Benefits in the Macrophage Response Due to Graphene Oxide Reduction by Thermal Treatment
Abstract
:1. Introduction
2. Results and Discussion
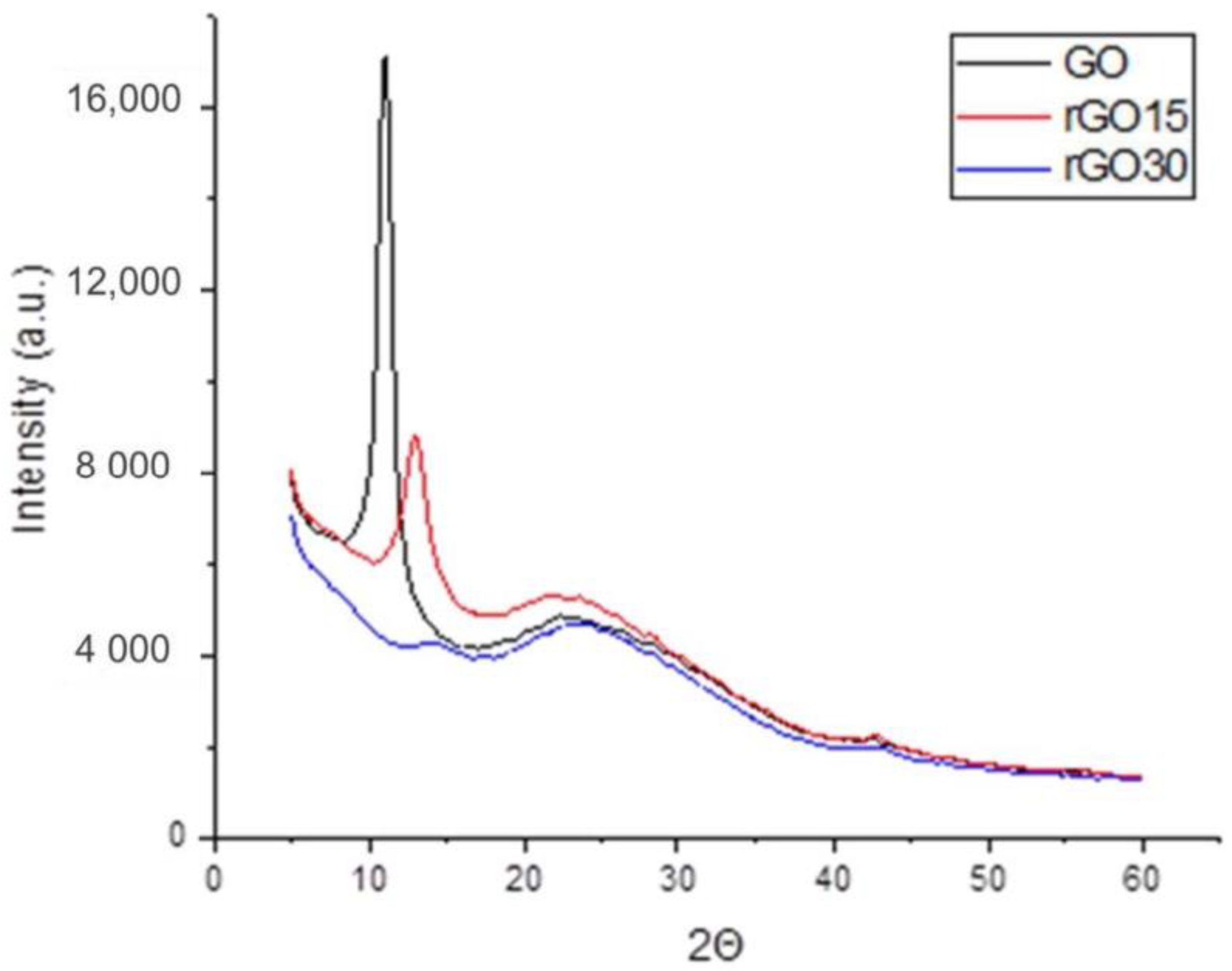
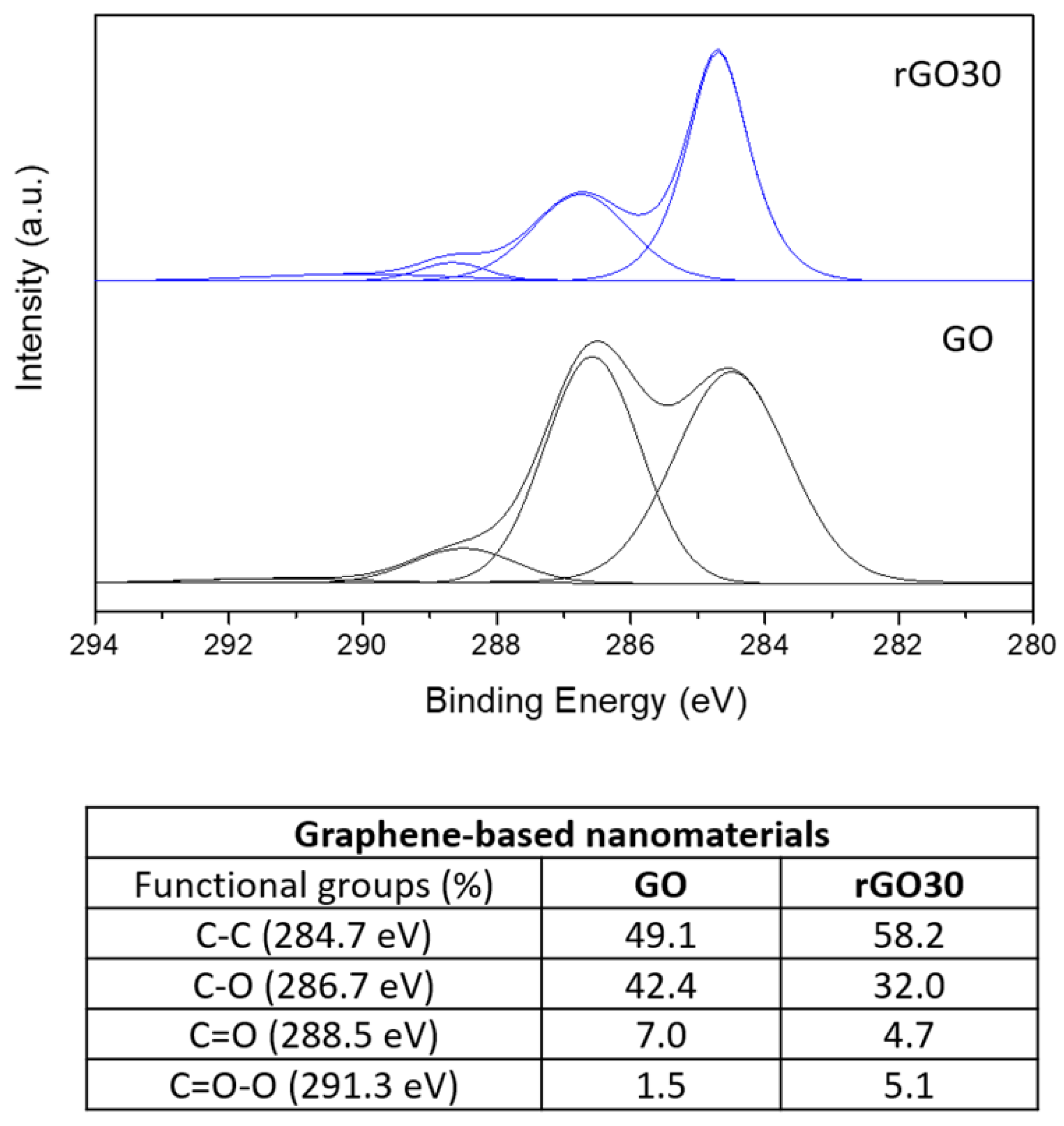
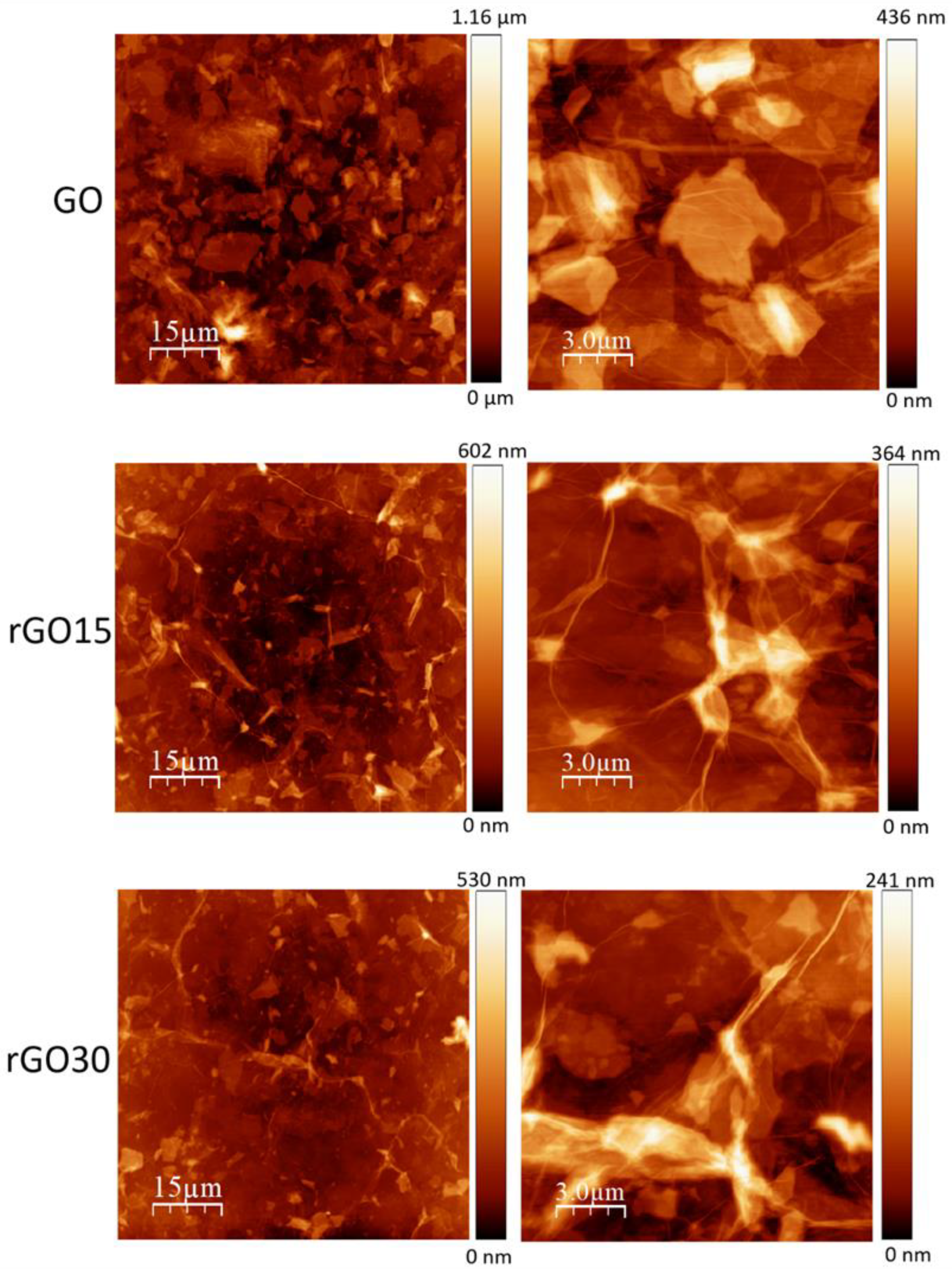
2.1. Structural and Morphological Analysis of GO, rGO15, and rGO30 Nanostructures
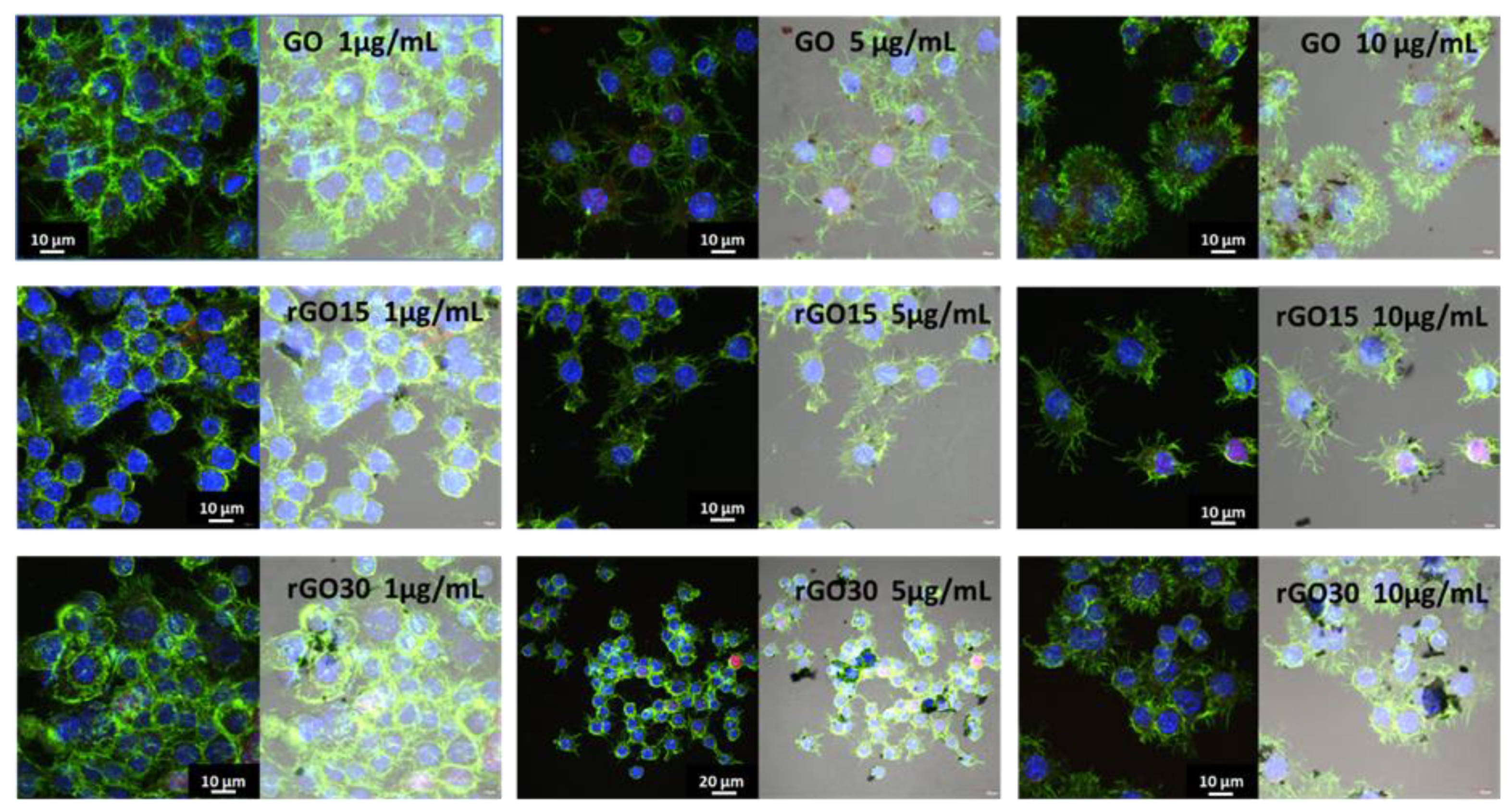
2.2. Uptake of GO, rGO15, and rGO30 by RAW-264.7 Macrophages
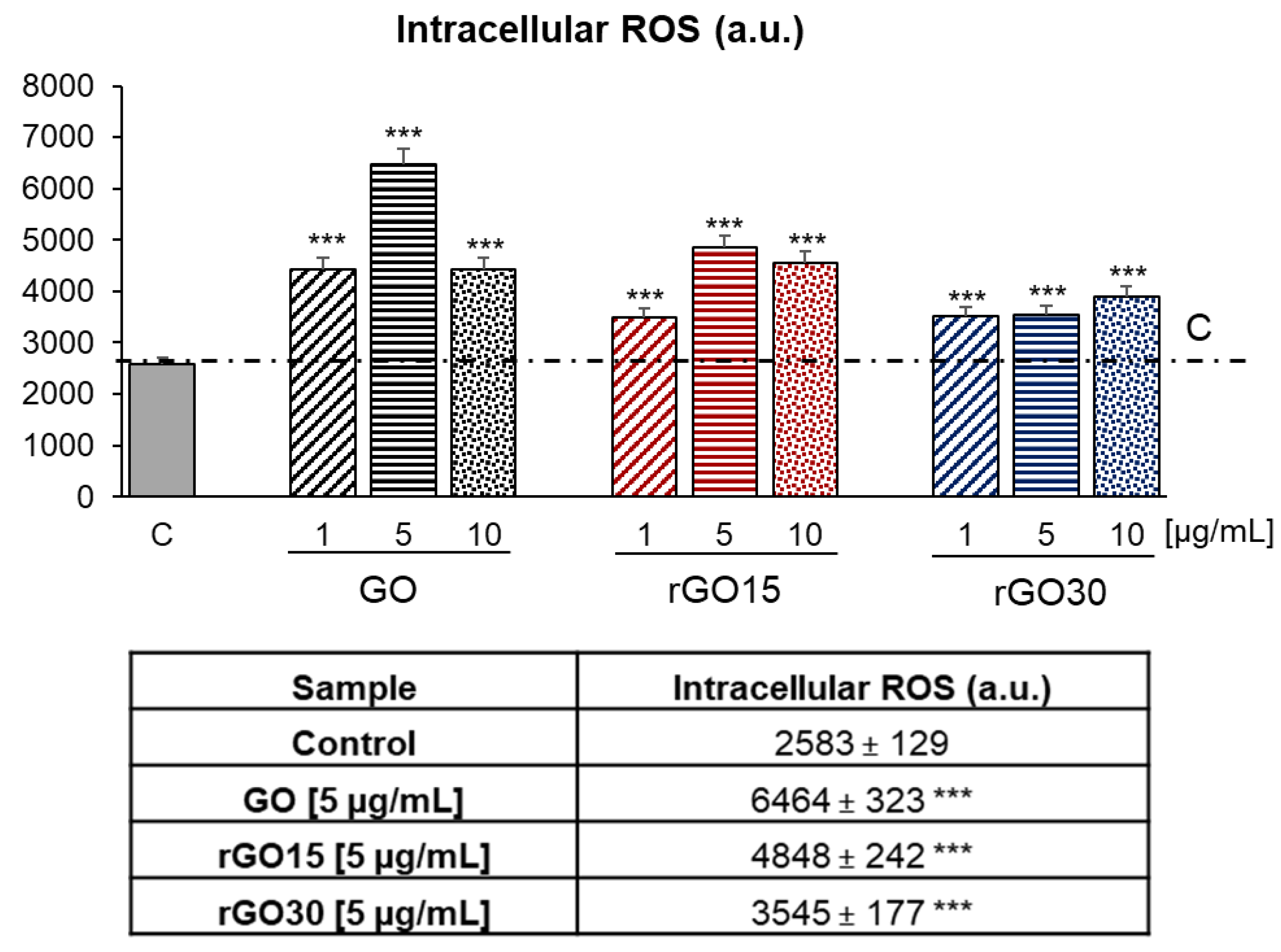
2.3. Intracellular Reactive Oxygen Species (ROS) Content of RAW-264.7 Macrophages after GO, rGO15, and rGO30 Uptake
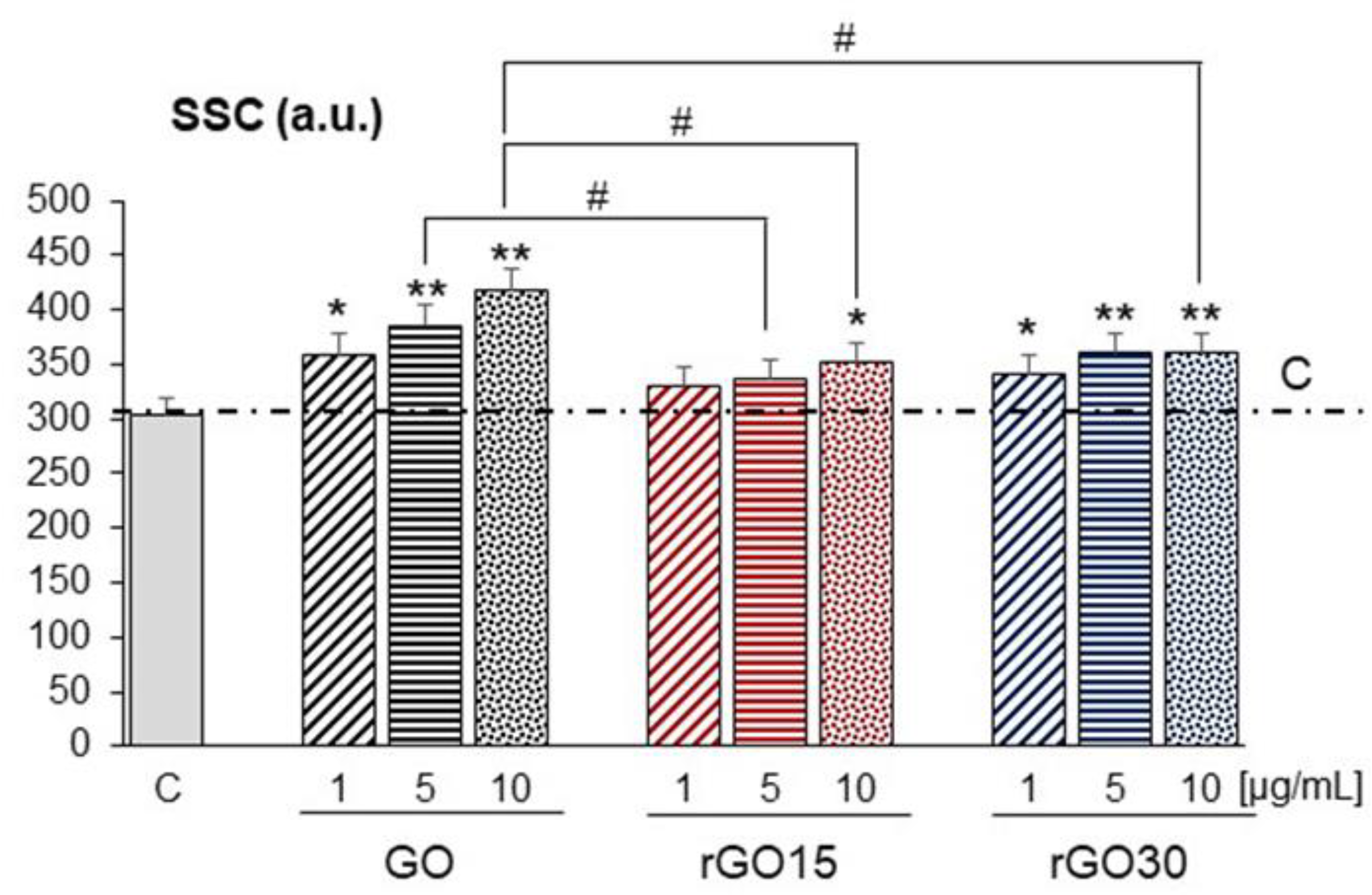
2.4. CD80 Expression by Macrophages after GO, rGO15, and rGO30 Uptake
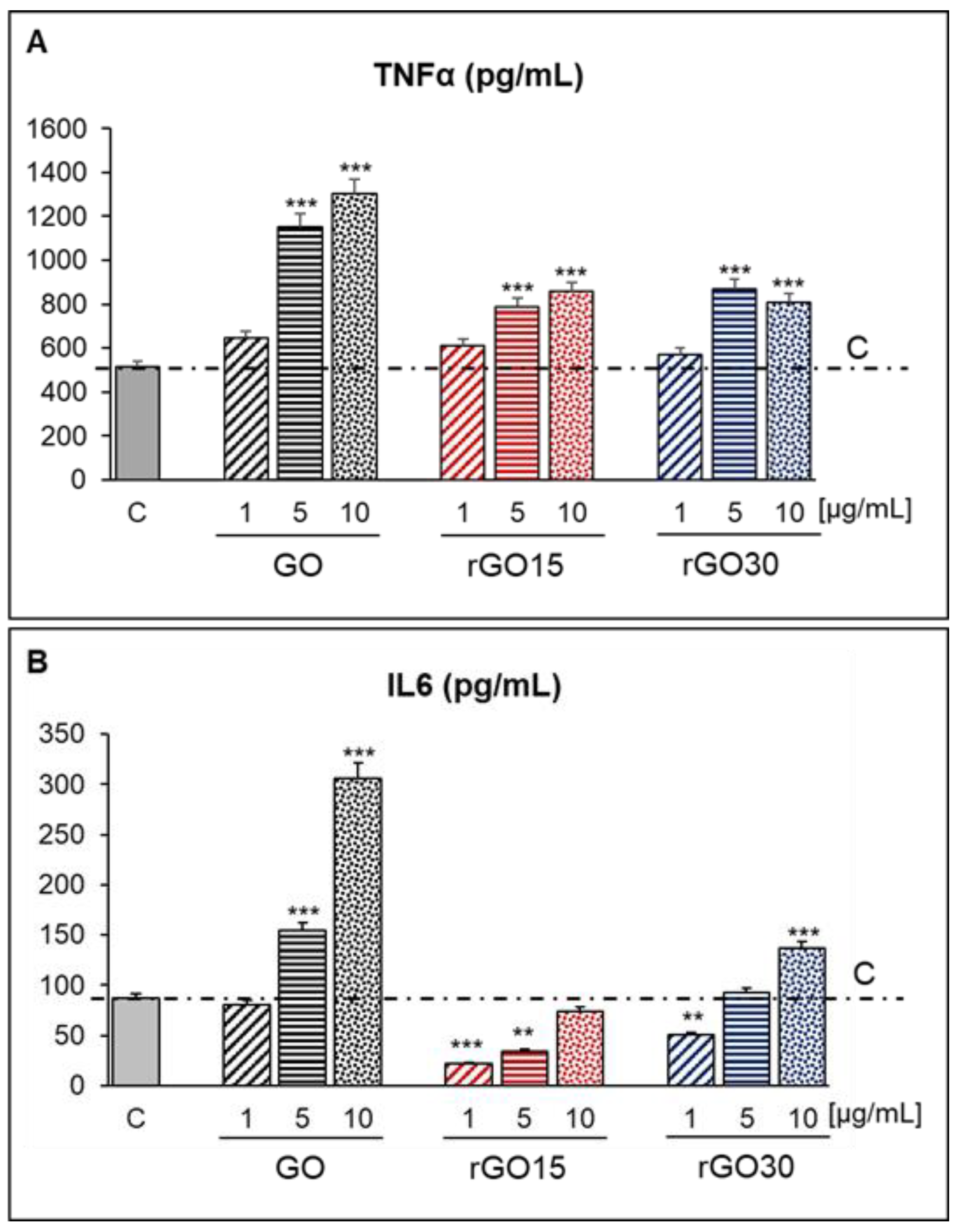
2.5. Detection of TNF-α and IL-6 as Inflammatory Cytokines
3. Materials and Methods
3.1. Preparation of GO, rGO15, and rGO30 Nanostructures
3.2. Morphological and Structural Characterization of GO, rGO15, and rGO30 Nanostructures
3.3. Culture of RAW-264.7 Macrophages for Treatment with GO, rGO15, and rGO30
3.4. Uptake of GO, rGO15, and rGO30 by RAW-264.7 Macrophages Evaluated by Flow Cytometry and Confocal and Phase Contrast Microscopy
3.5. Measurement of Intracellular Reactive Oxygen Species (ROS) Content of Macrophages by Flow Cytometry after GO, rGO15, and rGO30 Uptake
3.6. Detection of Macrophage M1 Proinflammatory Phenotype by Flow Cytometry after GO, rGO15, and rGO30 Uptake
3.7. Detection of TNF-α and IL-6 as Inflammatory Cytokines
3.8. Statistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K.A. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Asiri, A.M.; Tang, Z.W.; Du, D.; Lin, Y.H. Graphene based materials for biomedical applications. Mater. Today 2013, 16, 365–373. [Google Scholar] [CrossRef]
- Miao, W.; Shim, G.; Kim, G.; Lee, S.; Lee, H.J.; Kim, Y.B.; Byun, Y.; Oh, Y.K. Image-guided synergistic photothermal therapy using photoresponsive imaging agent-loaded graphene-based nanosheets. J. Control. Release 2015, 211, 28–36. [Google Scholar] [CrossRef]
- Bartelmess, J.; Quinn, S.J.; Giordani, S. Carbon nanomaterials: Multi-functional agents for biomedical fluorescence and Raman imaging. Chem. Soc. Rev. 2015, 44, 4672–4698. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Park, J.Y.; Miao, W.; Lee, J.; Oh, Y.K. Polyaptamer DNA nanothread-anchored, reduced graphene oxide nanosheets for targeted delivery. Biomaterials 2015, 48, 129–136. [Google Scholar] [CrossRef]
