Kcnj16 (Kir5.1) Gene Ablation Causes Subfertility and Increases the Prevalence of Morphologically Abnormal Spermatozoa
Abstract
1. Introduction
2. Results
2.1. Expression of Kir4.1, Kir4.2, and Kir5.1 Channels in Epididymal Ducts
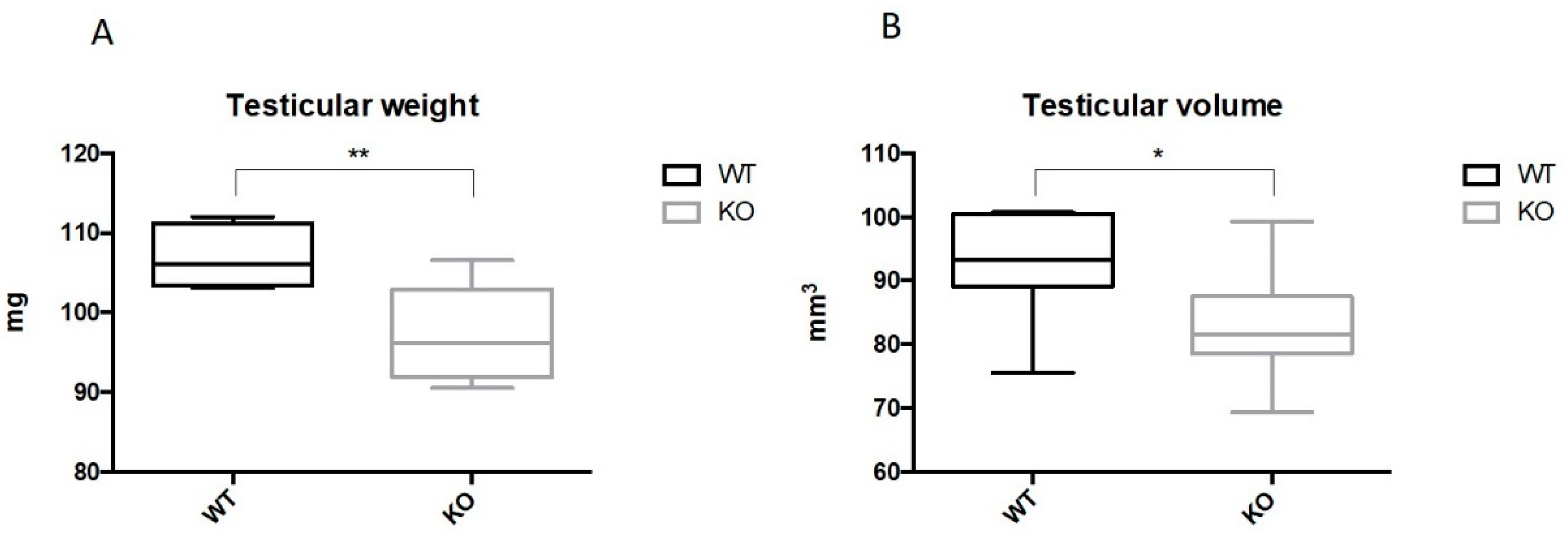
2.2. Ablation of Kir5.1 Gene Affects Fertility and Testicular Development
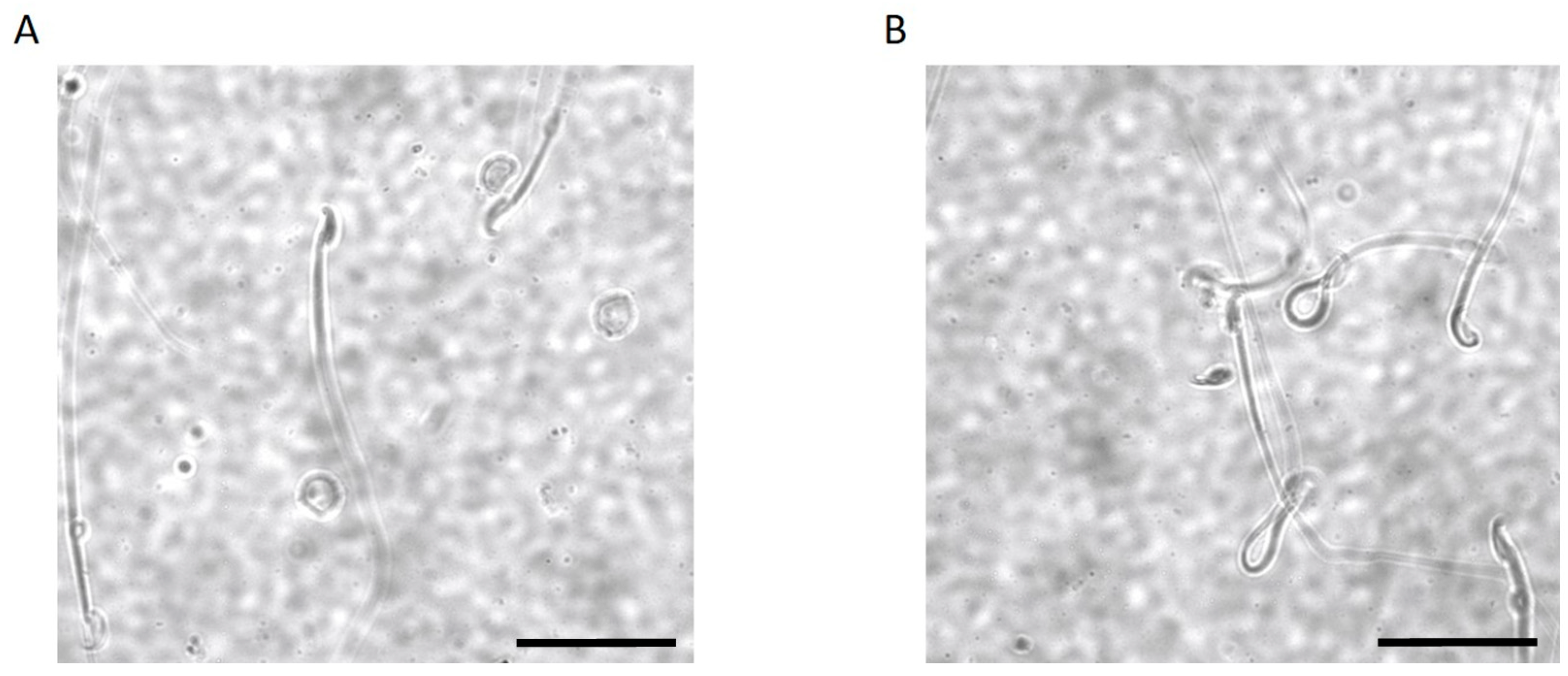
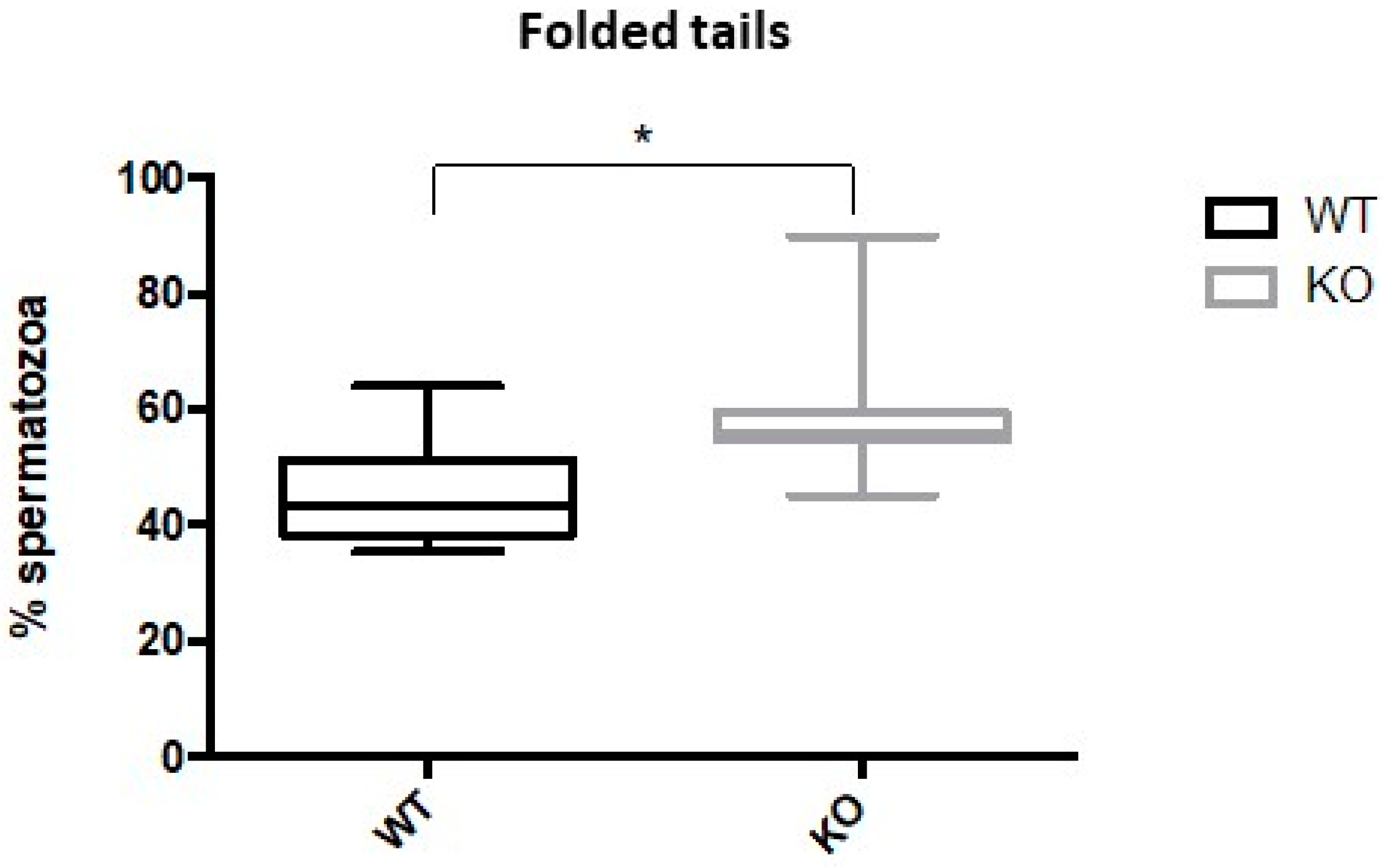
2.3. Kir5.1 KO Mice Showed Increased Percentage of Sperm with Abnormal Flagellar Morphology
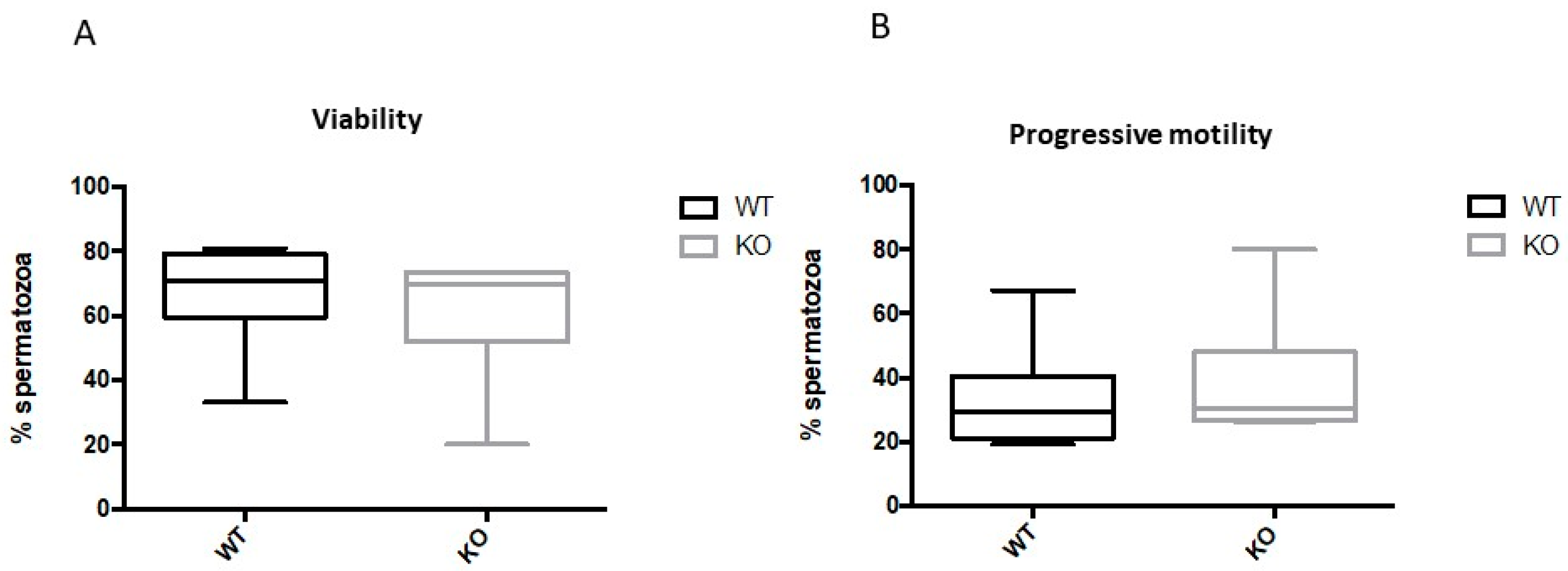
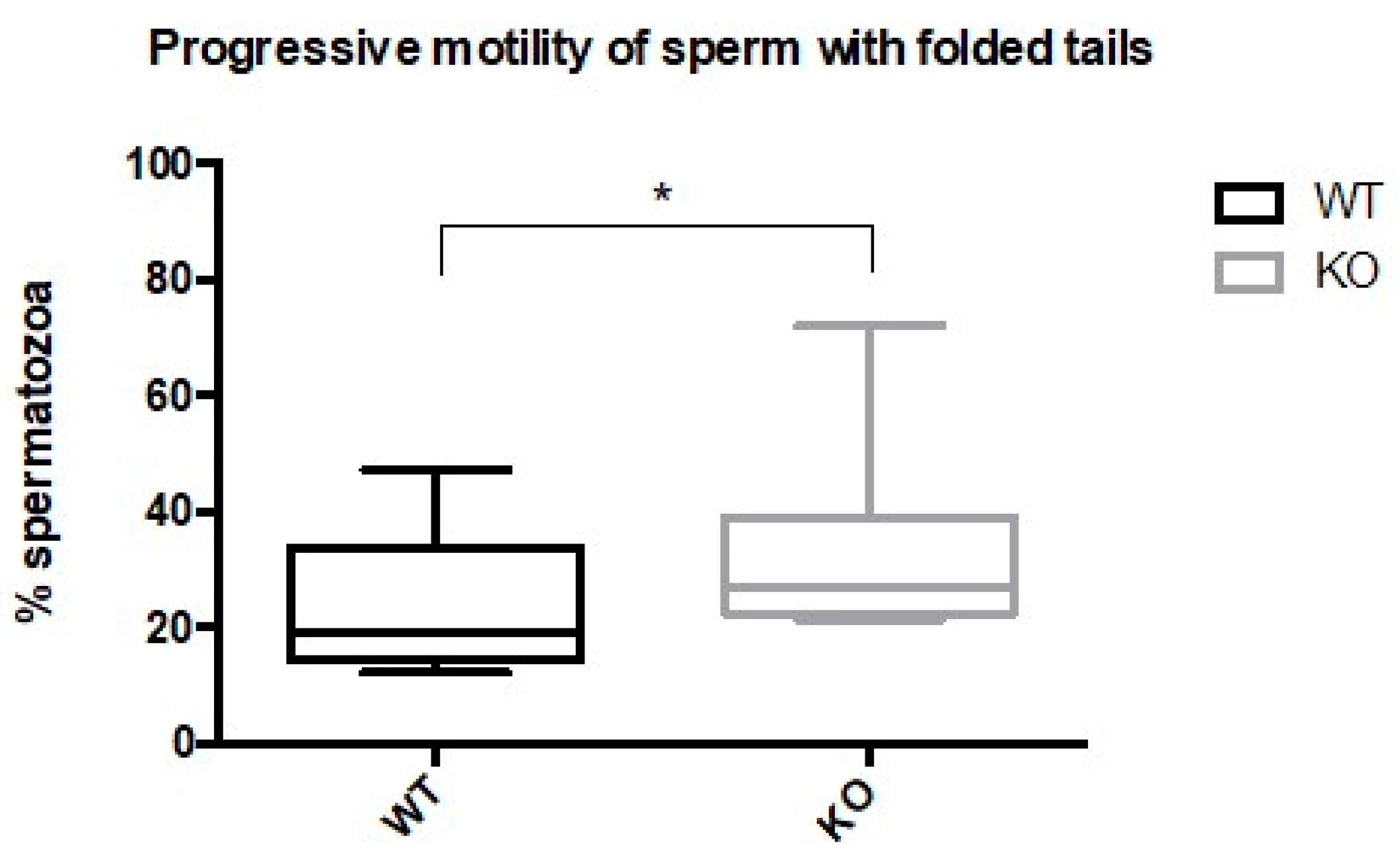
2.4. Spermatozoa from Kir5.1 KO Mice with Altered Flagellar Morphology Show Increased Motility
3. Discussion
4. Materials and Methods
4.1. Creation of a Colony, Mice Genotyping, and Breeding of Kir5.1 (Kcnj16) KO Mice
4.2. Fertility Assessment
4.3. Tissue Collection and Immunohistochemistry
4.4. Measurement of Testicular Volume
4.5. Measurements of pH in Epididymal Fluids Using pH Strips
4.6. Sperm Analysis
4.7. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hibino, H.; Inanobe, A.; Furutani, K.; Murakami, S.; Findlay, I.; Kurachi, Y. Inwardly Rectifying Potassium Channels: Their Structure, Function, and Physiological Roles. Physiol. Rev. 2010, 90, 291–366. [Google Scholar] [CrossRef]
- Bond, C.T.; Pessia, M.; Xia, X.M.; Lagrutta, A.; Kavanaugh, M.P.; Adelman, J.P. Cloning and expression of a family of inward rectifier potassium channels. Recept. Channels 1994, 2, 183–191, Erratum in 1994, 2, 350. [Google Scholar] [PubMed]
