Bio-Functionalized Chitosan for Bone Tissue Engineering
Abstract
1. Introduction
2. Results and Discussion
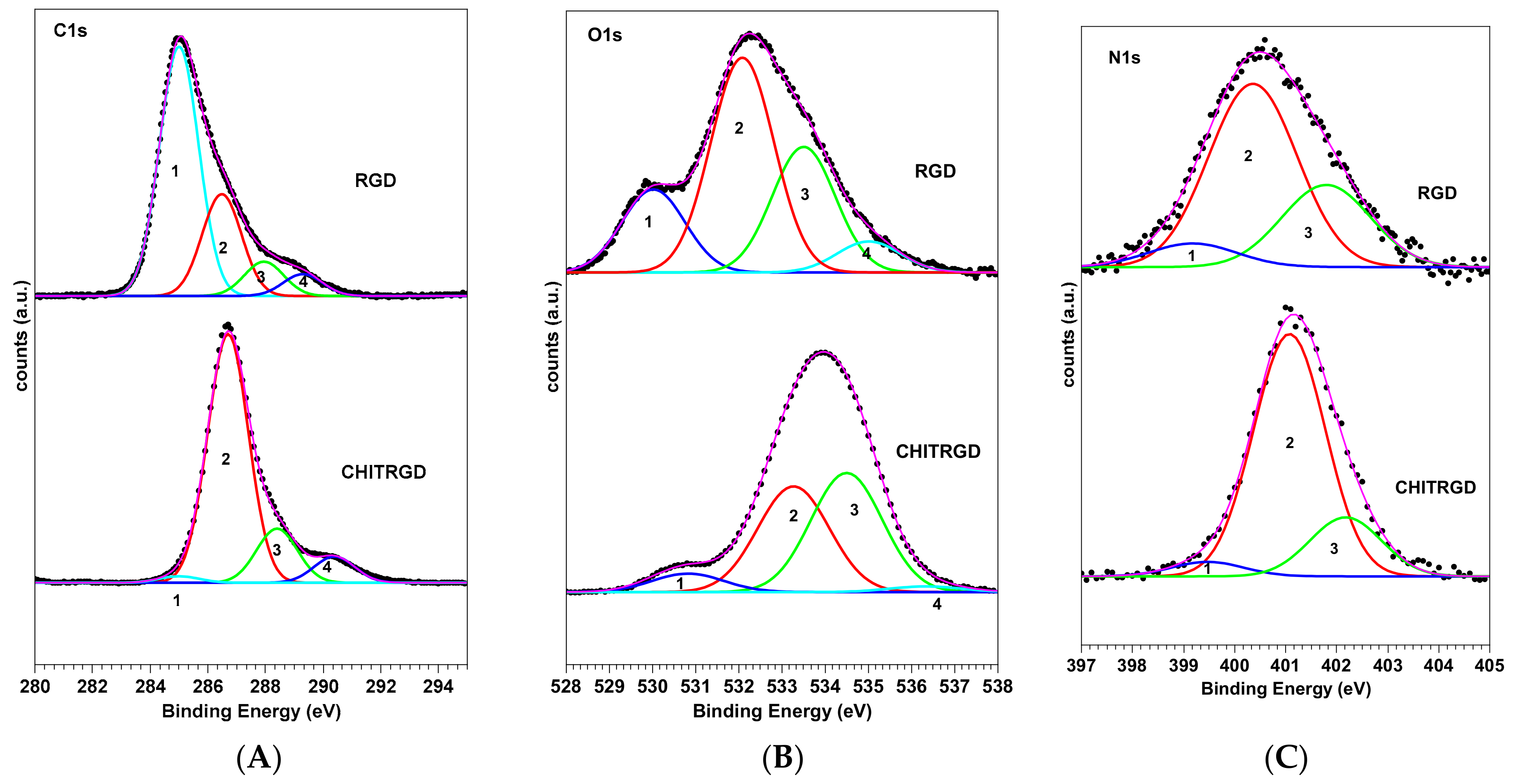
2.1. Chemical Composition and Biomolecule Stability: X-Ray Photoelectron Spectroscopy Investigation
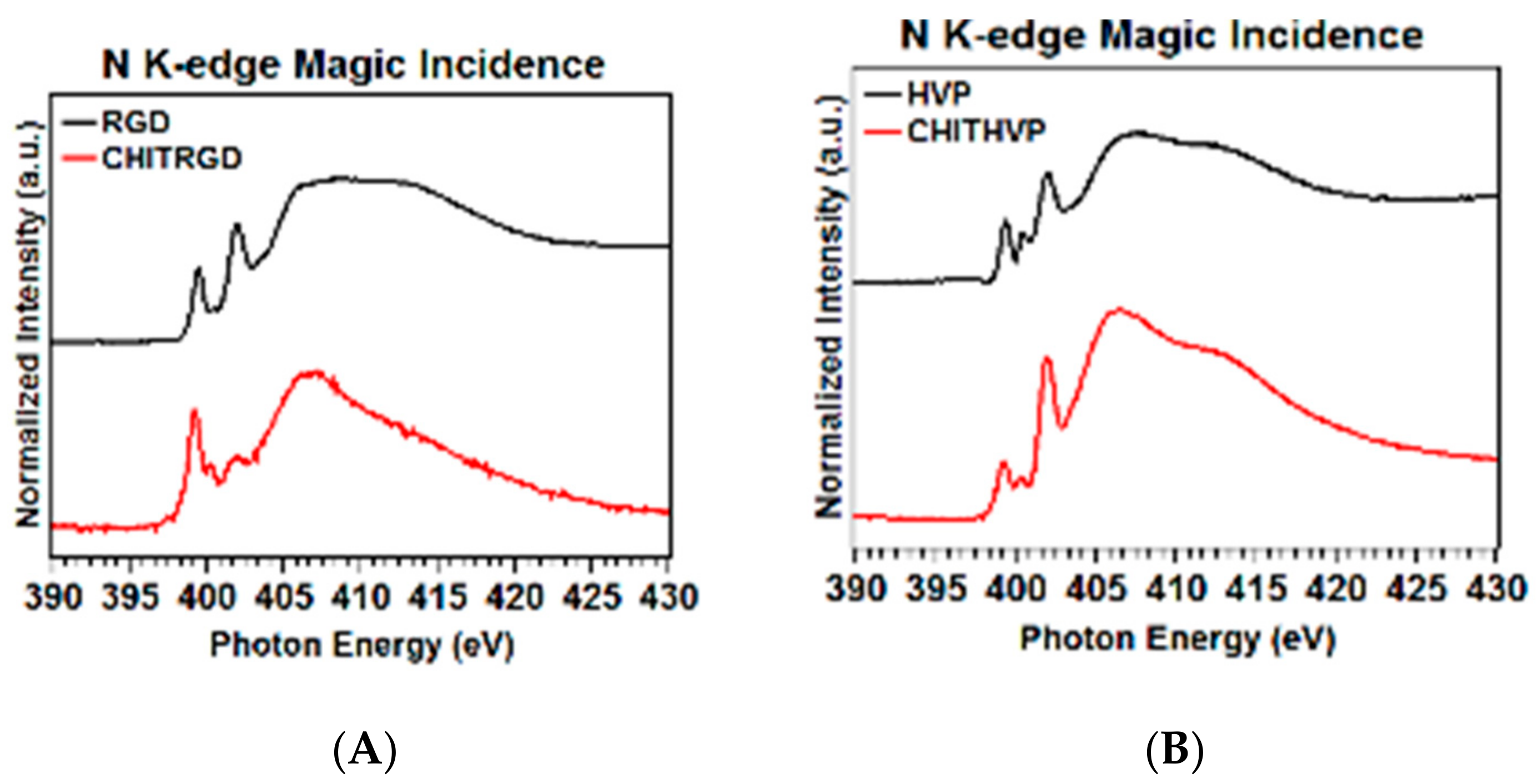
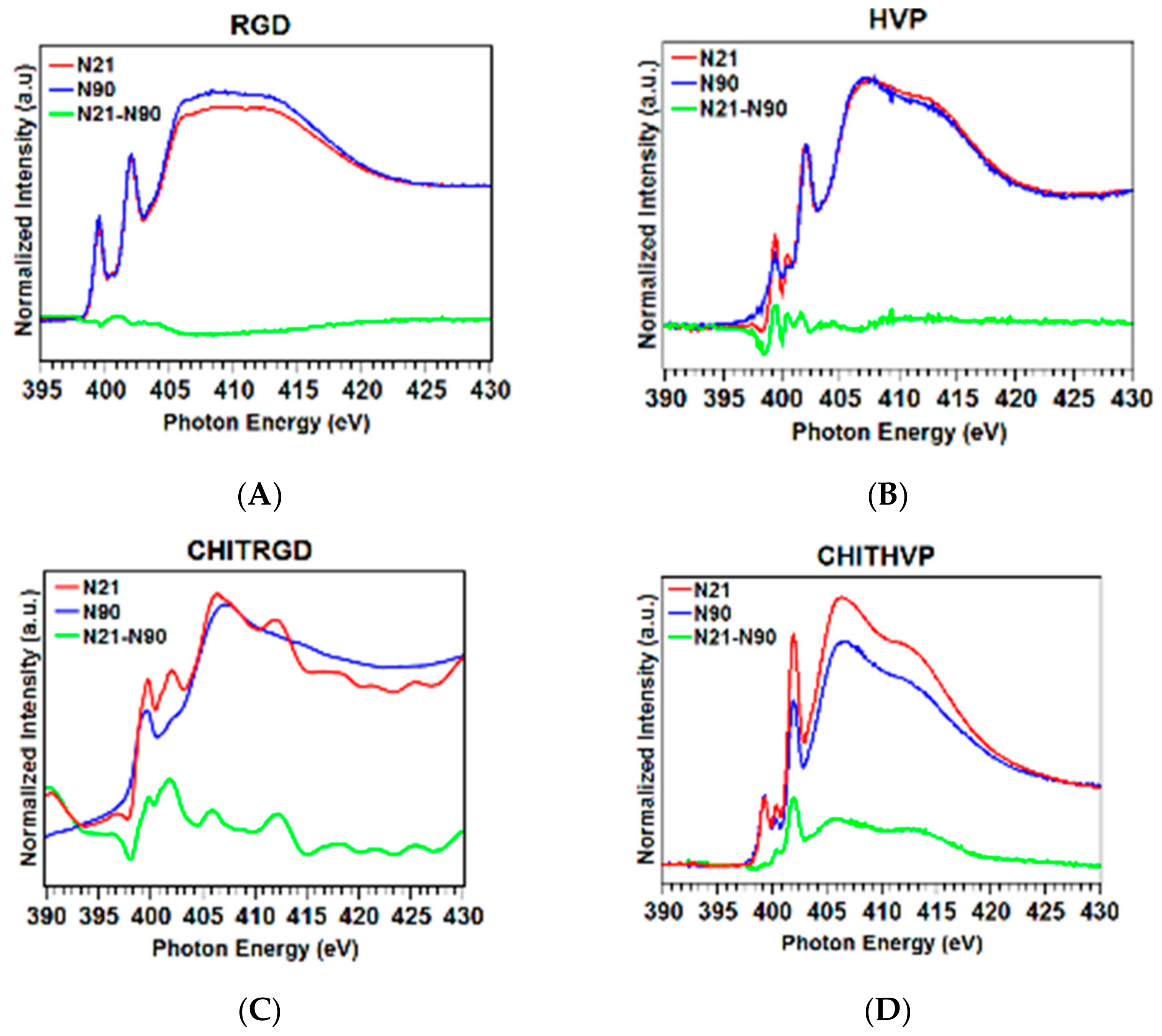
2.2. Molecular Structure and Organization: Near Edge X-ray Absorption Fine Structure Studies
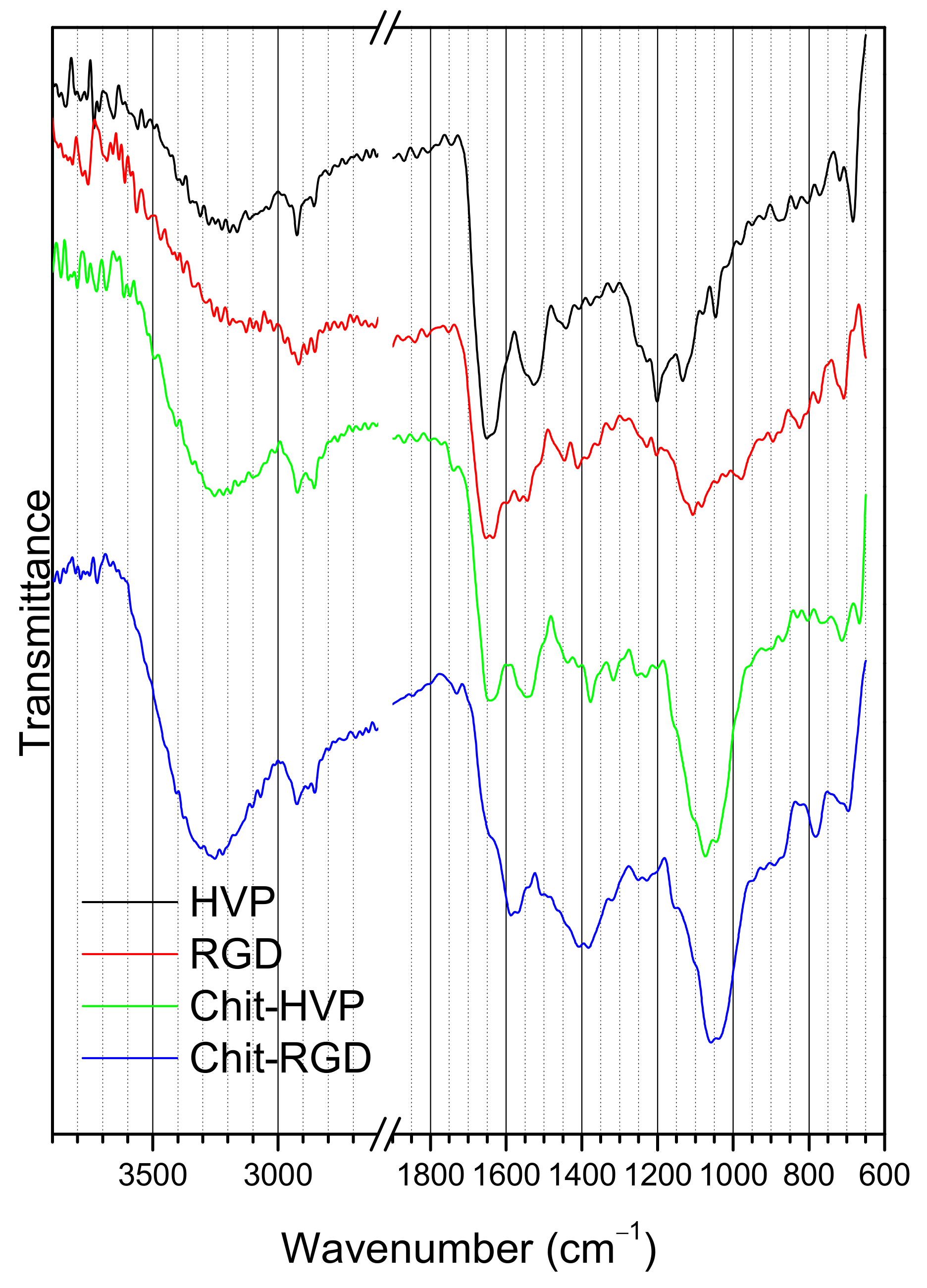
2.3. FTIR Spectroscopy
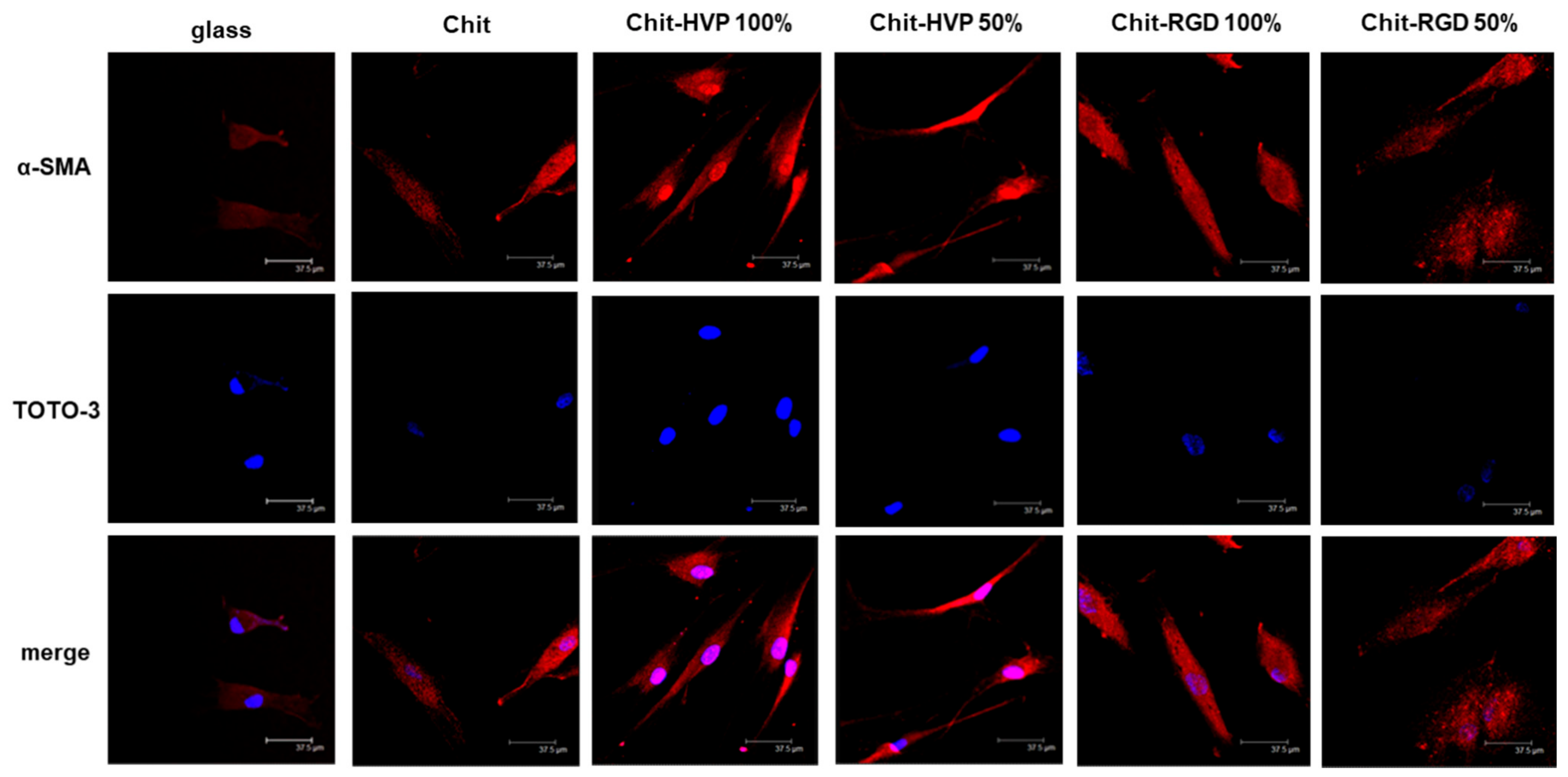
2.4. Functionalization of Chitosan with Biomimetic Peptides Affects Human Osteoblast Survival and Adhesion
2.5. HVP Functionalization of Chitosan Increases Proliferation of Human Osteoblast Cells
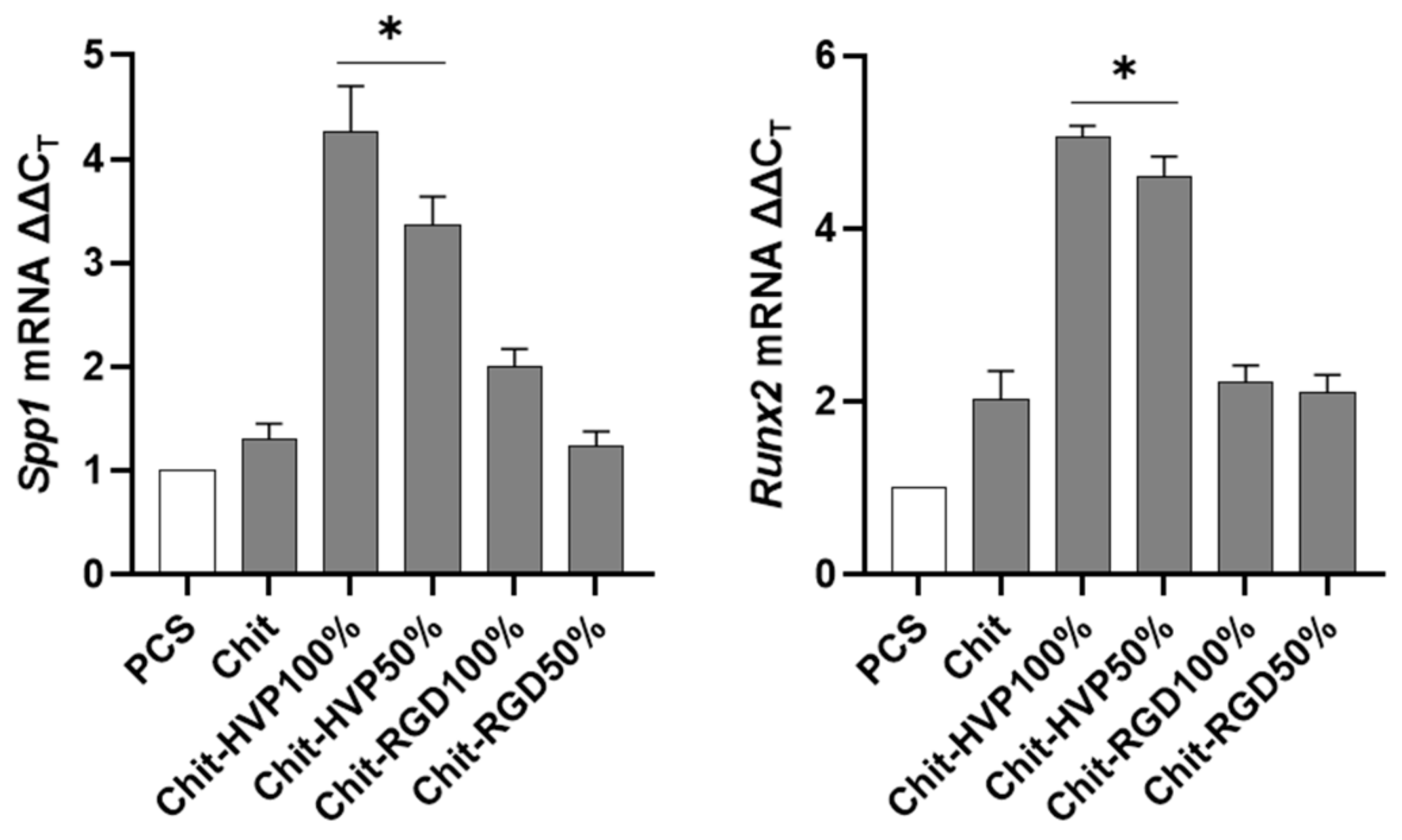
2.6. Chitosan Functionalization with HVP Supports Human Osteoblast Differentiation
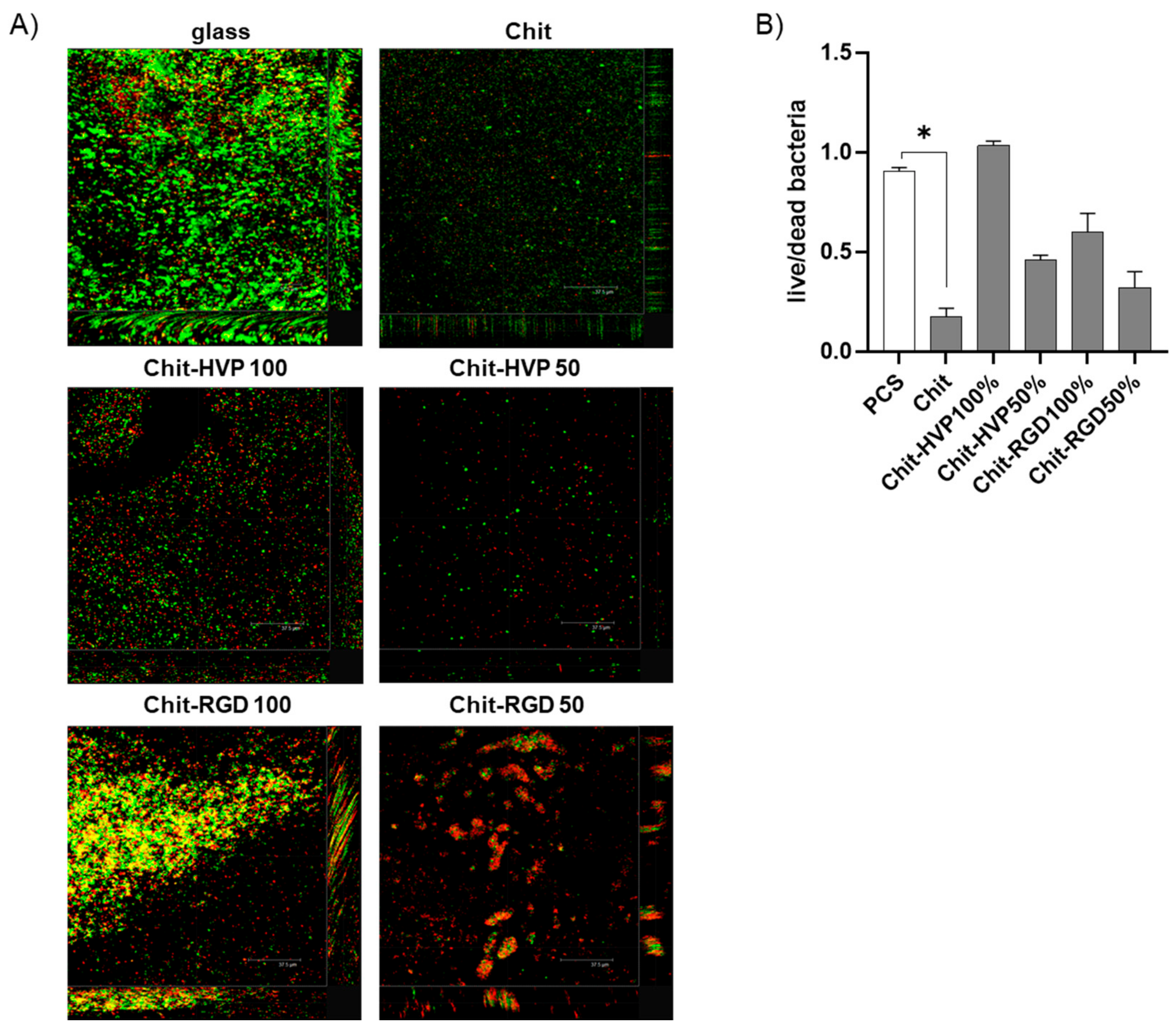
2.7. Chitosan Functionalization with Biomimetic Peptides Reduces the Antibacterial Activity
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Peptide Synthesis
- (1)
- GRGDSPK-Aldehyde
- (2)
- HVP-Aldehyde
3.2.2. Functionalization of Chitosan
3.2.3. Chemical Characterization
- (1)
- XPS
- (2)
- Near-Edge X-ray Absorption Fine Structure (NEXAFS)
