Mitochondrial Dynamics Markers and Related Signaling Molecules Are Important Regulators of Spermatozoa Number and Functionality
Abstract
1. Introduction
2. Results
2.1. Repeated Psychophysical Stress Increases the Level of Stress Hormones in Circulation, but Decreases Androgens Levels, as Well as the Functionality, ATP Level and Number of Spermatozoa
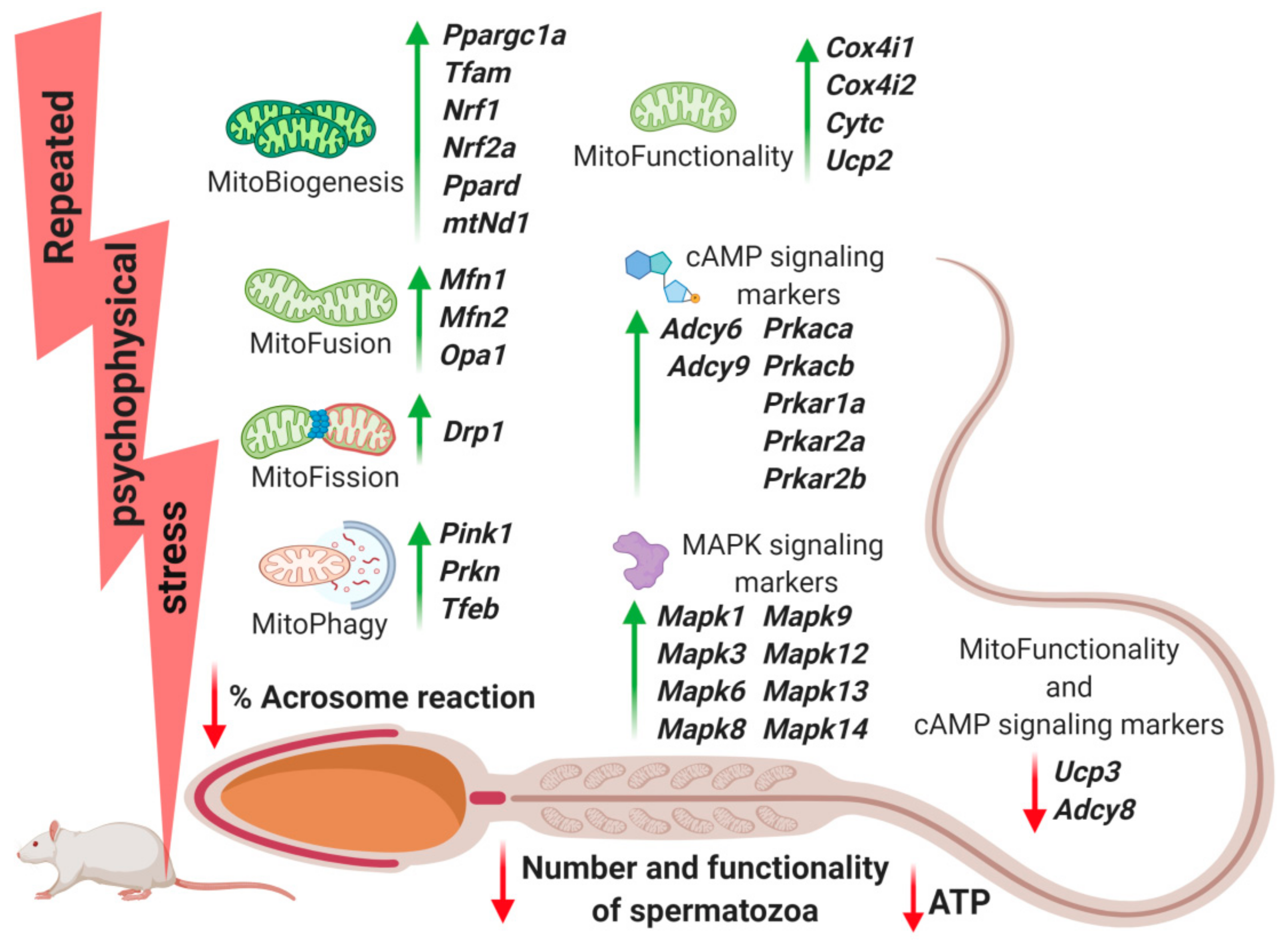
2.2. Transcriptional Profiles of Mitochondrial Dynamics and Functionality Markers Are Dramatically Changed in Spermatozoa from Stressed Rats
2.3. Transcriptional Profiles of Signaling Molecules Regulating Mitochondrial Dynamics and Functionality as Well as Number and Functionality of Spermatozoa, All of Which Are Important for Fertility, Are Dramatically Changed in Spermatozoa from Stressed Rats
3. Discussion
4. Materials and Methods
4.1. Statement of Institutional Review Board
4.2. Statement That the Authors Complied with ARRIVE Guidelines and Institutional Animal Care and Use Committee Guidelines
4.3. Animals and Experimental Model of Stress
4.4. Serum Hormones Measurement
4.5. Isolation of Spermatozoa and Assessment of Their Functionality (Capacitation and Acrosome Reaction)
4.6. Measurement of ATP Level
4.7. RNA Isolation and cDNA Synthesis
4.8. Relative Quantification of Gene Expression
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbagallo, F.; La Vignera, S.; Cannarella, R.; Aversa, A.; Calogero, A.E.; Condorelli, R.A. Evaluation of Sperm Mitochondrial Function: A Key Organelle for Sperm Motility. J. Clin. Med. 2020, 9, 363. [Google Scholar] [CrossRef]
- Choy, J.T.; Eisenberg, M.L. Male Infertility as a Window to Health. Fertil. Steril. 2018, 110, 810–814. [Google Scholar] [CrossRef]
- Bräuner, E.V.; Nordkap, L.; Priskorn, L.; Hansen, Å.M.; Bang, A.K.; Holmboe, S.A.; Schmidt, L.; Jensen, T.K.; Jørgensen, N. Psychological Stress, Stressful Life Events, Male Factor Infertility, and Testicular Function: A Cross-Sectional Study. Fertil. Steril. 2020, 113, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Nordkap, L.; Jensen, T.K.; Hansen, Å.M.; Lassen, T.H.; Bang, A.K.; Joensen, U.N.; Jensen, M.B.; Skakkebæk, N.E.; Jørgensen, N. Psychological Stress and Testicular Function: A Cross-Sectional Study of 1215 Danish Men. Fertil. Steril. 2016, 105, 174–187.e2. [Google Scholar] [CrossRef] [PubMed]
- Yazawa, H.; Sasagawa, I.; Ishigooka, M.; Nakada, T. Effect of Immobilization Stress on Testicular Germ Cell Apoptosis in Rats. Hum. Reprod. 1999, 14. [Google Scholar] [CrossRef] [PubMed]
- Nirupama, M.; Devaki, M.; Nirupama, R.; Yajurvedi, H.N. Chronic Intermittent Stress-Induced Alterations in the Spermatogenesis and Antioxidant Status of the Testis Are Irreversible in Albino Rat. J. Physiol. Biochem. 2013, 69, 59–68. [Google Scholar] [CrossRef]