- Lagrutta, A.A.; Bond, C.T.; Xia, X.M.; Pessia, M.; Tucker, S.; Adelman, J.P. Inward Rectifier Potassium Channels. Cloning, Expression and Structure-Function Studies. Jpn. Heart J. 1996, 37, 651–660. [Google Scholar] [CrossRef] [PubMed][Green Version]
- D’Adamo, M.C.; Shang, L.; Imbrici, P.; Brown, S.D.; Pessia, M.; Tucker, S.J. Genetic Inactivation of Kcnj16 Identifies Kir5.1 as an Important Determinant of Neuronal PCO2/pH Sensitivity. J. Biol. Chem. 2011, 286, 192–198. [Google Scholar] [CrossRef]
- Pessia, M.; Bond, C.; Kavanaugh, M.; Adelman, J. Contributions of the C-terminal domain to gating properties of inward rectifier potassium channels. Neuron 1995, 14, 1039–1045. [Google Scholar] [CrossRef]
- Pessia, M.; Tucker, S.J.; Lee, K.; Bond, C.T.; Adelman, J.P. Subunit positional effects revealed by novel heteromeric inwardly rectifying K+ channels. EMBO J. 1996, 15, 2980–2987. [Google Scholar] [CrossRef] [PubMed]
- Pessia, M.; Imbrici, P.; D’Adamo, M.C.; Salvatore, L.; Tucker, S.J. Differential pH sensitivity of Kir4.1 and Kir4.2 potassium channels and their modulation by heteropolymerisation with Kir5.1. J. Physiol. 2001, 532, 359–367. [Google Scholar] [CrossRef]
- Casamassima, M.; D’Adamo, M.C.; Pessia, M.; Tucker, S.J. Identification of a Heteromeric Interaction That Influences the Rectification, Gating, and pH Sensitivity of Kir4.1/Kir5.1 Potassium Channels. J. Biol. Chem. 2003, 278, 43533–43540. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Garay, C.; De la Vega-Beltrán, J.L.; Delgado, R.; Labarca, P.; Felix, R.; Darszon, A. Inwardly Rectifying K+ Channels in Spermatogenic Cells: Functional Expression and Implication in Sperm Capacitation. Dev. Biol. 2001, 234, 261–274. [Google Scholar] [CrossRef]