- (3)
- FT-IR
3.2.4. In Vitro ASSAYS
- (1)
- Human Osteoblast Cells’ Isolation and Culture
- (2)
- Cell Adhesion
- (3)
- Immunofluorescence
- (4)
- Proliferation Assay
- (5)
- Quantitative Real-Time Polymerase Chain Reaction
- (6)
- Alizarin Staining
- (7)
- Bacterial Cultures and Biofilm Staining
- (8)
- Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bagheri-Khoulenjani, S.; Taghizadeh, S.; Mirzadeh, H. An Investigation on the Short-Term Biodegradability of Chitosan with Various Molecular Weights and Degrees of Deacetylation. Carbohydr. Polym. 2009, 78, 773–778. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, T.; Jiang, C. Biomacromolecules As Carriers in Drug Delivery and Tissue Engineering. Acta Pharm. Sin. B 2018, 8, 34–50. [Google Scholar] [CrossRef] [PubMed]
- Palma, P.J.; Ramos, J.; Martins, J.B.; Diogenes, A.; Figueiredo, M.H.; Ferreira, P.; Viegas, C.; Santos, J.M. Histologic Evaluation of Regenerative Endodontic Procedures with the Use of Chitosan Scaffolds in Immature Dog Teeth with Apical Periodontitis. J. Endod. 2017, 43, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-M.; Lin, Y.-C.; Chen, C.-Y.; Hsieh, Y.-Y.; Liaw, C.-K.; Huang, S.-W.; Tsuang, Y.-H.; Chen, C.