β-Hexachlorocyclohexane Drives Carcinogenesis in the Human Normal Bronchial Epithelium Cell Line BEAS-2B
Abstract
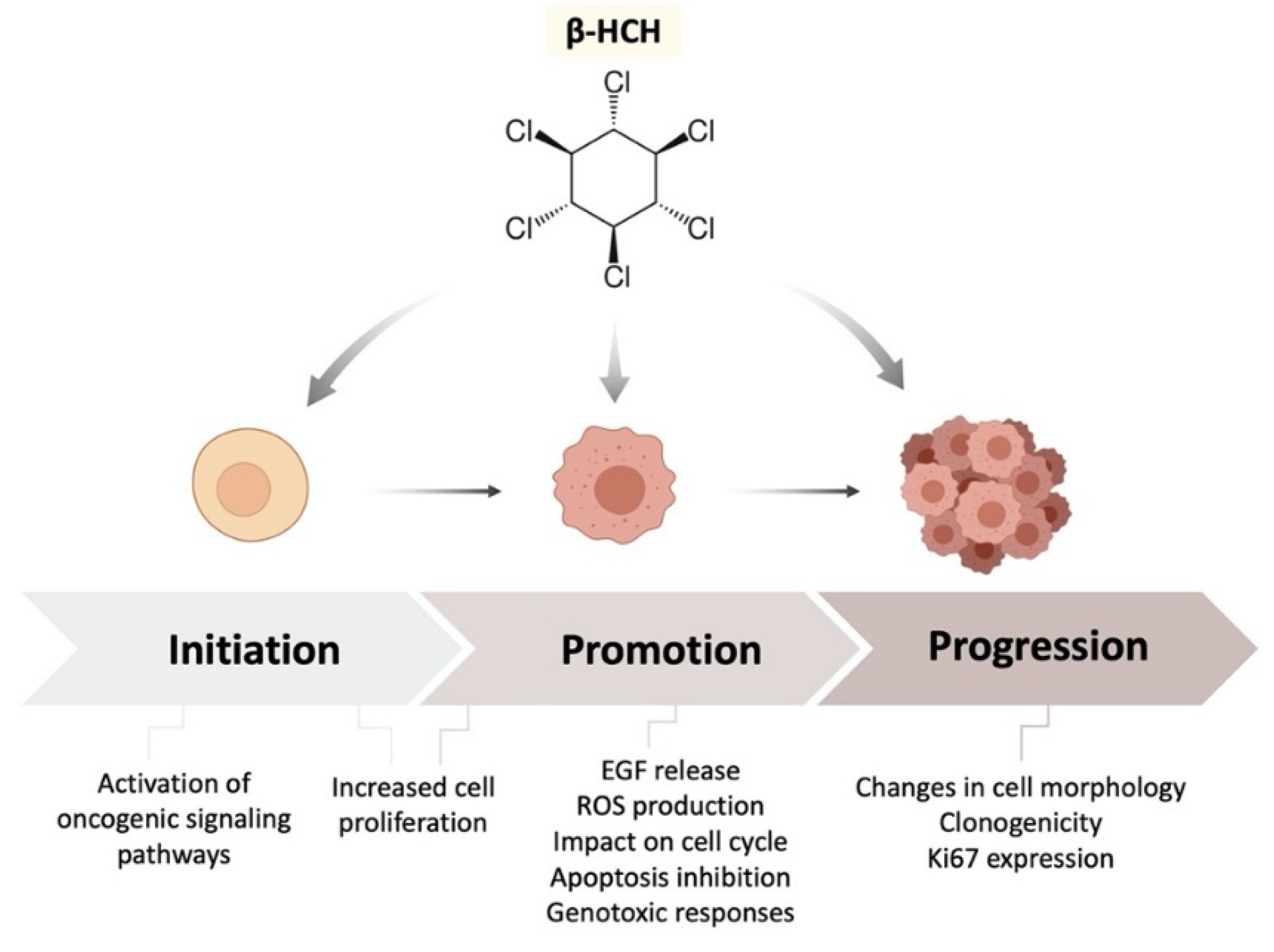
1. Introduction
2. Results
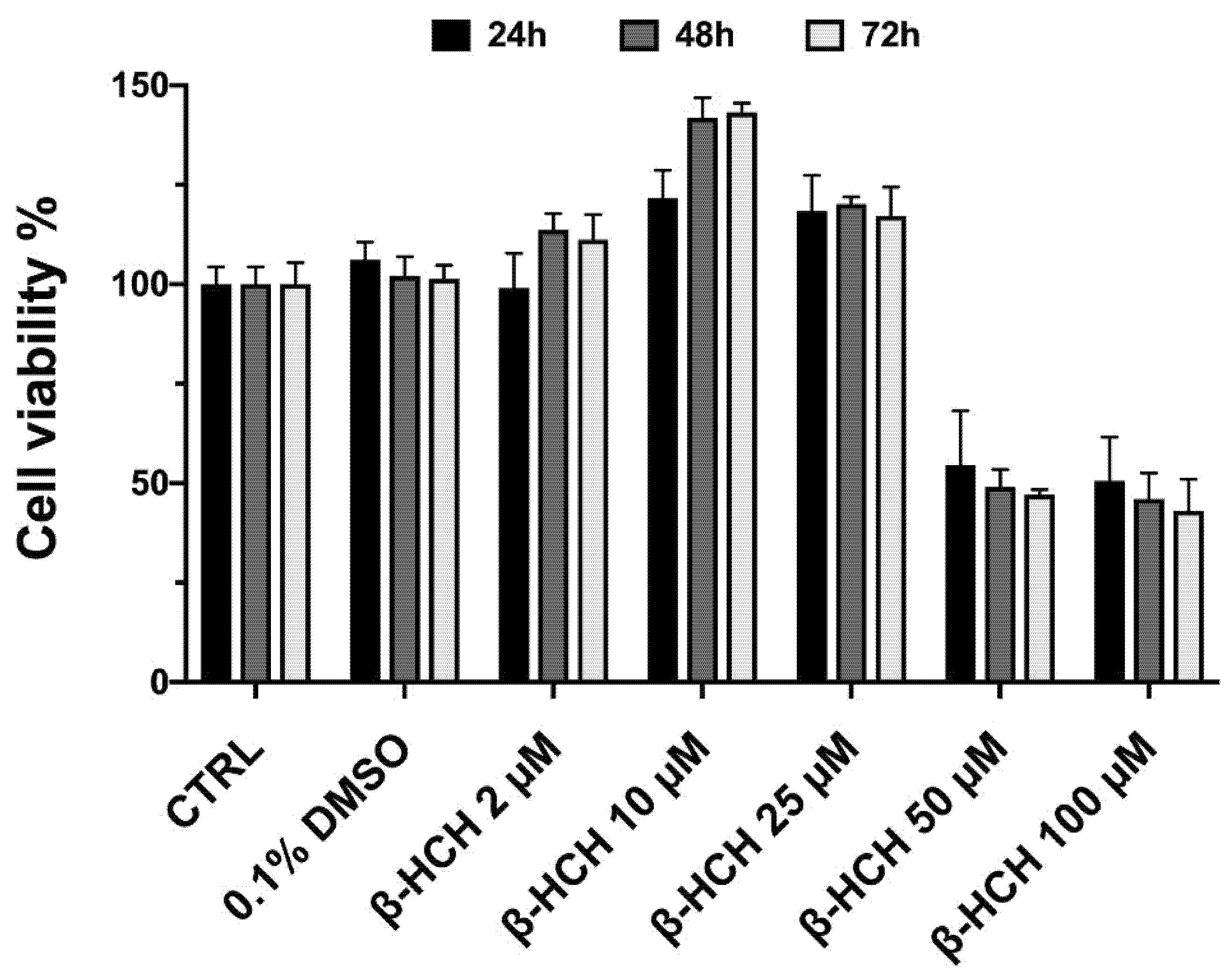
2.1. β-HCH Increases Cell Proliferation