- Visconti, P.E.; Krapf, D.; De La Vega-Beltrán, J.L.; Acevedo, J.J.; Darszon, A. Ion channels, phosphorylation and mammalian sperm capacitation. Asian J. Androl. 2011, 13, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.G.; Publicover, S.J.; Barratt, C.L.R.; Da Silva, S.J.M. Human sperm ion channel (dys)function: Implications for fertilization. Hum. Reprod. Updat. 2019, 25, 758–776. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, L.; D’Adamo, M.C.; Polishchuk, R.; Salmona, M.; Pessia, M. Localization and age-dependent expression of the inward rectifier K+ channel subunit Kir 5.1 in a mammalian reproductive system. FEBS Lett. 1999, 449, 146–152. [Google Scholar] [CrossRef]
- Franks, L.M.; Payne, J. The Influence of Age on Reproductive Capacity in C57bl Mice. J. Reprod. Fert. 1970, 21, 563–565. [Google Scholar] [CrossRef]
- Protein Atlas. Available online: https://www.proteinatlas.org (accessed on 18 January 2021).
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Orre, L.M.; Johansson, H.J.; Huss, M.; Boekel, J.; Vesterlund, M.; Fernandez-Woodbridge, A.; Branca, R.M.M.; Lehtiö, J. Discovery of coding regions in the human genome by integrated proteogenomics analysis workflow. Nat. Commun. 2018, 9, 903, Erratum in 2018, 9, 1852. [Google Scholar] [CrossRef]