-H.; Lin, F.-H. Thermosensitive Chitosan–Gelatin–Glycerol Phosphate Hydrogels as Collagenase Carrier for Tendon–Bone Healing in a Rabbit Model. Polymers 2020, 12, 436. [Google Scholar] [CrossRef] [PubMed]
- Hansson, A.; Hashom, N.; Falson, F.; Rousselle, P.; Olivier, J.; Borchard, E.G. In Vitro Evaluation of an RGD-Functionalized Chi-Tosan Derivative for Enhanced Cell adhesion. Carbohydr. Polym. 2012, 90, 1494–1500. [Google Scholar] [CrossRef]
- Wang, C.; Chen, B.; Zou, M.; Cheng, G. Cyclic RGD-Modified chitosan/Graphene Oxide Polymers for Drug Delivery and Cellular Imaging. Colloids Surf. B 2014, 122, 332–340. [Google Scholar] [CrossRef]
- Leedy, M.R.; Martin, H.J.; Andrew Norowski, P.; Jennings, J.A.; Haggard, W.O.; Bumgardner, J.D. Use of Chitosan as a Bioactive Implant Coating for Bone-Implant applications. Chitosan Biomater. 2011, 244, 129–165. [Google Scholar]
- Kjalarsdóttir, L.; Dýrfjörd, A.; Dagbjartsson, A.; Laxdal, E.H.; Örlygsson, G.; Gíslason, J.; Einarsson, J.M.; Ng, C.-H.; Jónsson, H. Bone Remodeling Effect of a Chitosan and Calcium Phosphate-Based Composite. Regen. Biomater. 2019, 6, 241–247. [Google Scholar] [CrossRef]