- Gonçalves, G.; Vila, M.; Portolés, M.T.; Vallet-Regí, M.; Gracio, J.; Marques, P.A.A.P. Nano-Graphene Oxide: A Potential Multifunctional Platform for Cancer Therapy. Adv. Healthcare Mater. 2013, 2, 1072–1090. [Google Scholar] [CrossRef] [PubMed]
- Raslan, A.; Saenz del Burgo, L.; Ciriza, J.; Pedraz, J.L. Graphene oxide and reduced graphene oxide-based scaffolds in regenerative medicine. Int. J. Pharm. 2020, 580, 119226. [Google Scholar] [CrossRef] [PubMed]
- López-Dolado, E.; González-Mayorga, A.; Portolés, M.T.; Feito, M.J.; Ferrer, M.L.; del Monte, F.; Gutiérrez, M.C.; Serrano, M.C. Subacute tissue response to 3D graphene oxide scaffolds implanted in the injured rat spinal cord. Adv. Healthc. Mater. 2015, 4, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Menaa, F.; Abdelghani, A.; Menaa, B. Graphene nanomaterials as biocompatible and conductive scaffolds for stem cells: Impact for tissue engineering and regenerative medicine. J. Tissue Eng. Regen. Med. 2015, 9, 1321–1338. [Google Scholar] [CrossRef]
- Girão, A.F.; Gonçalves, G.; Bhangra, K.S.; Phillips, J.B.; Knowles, J.; Hurietta, G.; Singh, M.K.; Bdkin, I.; Completo, A.; Marques, P.A.A.P. Electrostatic self-assembled graphene oxide-collagen scaffolds towards a three-dimensional microenvironment for biomimetic applications. RSC Adv. 2016, 6, 49039–49051. [Google Scholar] [CrossRef] [Green Version]
- Mohan, V.B.; Brown, R.; Jayaraman, K.; Bhattacharyya, D. Characterization of reduced graphene oxide: Effects of reduction variables on electrical conductivity. Mater. Sci. Eng. B 2015, 193, 49–60. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Meng, H.; Liu, S.; Zhang, C. Reduction pathway-dependent cytotoxicity of reduced graphene oxide. Environ. Sci. Nano 2018, 5, 1361–1371. [Google Scholar] [CrossRef]
- Wang, B.; Su, X.; Liang, J.; Yang, L.; Hu, Q.; Shan, X.; Wan, J.; Hu, Z. Synthesis of polymer-functionalized nanoscale graphene oxide with different surface charge and its cellular uptake, biosafety and immune responses in Raw 264.7 macrophages. Mater. Sci. Eng. C 2018, 90, 514–522. [Google Scholar] [CrossRef]