- Selye, H. A Syndrome Produced by Diverse Nocuous Agents. Nature 1936, 138, 32. [Google Scholar] [CrossRef]
- Chrousos, G.P. Stress and Disorders of the Stress System. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Manoli, I.; Alesci, S.; Blackman, M.R.; Su, Y.A.; Rennert, O.M.; Chrousos, G.P. Mitochondria as Key Components of the Stress Response. Trends Endocrinol. Metab. 2007, 18, 190–198. [Google Scholar] [CrossRef]
- Pitman, R.K.; Rasmusson, A.M.; Koenen, K.C.; Shin, L.M.; Orr, S.P.; Gilbertson, M.W.; Milad, M.R.; Liberzon, I. Biological Studies of Post-Traumatic Stress Disorder. Nat. Rev. Neurosci. 2012, 13, 769–787. [Google Scholar] [CrossRef]
- Sapolsky, R.M. The Influence of Social Hierarchy on Primate Health. Science 2005, 308, 648–652. [Google Scholar] [CrossRef]
- Selye, H. Stress and Disease. Science 1955, 122, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Gak, I.A.; Radovic, S.M.; Dukic, A.R.; Janjic, M.M.; Stojkov-Mimic, N.J.; Kostic, T.S.; Andric, S.A. Stress Triggers Mitochondrial Biogenesis to Preserve Steroidogenesis in Leydig Cells. Biochim. Biophys. Acta BBA Mol. Cell Res. 2015, 1853, 2217–2227. [Google Scholar] [CrossRef]
- Starovlah, I.M.; Radovic Pletikosic, S.M.; Kostic, T.S.; Andric, S.A. Reduced Spermatozoa Functionality during Stress Is the Consequence of Adrenergic-Mediated Disturbance of Mitochondrial Dynamics Markers. Sci. Rep. 2020, 10, 16813. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.R.; Kovacs, J.J.; Whalen, E.J.; Rajagopal, S.; Strachan, R.T.; Grant, W.; Towers, A.J.; Williams, B.; Lam, C.M.; Xiao, K.; et al. A Stress Response Pathway Regulates DNA Damage through Β2-Adrenoreceptors and β-Arrestin-1. Nature 2011, 477, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Mhaouty-Kodja, S.; Lozach, A.; Habert, R.; Tanneux, M.; Guigon, C.; Brailly-Tabard, S.; Maltier, J.-P.; Legrand-Maltier, C. Fertility and Spermatogenesis Are Altered in 1b-Adrenergic Receptor Knockout Male Mice. J. Endocrinol. 2007, 195, 281–292. [Google Scholar] [CrossRef][Green Version]
- Gallon, F.; Marchetti, C.; Jouy, N.; Marchetti, P. The Functionality of Mitochondria Differentiates Human Spermatozoa with High and Low Fertilizing Capability. Fertil. Steril. 2006, 86, 1526–1530. [Google Scholar] [CrossRef]
- Kao, S.-H.; Chao, H.-T.; Liu, H.-W.; Liao, T.-L.; Wei, Y.-H. Sperm Mitochondrial DNA Depletion in Men with Asthenospermia. Fertil. Steril. 2004, 82, 66–73. [Google Scholar] [CrossRef]
- Song, G.J.; Lewis, V. Mitochondrial DNA Integrity and Copy Number in Sperm from Infertile Men. Fertil. Steril. 2008, 90, 2238–2244. [Google Scholar] [CrossRef]