- Vaz, J.M.; Pezzoli, D.; Chevallier, P.; Campelo, C.S.; Candiani, G.; Mantovani, D. Antibacterial Coatings Based on Chitosan for Pharmaceutical and Biomedical Applications. Curr. Pharm. Des. 2018, 24, 866–885. [Google Scholar] [CrossRef]
- Morgenstern, M.; Kuehl, R.; Zalavras, C.G.; McNally, M.; Zimmerli, W.; Burch, M.A.; Vandendriessche, T.; Obremskey, W.T.; Verhofstad, M.H.J.; Metsemakers, W.J. The Influence of Duration of Infection on Outcome of Debridement and Implant Retention in Fracture-Related Infection. Bone Jt. J. 2021, 103-B, 213–221. [Google Scholar] [CrossRef]
- Schierholz, J.; Beuth, J. Implant Infections: A Haven for Opportunistic Bacteria. J. Hosp. Infect. 2001, 49, 87–93. [Google Scholar] [CrossRef]
- Verlee, S.; Mincke, E.C.; Stevens, V. Recent Developments in Antibacterial and Antifungal Chitosan and Its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef]
- Sadeghi, A.; Dorkoosh, F.; Avadi, M.; Saadat, P.; Rafiee-Tehrani, M.; Junginger, H. Preparation, Characterization and Antibacterial Activities of Chitosan, N-Trimethyl Chitosan (TMC) and N-Diethylmethyl Chitosan (DEMC) Nanoparticles Loaded with Insulin Using Both the Ionotropic Gelation and Polyelectrolyte Complexation Methods. Int. J. Pharm. 