- Tabish, T.A.; Pranjol, M.Z.I.; Hayat, H.; Rahat, A.A.M.; Abdullah, T.M.; Whatmore, J.L.; Zhang, S. In vitro toxic effects of reduced graphene oxide nanosheets on lung cancer cells. Nanotechnology 2017, 28, 504001. [Google Scholar] [CrossRef] [Green Version]
- Palejwala, A.H.; Fridley, J.S.; Mata, J.A.; Samuel, E.L.G.; Luerssen, T.G.; Perlaky, L.; Kent, T.A.; Tour, J.M.; Jea, A. Biocompatibility of reduced graphene oxide nanoscaffolds following acute spinal cord injury in rats. Surg. Neurol. Int. 2016, 7, 75. [Google Scholar]
- Wen, K.; Chen, Y.; Chuang, C.; Chang, H.; Lee, C.; Tai, N. Accumulation and toxicity of intravenously injected functionalized graphene oxide in mice. J. Appl. Toxicol. 2015, 35, 1211–1218. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.N.; Ratner, B.D.; Goodman, S.B.; Amar, S.; Badylak, S.F. Macrophage polarization: An opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials 2012, 33, 3792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feito, M.J.; Díez-Orejas, R.; Cicuéndez, M.; Casarrubios, L.; Rojo, J.M.; Portolés, M.T. Graphene oxide nanosheets modulate peritoneal macrophage polarization towards M1 and M2 phenotypes. Colloids Surf. B 2019, 176, 96–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Stout, R.D.; Suttles, J. Functional plasticity of macrophages: Reversible adaptation to changing microenvironments. J. Leukoc. Biol. 2004, 76, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.P.; Bondarenko, O.; Kohonen, P.; Andón, F.T.; Brzicová, T.; Gessner, I.; Mathur, S.; Bottini, M.; Calligari, P.; Stella, L.; et al. Macrophage sensing of single-walled carbon nanotubes via Toll-like receptors. Sci. Rep. 2018, 8, 1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, K.; Mukherjee, S.P.; Gallud, A.; Burkert, S.C.; Bistarelli, S.; Bellucci, S.; Bottini, M.; Star, A.; Fadeel, B. Biological interactions of carbon-based nanomaterials: From coronation to degradation. Nanomedicine 2016, 12, 333–351. [Google Scholar] [CrossRef] [Green Version]
- Kinaret, P.A.S.; Scala, G.; Federico, A.; Sund, J.; Greco, D. Carbon Nanomaterials Promote M1/M2 Macrophage Activation. Small 2020, 16, 1907609. [Google Scholar] [CrossRef] [Green Version]
- Harrison, B.S.; Atala, A. Carbon nanotube applications for tissue engineering. Biomaterials 2007, 28, 344–353. [Google Scholar] [CrossRef]