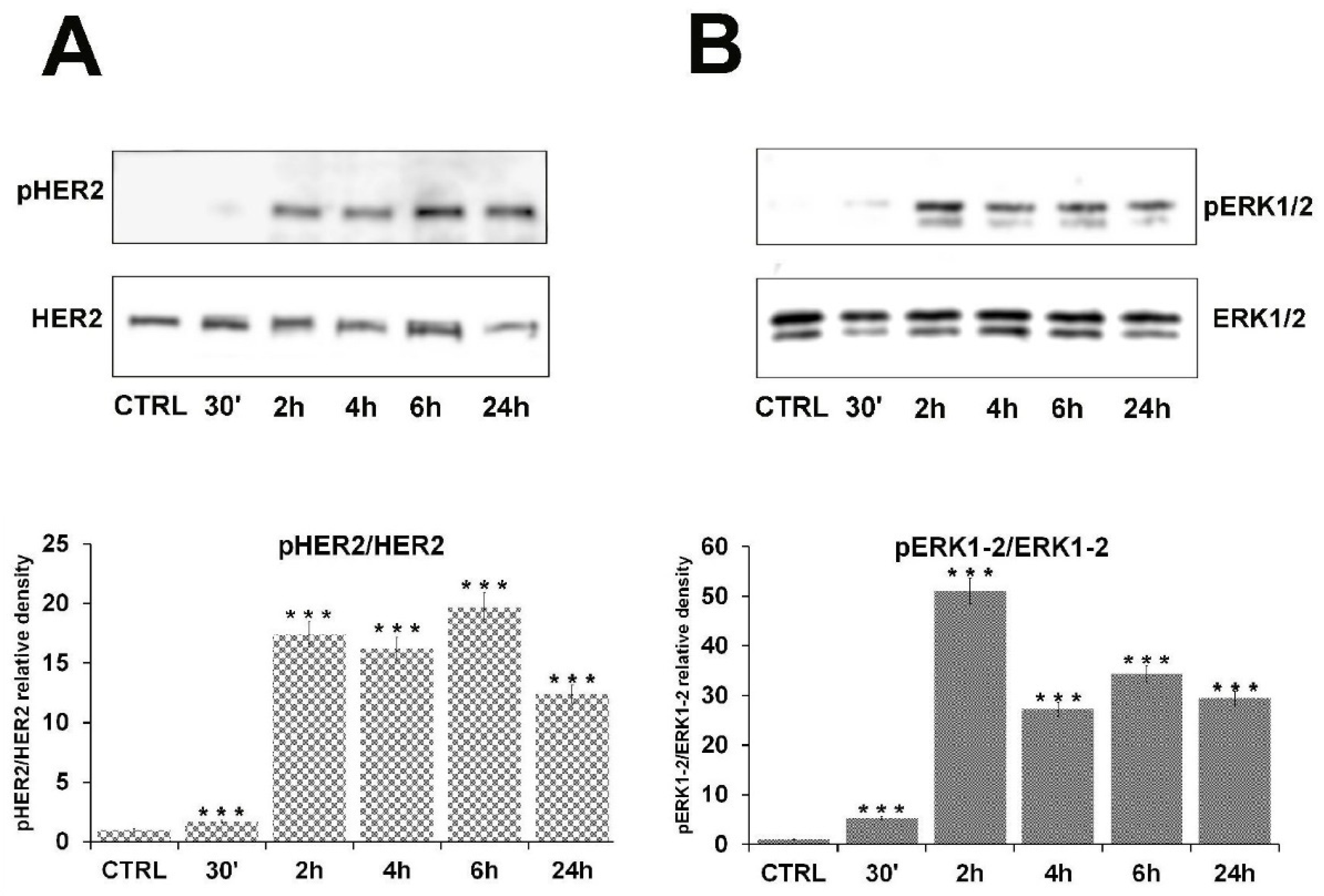
2.2. β-HCH Can Activate Oncogenic Signaling Pathways
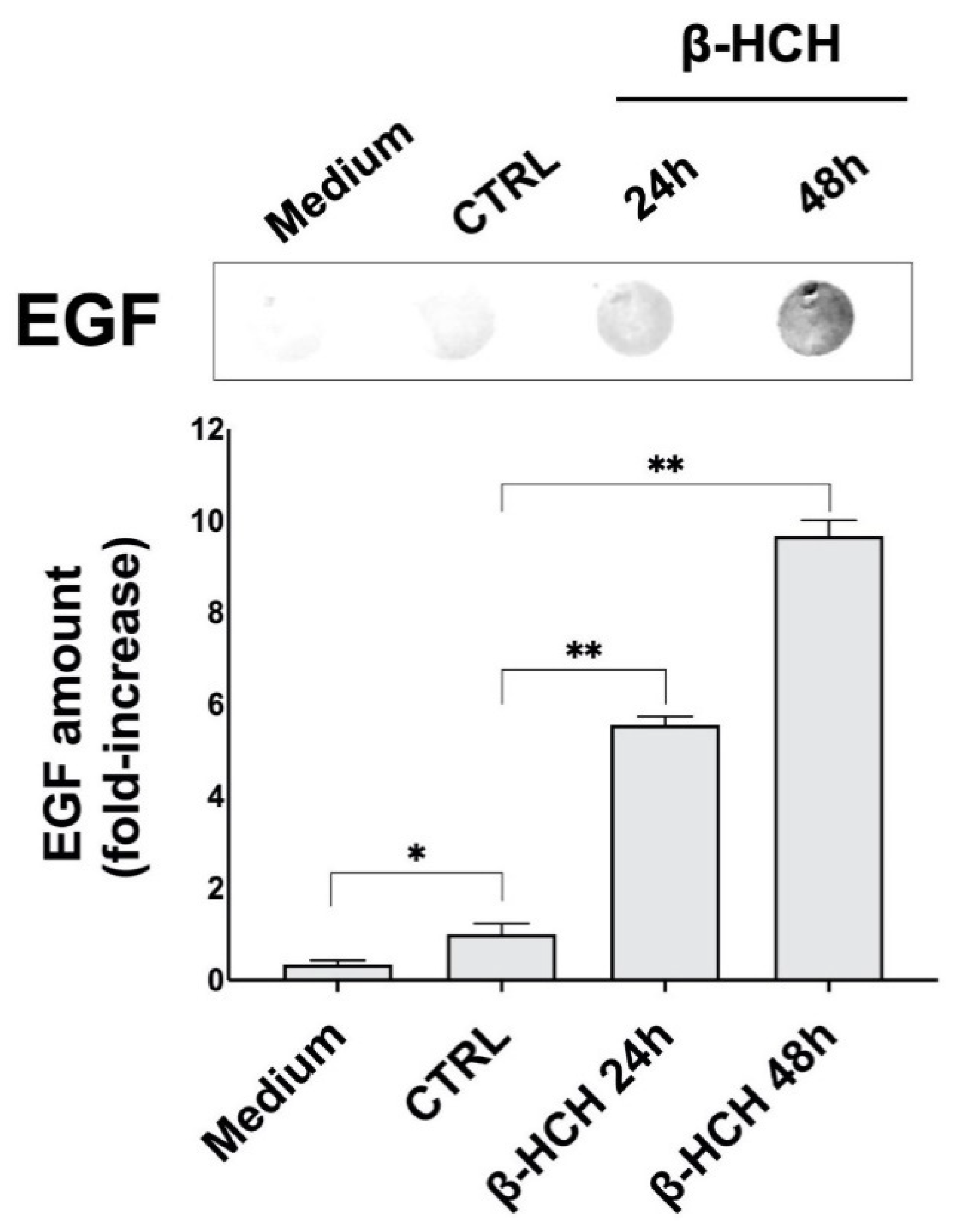
2.3. β-HCH Induces EGF Release
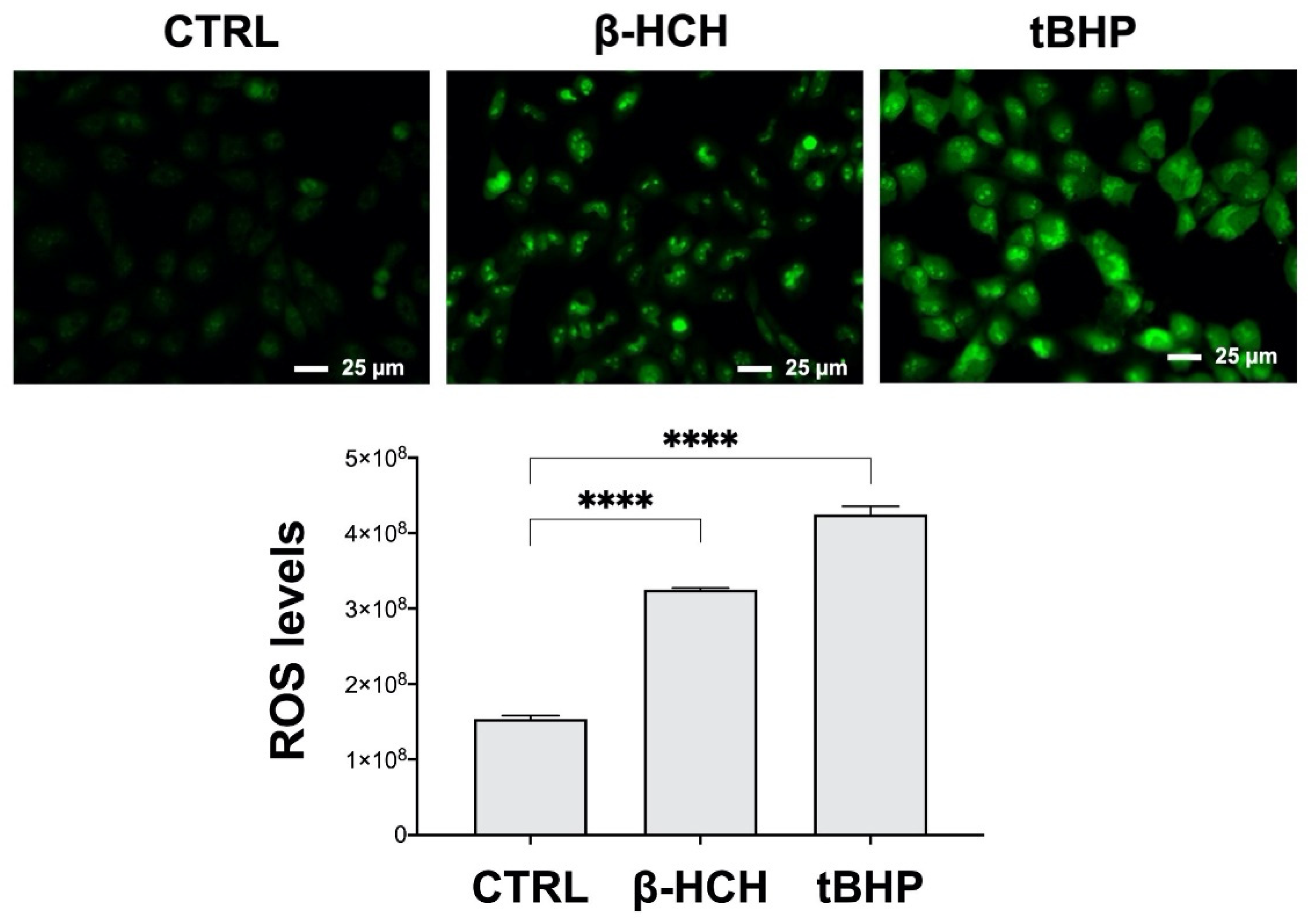
2.4. β-HCH Boosts ROS Production
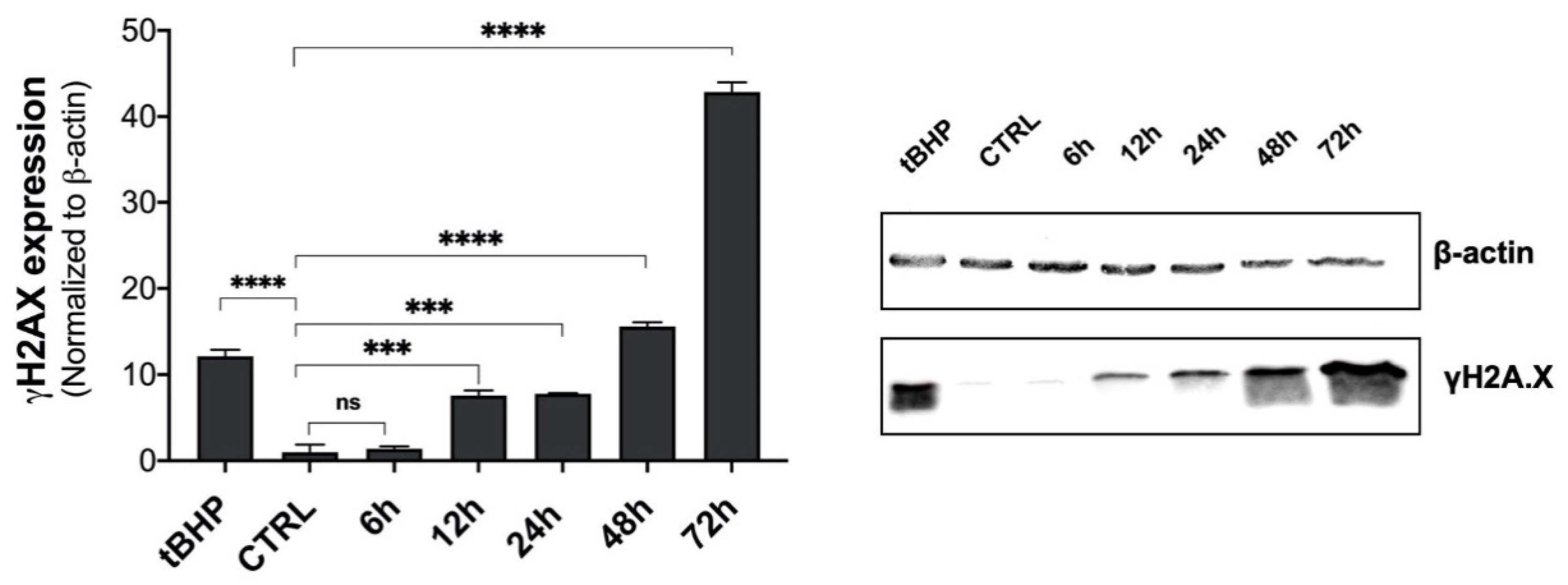