- Expression Atlas. Available online: https://www.ebi.ac.uk/gxa/experiments/E-GEOD-6872/Downloads?specific=true&geneQuery=%255B%257B%2522value%2522%253A%2522Hvcn1%2522%252C%2522category%2522%253A%2522symbol%2522%257D%255D&filterFactors=%257B%257D&cutoff=%257B%2522foldChange%2522%253A1%252C%2522pValue%2522%253A0.05%257D®ulation=%2522UP_DOWN%2522 (accessed on 18 January 2021).
- Tucker, S.J.; Imbrici, P.; Salvatore, L.; D’Adamo, M.C.; Pessia, M. pH Dependence of the Inwardly Rectifying Potassium Channel, Kir5.1, and Localization in Renal Tubular Epithelia. J. Biol. Chem. 2000, 275, 16404–16407. [Google Scholar] [CrossRef]
- Lishko, P.V.; Kirichok, Y.; Ren, D.; Navarro, B.; Chung, J.-J.; Clapham, D.E. The Control of Male Fertility by Spermatozoan Ion Channels. Annu. Rev. Physiol. 2012, 74, 453–475. [Google Scholar] [CrossRef]
- Arnoult, C.; Kazam, I.G.; Visconti, P.E.; Kopf, G.S.; Villaz, M.; Florman, H.M. Control of the low voltage-activated calcium channel of mouse sperm by egg ZP3 and by membrane hyperpolarization during capacitation. Proc. Natl. Acad. Sci. USA 1999, 96, 6757–6762. [Google Scholar] [CrossRef]
- Darszon, A.; Acevedo, J.J.; Galindo, B.E.; Hernández-González, E.O.; Nishigaki, T.; Treviño, C.L.; Wood, C.; Beltrán, C. Sperm channel diversity and functional multiplicity. Reproduction 2006, 131, 977–988. [Google Scholar] [CrossRef]
- Susko-Parrish, J.L.; First, N.L. Capacitation of Bovine Sperm by Heparin: Inhibitory Effect of Glucose and Role of Intracellular pH1. Biol. Reprod. 1989, 41, 683–699. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.S. Control of hyperactivation in sperm. Hum. Reprod. Update 2008, 14, 647–657. [Google Scholar] [CrossRef]