- Serrano, M.C.; Feito, M.J.; González-Mayorga, A.; Diez-Orejas, R.; Matesanz, M.C.; Portolés, M.T. Response of macrophages and neural cells in contact with reduced graphene oxide microfibers. Biomater. Sci. 2018, 6, 2987–2997. [Google Scholar] [CrossRef] [PubMed]
- Dolbin, A.V.; Khlistyuck, M.V.; Esel’son, V.B.; Gavrilko, V.G.; Vinnikov, N.A.; Basnukaeva, R.M.; Maluenda, I.; Maser, W.K.; Benito, A.M. The effect of the thermal reduction temperature on the structure and sorption capacity of reduced graphene oxide materials. Appl. Surf. Sci. 2016, 361, 213–220. [Google Scholar] [CrossRef]
- Huang, H.H.; De Silva, K.K.H.; Kumara, G.R.A.; Yoshimura, M. Structural evolution of hydrothermally derived reduced graphene oxide. Sci. Rep. 2018, 8, 6849. [Google Scholar] [CrossRef]
- Liu, J.; Cui, L.; Losic, D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef]
- Cai, W.; Piner, R.D.; Stadermann, F.J.; Park, S.; Shaibat, M.A.; Ishii, Y.; Yang, D.; Velamakanni, A.; An, S.J.; Stoller, M.; et al. Synthesis and solid-state NMR structural characterization of 13C-labeled graphite oxide. Science 2008, 321, 1815–1817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of graphite oxide revisited. J. Phys. Chem. B 1998, 102, 4477–4482. [Google Scholar] [CrossRef]
- Mattevi, C.; Eda, G.; Agnoli, S.; Miller, S.; Mkhoyan, K.A.; Celik, O.; Mastrogiovanni, D.; Granozzi, G.; Garfunkel, E.; Chhowalla, M. Evolution of electrical, chemical, and structural properties of transparent and conducting chemically derived graphene thin films. Adv. Funct. Mater. 2009, 19, 2577–2583. [Google Scholar] [CrossRef]
- Lipatov, A.; Guinel, M.J.-F.; Muratov, D.S.; Vanyushin, V.O.; Wilson, P.M.; Kolmakov, A.; Sinitskii, A. Low-temperature thermal reduction of graphene oxide: In situ correlative structural, thermal desorption, and electrical transport measurements. Appl. Phys. Lett. 2018, 112, 053103. [Google Scholar] [CrossRef]
- Girão, A.F.; Sousa, J.; Domínguez-Bajo, A.; González-Mayorga, A.; Bdikin, I.; Pujades-Otero, E.; Casañ-Pastor, N.; Hortigüela, M.J.; Otero-Irurueta, G.; Completo, A.; et al. 3D Reduced Graphene Oxide Scaffolds with a Combinatorial Fibrous-Porous Architecture for Neural Tissue Engineering. ACS Appl. Mater. Interfaces 2020, 12, 38962–38975. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Chen, C.M.; Li, Y.F.; Li, X.M.; Kong, Q.Q.; Wang, M.Z. Crumpled reduced graphene oxide by flame-induced reduction of graphite oxide for supercapacitive energy storage. J. Mater. Chem. A 2014, 2, 5730–5737. [Google Scholar] [CrossRef]