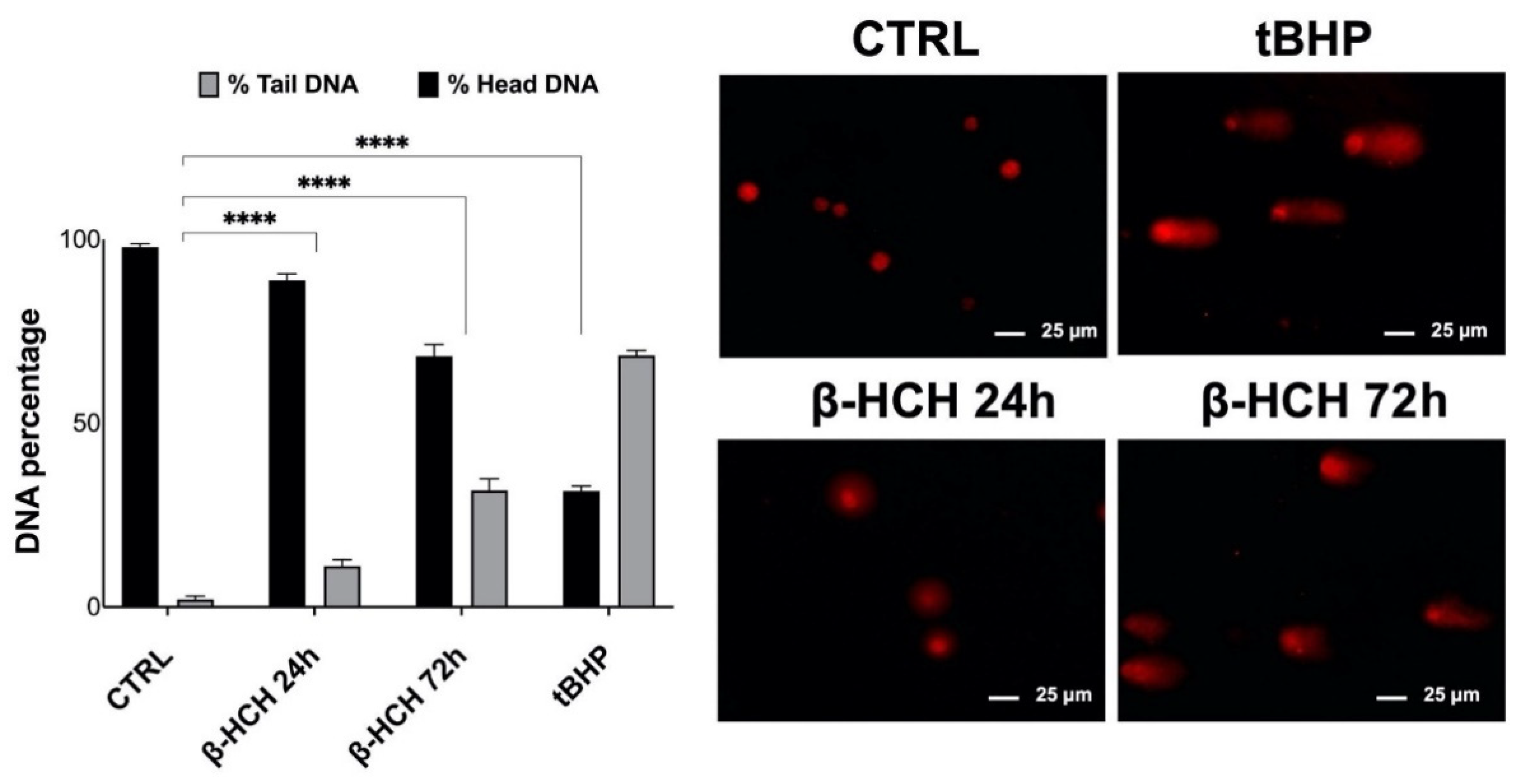
2.5. Genotoxic Responses to β-HCH
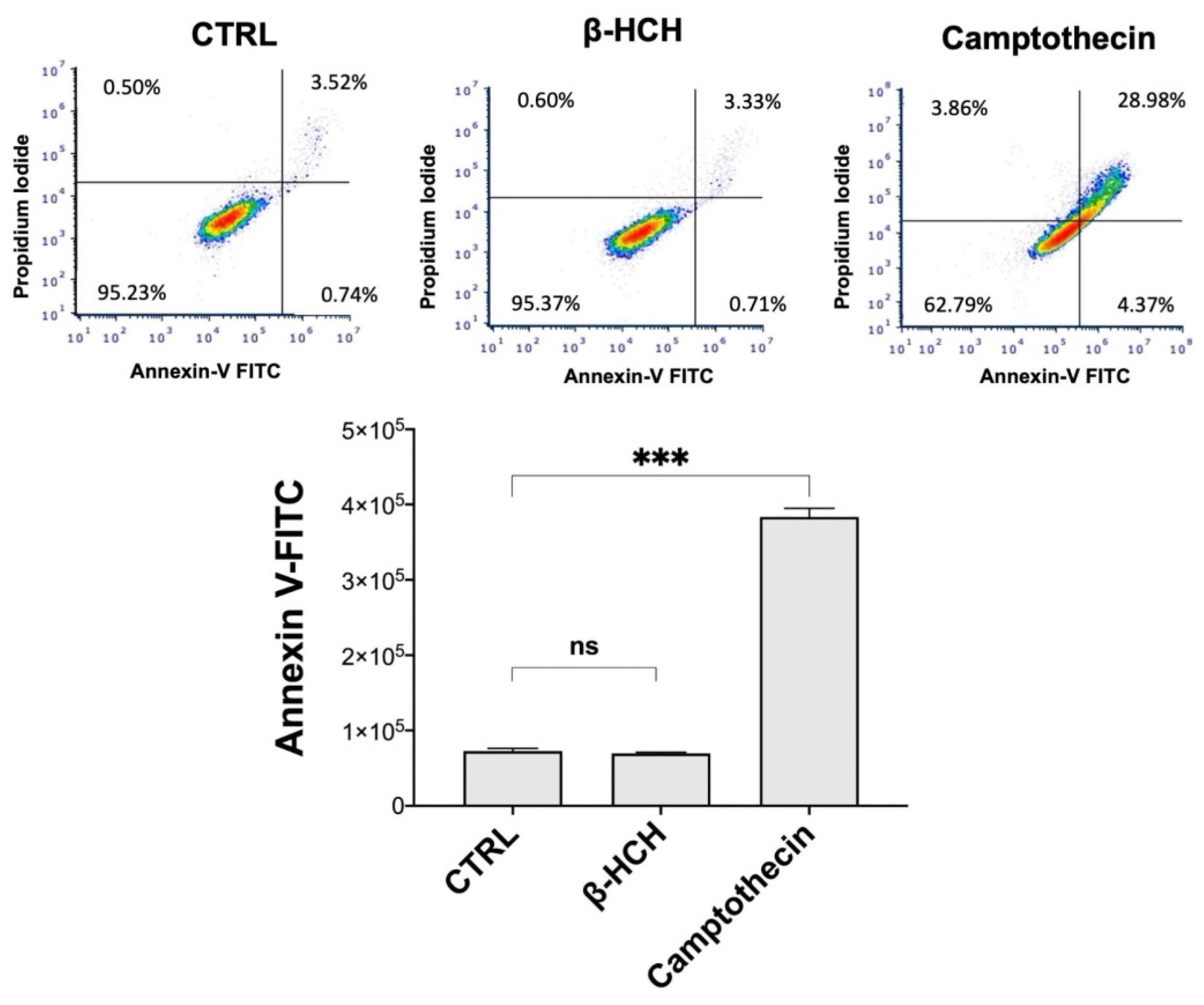
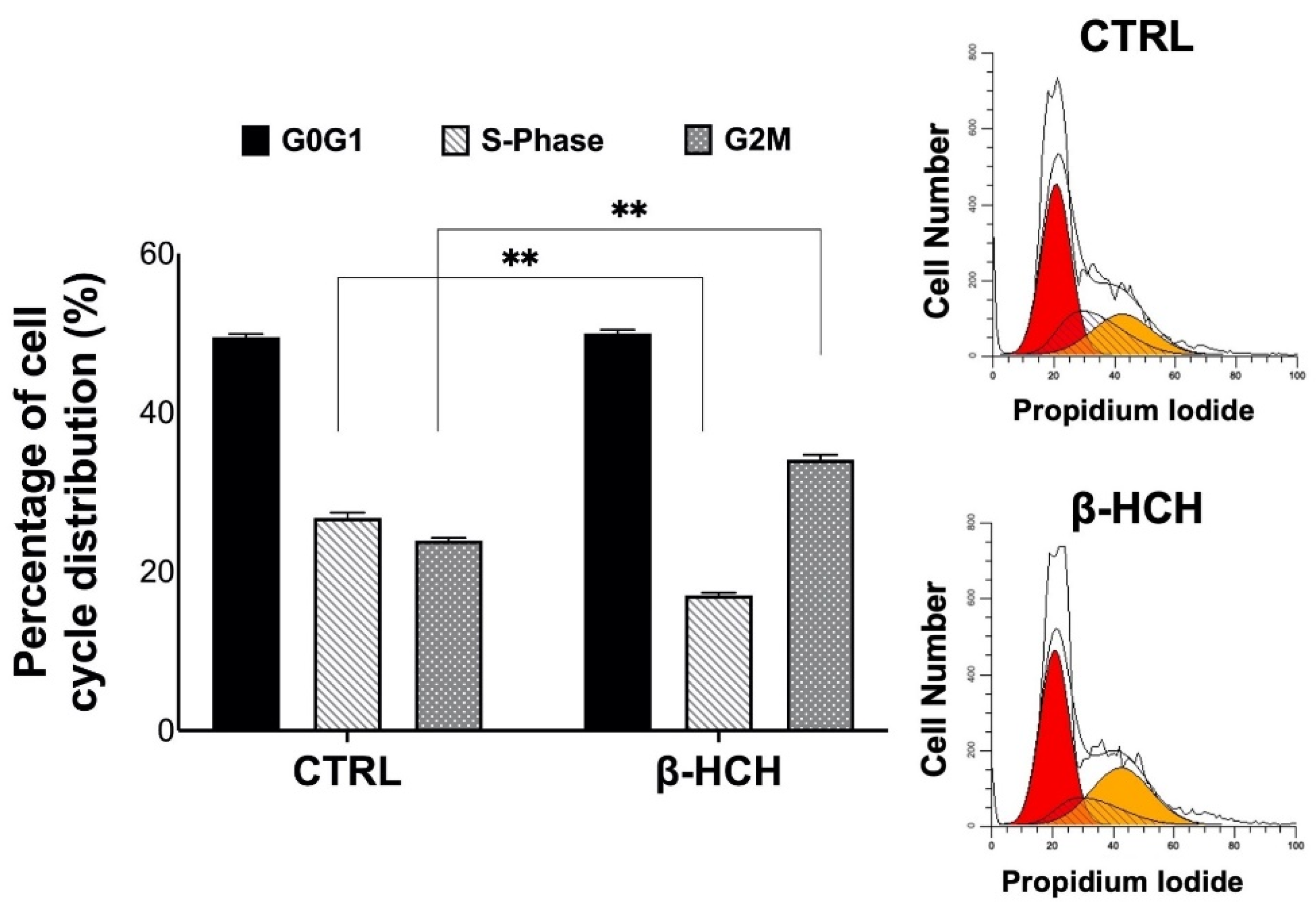
2.6. β-HCH’s Impact on Cell Cycle and Apoptosis
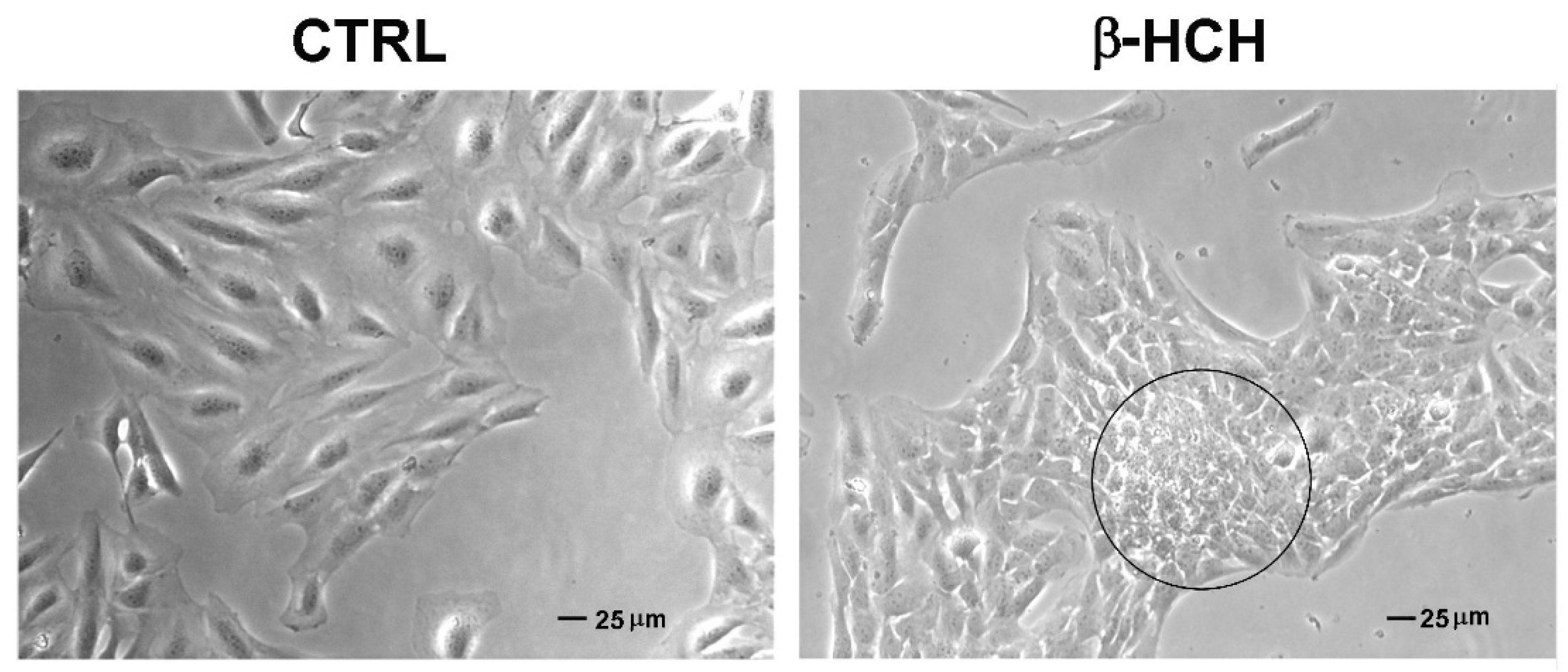
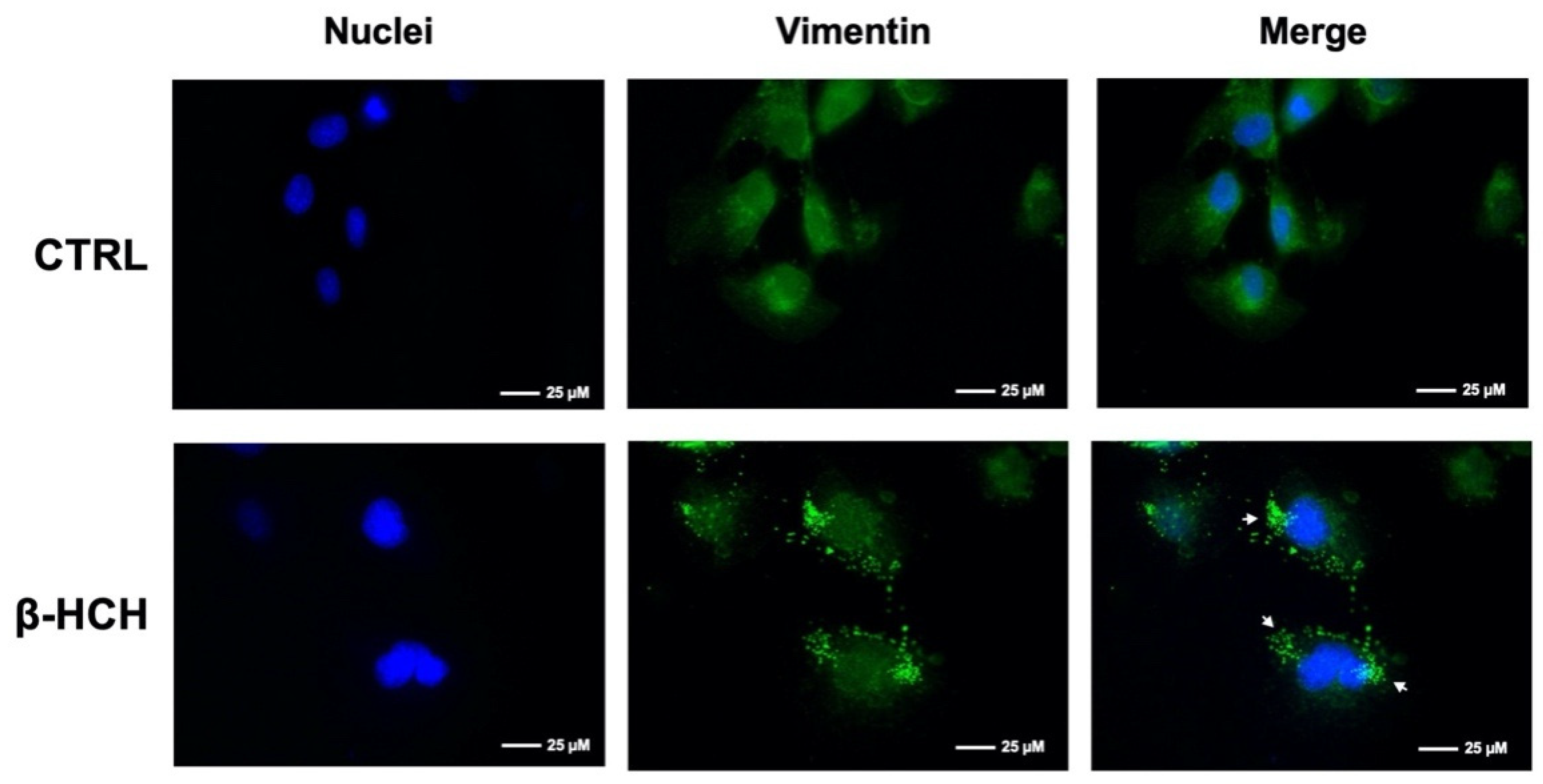