- Okuda, H.; Tsujimura, A.; Yamamoto, K.; Fukuhara, S.; Nakayama, J.; Kiuchi, H.; Takao, T.; Miyagawa, Y.; Nonomura, N.; Okuyama, A. Morphologic and Mitochondrial Characterization of Human Spermatogenic Cells Dispersed in Wet Preparation for Testicular Sperm Extraction: Establishment of a Microscopic Diagram of Developing Human Spermatogenic Cells. Fertil. Steril. 2011, 95, 2665–2668. [Google Scholar] [CrossRef]
- Pelliccione, F.; Micillo, A.; Cordeschi, G.; D’Angeli, A.; Necozione, S.; Gandini, L.; Lenzi, A.; Francavilla, F.; Francavilla, S. Altered Ultrastructure of Mitochondrial Membranes Is Strongly Associated with Unexplained Asthenozoospermia. Fertil. Steril. 2011, 95, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Carra, E.; Sangiorgi, D.; Gattuccio, F.; Rinaldi, A.M. Male Infertility and Mitochondrial DNA. Biochem. Biophys. Res. Commun. 2004, 322, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Gholinezhad, M.; Yousefnia-pasha, Y.; Colagar, A.H.; Mohammadoo-Khorasani, M.; Bidmeshkipour, A. Comparison of Large-Scale Deletions of the Sperm Mitochondrial DNA in Normozoospermic and Asthenoteratozoospermic Men. J. Cell. Biochem. 2019, 120, 1958–1968. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, Y.; Bai, H.; Liu, J.; Li, J.; Wu, B. Mitochondria-Targeted Antioxidant MitoTEMPO Improves the Post-Thaw Sperm Quality. Cryobiology 2018, 80, 26–29. [Google Scholar] [CrossRef]
- Paoli, D.; Gallo, M.; Rizzo, F.; Baldi, E.; Francavilla, S.; Lenzi, A.; Lombardo, F.; Gandini, L. Mitochondrial Membrane Potential Profile and Its Correlation with Increasing Sperm Motility. Fertil. Steril. 2011, 95, 2315–2319. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, W.; Zou, P.; Jiang, F.; Zeng, Y.; Chen, Q.; Sun, L.; Yang, H.; Zhou, N.; Wang, X.; et al. Mitochondrial Functionality Modifies Human Sperm Acrosin Activity, Acrosome Reaction Capability and Chromatin Integrity. Hum. Reprod. 2019, 34, 3–11. [Google Scholar] [CrossRef]
- Malić Vončina, S.; Golob, B.; Ihan, A.; Kopitar, A.N.; Kolbezen, M.; Zorn, B. Sperm DNA Fragmentation and Mitochondrial Membrane Potential Combined Are Better for Predicting Natural Conception than Standard Sperm Parameters. Fertil. Steril. 2016, 105, 637–644.e1. [Google Scholar] [CrossRef]
- Amaral, A.; Ramalho-Santos, J.; St John, J.C. The Expression of Polymerase Gamma and Mitochondrial Transcription Factor A and the Regulation of Mitochondrial DNA Content in Mature Human Sperm. Hum. Reprod. 2007, 22, 1585–1596. [Google Scholar] [CrossRef]
- Borges, E.; Zanetti, B.F.; Setti, A.S.; Braga, D.P.d.A.F.; Provenza, R.R.; Iaconelli, A. Sperm DNA Fragmentation Is Correlated with Poor Embryo Development, Lower Implantation Rate, and Higher Miscarriage Rate in Reproductive Cycles of Non–Male Factor Infertility. Fertil. Steril. 2019, 112, 483–490. [Google Scholar] [CrossRef]