- Zeng, Y.; Oberdorf, J.; Florman, H.M. pH Regulation in Mouse Sperm: Identification of Na+-, Cl−-, and [formula] Dependent and Arylaminobenzoate-Dependent Regulatory Mechanisms and Characterization of Their Roles in Sperm Capacitation. Dev. Biol. 1996, 173, 510–520. [Google Scholar] [CrossRef]
- Lishko, P.V.; Botchkina, I.L.; Fedorenko, A.; Kirichok, Y. Acid Extrusion from Human Spermatozoa Is Mediated by Flagellar Voltage-Gated Proton Channel. Cell 2010, 140, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Gadêlha, H.; Hernández-Herrera, P.; Montoya, F.; Darszon, A.; Corkidi, G. Human sperm uses asymmetric and anisotropic flagellar controls to regulate swimming. Sci. Adv. 2020, 6, eaba5168. [Google Scholar] [CrossRef]
- Ramsey, I.S.; Ruchti, E.; Kaczmarek, J.S.; Clapham, D.E. Hv1 proton channels are required for high-level NADPH oxidase-dependent superoxide production during the phagocyte respiratory burst. Proc. Natl. Acad. Sci. USA 2009, 106, 7642–7647. [Google Scholar] [CrossRef] [PubMed]
- Brenker, C.; Zhou, Y.; Müller, A.; Echeverry, F.A.; Troetschel, C.; Poetsch, A.; Xia, X.-M.; Bönigk, W.; Lingle, C.J.; Kaupp, U.B.; et al. The Ca2+-activated K+ current of human sperm is mediated by Slo3. eLife 2014, 3, e01438. [Google Scholar] [CrossRef] [PubMed]
- Borland, R.M.; Biggers, J.D.; Lechene, C.P.; Taymor, M.L. Elemental composition of fluid in the human Fallopian tube. Reproduction 1980, 58, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Connors, N.C.; Kofuji, P. Dystrophin Dp71 Is Critical for the Clustered Localization of Potassium Channels in Retinal Glial Cells. J. Neurosci. 2002, 22, 4321–4327. [Google Scholar] [CrossRef] [PubMed]