- Chang, C.; Song, Z.; Lin, J.; Xu, Z. How graphene crumples are stabilized? RSC Adv. 2013, 3, 2720–2726. [Google Scholar] [CrossRef]
- Zhang, H.B.; Wang, J.W.; Yan, Q.; Zheng, W.G.; Chen, C.; Yu, Z.Z. Vacuum-assisted synthesis of graphene from thermal exfoliation and reduction of graphite oxide. J. Mater. Chem. 2011, 21, 5392–5397. [Google Scholar] [CrossRef]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle Uptake: The Phagocyte Problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef] [Green Version]
- Udall, J.N.; Moscicki, R.A.; Preffer, F.I.; Ariniello, P.D.; Carter, E.A.; Bhan, A.K.; Bloch, K.J. Flow cytometry: A new approach to the isolation and characterization of Kupffer cells. In Recent Advances in Mucosal Immunology; Advances in Experimental Medicine and Biology; Mestecky, J., McGhee, J.R., Bienenstock, J., Ogra, P.L., Eds.; Springer: Boston, MA, USA, 1987; Volume 216A, pp. 821–827. [Google Scholar]
- Déciga-Alcaraz, A.; Medina-Reyes, E.I.; Delgado-Buenrostro, N.L.; Rodríguez-Ibarra, C.; Ganem-Rondero, A.; Vázquez-Zapién, G.J.; Mata-Miranda, M.M.; Limón-Pacheco, J.H.; García-Cuéllar, C.M.; Sánchez-Pérez, Y.; et al. Toxicity of engineered nanomaterials with different physicochemical properties and the role of protein corona on cellular uptake and intrinsic ROS production. Toxicology 2020, 442, 152545. [Google Scholar] [CrossRef]
- Gratton, S.E.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Liu, R.; Wang, X.; Liu, Q.; Chen, Y.; Valle, R.P.; Zuo, Y.Y.; Xia, T.; Liu, S. Crucial role of lateral size for graphene oxide in activating macrophages and stimulating proinflammatory responses in cells and animals. ACS Nano 2015, 9, 10498–10515. [Google Scholar] [CrossRef]
- Yue, H.; Wei, W.; Yue, Z.; Wang, B.; Luo, N.; Gao, Y.; Ma, D.; Ma, G.; Su, Z. The role of the lateral dimension of graphene oxide in the regulation of cellular responses. Biomaterials 2012, 33, 4013–4021. [Google Scholar] [CrossRef]
- Chatterjee, N.; Eom, H.J.; Choi, J. A systems toxicology approach to the Surface functionality control of graphene–cell interactions. Biomaterials 2014, 35, 1109–1127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wei, P.; Zhou, Z.; Wei, T. Interactions of graphene with mammalian cells: Molecular mechanisms and biomedical insights. Adv. Drug Deliv. Rev. 2016, 105, 145–162. [Google Scholar] [CrossRef] [Green Version]