- Faja, F.; Carlini, T.; Coltrinari, G.; Finocchi, F.; Nespoli, M.; Pallotti, F.; Lenzi, A.; Lombardo, F.; Paoli, D. Human Sperm Motility: A Molecular Study of Mitochondrial DNA, Mitochondrial Transcription Factor A Gene and DNA Fragmentation. Mol. Biol. Rep. 2019, 46, 4113–4121. [Google Scholar] [CrossRef]
- Aparicio, I.M.; Espino, J.; Bejarano, I.; Gallardo-Soler, A.; Campo, M.L.; Salido, G.M.; Pariente, J.A.; Peña, F.J.; Tapia, J.A. Autophagy-Related Proteins Are Functionally Active in Human Spermatozoa and May Be Involved in the Regulation of Cell Survival and Motility. Sci. Rep. 2016, 6, 33647. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qian, H.; Huang, X.; Li, J.; Zhang, J.; Zhu, N.; Chen, H.; Zhu, C.; Wang, J.; Zhang, P.; et al. UCP2 Mitigates the Loss of Human Spermatozoa Motility by Promoting MROS Elimination. Cell. Physiol. Biochem. 2018, 50, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Ni, K.; Shang, J.; Zhang, X.; Xiong, C.; Meng, T. Expression of Mitofusin 2 in Human Sperm and Its Relationship to Sperm Motility and Cryoprotective Potentials. Exp. Biol. Med. 2018, 243, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.V.; Freitas, M.J.; Correia, B.R.; Korrodi-Gregório, L.; Patrício, A.; Pelech, S.; Fardilha, M. Profiling Signaling Proteins in Human Spermatozoa: Biomarker Identification for Sperm Quality Evaluation. Fertil. Steril. 2015, 104, 845–856.e8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dominy, J.E.; Puigserver, P. Mitochondrial Biogenesis through Activation of Nuclear Signaling Proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, a015008. [Google Scholar] [CrossRef] [PubMed]
- Markaki, M.; Tavernarakis, N. Mitochondrial Turnover and Homeostasis in Ageing and Neurodegeneration. FEBS Lett. 2020, 594, 2370–2379. [Google Scholar] [CrossRef]
- Scarpulla, R.C. Transcriptional Paradigms in Mammalian Mitochondrial Biogenesis and Function. Physiol. Rev. 2008, 88, 611–638. [Google Scholar] [CrossRef]
- Scarpulla, R.C.; Vega, R.B.; Kelly, D.P. Transcriptional Integration of Mitochondrial Biogenesis. Trends Endocrinol. Metab. 2012, 23, 459–466. [Google Scholar] [CrossRef]
- Finkel, T. A Clean Energy Programme. Nature 2006, 444, 151–152. [Google Scholar] [CrossRef]
- Pyakurel, A.; Savoia, C.; Hess, D.; Scorrano, L. Extracellular Regulated Kinase Phosphorylates Mitofusin 1 to Control Mitochondrial Morphology and Apoptosis. Mol. Cell 2015, 58, 244–254. [Google Scholar] [CrossRef]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient Control of Glucose Homeostasis through a Complex of PGC-1alpha and SIRT1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef] [PubMed]