2.7. β-HCH Affects Cellular Morphology
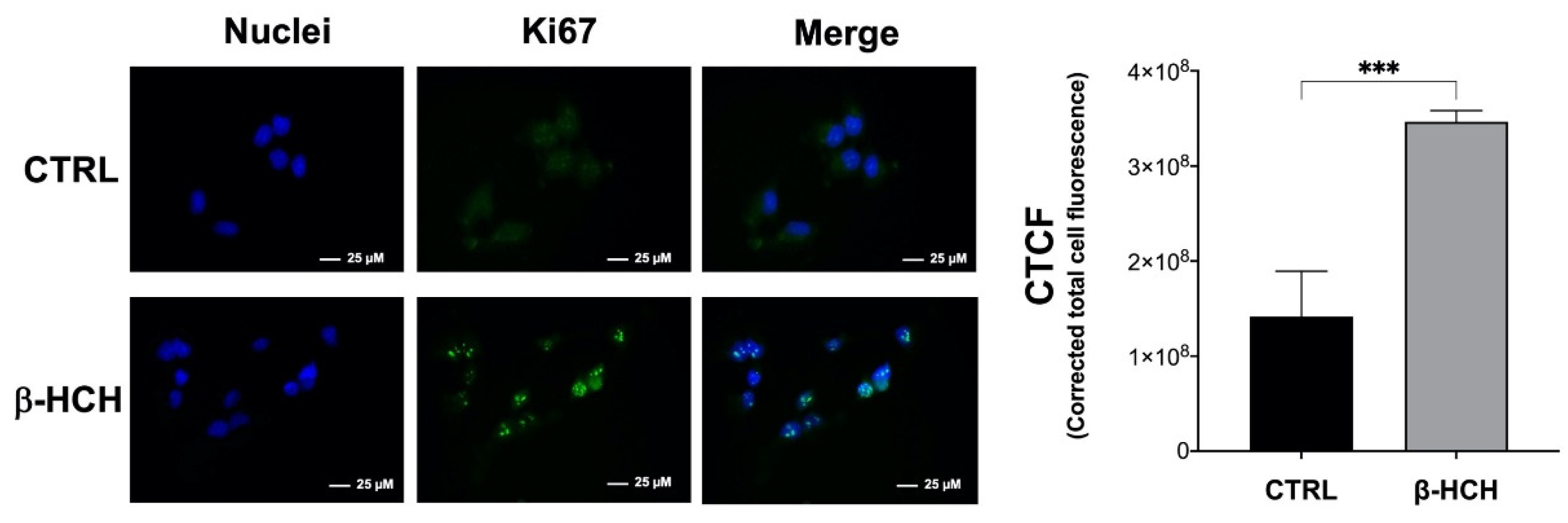
2.8. β-HCH Modulates the Expression of the Proliferative Marker Ki67
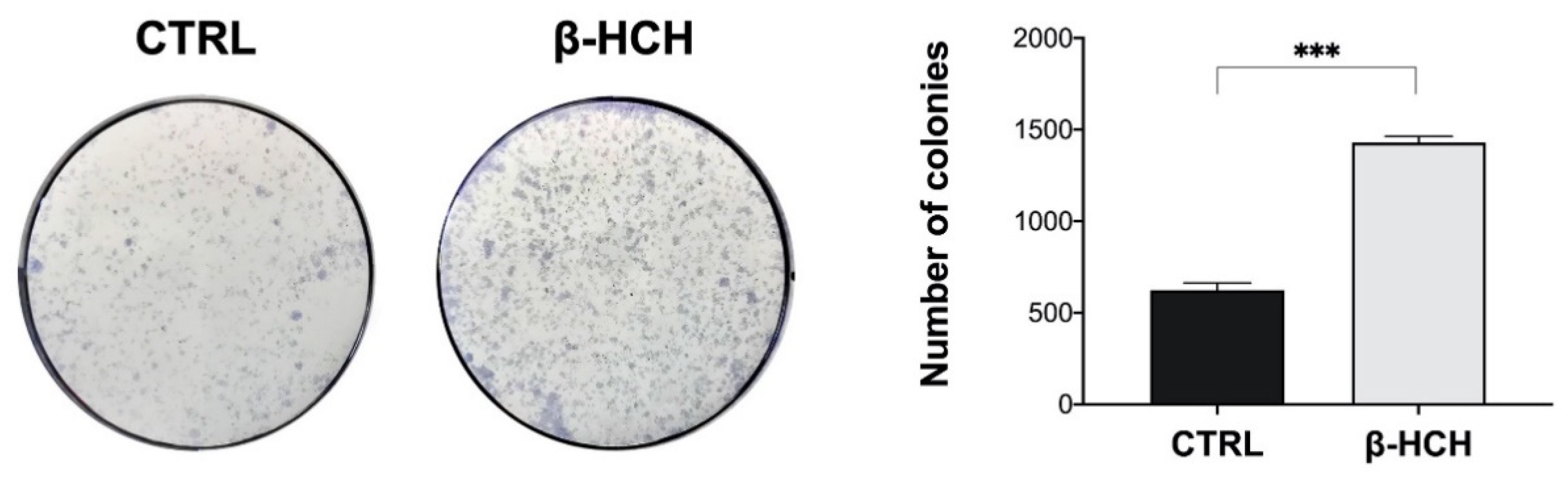
2.9. β-HCH Induces Colony Formation
3. Discussion
- Primary determining factors, which comprise chemical substances, physical agents, and the outcomes of viral-induced carcinogenic transformation. All these three components especially act on signaling pathways and nucleic acids (DNA and RNA), hence the modern notion of cancer as a molecular disease;
- Secondary determining factors, represented by hereditary determinism;
- Favoring factors, which are risk factors whose occasional or systematic intervention has been observed in the incidence of malignant tumors. In this group, some geographic factors, nutrition factors, sex, age, etc., can be mentioned.
