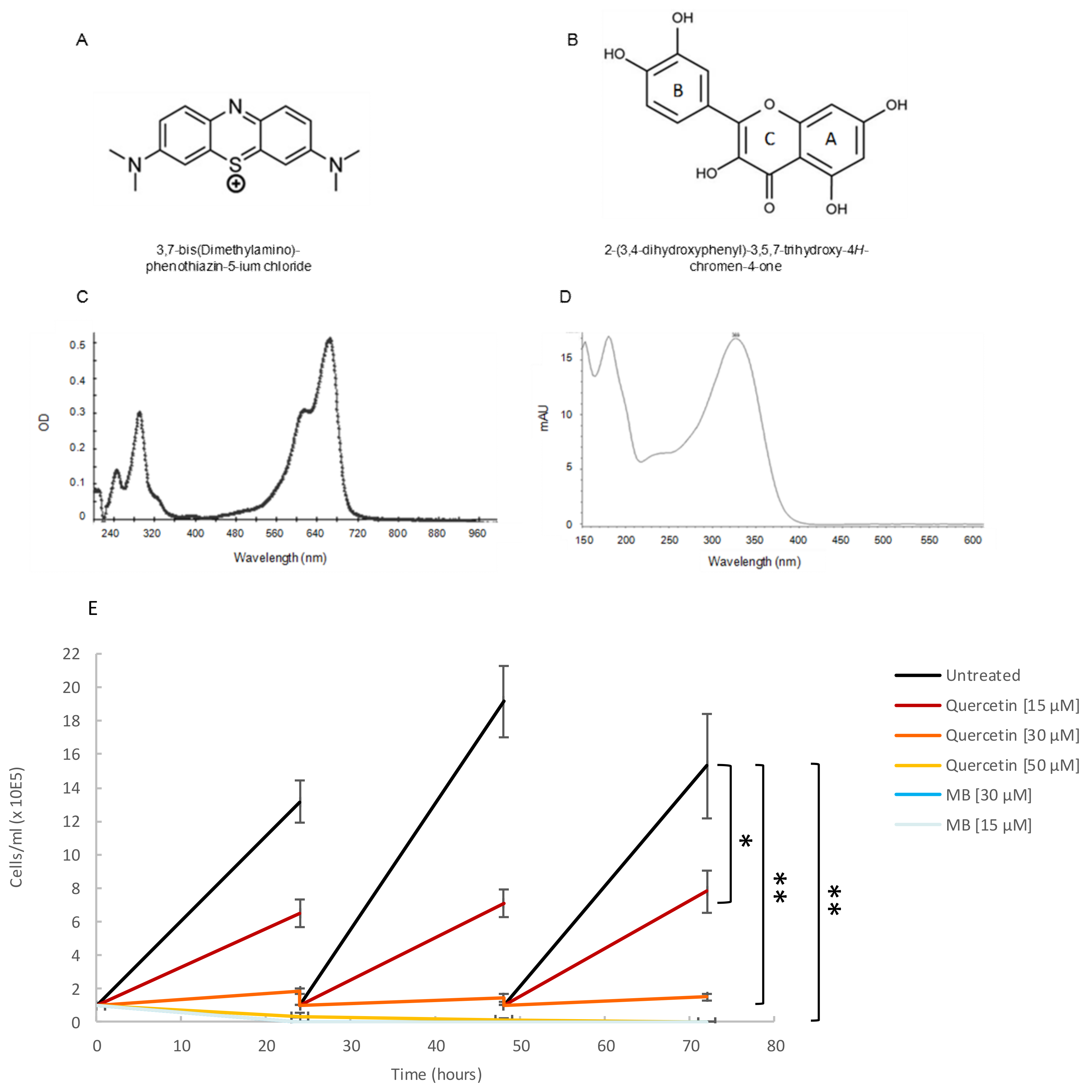
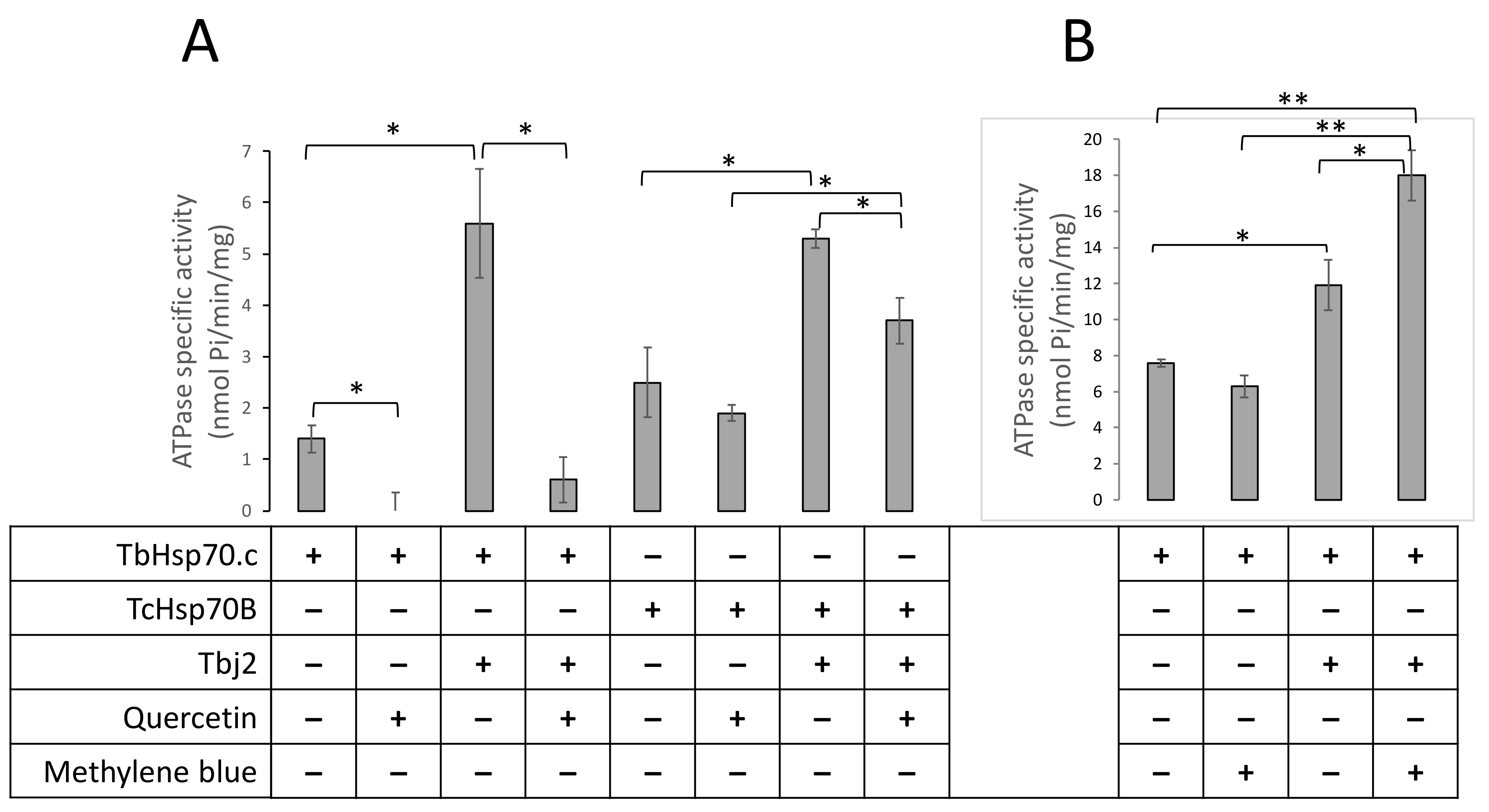
2.5. Both Quercetin and Methylene Blue Are Lethal to T. Brucei Parasites and Modulate the Activity of TbHsp70.c
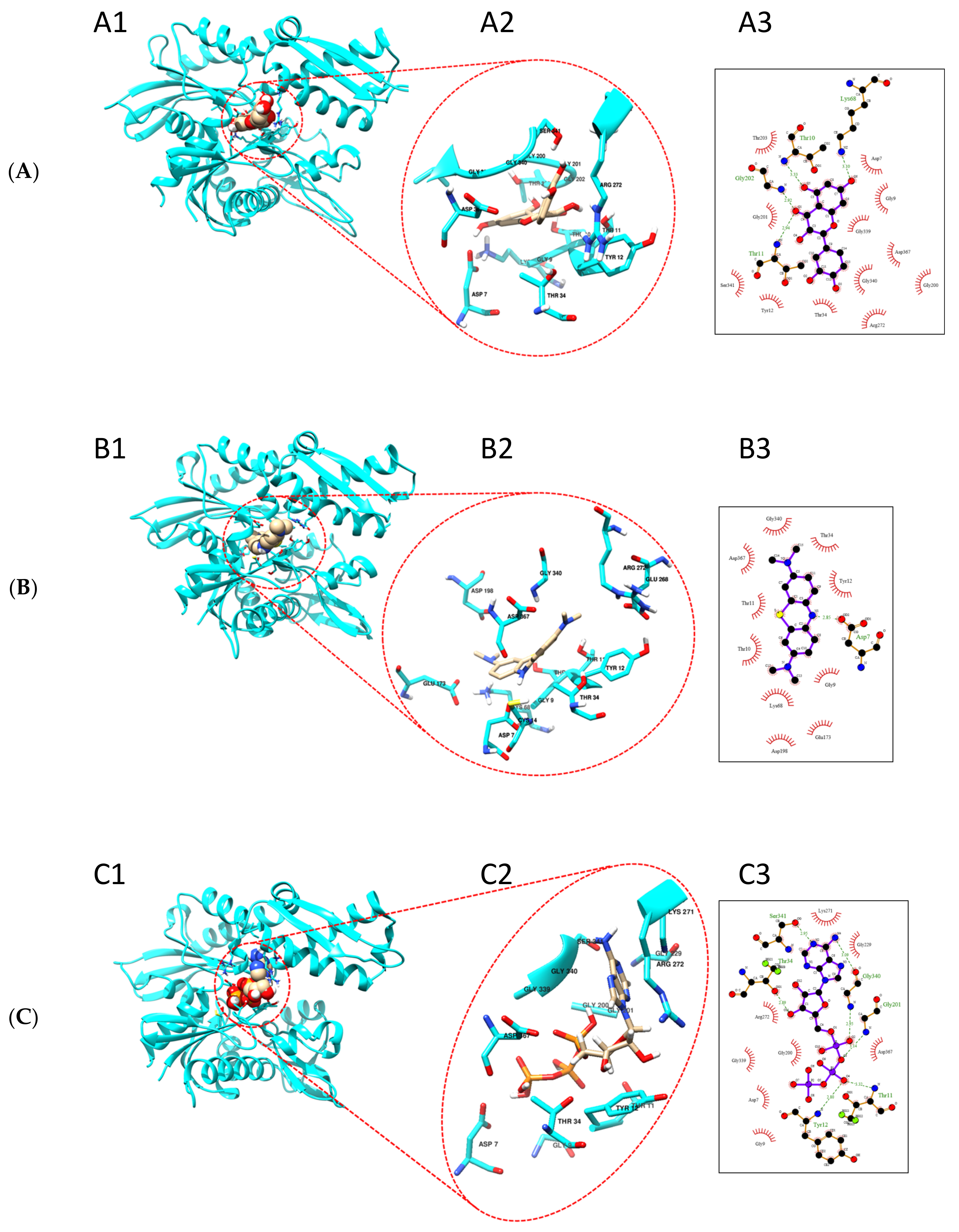
2.6. Quercetin and Methylene Blue Docks to the Nucleotide Binding Pocket of TbHsp70.c
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. T. brucei Culture
4.3. Investigation of the Subcellular Localization of TbHsp70.c Using Anti-TbHsp70.c Peptide Antibodies
4.4. Determination of Distinct Features of TbHsp70.c
4.5. Evaluation of the Interaction between the Cytosolic TbHsp70 Proteins and TbSTi1
4.6. Analysis of Quercetin and Methylene Blue on Parasite Growth
4.7. Analysis of Quercetin and Methylene Blue on TbHsp70.c-Tbj2 Chaperone Activity
4.8. Quercetin and Methylene Blue Are Predicted to Bind the ATPase Domain of TbHsp70.c
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Fact Sheet: Trypanosomiasis Human African (Sleeping Sickness). 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/trypanosomiasis-human-african-(sleeping-sickness) (accessed on 24 April 2021).
