Pigmentation Levels Affect Melanoma Responses to Coriolus versicolor Extract and Play a Crucial Role in Melanoma-Mononuclear Cell Crosstalk
Abstract
1. Introduction
2. Results
2.1. Pigmented Melanoma Cells Are Resistant to Cytotoxic Activity of CV Extract
2.2. Inhibition of Melanogenesis Enhances Melanoma Sensitivity to the Killing Action of CV Extract
2.3. CV Extract Induces a Necroptotic Death Pathway in the Depigmented Cells
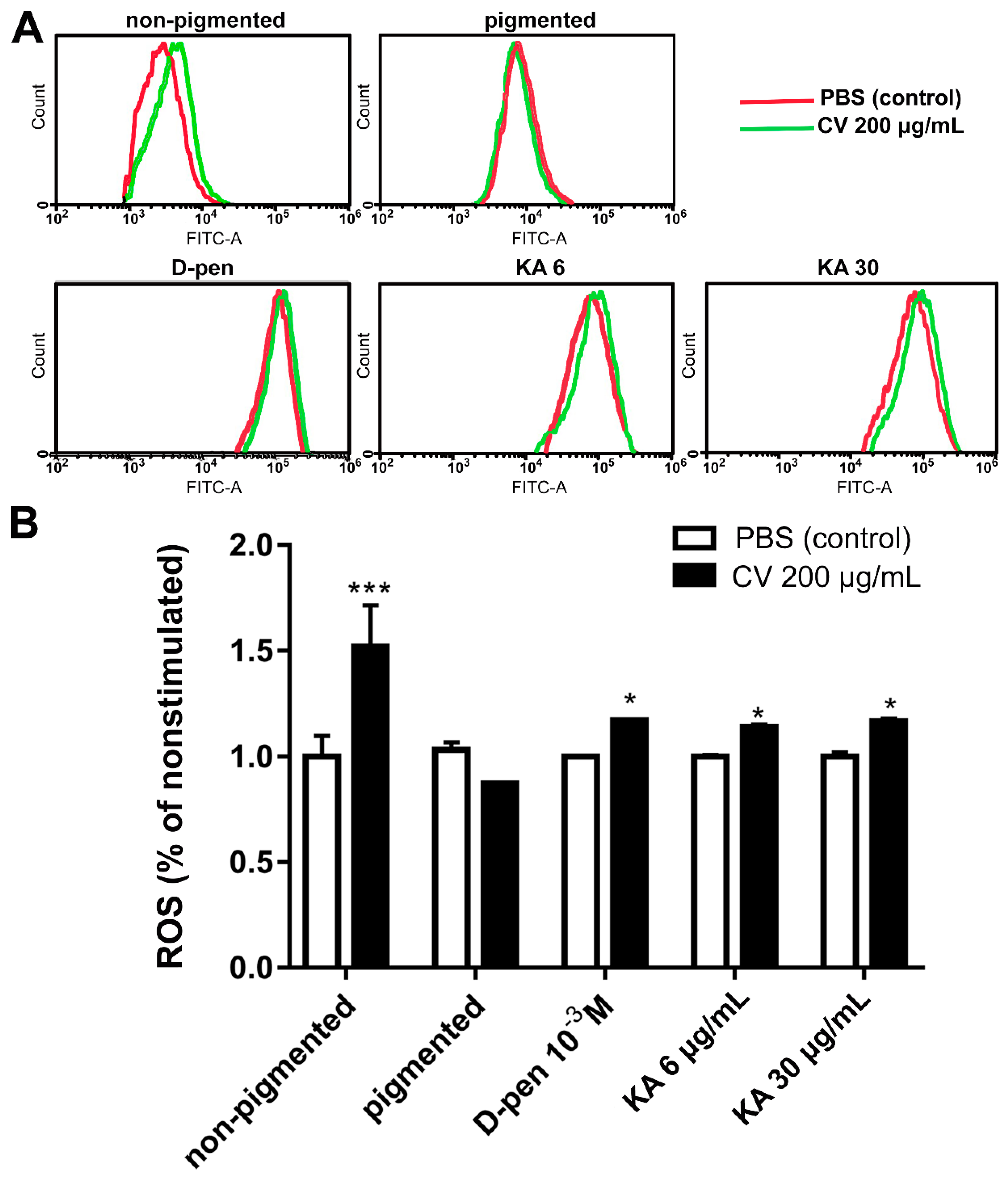
2.4. Depigmentation of Melanoma Cells Partially Restores CV-Induced Intracellular ROS Generation
2.5. Pharmacological Inhibition of Melanogenesis in Melanoma Cells Increases the Reactivity of Co-Cultured Human PBMCs
3. Discussion
4. Materials and Methods
4.1. Chemicals/Reagents
4.2. Cell Culture
4.3. Melanogenesis Inhibition and Depigmentation
4.4. Measurement of Melanin Content
4.5. Cell Viability Assay
4.6. Ki67 and Integrin Expression in Melanoma Cells
4.7. Measurement of Intracellular ROS
4.8. Co-Cultures of PBMCs and Melanoma Cells for the qPCR Assay
4.9. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Brożyna, A.; Zbytek, B.; Granese, J.; Carlson, J.A.; Ross, J.; Slominski, A. Mechanism of UV-related carcinogenesis and its contribution to nevi/melanoma. Expert Rev. Dermatol. 2007, 2, 451–469. [Google Scholar] [CrossRef]
- Domingues, B.; Lopes, J.M.; Soares, P.; Populo, H. Melanoma treatment in review. ImmunoTargets Ther. 2018, 7, 35–49. [Google Scholar] [CrossRef]
- Hayward, N.; Wilmott, J.; Waddell, N.; Johansson, P.A.; Field, M.A.; Nones, K.; Patch, A.-M.; Kakavand, H.; Alexandrov, L.B.; Burke, H.; et al. Whole-genome landscapes of major melanoma subtypes. Nat. Cell Biol. 2017, 545, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Mattia, G.; Puglisi, R.; Ascione, B.; Malorni, W.; Carè, A.; Matarrese, P. Cell death-based treatments of melanoma:conventional treatments and new therapeutic strategies. Cell Death Dis. 2018, 9, 112. [Google Scholar] [CrossRef]
- Yang, K.; Oak, A.S.; Slominski, R.M.; Brożyna, A.A.; Slominski, A.T. Current Molecular Markers of Melanoma and Treatment Targets. Int. J. Mol. Sci. 2020, 21, 3535. [Google Scholar] [CrossRef] [PubMed]
- Leiter, U.; Eigentler, T.; Garbe, C. Epidemiology of Skin Cancer. Adv. Exp. Med. Biol. 2014, 810, 120–140. [Google Scholar] [CrossRef] [PubMed]
- Brożyna, A.A.; Jóźwicki, W.; Roszkowski, K.; Filipiak, J.; Slominski, A.T. Melanin content in melanoma metastases affects the outcome of radiotherapy. Oncotarget 2016, 7, 17844–17853. [Google Scholar] [CrossRef]