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Viability
4.3. Time Course Assay
4.4. Observation of Morphological Changes
4.5. Protein Extraction and Immunoblotting
4.6. Reactive Oxygen Species (ROS) Detection
4.7. Determination of Apoptosis
4.8. Dot Blot
4.9. Immunofluorescence
4.10. Cell Cycle Analysis
4.11. Comet Assay
4.12. Colony Formation Assay
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Casey, S.C.; Vaccari, M.; Al-Mulla, F.; Al-Temaimi, R.; Amedei, A.; Barcellos-Hoff, M.H.; Brown, D.G.; Chapellier, M.; Christopher, J.; Curran, C.S.; et al. The effect of environmental chemicals on the tumor microenvironment. Carcinogenesis 2015, 36, S160–S183. [Google Scholar] [CrossRef]
- Fu, X.; Xu, J.; Zhang, R.; Yu, J. The association between environmental endocrine disruptors and cardiovascular diseases: A systematic review and meta-analysis. Environ. Res. 2020, 187, 109464. [Google Scholar] [CrossRef]
- Thong, T.; Forté, C.A.; Hill, E.M.; Colacino, J.A. Environmental exposures, stem cells, and cancer. Pharmacol. Ther. 2019, 204, 107398. [Google Scholar] [CrossRef]
- Sharma, S.; Wakode, S.; Sharma, A.; Nair, N.; Dhobi, M.; Wani, M.A.; Pottoo, F.H. Effect of environmental toxicants on neuronal functions. Environ. Sci. Pollut. Res. 2020, 27, 44906–44921. [Google Scholar] [CrossRef]
- Blackadar, C.B. Historical review of the causes of cancer. World J. Clin. Oncol. 2016, 7, 54–86. [Google Scholar] [CrossRef] [PubMed]
- Yamagiwa, K.; Ichikawa, K. Experimental Study of the Pathogenesis of Carcinoma. CA Cancer J. Clin. 1977, 27, 174–181. [Google Scholar] [CrossRef]
- Zheng, W.; McLaughlin, J.K.; Chow, W.-H.; Chien, H.T.C.; Blot, W.J. Risk Factors for Cancers of the Nasal Cavity and Paranasal Sinuses among White Men in the United States. Am. J. Epidemiol. 1993, 138, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Ward, E.; Carpenter, A.; Markowitz, S.; Roberts, D.; Halperin, W. Excess Number of Bladder Cancers in Workers Exposed to Ortho-Toluidine and Aniline. J. Natl. Cancer Inst. 1991, 83, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Ledda, C.; Rapisarda, V. Occupational and Environmental Carcinogenesis. Cancers 2020, 12, 2547. [Google Scholar] [CrossRef] [PubMed]
- Kahn, L.G.; Philippat, C.; Nakayama, S.F.; Slama, R.; Trasande, L. Endocrine-disrupting chemicals: Implications for human health. Lancet Diabetes Endocrinol. 2020, 8, 703–718. [Google Scholar] [CrossRef]
- Saravi, S.S.S.; Dehpour, A.R. Potential role of organochlorine pesticides in the pathogenesis of neurodevelopmental, neurodegenerative, and neurobehavioral disorders: A review. Life Sci. 2016, 145, 255–264. [Google Scholar] [CrossRef]
- Jayaraj, R.; Megha, P.; Sreedev, P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef]
- Oliveira, P.A.; Colaço, A.; Chaves, R.; Guedes-Pinto, H.; De-La-Cruz, P.; Louis, F.; Lopes, C. Chemical carcinogenesis. An. Acad. Bras. Cienc. 2007, 79, 593–616. [Google Scholar] [CrossRef] [PubMed]
- Burgio, E.; Piscitelli, P.; Colao, A. Environmental Carcinogenesis and Transgenerational Transmission of Carcinogenic Risk: From Genetics to Epigenetics. Int. J. Environ. Res. Public Health 2018, 15, 1791. [Google Scholar] [CrossRef] [PubMed]
- Olivero-Verbel, J.; Guerrero-Castilla, A.; Ramos, N.R. Biochemical Effects Induced by the Hexachlorocyclohexanes. Rev. Environ. Contam. Toxicol. 2011, 212, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Vijgen, J.; Abhilash, P.C.; Li, Y.F.; Lal, R.; Forter, M.; Torres, J.P.M.; Singh, N.; Yunus, M.; Tian, C.; Schäffer, A.; et al. Hexachlorocyclohexane (HCH) as new Stockholm Convention POPs—A global perspective on the management of Lindane and its waste isomers. Environ. Sci. Pollut. Res. 2011, 18, 152–162. [Google Scholar] [CrossRef]
- Vijgen, J.; de Borst, B.; Weber, R.; Stobiecki, T.; Forter, M. HCH and lindane contaminated sites: European and global need for a permanent solution for a long-time neglected issue. Environ. Pollut. 2019, 248, 696–705. [Google Scholar] [CrossRef]
- Li, Y.-F.; Macdonald, R.; Jantunen, L.; Harner, T.; Bidleman, T.; Strachan, W. The transport of β-hexachlorocyclohexane to the western Arctic Ocean: A contrast to α-HCH. Sci. Total Environ. 2002, 291, 229–246. [Google Scholar] [CrossRef]
- Rubini, E.; Altieri, F.; Chichiarelli, S.; Giamogante, F.; Carissimi, S.; Paglia, G.; Macone, A.; Eufemi, M. STAT3, a Hub Protein of Cellular Signaling Pathways, Is Triggered by β-Hexaclorocyclohexane. Int. J. Mol. Sci. 2018, 19, 2108. [Google Scholar] [CrossRef] [PubMed]
- Rubini, E.; Paglia, G.; Cannella, D.; Macone, A.; Di Sotto, A.; Gullì, M.; Altieri, F.; Eufemi, M. β-Hexachlorocyclohexane: A Small Molecule with a Big Impact on Human Cellular Biochemistry. Biomedicines 2020, 8, 505. [Google Scholar] [CrossRef]
- Narduzzi, S.; Porta, D.; Fantini, F.; Blasetti, F.; Davoli, M.; Forastiere, F. Sorveglianza Sanitaria ed Epidemiologica della Popolazione Residente in Prossimità del Fiume Sacco, Rapporto Tecnico Attività 2013–2015; Dipartimento di Epidemiologia del Servizio Sanitario Regionale-Regione Lazio: Roma, Italy, 2016. [Google Scholar]
- Malarkey, D.E.; Hoenerhoff, M.; Maronpot, R.R. Carcinogenesis: Mechanisms and Manifestations. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 107–146. ISBN 9780124157590. [Google Scholar]
- Kakehashi, A.; Wei, M.; Fukushima, S.; Wanibuchi, H. Oxidative Stress in the Carcinogenicity of Chemical Carcinogens. Cancers 2013, 5, 1332–1354. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Badana, A.K.; Murali Mohan, G.; Shailender, G.; Malla, R.R. Reactive Oxygen Species: A Key Constituent in Cancer Survival. Biomark. Insights 2018, 13, 1–9. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, X.; Tang, M.; Li, L.; Lei, Y.; Cheng, P.; Guo, W.; Zheng, Y.; Wang, W.; Luo, N.; et al. The role of ROS and subsequent DNA-damage response in PUMA-induced apoptosis of ovarian cancer cells. Oncotarget 2017, 8, 23492–23506. [Google Scholar] [CrossRef] [PubMed]