- Delespaux, V.; de Koning, H.P. Drugs and drug resistance in African trypanosomiasis. Drug Resist. Updat. 2007, 10, 30–50. [Google Scholar] [CrossRef]
- Folgueira, C.; Requena, J.M. A postgenomic view of the heat shock proteins in kinetoplastids. FEMS Microbiol Rev. 2007, 31, 359–377. [Google Scholar] [CrossRef]
- Arndt, V.; Rogon, C.; Höhfeld, J. To be, or not to be—Molecular chaperones in protein degradation. Cell Mol. Life Sci. 2007, 64, 2525–2541. [Google Scholar] [CrossRef]
- Kon, M.; Cuervo, A.M. Chaperone-mediated autophagy in health and disease. FEBS Lett. 2010, 584, 1399–1404. [Google Scholar] [CrossRef] [Green Version]
- Zininga, T.; Ramatsui, L.; Makhado, P.B.; Makumire, S.; Achilinou, I.; Hoppe, H.; Dirr, H.W.; Shonhai, A. (-)-Epigallocatechin-3gallate inhibits the chaperone activity of Plasmodium falciparum Hsp70 chaperones and abrogates their association with functional partners. Molecules 2017, 22, 2139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreassend, S.K.; Bentley, S.J.; Blatch, G.L.; Boshoff, A.; Keyzers, R.A. Screening for Small Molecule Modulators of Trypanosoma brucei Hsp70 Chaperone Activity Based upon Alcyonarian Coral-Derived Natural Products. Mar. Drugs 2020, 18, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shonhai, A. Plasmodial heat shock proteins: Targets for chemotherapy. FEMS Immunol. Med. Microbiol. 2010, 58, 61–74. [Google Scholar] [CrossRef]
- Mayer, M.P. Hsp70 chaperone dynamics and molecular mechanism. Trends Biochem. Sci. 2013, 38, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Zininga, T.; Ramatsui, L.; Shonhai, A. Heat Shock Proteins as Immunomodulants. Molecules 2018, 23, 2846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bentley, S.J.; Boshoff, A. Trypanosoma brucei J-Protein 2 Functionally Co-Operates with the Cytosolic Hsp70 and Hsp70.4 Proteins. Int. J. Mol. Sci. 2019, 20, 5843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebepe, C.M.; Matambanadzo, P.R.; Makhoba, X.H.; Achilonu, I.; Zininga, T.; Shonhai, A. Comparative Characterization of Plasmodium falciparum Hsp70-1 Relative to E. coli DnaK Reveals the Functional Specificity of the Parasite Chaperone. Biomolecules 2020, 10, 856. [Google Scholar] [CrossRef]
- Makumire, S.; Dongola, T.H.; Chakafana, G.; Tshikonwane, L.; Chauke, C.T.; Maharaj, T.; Zininga, T.; Shonhai, A. Mutation of GGMP Repeat Segments of Plasmodium falciparum Hsp70-1 Compromises Chaperone Function and Hop Co-Chaperone Binding. Int. J. Mol. Sci. 2021, 22, 2226. [Google Scholar] [CrossRef]
- Cockburn, I.L.; Boshoff, A.; Pesce, E.R.; Blatch, G.L. Selective modulation of plasmodial Hsp70s by small molecules with antimalarial activity. Biol. Chem. 2014, 395, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhao, X.; Burkholder, W.F.; Gragerov, A.; Ogata, C.M.; Gottesman, M.E.; Hendrickson, W.A. Structural analysis of substrate binding by the molecular chaperone DnaK. Science 1996, 272, 1606–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gragerov, A.; Gottesman, M.E. Different peptide binding specificities of Hsp70 family members. J. Mol. Biol. 1994, 241, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Chakafana, G.; Zininga, T.; Shonhai, A. The Link That Binds: The Linker of Hsp70 as a Helm of the Protein’s Function. Biomolecules 2019, 9, 543. [Google Scholar] [CrossRef] [Green Version]