- Rigel, D.S.; Russak, J.; Friedman, R. The Evolution of Melanoma Diagnosis: 25 Years Beyond the ABCDs. CA Cancer J. Clin. 2010, 60, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, N.; Della Corte, M.; Pelaia, C.; Piazzetta, G.; Lobello, N.; Del Duca, E.; Bennardo, L.; Nisticò, S. Primary Mucosal Melanoma Presenting with a Unilateral Nasal Obstruction of the Left Inferior Turbinate. Medicina 2021, 57, 359. [Google Scholar] [CrossRef] [PubMed]
- Vandyck, H.H.; Hillen, L.M.; Bosisio, F.M.; Oord, J.V.D.; Hausen, A.Z.; Winnepenninckx, V. Rethinking the biology of metastatic melanoma: A holistic approach. Cancer Metastasis Rev. 2021, 1–22. [Google Scholar] [CrossRef]
- Brożyna, A.A.; Van Middlesworth, L.; Slominski, A.T. Inhibition of melanogenesis as a radiation sensitizer for melanoma therapy. Int. J. Cancer 2008, 123, 1448–1456. [Google Scholar] [CrossRef]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin Pigmentation in Mammalian Skin and Its Hormonal Regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zbytek, B.; Slominski, R. Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Int. J. Cancer 2009, 124, 1470–1477. [Google Scholar] [CrossRef]
- Śniegocka, M.; Podgórska, E.; Płonka, P.M.; Elas, M.; Romanowska-Dixon, B.; Szczygieł, M.; Żmijewski, M.A.; Cichorek, M.; Markiewicz, A.; Brożyna, A.A.; et al. Transplantable Melanomas in Hamsters and Gerbils as Models for Human Melanoma. Sensitization in Melanoma Radiotherapy—From Animal Models to Clinical Trials. Int. J. Mol. Sci. 2018, 19, 1048. [Google Scholar] [CrossRef]
- Korner, A.; Pawelek, J. Mammalian tyrosinase catalyzes three reactions in the biosynthesis of melanin. Science 1982, 217, 1163–1165. [Google Scholar] [CrossRef]
- Pawelek, J.M.; Körner, A.M. The biosynthesis of mammalian melanin. Am. Sci. 1982, 70, 136–145. [Google Scholar]
- Plonka, P.M.; Passeron, T.; Brenner, M.; Tobin, D.J.; Shibahara, S.; Thomas, A.; Slominski, A.; Kadekaro, A.L.; Hershkovitz, D.; Peters, E.; et al. What are melanocytes really doing all day long…? Exp. Dermatol. 2009, 18, 799–819. [Google Scholar] [CrossRef] [PubMed]
- Różanowska, M.; Sarna, T.; Land, E.J.; Truscott, T. Free radical scavenging properties of melanin. Free. Radic. Biol. Med. 1999, 26, 518–525. [Google Scholar] [CrossRef]
- Serrone, L.; Hersey, P. The chemoresistance of human malignant melanoma. Melanoma Res. 1999, 9, 51–58. [Google Scholar] [CrossRef]
- Soengas, M.S.; Lowe, S.W. Apoptosis and melanoma chemoresistance. Oncogene 2003, 22, 3138–3151. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.; Hearing, V.J. The Protective Role of Melanin against UV Damage in Human Skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Kollias, N.; Sayre, R.M.; Zeise, L.; Chedekel, M.R. New trends in photobiology. J. Photochem. Photobiol. B Biol. 1991, 9, 135–160. [Google Scholar] [CrossRef]