2008, 355, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Brun, P.; Zamuner, A.; Peretti, A.; Conti, J.; Messina, G.M.L.; Marletta, G.; Dettin, M. 3D Synthetic Peptide-Based Architectures for the Engineering of the Enteric Nervous System. Sci. Rep. 2019, 9, 5583. [Google Scholar] [CrossRef]
- Zamuner, A.; Brun, P.; Scorzeto, M.; Sica, G.; Castagliuolo, I.; Dettin, M. Smart Biomaterials: Surfaces Functionalized with Proteo-Lytically Stable Osteoblast-Adhesive peptides. Bioact. Mater. 2017, 2, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Brun, P.; Scorzeto, M.; Vassanelli, S.; Castagliuolo, I.; Palù, G.; Ghezzo, F.; Messina, G.M.; Iucci, G.; Battaglia, V.; Sivolella, S.; et al. Mechanisms Underlying the Attachment and Spreading of Human Osteoblasts: From Transient Interactions to Focal Adhesions on Vitronectin-Grafted Bioactive Surfaces. Acta Biomater. 2013, 9, 6105–6115. [Google Scholar] [CrossRef]
- Secchi, V.; Franchi, S.; Santi, M.; Dettin, M.; Zamuner, A.; Iucci, G.; Battocchio, C. Self-Assembling Behavior of Cysteine-Modified Oligopeptides: An XPS and NEXAFS Study. J. Phys. Chem. C 2018, 122, 6236–6239. [Google Scholar] [CrossRef]