- Nagelkerke, A.; Span, P.N. Staining Against Phospho-H2AX (γ-H2AX) as a Marker for DNA Damage and Genomic Instability in Cancer Tissues and Cells. In Chemistry and Biology of Pteridines and Folates; Springer: Berlin/Heidelberg, Germany, 2016; Volume 899, pp. 1–10. [Google Scholar]
- Speit, G.; Rothfuss, A. The Comet Assay: A Sensitive Genotoxicity Test for the Detection of DNA Damage and Repair. Methods Mol. Biol. 2012, 920, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, E.; Castle, J.; Quirk, A.; Taylor, C.D.; Xu, W.; Prasad, A. Cellular morphological features are predictive markers of cancer cell state. Comput. Biol. Med. 2020, 126, 104044. [Google Scholar] [CrossRef]
- Acheva, A.; Haghdoost, S.; Sollazzo, A.; Launonen, V.; Kämäräinen, M. Presence of Stromal Cells Enhances Epithelial-to-Mesenchymal Transition (EMT) Induction in Lung Bronchial Epithelium after Protracted Exposure to Oxidative Stress of Gamma Radiation. Oxidative Med. Cell. Longev. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Morrow, C.S.; Moore, D.L. Vimentin’s side gig: Regulating cellular proteostasis in mammalian systems. Cytoskeleton 2020, 77, 515–523. [Google Scholar] [CrossRef]
- Mrouj, K.; Andrés-Sánchez, N.; Dubra, G.; Singh, P.; Sobecki, M.; Chahar, D.; Al Ghoul, E.; Aznar, A.B.; Prieto, S.; Pirot, N.; et al. Ki-67 regulates global gene expression and promotes sequential stages of carcinogenesis. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Rafehi, H.; Orlowski, C.; Georgiadis, G.T.; Ververis, K.; El-Osta, A.; Karagiannis, T.C. Clonogenic Assay: Adherent Cells. J. Vis. Exp. 2011, 49, e2573. [Google Scholar] [CrossRef]
- Faltas, B. Cancer is an ancient disease: The case for better palaeoepidemiological and molecular studies. Nat. Rev. Cancer 2010, 11, 76. [Google Scholar] [CrossRef]
- Klaunig, J. Chapter 8 – Carcinogenesis. In An Introduction to Interdisciplinary Toxicology, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 97–110. ISBN 9780128136027. [Google Scholar]
- Madia, F.; Worth, A.; Whelan, M.; Corvi, R. Carcinogenicity assessment: Addressing the challenges of cancer and chemicals in the environment. Environ. Int. 2019, 128, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, H.N.; Manoj, M.J.; Ghosh, D.; Chetan, G.K. Environmental pollutants leading to carcinogenesis: Process of natural selection of human cells due to chronic inflammation and sustained stress environment. Int. J. Environ. Sci. Technol. 2015, 12, 2415–2426. [Google Scholar] [CrossRef][Green Version]
- Krewski, D.; Bird, M.; Al-Zoughool, M.; Birkett, N.; Billard, M.; Milton, B.; Rice, J.M.; Grosse, Y.; Cogliano, V.J.; Hill, M.A.; et al. Key characteristics of 86 agents known to cause cancer in humans. J. Toxicol. Environ. Health Part B 2019, 22, 244–263. [Google Scholar] [CrossRef]
- Caudle, W.M. Occupational exposures and parkinsonism. Neurocutan. Syndr. 2015, 131, 225–239. [Google Scholar]
- Kripke, M.; Brody, J.G.; Hawk, E.; Hernandez, A.B.; Hoppin, P.J.; Jacobs, M.M.; Rudel, R.A.; Rebbeck, T.R. Rethinking Environmental Carcinogenesis. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1870–1875. [Google Scholar] [CrossRef] [PubMed]
- Cocchiola, R.; Rubini, E.; Altieri, F.; Chichiarelli, S.; Paglia, G.; Romaniello, D.; Carissimi, S.; Giorgi, A.; Giamogante, F.; Macone, A.; et al. STAT3 Post-Translational Modifications Drive Cellular Signaling Pathways in Prostate Cancer Cells. Int. J. Mol. Sci. 2019, 20, 1815. [Google Scholar] [CrossRef] [PubMed]
- McCloy, R.; Rogers, S.; Caldon, C.E.; Lorca, T.; Castro, A.; Burgess, A. Partial inhibition of Cdk1 in G2phase overrides the SAC and decouples mitotic events. Cell Cycle 2014, 13, 1400–1412. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Yang, C. Evaluating In Vitro DNA Damage Using Comet Assay. J. Vis. Exp. 2017, 128, 56450. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Chattopadhyay, N.; Liu, W.J.; Chan, C.; Pignol, J.-P.; Reilly, R.M. Optimized digital counting colonies of clonogenic assays using ImageJ software and customized macros: Comparison with manual counting. Int. J. Radiat. Biol. 2011, 87, 1135–1146. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubini, E.; Minacori, M.; Paglia, G.; Altieri, F.; Chichiarelli, S.; Romaniello, D.; Eufemi, M. β-Hexachlorocyclohexane Drives Carcinogenesis in the Human Normal Bronchial Epithelium Cell Line BEAS-2B. Int. J. Mol. Sci. 2021, 22, 5834. https://doi.org/10.3390/ijms22115834
Rubini E, Minacori M, Paglia G, Altieri F, Chichiarelli S, Romaniello D, Eufemi M. β-Hexachlorocyclohexane Drives Carcinogenesis in the Human Normal Bronchial Epithelium Cell Line BEAS-2B. International Journal of Molecular Sciences. 2021; 22(11):5834. https://doi.org/10.3390/ijms22115834
Chicago/Turabian StyleRubini, Elisabetta, Marco Minacori, Giuliano Paglia, Fabio Altieri, Silvia Chichiarelli, Donatella Romaniello, and Margherita Eufemi. 2021. "β-Hexachlorocyclohexane Drives Carcinogenesis in the Human Normal Bronchial Epithelium Cell Line BEAS-2B" International Journal of Molecular Sciences 22, no. 11: 5834. https://doi.org/10.3390/ijms22115834
APA StyleRubini, E., Minacori, M., Paglia, G., Altieri, F., Chichiarelli, S., Romaniello, D., & Eufemi, M. (2021). β-Hexachlorocyclohexane Drives Carcinogenesis in the Human Normal Bronchial Epithelium Cell Line BEAS-2B. International Journal of Molecular Sciences, 22(11), 5834. https://doi.org/10.3390/ijms22115834