- Meyskens, F.L.; Farmer, P.J.; Yang, S.; Anton-Culver, H. New Perspectives on Melanoma Pathogenesis and Chemoprevention. Methods Mol. Biol. 2007, 174, 191–195. [Google Scholar] [CrossRef]
- Slominski, A.; Paus, R.; Mihm, M.C. Inhibition of melanogenesis as an adjuvant strategy in the treatment of melanotic melanomas: Selective review and hypothesis. Anticancer Res. 1998, 18, 3709–3715. [Google Scholar] [PubMed]
- Janjetovic, Z.; Brozyna, A.A.; Tuckey, R.C.; Kim, T.-K.; Nguyen, M.N.; Jozwicki, W.; Pfeffer, S.R.; Pfeffer, L.; Slominski, A.T. High basal NF-κB activity in nonpigmented melanoma cells is associated with an enhanced sensitivity to vitamin D3 derivatives. Br. J. Cancer 2011, 105, 1874–1884. [Google Scholar] [CrossRef]
- Slominski, A.; Goodman-Snitkoff, G.G. Dopa inhibits induced proliferative activity of murine and human lymphocytes. Anticancer Res. 1992, 12, 753–756. [Google Scholar] [PubMed]
- Li, W.; Slominski, R.; Slominski, A.T. High-resolution magic angle spinning nuclear magnetic resonance analysis of metabolic changes in melanoma cells after induction of melanogenesis. Anal. Biochem. 2009, 386, 282–284. [Google Scholar] [CrossRef]
- Scisłowski, P.W.; Słomiński, A.; Bomirski, A. Biochemical characterization of three hamster melanoma variants—II. Glycolysis and oxygen consumption. Int. J. Biochem. 1984, 16, 327–331. [Google Scholar] [CrossRef]
- Scisłowski, P.W.; Słomiński, A.; Bomirski, A.; Zydowo, M. Metabolic characterization of three hamster melanoma variants. Neoplasma 1985, 32, 593–598. [Google Scholar]
- Slominski, A.; Kim, T.-K.; Brożyna, A.; Janjetovic, Z.; Brooks, D.; Schwab, L.; Skobowiat, C.; Jóźwicki, W.; Seagroves, T. The role of melanogenesis in regulation of melanoma behavior: Melanogenesis leads to stimulation of HIF-1α expression and HIF-dependent attendant pathways. Arch. Biochem. Biophys. 2014, 563, 79–93. [Google Scholar] [CrossRef]
- Ma, L.-W.; Nielsen, K.P.; Iani, V.; Moan, J. A New Method for Photodynamic Therapy of Melanotic Melanoma—Effects of Depigmentation with Violet Light Photodynamic Therapy. J. Environ. Pathol. Toxicol. Oncol. 2007, 26, 165–172. [Google Scholar] [CrossRef]
- Nelson, J.S.; McCullough, J.L.; Berns, M.W. Photodynamic Therapy of Human Malignant Melanoma Xenografts in Athymic Nude Mice1. J. Natl. Cancer Inst. 1988, 80, 56–60. [Google Scholar] [CrossRef]
- Cichorek, M. Camptothecin-induced death of amelanotic and melanotic melanoma cells in different phases of cell cycle. Neoplasma 2011, 58, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Cichorek, M.; Kozłowska, K.; Bryl, E. The activity of caspases in spontaneous and camptothecin-induced death of melanotic and amelanotic melanoma cell. Cancer Biol. Ther. 2007, 6, 346–353. [Google Scholar] [CrossRef]