- Laufen, T.; Mayer, M.P.; Beisel, C.; Klostermeier, D.; Mogk, A.; Reinstein, J.; Bukau, B. Mechanism of regulation of Hsp70 chaperones by DnaJ cochaperones. Proc. Natl. Acad. Sci. USA 1999, 96, 5452–5457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zininga, T.; Makumire, S.; Gitau, G.W.; Njunge, J.M.; Pooe, O.J.; Klimek, H.; Scheurr, R.; Raifer, H.; Prinsloo, E.; Przyborski, J.M.; et al. Plasmodium falciparum Hop (PfHop) Interacts with the Hsp70 Chaperone in a Nucleotide-Dependent Fashion and Exhibits Ligand Selectivity. PLoS ONE 2015, 10, e0135326. [Google Scholar] [CrossRef] [Green Version]
- Makumire, S.; Zininga, T.; Vahokoski, J.; Kursula, I.; Shonhai, A. Biophysical analysis of Plasmodium falciparum Hsp70-Hsp90 organising protein (PfHop) reveals a monomer that is characterised by folded segments connected by flexible linkers. PLoS ONE 2020, 15, e0226657. [Google Scholar] [CrossRef]
- Bentley, S.J.; Jamabo, M.; Boshoff, A. The Hsp70/J-protein machinery of the African trypanosome, Trypanosoma brucei. Cell Stress Chaperon. 2019, 24, 125–148. [Google Scholar] [CrossRef] [PubMed]
- Burger, A.; Ludewig, M.H.; Boshoff, A. Investigating the Chaperone Properties of a Novel Heat Shock Protein, Hsp70.c, from Trypanosoma brucei. J. Parasitol. Res. 2014, 2014, 172582. [Google Scholar] [CrossRef] [Green Version]
- Louw, C.A.; Ludewig, M.H.; Mayer, J.; Blatch, G.L. The Hsp70 chaperones of the Tritryps are characterized by unusual features and novel members. Parasitol. Int. 2010, 59, 497–505. [Google Scholar] [CrossRef]
- Dean, S.; Sunter, J.D.; Wheeler, R.J. TrypTag.org: A Trypanosome Genome-wide Protein Localization Resource. Trends Parasitol. 2017, 33, 80–82. [Google Scholar] [CrossRef] [Green Version]
- Ludewig, M.H.; Boshoff, A.; Horn, D.; Blatch, G.L. Trypanosoma brucei J protein 2 is a stress inducible and essential Hsp40. Int. J. Biochem. Cell Biol. 2015, 60, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Alsford, S.; Turner, D.J.; Obado, S.O.; Sanchez-Flores, A.; Glover, L.; Berriman, M.; Hertz-Fowler, C.; Horn, D. High-throughput phenotyping using parallel sequencing of RNA interference targets in the African trypanosome. Genome Res. 2011, 21, 915–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, M.; Mayer, M.P.; Bukau, B. Allosteric regulation of Hsp70 chaperones involves a conserved interdomain linker. J. Biol. Chem. 2006, 281, 38705–38711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Leu, J.I.; Murphy, M.E.; George, D.L.; Marmorstein, R. Crystal structure of the stress-inducible human heat shock protein 70 substrate-binding domain in complex with peptide substrate. PLoS ONE 2014, 9, e103518. [Google Scholar] [CrossRef] [Green Version]
- DeLano, W.L. The PyMOL Molecular Graphics System; Delano Scientific: San Carlos, CA, USA, 2002. [Google Scholar]
- Day, J.; Passecker, A.; Beck, H.-P.; Vakonakis, I. The Plasmodium falciparum Hsp70-x chaperone assists the heat stress response of the malaria parasite. FASEB J. 2019, 33, 14611–14624. [Google Scholar] [CrossRef]
- Lehane, A.M.; Saliba, K.J. Common dietary flavonoids inhibit the growth of the intraerythrocytic malaria parasite. BMC Res. Notes 2008, 1, 26. [Google Scholar] [CrossRef] [Green Version]
- Wadi, I.; Pillai, C.R.; Anvikar, A.R.; Sinha, A.; Nath, M.; Valecha, N. Methylene blue induced morphological deformations in Plasmodium falciparum gametocytes: Implications for transmission-blocking. Malar. J. 2018, 17, 11. [Google Scholar] [CrossRef] [Green Version]
- Kajander, T.; Sachs, J.N.; Goldman, A.; Regan, L. Electrostatic interactions of Hsp-organizing protein tetratricopeptide domains with Hsp70 and Hsp90: Computational analysis and protein engineering. J. Biol. Chem. 2009, 284, 25364–25374. [Google Scholar] [CrossRef] [Green Version]