- García-Bueno, B.; Madrigal, J.L.M.; Pérez-Nievas, B.G.; Leza, J.C. Stress Mediators Regulate Brain Prostaglandin Synthesis and Peroxisome Proliferator-Activated Receptor-Gamma Activation after Stress in Rats. Endocrinology 2008, 149, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.; Brück, P.; Mikes, Z.; Küpper, J.-H.; Klingenspor, M.; Wiesner, R.J. Glucocorticoid Hormone Stimulates Mitochondrial Biogenesis Specifically in Skeletal Muscle. Endocrinology 2002, 143, 177–184. [Google Scholar] [CrossRef]
- Krauss, S.; Zhang, C.-Y.; Lowell, B.B. The Mitochondrial Uncoupling-Protein Homologues. Nat. Rev. Mol. Cell Biol. 2005, 6, 248–261. [Google Scholar] [CrossRef]
- Echave, P.; Machado-da-Silva, G.; Arkell, R.S.; Duchen, M.R.; Jacobson, J.; Mitter, R.; Lloyd, A.C. Extracellular Growth Factors and Mitogens Cooperate to Drive Mitochondrial Biogenesis. J. Cell Sci. 2009, 122, 4516–4525. [Google Scholar] [CrossRef] [PubMed]
- Boguenet, M.; Bouet, P.-E.; Spiers, A.; Reynier, P.; May-Panloup, P. Mitochondria: Their role in spermatozoa and in male infertility. Hum. Reprod. Update 2021. [Google Scholar] [CrossRef]
- Starovlah, I.M.; Sava, R.M.; Marinovic, M.A.; Kostic, T.S.; Andric, S.A. Psychophysical Stress Disturbs Expression of Mitochondrial Biogenesis Markers in Hypothalamus and Adenohypophysis. Biol. Serbica 2017, 39, 43–51. [Google Scholar]
- Hou, G.; Xiong, W.; Wang, M.; Chen, X.; Yuan, T. Chronic Stress Influences Sexual Motivation and Causes Damage to Testicular Cells in Male Rats. J. Sex. Med. 2014, 11, 653–663. [Google Scholar] [CrossRef]
- Mehfooz, A.; Wei, Q.; Zheng, K.; Fadlalla, M.B.; Maltasic, G.; Shi, F. Protective Roles of Rutin against Restraint Stress on Spermatogenesis in Testes of Adult Mice. Tissue Cell 2018, 50, 133–143. [Google Scholar] [CrossRef]
- Demirci, T.; Sahin, E. The Effect of Chronic Stress and Obesity on Sperm Quality and Testis Histology in Male Rats: A Morphometric and Immunohistochemical Study. Histol. Histopathol. 2019, 34, 287–302. [Google Scholar]
- Zou, P.; Wang, X.; Yang, W.; Liu, C.; Chen, Q.; Yang, H.; Zhou, N.; Zeng, Y.; Chen, H.; Zhang, G.; et al. Mechanisms of Stress-Induced Spermatogenesis Impairment in Male Rats Following Unpredictable Chronic Mild Stress (UCMS). Int. J. Mol. Sci. 2019, 20, 4470. [Google Scholar] [CrossRef]
- De Souza, M.J.; Arce, J.C.; Pescatello, L.S.; Scherzer, H.S.; Luciano, A.A. Gonadal Hormones and Semen Quality in Male Runners. A Volume Threshold Effect of Endurance Training. Int. J. Sports Med. 1994, 15, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Fenster, L.; Katz, D.F.; Wyrobek, A.J.; Pieper, C.; Rempel, D.M.; Oman, D.; Swan, S.H. Effects of Psychological Stress on Human Semen Quality. J. Androl. 1997, 18, 194–202. [Google Scholar] [PubMed]