- Sharma, K.V.; Davids, L.M. Depigmentation in melanomas increases the efficacy of hypericin-mediated photodynamic-induced cell death. Photodiagnosis Photodyn. Ther. 2012, 9, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Pawlikowska, M.; Piotrowski, J.; Jędrzejewski, T.; Kozak, W.; Slominski, A.T.; Brożyna, A.A. Coriolus versicolor -derived protein-bound polysaccharides trigger the caspase-independent cell death pathway in amelanotic but not melanotic melanoma cells. Phytother. Res. 2020, 34, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.H.; Rashedi, I.; Keating, A. Immunomodulatory Properties of Coriolus versicolor: The Role of Polysaccharopeptide. Front. Immunol. 2017, 8, 1087. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Trametes versicolor (Synn. Coriolus versicolor) Polysaccharides in Cancer Therapy: Targets and Efficacy. Biomedicines 2020, 8, 135. [Google Scholar] [CrossRef]
- Georgescu, S.R.; Tampa, M.; Mitran, C.I.; Mitran, M.I.; Caruntu, C.; Caruntu, A.; Lupu, M.; Matei, C.; Constantin, C.; Neagu, M. Tumour Microenvironment in Skin Carcinogenesis. Adv. Exp. Med. Biol. 2020, 1226, 123–142. [Google Scholar] [CrossRef]
- Kycler, W.; Grodecka-Gazdecka, S.; Bręborowicz, J.; Filas, V.; Teresiak, M. Prognostic factors in melanoma. Rep. Pract. Oncol. Radiother. 2006, 11, 39–48. [Google Scholar] [CrossRef][Green Version]
- Pawlikowska, M.; Jędrzejewski, T.; Brożyna, A.A.; Wrotek, S. Protein-Bound Polysaccharides from Coriolus Versicolor Induce RIPK1/RIPK3/MLKL-Mediated Necroptosis in ER-Positive Breast Cancer and Amelanotic Melanoma Cells. Cell. Physiol. Biochem. 2020, 54, 591–604. [Google Scholar] [CrossRef]
- Chen, D.; Yu, J.; Zhang, L. Necroptosis: An alternative cell death program defending against cancer. Biochim. Biophys. Acta 2016, 1865, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Degterev, A.; Hitomi, J.; Germscheid, M.; Ch’En, I.L.; Korkina, O.; Teng, X.; Abbott, D.; Cuny, G.D.; Yuan, C.; Wagner, G.; et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 2008, 4, 313–321. [Google Scholar] [CrossRef]
- Sun, L.; Wang, H.; Wang, Z.; He, S.; Chen, S.; Liao, D.; Wang, L.; Yan, J.; Liu, W.; Lei, X.; et al. Mixed Lineage Kinase Domain-like Protein Mediates Necrosis Signaling Downstream of RIP3 Kinase. Cell 2012, 148, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Zong, A.; Cao, H.; Wang, F. Anticancer polysaccharides from natural resources: A review of recent research. Carbohydr. Polym. 2012, 90, 1395–1410. [Google Scholar] [CrossRef]