- Dalloz, C.; Sarig, R.; Fort, P.; Yaffe, D.; Bordais, A.; Pannicke, T.; Grosche, J.; Mornet, D.; Reichenbach, A.; Sahel, J.-A.; et al. Targeted inactivation of dystrophin gene product Dp71: Phenotypic impact in mouse retina. Hum. Mol. Genet. 2003, 12, 1543–1554. [Google Scholar] [CrossRef]
- Hernández-González, E.O.; Mornet, D.; Rendon, A.; Martínez-Rojas, D. Absence of Dp71 in mdx3cv mouse spermatozoa alters flagellar morphology and the distribution of ion channels and nNOS. J. Cell Sci. 2005, 118, 137–145. [Google Scholar] [CrossRef][Green Version]
- Datta, J.; Palmer, M.; Tanton, C.; Gibson, L.; Jones, K.; MacDowall, W.; Glasier, A.; Sonnenberg, P.; Field, N.; Mercer, C.; et al. Prevalence of infertility and help seeking among 15 000 women and men. Hum. Reprod. 2016, 31, 2108–2118. [Google Scholar] [CrossRef]
- Brown, S.G.; Publicover, S.J.; Mansell, S.A.; Lishko, P.V.; Williams, H.L.; Ramalingam, M.; Wilson, S.M.; Barratt, C.L.; Sutton, K.A.; Da Silva, S.M. Depolarization of sperm membrane potential is a common feature of men with subfertility and is associated with low fertilization rate at IVF. Hum. Reprod. 2016, 31, 1147–1157. [Google Scholar] [CrossRef]
- Tan, Y.-Q.; Wang, W.-L.; Tu, C.-F. Insight on multiple morphological abnormalities of sperm flagella in male infertility: What is new? Asian J. Androl. 2020, 22, 236–245. [Google Scholar] [CrossRef]