- Elezaj, S.; Gashi, Z.; Zeqiraj, A.; Grabanica, D.; Shllaku, A.; Gruda, B.; Musaj, V. Treatment of Infertility in Men with Post-Traumatic Stress Disorder (PTSD) with the Method of Intrauterine Insemination. Med. Arch. 2015, 69, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lin, Q.; Wen, J.; Lin, W.; Liang, J.; Huang, H.; Li, L.; Huang, J.; Chen, F.; Liu, D.; et al. Whole Genome Bisulfite Sequencing of Human Spermatozoa Reveals Differentially Methylated Patterns from Type 2 Diabetic Patients. J. Diabetes Investig. 2020, 11, 856–864. [Google Scholar] [CrossRef]
- Gawish, R.A.; Fahmy, H.A.; Abd El Fattah, A.I.; Nada, A.S. The Potential Effect of Methylseleninic Acid (MSA) against γ-Irradiation Induced Testicular Damage in Rats: Impact on JAK/STAT Pathway. Arch. Biochem. Biophys. 2020, 679, 108205. [Google Scholar] [CrossRef]
- Larsson, N.G.; Garman, J.D.; Oldfors, A.; Barsh, G.S.; Clayton, D.A. A Single Mouse Gene Encodes the Mitochondrial Transcription Factor A and a Testis-Specific Nuclear HMG-Box Protein. Nat. Genet. 1996, 13, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Varuzhanyan, G.; Rojansky, R.; Sweredoski, M.J.; Graham, R.L.; Hess, S.; Ladinsky, M.S.; Chan, D.C. Mitochondrial Fusion Is Required for Spermatogonial Differentiation and Meiosis. eLife 2019, 8, e51601. [Google Scholar] [CrossRef] [PubMed]
- Binder, N.K.; Sheedy, J.R.; Hannan, N.J.; Gardner, D.K. Male Obesity Is Associated with Changed Spermatozoa Cox4i1 MRNA Level and Altered Seminal Vesicle Fluid Composition in a Mouse Model. Mol. Hum. Reprod. 2015, 21, 424–434. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Zhang, X.; Qin, J.; Wan, Y.; Hu, Y.; Liu, T.; Li, J.; Dong, W.; Du, E.; Pan, C.; et al. The Histone Methyltransferase ESET Is Required for the Survival of Spermatogonial Stem/Progenitor Cells in Mice. Cell Death Dis. 2014, 5, e1196. [Google Scholar] [CrossRef]
- Zhang, W.-D.; Zhang, Z.; Jia, L.-T.; Zhang, L.-L.; Fu, T.; Li, Y.-S.; Wang, P.; Sun, L.; Shi, Y.; Zhang, H.-Z. Oxygen Free Radicals and Mitochondrial Signaling in Oligospermia and Asthenospermia. Mol. Med. Rep. 2014, 10, 1875–1880. [Google Scholar] [CrossRef][Green Version]
- Finkelstein, M.; Etkovitz, N.; Breitbart, H. Ca2+ Signaling in Mammalian Spermatozoa. Mol. Cell. Endocrinol. 2020, 516, 110953. [Google Scholar] [CrossRef] [PubMed]
- Schuh, S.M.; Carlson, A.E.; McKnight, G.S.; Conti, M.; Hille, B.; Babcock, D.F. Signaling Pathways for Modulation of Mouse Sperm Motility by Adenosine and Catecholamine Agonists. Biol. Reprod. 2006, 74, 492–500. [Google Scholar] [CrossRef]