- Jędrzejewski, T.; Pawlikowska, M.; Piotrowski, J.; Kozak, W. Protein-bound polysaccharides from Coriolus versicolor attenuate LPS-induced synthesis of pro-inflammatory cytokines and stimulate PBMCs proliferation. Immunol. Lett. 2016, 178, 140–147. [Google Scholar] [CrossRef]
- Jędrzejewski, T.; Pawlikowska, M.; Sobocińska, J.; Wrotek, S. Protein-Bound Polysaccharides from Coriolus Versicolor Fungus Disrupt the Crosstalk Between Breast Cancer Cells and Macrophages through Inhibition of Angiogenic Cytokines Production and Shifting Tumour-Associated Macrophages from the M2 to M1 Subtype. Cell. Physiol. Biochem. 2020, 54, 615–628. [Google Scholar] [CrossRef]
- Pawlikowska, M.; Jędrzejewski, T.; Piotrowski, J.; Kozak, W. Fever-range hyperthermia inhibits cells immune response to protein-bound polysaccharides derived from Coriolus versicolor extract. Mol. Immunol. 2016, 80, 50–57. [Google Scholar] [CrossRef]
- Jędrzejewski, T.; Sobocińska, J.; Pawlikowska, M.; Dzialuk, A.; Wrotek, S. Extract from the Coriolus versicolor Fungus as an Anti-Inflammatory Agent with Cytotoxic Properties against Endothelial Cells and Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 9063. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, B.; Feng, Z.; Yu, S.; Bao, Y. A study on immunomodulatory mechanism of Polysaccharopeptide mediated by TLR4 signaling pathway. BMC Immunol. 2015, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.-C.; Wu, P.; Park, S.; Wu, J.M. Induction of cell cycle changes and modulation of apoptogenic/anti-apoptotic and extracellular signaling regulatory protein expression by water extracts of I’m-Yunity™ (PSP). BMC Complement. Altern. Med. 2006, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Medina, E.; Berruguilla, E.; Romero, I.; Algarra, I.; Collado, A.; Garrido, F.; Garcia-Lora, A. The immunomodulator PSK induces in vitro cytotoxic activity in tumour cell lines via arrest of cell cycle and induction of apoptosis. BMC Cancer 2008, 8, 78. [Google Scholar] [CrossRef]
- Ricciardi, M.R.; Licchetta, R.; Mirabilii, S.; Scarpari, M.; Parroni, A.; Fabbri, A.A.; Cescutti, P.; Reverberi, M.; Fanelli, C.; Tafuri, A. Preclinical Antileukemia Activity of Tramesan: A Newly Identified Bioactive Fungal Metabolite. Oxidative Med. Cell. Longev. 2017, 2017, 5061639. [Google Scholar] [CrossRef]
- Hirahara, N.; Edamatsu, T.; Fujieda, A.; Fujioka, M.; Wada, T.; Tajima, Y. Protein-bound polysaccharide-K induces apoptosis via mitochondria and p38 mitogen-activated protein kinase-dependent pathways in HL-60 promyelomonocytic leukemia cells. Oncol. Rep. 2013, 30, 99–104. [Google Scholar] [CrossRef][Green Version]
- Yang, X.; Sit, W.-H.; Chan, D.K.-O.; Wan, J.M.-F. The cell death process of the anticancer agent polysaccharide-peptide (PSP) in human promyelocytic leukemic HL-60 cells. Oncol. Rep. 2005, 13, 1201–1210. [Google Scholar] [CrossRef]