- Hildebrand, M.S.; Avenarius, M.R.; Fellous, M.; Zhang, Y.; Meyer, N.C.; Auer, J.; Serres, C.; Kahrizi, K.; Najmabadi, H.; Beckmann, J.S.; et al. Genetic male infertility and mutation of CATSPER ion channels. Eur. J. Hum. Genet. 2010, 18, 1178–1184. [Google Scholar] [CrossRef]
- Dirami, T.; Rode, B.; Jollivet, M.; Da Silva, N.; Escalier, D.; Gaitch, N.; Norez, C.; Tuffery, P.; Wolf, J.-P.; Becq, F.; et al. Missense Mutations in SLC26A8, Encoding a Sperm-Specific Activator of CFTR, Are Associated with Human Asthenozoospermia. Am. J. Hum. Genet. 2013, 92, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.F.; Toure, A.; Metzler-Guillemain, C.; Mitchell, M.J.; Arnoult, C.; Coutton, C. Genetic abnormalities leading to qualitative defects of sperm morphology or function. Clin. Genet. 2017, 91, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Nistal, M.; Paniagua, R.; Queizán, A. Histologic lesions in undescended ectopic obstructed testes. Fertil. Steril. 1985, 43, 455–462. [Google Scholar] [CrossRef]
- Love, C.C.; Garcia, M.C.; Riera, F.R.; Kenney, R.M. Evaluation of measures taken by ultrasonography and caliper to estimate testicular volume and predict daily sperm output in the stallion. J. Reprod. Fertil. Suppl. 1991, 44, 99–105. [Google Scholar] [PubMed]
- Brêtas, S.; Tatsuo, E.S.; Brêtas, M.O.O.; Brêtas, C.O. Measurement of testicular volume in Wistar rats using a caliper and ultrasonography in experimental surgery. Acta Cir. Bras. 2016, 31, 479–485. [Google Scholar] [CrossRef]