- Wertheimer, E.; Krapf, D.; de la Vega-Beltran, J.L.; Sánchez-Cárdenas, C.; Navarrete, F.; Haddad, D.; Escoffier, J.; Salicioni, A.M.; Levin, L.R.; Buck, J.; et al. Compartmentalization of Distinct CAMP Signaling Pathways in Mammalian Sperm. J. Biol. Chem. 2013, 288, 35307–35320. [Google Scholar] [CrossRef] [PubMed]
- Capkova, J.; Kubatova, A.; Ded, L.; Tepla, O.; Peknicova, J. Evaluation of the Expression of Sperm Proteins in Normozoospermic and Asthenozoospermic Men Using Monoclonal Antibodies. Asian J. Androl. 2016, 18, 108–113. [Google Scholar] [PubMed]
- Yadav, S.K.; Pandey, A.; Kumar, L.; Devi, A.; Kushwaha, B.; Vishvkarma, R.; Maikhuri, J.P.; Rajender, S.; Gupta, G. The Thermo-Sensitive Gene Expression Signatures of Spermatogenesis. Reprod. Biol. Endocrinol. RBE 2018, 16, 56. [Google Scholar] [CrossRef] [PubMed]
- Orta, G.; de la Vega-Beltran, J.L.; Martín-Hidalgo, D.; Santi, C.M.; Visconti, P.E.; Darszon, A. CatSper Channels Are Regulated by Protein Kinase A. J. Biol. Chem. 2018, 293, 16830–16841. [Google Scholar] [CrossRef]
- Jia, Y.; Castellanos, J.; Wang, C.; Sinha-Hikim, I.; Lue, Y.; Swerdloff, R.S.; Sinha-Hikim, A.P. Mitogen-Activated Protein Kinase Signaling in Male Germ Cell Apoptosis in the Rat1. Biol. Reprod. 2009, 80, 771–780. [Google Scholar] [CrossRef]
- Salgado-Lucio, M.L.; Ramírez-Ramírez, D.; Jorge-Cruz, C.Y.; Roa-Espitia, A.L.; Hernández-González, E.O. FAK Regulates Actin Polymerization during Sperm Capacitation via the ERK2/GEF-H1/RhoA Signaling Pathway. J. Cell Sci. 2020, 133, jcs239186. [Google Scholar] [CrossRef] [PubMed]
- Gesquiere, L.R.; Learn, N.H.; Simao, M.C.M.; Onyango, P.O.; Alberts, S.C.; Altmann, J. Life at the Top: Rank and Stress in Wild Male Baboons. Science 2011, 333, 357–360. [Google Scholar] [CrossRef]
- Medar, M.L.J.; Marinkovic, D.Z.; Kojic, Z.; Becin, A.P.; Starovlah, I.M.; Kravic-Stevovic, T.; Andric, S.A.; Kostic, T.S. Dependence of Leydig Cell’s Mitochondrial Physiology on Luteinizing Hormone Signaling. Life 2021, 11, 19. [Google Scholar] [CrossRef] [PubMed]



| Group | Control | 1×3hIMO | 10×3hIMO | |
|---|---|---|---|---|
| Transcript | ||||
| Ppargc1a | 1.0 ± 0.07 | 0.91 ± 0.30 | 2.74 *,# ± 0.52  | |
| Tfam | 1.0 ± 0.02 | 1.19 ± 0.04 | 1.94 *,# ± 0.29  | |
| Nrf1 | 1.0 ± 0.02 | 1.14 ± 0.06 | 2.33 * ± 0.54  | |
| Nrf2a | 1.0 ± 0.06 | 1.18 ± 0.04 | 1.82 *,# ± 0.08  | |
| Ppard | 1.0 ± 0.01 | 1.92 * ± 0.01  | 1.86 * ± 0.15  | |
| mtNd1 | 1.0 ± 0.02 | 1.20 ± 0.01 | 7.03 *,# ± 0.16  | |
| Mfn1 | 1.0 ± 0.01 | 1.00 ± 0.08 | 3.28 *,# ± 0.69  | |
| Mfn2 | 1.0 ± 0.04 | 0.81 ± 0.01 | 2.88 *,# ± 0.37  | |
| Opa1 | 1.0 ± 0.06 | 1.27 ± 0.01 | 1.63 * ± 0.08  | |
| Drp1 | 1.0 ± 0.01 | 0.98 ± 0.03 | 3.23 *,# ± 0.39  | |