- Pinner, S.; Jordan, P.; Sharrock, K.; Bazley, L.; Collinson, L.; Marais, R.; Bonvin, E.; Goding, C.; Sahai, E. Intravital Imaging Reveals Transient Changes in Pigment Production and Brn2 Expression during Metastatic Melanoma Dissemination. Cancer Res. 2009, 69, 7969–7977. [Google Scholar] [CrossRef]
- Aoudjit, F.; Vuori, K. Integrin signaling inhibits paclitaxel-induced apoptosis in breast cancer cells. Oncogene 2001, 20, 4995–5004. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Cabello, C.M.; Lamore, S.D.; Lesson, J.L.; Wondrak, G.T. D-penicillamine targets metastatic melanoma cells with induction of the unfolded protein response (UPR) and Noxa (PMAIP1)-dependent mitochondrial apoptosis. Apoptosis 2012, 17, 1079–1094. [Google Scholar] [CrossRef] [PubMed]
- Sciegienka, S.J.; Solst, S.R.; Falls, K.C.; Schoenfeld, J.D.; Klinger, A.R.; Ross, N.L.; Rodman, S.; Spitz, D.R.; Fath, M.A. d-penicillamine combined with inhibitors of hydroperoxide metabolism enhances lung and breast cancer cell responses to radiation and carboplatin via H2O2-mediated oxidative stress. Free. Radic. Biol. Med. 2017, 108, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.K.; Koketsu, M.; Lee, K.; Choi, S.Y.; Park, J.-H.; Ishihara, H.; Kim, S.Y. Inhibition of Tyrosinase Activity by N,N-Unsubstituted Selenourea Derivatives. Biol. Pharm. Bull. 2005, 28, 838–840. [Google Scholar] [CrossRef] [PubMed]
- Momtaz, S.; Mapunya, B.; Houghton, P.; Edgerly, C.; Hussein, A.; Naidoo, S.; Lall, N. Tyrosinase inhibition by extracts and constituents of Sideroxylon inerme L. stem bark, used in South Africa for skin lightening. J. Ethnopharmacol. 2008, 119, 507–512. [Google Scholar] [CrossRef]
- Eisenhofer, G.; Tian, H.; Holmes, C.; Matsunaga, J.; Roffler-Tarlov, S.; Hearing, V.J. Tyrosinase: A developmentally specific major determinant of peripheral dopamine. FASEB J. 2003, 17, 1248–1255. [Google Scholar] [CrossRef]
- Lajis, A.F.B.; Hamid, M.; Ariff, A.B. Depigmenting Effect of Kojic Acid Esters in Hyperpigmented B16F1 Melanoma Cells. J. Biomed. Biotechnol. 2012, 2012, 952452. [Google Scholar] [CrossRef] [PubMed]
- Belizário, J.; Cordeiro, L.; Enns, S. Necroptotic Cell Death Signaling and Execution Pathway: Lessons from Knockout Mice. Mediat. Inflamm. 2015, 2015, 128076. [Google Scholar] [CrossRef]
- Qiu, X.; Zhang, Y.; Han, J. RIP3 is an upregulator of aerobic metabolism and the enhanced respiration by necrosomal RIP3 feeds back on necrosome to promote necroptosis. Cell Death Differ. 2018, 25, 821. [Google Scholar] [CrossRef] [PubMed]