- Yeung, C.-H.; Breton, S.; Setiawan, I.; Xu, Y.; Lang, F.; Cooper, T.G. Increased luminal pH in the epididymis of infertile c-ros knockout mice and the expression of sodium-hydrogen exchangers and vacuolar proton pump H+-ATPase. Mol. Reprod. Dev. 2004, 68, 159–168. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poli, G.; Hasan, S.; Belia, S.; Cenciarini, M.; Tucker, S.J.; Imbrici, P.; Shehab, S.; Pessia, M.; Brancorsini, S.; D'Adamo, M.C. Kcnj16 (Kir5.1) Gene Ablation Causes Subfertility and Increases the Prevalence of Morphologically Abnormal Spermatozoa. Int. J. Mol. Sci. 2021, 22, 5972. https://doi.org/10.3390/ijms22115972
Poli G, Hasan S, Belia S, Cenciarini M, Tucker SJ, Imbrici P, Shehab S, Pessia M, Brancorsini S, D'Adamo MC. Kcnj16 (Kir5.1) Gene Ablation Causes Subfertility and Increases the Prevalence of Morphologically Abnormal Spermatozoa. International Journal of Molecular Sciences. 2021; 22(11):5972. https://doi.org/10.3390/ijms22115972
Chicago/Turabian StylePoli, Giulia, Sonia Hasan, Silvia Belia, Marta Cenciarini, Stephen J. Tucker, Paola Imbrici, Safa Shehab, Mauro Pessia, Stefano Brancorsini, and Maria Cristina D'Adamo. 2021. "Kcnj16 (Kir5.1) Gene Ablation Causes Subfertility and Increases the Prevalence of Morphologically Abnormal Spermatozoa" International Journal of Molecular Sciences 22, no. 11: 5972. https://doi.org/10.3390/ijms22115972
APA StylePoli, G., Hasan, S., Belia, S., Cenciarini, M., Tucker, S. J., Imbrici, P., Shehab, S., Pessia, M., Brancorsini, S., & D'Adamo, M. C. (2021). Kcnj16 (Kir5.1) Gene Ablation Causes Subfertility and Increases the Prevalence of Morphologically Abnormal Spermatozoa. International Journal of Molecular Sciences, 22(11), 5972. https://doi.org/10.3390/ijms22115972