| Pink1 | 1.0 ± 0.00 | 0.70 ± 0.01 | 2.31 *,# ± 0.18  | |
| Prkn | 1.0 ± 0.05 | 0.75 ± 0.23 | 1.45 *,# ± 0.15  | |
| Tfeb | 1.0 ± 0.01 | 1.28 ± 0.08 | 2.61 *,# ± 0.59  | |
| Cox4i1 | 1.0 ± 0.02 | 1.15 ± 0.02 | 2.75 *,# ± 0.17  | |
| Cox4i2 | 1.0 ± 0.02 | 0.64 ± 0.19 | 2.78 *,# ± 0.68  | |
| Cytc | 1.0 ± 0.01 | 1.25 ± 0.04 | 1.60 * ± 0.08  | |
| Ucp2 | 1.0 ± 0.02 | 1.23 ± 0.01 | 3.22 *,# ± 0.59  | |
| Ucp3 | 1.0 ± 0.06 | 0.71 ± 0.03 | 0.20 *,# ± 0.02  | |
| Adcy6 | 1.0 ± 0.02 | 0.52 * ± 0.01  | 3.13 *,# ± 0.38  | |
| Adcy8 | 1.0 ± 0.01 | 0.43 * ± 0.06  | 0.43 * ± 0.11  | |
| Adcy9 | 1.0 ± 0.01 | 0.85 ± 0.00 | 2.70 *,# ± 0.45  | |
| Prkaca | 1.0 ± 0.02 | 0.77 ± 0.05 | 1.70 *,# ± 0.19  | |
| Prkacb | 1.0 ± 0.02 | 0.96 ± 0.02 | 2.43 *,# ± 0.54  | |
| Prkar1a | 1.0 ± 0.01 | 0.96 ± 0.02 | 2.29 *,# ± 0.30  | |
| Prkar2a | 1.0 ± 0.02 | 0.63 ± 0.06 | 1.89 *,# ± 0.20  | |
| Prkar2b | 1.0 ± 0.02 | 0.68 ± 0.05 | 3.90 *,# ± 0.51  | |
| Mapk1 | 1.0 ± 0.01 | 1.89 * ± 0.09  | 3.33 *,# ± 0.65  | |
| Mapk3 | 1.0 ± 0.02 | 0.72 ± 0.04 | 1.70 *,# ± 0.29  | |
| Mapk6 | 1.0 ± 0.02 | 1.72 * ± 0.06  | 1.58 * ± 0.19  | |
| Mapk8 | 1.0 ± 0.01 | 1.56 * ± 0.03  | 3.22 *,# ± 0.56  | |
| Mapk9 | 1.0 ± 0.02 | 1.02 ± 0.01 | 1.82 *,# ± 0.23  | |
| Mapk12 | 1.0 ± 0.01 | 0.95 ± 0.05 | 2.39 *,# ± 0.48  | |
| Mapk13 | 1.0 ± 0.02 | 0.61 ± 0.04 | 3.25 *,# ± 0.56  | |
| Mapk14 | 1.0 ± 0.01 | 0.83 ± 0.01 | 1.77 *,# ± 0.34  | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starovlah, I.M.; Radovic Pletikosic, S.M.; Kostic, T.S.; Andric, S.A. Mitochondrial Dynamics Markers and Related Signaling Molecules Are Important Regulators of Spermatozoa Number and Functionality. Int. J. Mol. Sci. 2021, 22, 5693. https://doi.org/10.3390/ijms22115693
Starovlah IM, Radovic Pletikosic SM, Kostic TS, Andric SA. Mitochondrial Dynamics Markers and Related Signaling Molecules Are Important Regulators of Spermatozoa Number and Functionality. International Journal of Molecular Sciences. 2021; 22(11):5693. https://doi.org/10.3390/ijms22115693
Chicago/Turabian StyleStarovlah, Isidora M., Sava M. Radovic Pletikosic, Tatjana S. Kostic, and Silvana A. Andric. 2021. "Mitochondrial Dynamics Markers and Related Signaling Molecules Are Important Regulators of Spermatozoa Number and Functionality" International Journal of Molecular Sciences 22, no. 11: 5693. https://doi.org/10.3390/ijms22115693
APA StyleStarovlah, I. M., Radovic Pletikosic, S. M., Kostic, T. S., & Andric, S. A. (2021). Mitochondrial Dynamics Markers and Related Signaling Molecules Are Important Regulators of Spermatozoa Number and Functionality. International Journal of Molecular Sciences, 22(11), 5693. https://doi.org/10.3390/ijms22115693