- Secchi, V.; Franchi, S.; Santi, M.; Vladescu, A.; Braic, M.; Skála, T.; Nováková, J.; Dettin, M.; Zamuner, A.; Iucci, G.; et al. Biocompatible Materials Based on Self-Assembling Peptides on Ti25Nb10Zr Alloy: Molecular Structure and Organization in-Vestigated by Synchrotron Radiation Induced techniques. Nanomaterials 2018, 8, 148. [Google Scholar] [CrossRef]
- Battocchio, C.; Concolato, S.; De Santis, S.; Fahlman, M.; Iucci, G.; Santi, M.; Sotgiu, G.; Orsini, M. Chitosan Functionalization of Titanium and Ti6Al4V Alloy with Chloroacetic Acid as Linker Agent. Mater. Sci. Eng. C 2019, 99, 1133–1140. [Google Scholar] [CrossRef]
- Skwarczynska, A.; Kamińska, M.; Owczarz, P.; Bartoszek, N.; Walkowiak, B.; Modrzejewska, Z. The Structural (FTIR, XRD, and XPS) and Biological Studies of Thermosensitive Chitosan Chloride Gels With β-Glycerophosphate Disodium. J. Appl. Polym. Sci. 2018, 135. [Google Scholar] [CrossRef]
- Solomon, D.; Lehmann, J.; Kinyangi, J.; Liang, B.; Heymann, K.; Dathe, L.; Hanley, K.; Wirick, S.; Jacobsen, C. Carbon (1s) NEXAFS Spectroscopy of Biogeochemically Relevant Reference Organic Compounds. Soil Sci. Soc. Am. J. 2009, 73, 1817–1830. [Google Scholar] [CrossRef]
- Polzonetti, G.; Battocchio, C.; Iucci, G.; Dettin, M.; Gambaretto, R.; Di Bello, C.; Carravetta, V. Thin Films of a Self-Assembling Peptide on TiO2 and Au Studied by NEXAFS, XPS and IR Spectroscopies. Mater. Sci. Eng. C 2006, 26, 929–934. [Google Scholar] [CrossRef]