- Boda, C.; Enanga, B.; Courtioux, B.; Breton, J.-C.; Bouteille, B. Trypanocidal activity of methylene blue; evidence for in vitro efficacy and in vivo failure. Chemotherapy 2006, 52, 16–19. [Google Scholar] [CrossRef]
- Jinwal, U.K.; Koren, J.; O’Leary, J.C.; Jones, J.R.; Abisambra, J.F.; Dickey, C.A. Hsp70 ATPase Modulators as Therapeutics for Alzheimer’s and other Neurodegenerative Diseases. Mol. Cell Pharm. 2010, 2, 43–46. [Google Scholar] [PubMed]
- Chakafana, G.; Zininga, T.; Shonhai, A. Comparative structure-function features of Hsp70s of Plasmodium falciparum and human origins. Bioph. Rev. 2019, 11, 591–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reuner, B.; Vassella, E.; Yutzy, B.; Boshart, M. Cell density triggers slender to stumpy differentiation of Trypanosoma brucei bloodstream forms in culture. Mol. Biochem. Parasitol. 1997, 90, 269–280. [Google Scholar] [CrossRef]
- Jiang, J.; Prasad, K.; Lafer, E.M.; Sousa, R. Structural basis of interdomain communication in the Hsc70 chaperone. Mol. Cell. 2005, 20, 513–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eswar, N.; Webb, B.; Marti-Renom, M.A.; Madhusudhan, M.; Eramian, D.; Shen, M.-Y.; Pieper, U.; Sali, A. Comparative Protein Structure Modeling Using Modeller. Curr. Protoc. Bioinform. 2006, 15, 5.6.1–5.6.30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sippl, M.J. Recognition of errors in three-dimensional structures of proteins. Proteins 1993, 17, 355–362. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Q.; Chen, X.Z. Detecting protein-protein interactions by Far western blotting. Nat. Protoc. 2007, 2, 3278–3284. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [Green Version]
- Schwede, T.; Kopp, J.; Guex, N.; Peitsch, M.C. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003, 31, 3381–3385. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, G.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and AutoDockTools4: Automated docking with selective receptor flexiblity. J. Computational Chemistry 2009, 16, 2785–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burger, A.; Macucule-Tinga, P.; Bentley, S.J.; Ludewig, M.H.; Mhlongo, N.N.; Shonhai, A.; Boshoff, A. Characterization of an Atypical Trypanosoma brucei Hsp70 Demonstrates Its Cytosolic-Nuclear Localization and Modulation by Quercetin and Methylene Blue. Int. J. Mol. Sci. 2021, 22, 6776. https://doi.org/10.3390/ijms22136776
Burger A, Macucule-Tinga P, Bentley SJ, Ludewig MH, Mhlongo NN, Shonhai A, Boshoff A. Characterization of an Atypical Trypanosoma brucei Hsp70 Demonstrates Its Cytosolic-Nuclear Localization and Modulation by Quercetin and Methylene Blue. International Journal of Molecular Sciences. 2021; 22(13):6776. https://doi.org/10.3390/ijms22136776
Chicago/Turabian StyleBurger, Adélle, Paula Macucule-Tinga, Stephen John Bentley, Michael Hans Ludewig, Ndumiso Nhlakanipho Mhlongo, Addmore Shonhai, and Aileen Boshoff. 2021. "Characterization of an Atypical Trypanosoma brucei Hsp70 Demonstrates Its Cytosolic-Nuclear Localization and Modulation by Quercetin and Methylene Blue" International Journal of Molecular Sciences 22, no. 13: 6776. https://doi.org/10.3390/ijms22136776
APA StyleBurger, A., Macucule-Tinga, P., Bentley, S. J., Ludewig, M. H., Mhlongo, N. N., Shonhai, A., & Boshoff, A. (2021). Characterization of an Atypical Trypanosoma brucei Hsp70 Demonstrates Its Cytosolic-Nuclear Localization and Modulation by Quercetin and Methylene Blue. International Journal of Molecular Sciences, 22(13), 6776. https://doi.org/10.3390/ijms22136776