- Linares, J.; Matesanz, M.C.; Vila, M.; Feito, M.J.; Goncalves, G.; Vallet-Regi, M.; Marques, P.A.; Portoles, M.T. Endocytic mechanisms of graphene oxide nanosheets in osteoblasts, hepatocytes and macrophages. ACS Appl. Mater. Interfaces 2014, 6, 13697–13706. [Google Scholar] [CrossRef] [PubMed]
- Wan, B.; Wang, Z.X.; Lv, Q.Y.; Dong, P.X.; Zhao, L.X.; Yang, Y.; Guo, L.H. Single-walled carbon nanotubes and graphene oxides induce autophagosome accumulation and lysosome impairment in primarily cultured murine peritoneal macrophages. Toxicol. Lett. 2013, 221, 118–127. [Google Scholar] [CrossRef]
- Saleem, J.; Wang, L.; Chen, C. Immunological effects of graphene family nanomaterials. NanoImpact 2017, 5, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.; Li, Y.; Tjong, S.C. Graphene Nanomaterials: Synthesis, Biocompatibility, and Cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564. [Google Scholar] [CrossRef] [Green Version]
- Julier, Z.; Park, A.J.; Briquez, P.S.; Martino, M.M. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017, 53, 13–28. [Google Scholar] [CrossRef]
- Sridharan, R.; Cameron, A.; Kelly, D.; Kearney, C.; O’Brien, F. Biomaterial based modulation of macrophage polarization: A review and suggested design principles. Mater. Today 2015, 18, 313–325. [Google Scholar] [CrossRef]
- Tan, H.-Y.; Wang, N.; Li, S.; Hong, M.; Wang, X.; Feng, Y. The reactive oxygen species in macrophage polarization: Reflecting its dual role in progression and treatment of human diseases. Oxid. Med. Cell. Longev. 2016, 2795090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahamed, M.; Akhtar, M.J.; Khan, M.A.M.; Alhadlaq, H.A. Reduced graphene oxide mitigates cadmium-induced cytotoxicity and oxidative stress in HepG2 cells. Food Chem. Toxicol. 2020, 143, 111515. [Google Scholar] [CrossRef]
- Italiani, P.; Boraschi, D. From monocytes to M1/M2 macrophages: Phenotypical vs. functional differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.Y. M1 and M2 polarization of macrophages: A mini-review. Med. Biol. Sci. Eng. 2019, 2, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Marco, O.; Yanal, G.; Bala, P.A.; Klaus, L. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2 (LPS–) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Jiménez-Uribe, A.P.; Valencia-Martínez, H.; Carballo-Uicab, G.; Vallejo-Castillo, L.; Medina-Rivero, E.; Chacón-Salinas, R.; Pavón, L.; Velasco-Velázquez, M.A.; Mellado-Sánchez, G.; Estrada-Parra, S.; et al. CD80 Expression Correlates with IL-6 Production in THP-1-Like Macrophages Costimulated with LPS and Dialyzable Leukocyte Extract (Transferon®). J. Immunol. Res. 2019, 2019, 2198508. [Google Scholar] [CrossRef]
- Cong, H.P.; Chen, J.F.; Yu, S.H. Graphene-based macroscopic assembles and architectures: An emerging material system. Chem. Soc. Rev. 2014, 43, 7295–7325. [Google Scholar] [CrossRef]
- Thangavel, P.; Kannan, R.; Ramachandran, B.; Moorthy, G.; Suguna, L.; Muthuvijayan, V. Development of reduced graphene oxide (rGO)-isabgol nanocomposite dressings for enhanced vascularization and accelerated wound healing in normal and diabetic rats. J. Colloid Interface Sci. 2018, 517, 251–264. [Google Scholar] [CrossRef]
- López-Dolado, E.; González-Mayorga, A.; Gutiérrez, M.C.; Serrano, M.C. Immunomodulatory and angiogenic responses induced by graphene oxide scaffolds in chronic spinal hemisected rats. Biomaterials 2016, 99, 72–81. [Google Scholar] [CrossRef]
- Gohari, P.H.M.; Nazarpak, M.H.; Solati-Hashjin, M. The effect of adding reduced graphene oxide to electrospun polycaprolactone scaffolds on MG-63 cells activity. Mater. Today Commun. 2021, 27, 102287. [Google Scholar] [CrossRef]
- Magaz, A.; Li, X.; Gough, J.E.; Blaker, J.J. Graphene oxide and electroactive reduced graphene oxide-based composite fibrous scaffolds for engineering excitable nerve tissue. Mater. Sci. Eng. C 2021, 119, 111632. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Mei, N. Assessment of the toxic potential of graphene family nanomaterials. J. Food Drug Anal. 2014, 22, 105–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicuéndez, M.; Fernandes, M.; Ayán-Varela, M.; Oliveira, H.; Feito, M.J.; Diez-Orejas, R.; Paredes, J.I.; Villar-Rodil, S.; Vila, M.; Portolés, M.T.; et al. Macrophage inflammatory and metabolic responses to graphene-based nanomaterials differing in size and functionalization. Colloids Surf. B 2020, 186, 110709. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, S.S.; Bengtson, S.; Williams, A.; Jacobsen, N.R.; Troelsen, J.T.; Halappanavar, S.; Vogel, U. A transcriptomic overview of lung and liver changes one day after pulmonary exposure to graphene and graphene oxide. Toxicol. Appl. Pharmacol. 2021, 410, 115343. [Google Scholar] [CrossRef]