- Li, H.; Jiang, J.; Luo, Y. Identification of the Smallest Peptide with a Zwitterion as the Global Minimum: A First-Principles Study on Arginine-Containing Peptides. Phys. Chem. Chem. Phys. 2017, 19, 12117–12126. [Google Scholar] [CrossRef]
- Feyer, V.; Plekan, O.; Ptasińska, S.; Iakhnenko, M.; Tsud, N.; Prince, K.C. Adsorption of Histidine and a Histidine Tripeptide on Au(111) and Au(110) from Acidic Solution. J. Phys. Chem. C 2012, 116, 22960–22966. [Google Scholar] [CrossRef]
- Stöhr, J. NEXAFS Spectroscopy; Springer Series in Surface Sciences; Springer: Berlin/Heidelberg, Germany, 1992. [Google Scholar] [CrossRef]
- Haris, P.I.; Chapman, D. The Conformational Analysis of Peptides Using Fourier Transform IR Spectroscopy. Biopolymers 1995, 37, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Nevskaya, N.; Chirgadze, Y. Infrared Spectra and Resonance Interactions of amide-I and II Vibrations of α-helix. Biopolymers 1976, 15, 637–648. [Google Scholar] [CrossRef] [PubMed]
- NIST Database. Available online: https://webbook.nist.gov/Chemistry/ (accessed on 10 December 2020).