- Ercolano, G.; Garcia-Garijo, A.; Salomé, B.; Gomez-Cadena, A.; Vanoni, G.; Mastelic-Gavillet, B.; Ianaro, A.; Speiser, D.E.; Romero, P.; Trabanelli, S.; et al. Immunosuppressive Mediators Impair Proinflammatory Innate Lymphoid Cell Function in Human Malignant Melanoma. Cancer Immunol. Res. 2020, 8, 556–564. [Google Scholar] [CrossRef]
- Parmiani, G.; Castelli, C.; Rivoltini, L.; Casati, C.; Tully, G.A.; Novellino, L.; Patuzzo, A.; Tosi, D.; Anichini, A.; Santinami, M. Immunotherapy of melanoma. Semin. Cancer Biol. 2003, 13, 391–400. [Google Scholar] [CrossRef]
- Paulos, C.M.; Kaiser, A.; Wrzesinski, C.; Hinrichs, C.S.; Cassard, L.; Boni, A.; Muranski, P.; Sanchez-Perez, L.; Palmer, D.C.; Yu, Z.; et al. Toll-like Receptors in Tumor Immunotherapy. Clin. Cancer Res. 2007, 13, 5280–5289. [Google Scholar] [CrossRef] [PubMed]
- Shah, G.D.; Chapman, P.B. Adjuvant Therapy of Melanoma. Cancer J. 2007, 13, 217–222. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Kirkwood, J.M. Management of Metastatic Melanoma. Semin. Oncol. 2007, 34, 532–545. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J.; Mazurkiewicz, J.E.; Matsuoka, L.; Dietrich, J.; Lawrence, K.; Gorbani, A.; Paus, R. Detection of proopiomelanocortin-derived antigens in normal and pathologic human skin. J. Lab. Clin. Med. 1993, 122, 658–666. [Google Scholar]
- Garbe, C.; Krasagakis, K. Effects of interferons and cytokines on melanoma cells. J. Investig. Dermatol. 1993, 100, 239–244. [Google Scholar] [CrossRef][Green Version]
- Atkins, M.B.; Lotze, M.T.; Dutcher, J.P.; Fisher, R.I.; Weiss, G.; Margolin, K.; Abrams, J.; Sznol, M.; Parkinson, D.; Hawkins, M.; et al. High-Dose Recombinant Interleukin 2 Therapy for Patients With Metastatic Melanoma: Analysis of 270 Patients Treated Between 1985 and 1993. J. Clin. Oncol. 1999, 17, 2105. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Tykodi, S.S.; Thompson, J.A. Treatment of metastatic melanoma: An overview. Oncology 2009, 23, 488–496. [Google Scholar] [PubMed]
- Dillman, R.O.; DePriest, C.; McClure, S.E. High-dose IL2 in metastatic melanoma: Better survival in patients immunized with antigens from autologous tumor cell lines. Cancer Biother. Radiopharm. 2014, 29, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Greaney, S.K.; Algazi, A.P.; Tsai, K.K.; Takamura, K.T.; Chen, L.; Twitty, C.G.; Zhang, L.; Paciorek, A.; Pierce, R.H.; Le, M.H.; et al. Intratumoral Plasmid IL12 Electroporation Therapy in Patients with Advanced Melanoma Induces Systemic and Intratumoral T-cell Responses. Cancer Immunol. Res. 2020, 8, 246–254. [Google Scholar] [CrossRef]
- Heinzerling, L.; Burg, G.; Dummer, R.; Maier, T.; Oberholzer, P.A.; Schultz, J.; Elzaouk, L.; Pavlovic, J.; Moelling, K. Intratumoral Injection of DNA Encoding Human Interleukin 12 into Patients with Metastatic Melanoma: Clinical Efficacy. Hum. Gene Ther. 2005, 16, 35–48. [Google Scholar] [CrossRef]