- Greulich, C.; Diendorf, J.; Simon, T.; Eggeler, G.; Epple, M. Uptake and intracellular distribution of silver nanoparticles in human mesenchymal stem cells. Acta Biomater. 2011, 7, 347–354. [Google Scholar] [CrossRef]
- Suzuki, H.; Toyooka, T.; Ibuki, Y. Simple and easy method to evaluate uptake potential of nanoparticles in mammalian cells using a flow cytometric light scatter analysis. Environ. Sci. Technol. 2007, 41, 3018–3024. [Google Scholar] [CrossRef]
- Burastero, S.E.; Magnani, Z.; Confetti, C.; Abbruzzese, L.; Oddera, S.; Balbo, P.; Rossi, G.A.; Crimi, E. Increased expression of the CD80 accessory molecule by alveolar macrophages in asthmatic subjects and its functional involvement in allergen presentation to autologous TH2 lymphocytes. J. Allergy Clin. Immunol. 1999, 103, 1136–1142. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cicuéndez, M.; Casarrubios, L.; Barroca, N.; Silva, D.; Feito, M.J.; Diez-Orejas, R.; Marques, P.A.A.P.; Portolés, M.T. Benefits in the Macrophage Response Due to Graphene Oxide Reduction by Thermal Treatment. Int. J. Mol. Sci. 2021, 22, 6701. https://doi.org/10.3390/ijms22136701
Cicuéndez M, Casarrubios L, Barroca N, Silva D, Feito MJ, Diez-Orejas R, Marques PAAP, Portolés MT. Benefits in the Macrophage Response Due to Graphene Oxide Reduction by Thermal Treatment. International Journal of Molecular Sciences. 2021; 22(13):6701. https://doi.org/10.3390/ijms22136701
Chicago/Turabian StyleCicuéndez, Mónica, Laura Casarrubios, Nathalie Barroca, Daniela Silva, María José Feito, Rosalía Diez-Orejas, Paula A. A. P. Marques, and María Teresa Portolés. 2021. "Benefits in the Macrophage Response Due to Graphene Oxide Reduction by Thermal Treatment" International Journal of Molecular Sciences 22, no. 13: 6701. https://doi.org/10.3390/ijms22136701
APA StyleCicuéndez, M., Casarrubios, L., Barroca, N., Silva, D., Feito, M. J., Diez-Orejas, R., Marques, P. A. A. P., & Portolés, M. T. (2021). Benefits in the Macrophage Response Due to Graphene Oxide Reduction by Thermal Treatment. International Journal of Molecular Sciences, 22(13), 6701. https://doi.org/10.3390/ijms22136701