- Tortora, L.; Concolato, S.; Urbini, M.; Giannitelli, S.M.; Basoli, F.; Rainer, A.; Trombetta, M.; Orsini, M.; Mozetic, P. Functionali-Zation of poly(ε-Caprolactone) Surface with Lactose-Modified Chitosan via Alkaline Hydrolysis: ToF-SIMS characterization. Biointerphases 2016, 11, 02A32. [Google Scholar] [CrossRef]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, E.P. Application of Spec-Troscopic Methods for Structural Analysis of Chitin and chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef]
- Secchi, V.; Franchi, S.; Ciccarelli, D.; Dettin, M.; Zamuner, A.; Serio, A.; Iucci, G.; Battocchio, C. Biofunctionalization of TiO2 Surfaces with Self-Assembling Layers of Oligopeptides Covalently Grafted to Chitosan. ACS Biomater. Sci. Eng. 2019, 5, 2190–2199. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Dugina, V.; Ballestrem, C.; Wehrle-Haller, B.; Chaponnier, E.C. α-Smooth Muscle Actin Is Crucial for Focal Adhesion Maturation in myofibroblasts. Mol. Biol. Cell 2003, 14, 2508–2519. [Google Scholar] [CrossRef]
- Ho, M.-H.; Liao, M.-H.; Lin, Y.-L.; Lai, C.-H.; Lin, P.-I.; Chen, R.-M. Improving Effects of Chitosan Nanofiber Scaffolds on Osteoblast Proliferation and maturation. Int. J. Nanomed. 2014, 9, 4293–4304. [Google Scholar]
- Si, J.; Wang, C.; Zhang, D.; Wang, B.; Hou, W.; Zhou, Y. Osteopontin in Bone Metabolism and Bone Diseases. Med. Sci. Monit. 2020, 26, e919159-1. [Google Scholar] [CrossRef] [PubMed]
- Paredes, R.; Arriagada, G.; Cruzat, F.; Villagra, A.; Olate, J.; Zaidi, K.; Van Wijnen, A.; Lian, J.B.; Stein, G.S.; Stein, J.L.; et al. Bone-Specific Transcription Factor Runx2 Interacts with the 1α,25-Dihydroxyvitamin D3 Receptor To Up-Regulate Rat Osteocalcin Gene Expression in Osteoblastic Cells. Mol. Cell. Biol. 2004, 24, 8847–8861. [Google Scholar] [CrossRef] [PubMed]
- No, H.K.; Park, N.Y.; Lee, S.H.; Meyers, S.P. Antibacterial Activity of Chitosans and Chitosan Oligomers with Different Molecular Weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]





| Gene (Accession #) | Sequence | |
|---|---|---|
| Gapdh (NM_001289726) | Fw: 5′-agtgccagcctcgtcccgta-3′ | Rv: 5′-caggcgcccaatacggccaa-3′ |
| Spp1 (NM_000582) | Fw: 5′-aagtttcgcagacctgacatc-3′ | Rv: 5′-ggctgtcccaatcagaagg-3′ |
| Runx2 (NM_001024630) | Fw: 5′-cagtgacaccatgtcagcaa-3′ | Rv: 5′-gctcacgtcgctcattttg-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brun, P.; Zamuner, A.; Battocchio, C.; Cassari, L.; Todesco, M.; Graziani, V.; Iucci, G.; Marsotto, M.; Tortora, L.; Secchi, V.; et al. Bio-Functionalized Chitosan for Bone Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 5916. https://doi.org/10.3390/ijms22115916
Brun P, Zamuner A, Battocchio C, Cassari L, Todesco M, Graziani V, Iucci G, Marsotto M, Tortora L, Secchi V, et al. Bio-Functionalized Chitosan for Bone Tissue Engineering. International Journal of Molecular Sciences. 2021; 22(11):5916. https://doi.org/10.3390/ijms22115916
Chicago/Turabian StyleBrun, Paola, Annj Zamuner, Chiara Battocchio, Leonardo Cassari, Martina Todesco, Valerio Graziani, Giovanna Iucci, Martina Marsotto, Luca Tortora, Valeria Secchi, and et al. 2021. "Bio-Functionalized Chitosan for Bone Tissue Engineering" International Journal of Molecular Sciences 22, no. 11: 5916. https://doi.org/10.3390/ijms22115916
APA StyleBrun, P., Zamuner, A., Battocchio, C., Cassari, L., Todesco, M., Graziani, V., Iucci, G., Marsotto, M., Tortora, L., Secchi, V., & Dettin, M. (2021). Bio-Functionalized Chitosan for Bone Tissue Engineering. International Journal of Molecular Sciences, 22(11), 5916. https://doi.org/10.3390/ijms22115916