- Heinzerling, L.; Dummer, R.; Pavlovic, J.; Schultz, J.; Burg, G.; Moelling, K. Tumor regression of human and murine melanoma after intratumoral injection of IL-12-encoding plasmid DNA in mice. Exp. Dermatol. 2002, 11, 232–240. [Google Scholar] [CrossRef]
- Flørenes, V.A.; Lu, C.; Bhattacharya, N.; Rak, J.; Sheehan, C.; Slingerland, J.M.; Kerbel, R.S. Interleukin-6 dependent induction of the cyclin dependent kinase inhibitor p21WAF1/CIP1 is lost during progression of human malignant melanoma. Oncogene 1999, 18, 1023–1032. [Google Scholar] [CrossRef]
- Minichsdorfer, C.; Wasinger, C.; Sieczkowski, E.; Atil, B.; Hohenegger, M. Tocilizumab unmasks a stage-dependent interleukin-6 component in statin-induced apoptosis of metastatic melanoma cells. Melanoma Res. 2015, 25, 284–294. [Google Scholar] [CrossRef]
- Mohagheghpour, N.; Waleh, N.; Garger, S.J.; Dousman, L.; Grill, L.K.; Tusé, D. Synthetic Melanin Suppresses Production of Proinflammatory Cytokines. Cell. Immunol. 2000, 199, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Ermak, G.; Wortsman, J. Modification of melanogenesis in cultured human melanoma cells. Vitr. Cell. Dev. Biol. Anim. 1999, 35, 564–565. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlikowska, M.; Jędrzejewski, T.; Slominski, A.T.; Brożyna, A.A.; Wrotek, S. Pigmentation Levels Affect Melanoma Responses to Coriolus versicolor Extract and Play a Crucial Role in Melanoma-Mononuclear Cell Crosstalk. Int. J. Mol. Sci. 2021, 22, 5735. https://doi.org/10.3390/ijms22115735
Pawlikowska M, Jędrzejewski T, Slominski AT, Brożyna AA, Wrotek S. Pigmentation Levels Affect Melanoma Responses to Coriolus versicolor Extract and Play a Crucial Role in Melanoma-Mononuclear Cell Crosstalk. International Journal of Molecular Sciences. 2021; 22(11):5735. https://doi.org/10.3390/ijms22115735
Chicago/Turabian StylePawlikowska, Małgorzata, Tomasz Jędrzejewski, Andrzej T. Slominski, Anna A. Brożyna, and Sylwia Wrotek. 2021. "Pigmentation Levels Affect Melanoma Responses to Coriolus versicolor Extract and Play a Crucial Role in Melanoma-Mononuclear Cell Crosstalk" International Journal of Molecular Sciences 22, no. 11: 5735. https://doi.org/10.3390/ijms22115735
APA StylePawlikowska, M., Jędrzejewski, T., Slominski, A. T., Brożyna, A. A., & Wrotek, S. (2021). Pigmentation Levels Affect Melanoma Responses to Coriolus versicolor Extract and Play a Crucial Role in Melanoma-Mononuclear Cell Crosstalk. International Journal of Molecular Sciences, 22(11), 5735. https://doi.org/10.3390/ijms22115735






