All chemicals, reagents, and solvents were analytical grade, purchased from commercial suppliers (i.e., Aladdin, Adamas, and Acros).
The reactions were monitored via thin-layer chromatography (TLC) performed on HSGF254 plates and visualized under UV light. Melting points were measured with an X4-A microscopic melting point apparatus. 1HNMR spectra were recorded on a BrukerBioSpin GmbH spectrometer at 300 and 400 MHz; 13CNMR spectra were recorded on a BrukerBioSpin GmbH spectrometer at 75 and 100 MHz; the coupling constants are given in Hz. Mass (HRMS) analysis was obtained using Agilent 6200 Accurate-Mass TOF LC/MS system with Electrospray Ionization (ESI). HPLC analysis was obtained using SHIMADZULC-20AB.
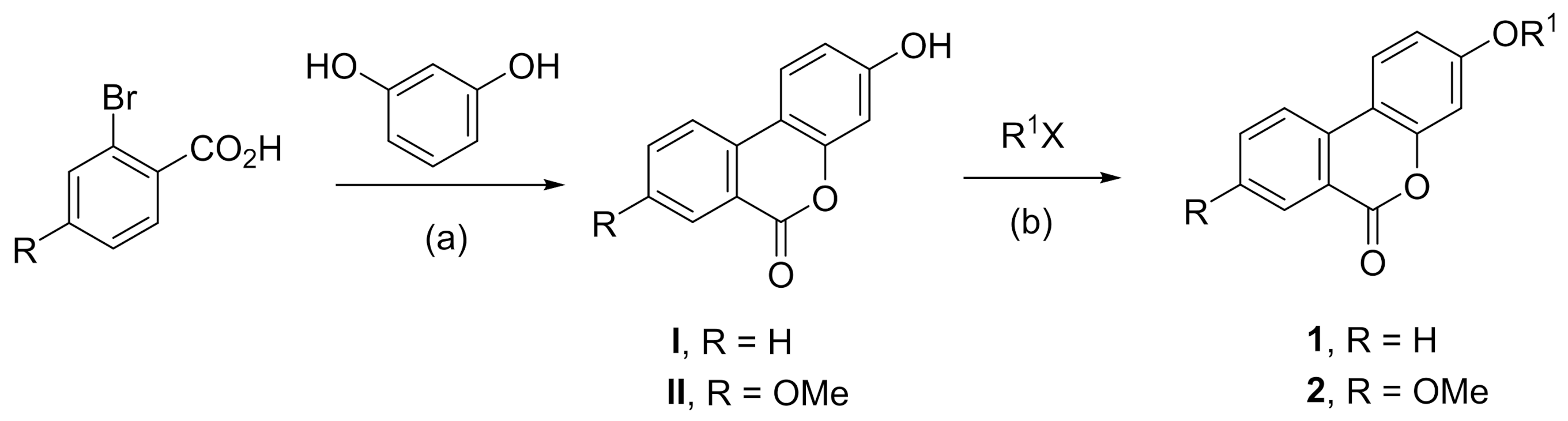
3.1.2. General Procedures for Synthesis of Compounds 1a–u [28]
The anhydrous DMF (30 mL) was added to a 250 mL round bottom flask, compound I (9.4 mmol, 2.0 g), anhydrous K2CO3 (12.2 mmol, 1.7 g), and halide (12.2 mmol) were added, and the temperature was controlled at 80~120 °C; the reaction was stirred for 24 h. The reaction was monitored by TLC (PE:EA = 3:1). When the reaction was over, the reaction solution was added to the ice-water mixture to obtain brown crude solid. After suction filtration and drying, the brown crude solid was purified by column chromatography to obtain compounds 1a–u.
3-Methoxy-6H-benzo[c]chromen-6-one (1a): Using iodomethane as the starting material, the desired white solid 1a was isolated (1.30 g, 61%). M.p. 131.5–132.8 °C. 1H NMR (400 MHz, DMSO-d6) δ = 8.35 (d, J = 8.0 Hz, 1H), 8.29 (d, J = 12.0 Hz, 1H), 8.22 (dd, J = 8.0 Hz, 1H), 7.95–7.90 (m, 1H), 7.62 (t, J = 6.0 Hz, 1H), 7.06–6.99 (m, 2H), 3.88 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ = 161.67, 160.96, 152.54, 135.78, 135.19, 130.14, 128.53, 125.17, 122.40, 119.68, 112.78, 111.09, 101.91, 56.23; HRMS (ESI) m/z calcd for C14H11O3+[M + H]+: 227.0630; found: 227.0626. Chromatographic purity: 98.2% (HPLC).
Ethoxy-6H-benzo[c]chromen-6-one (1b): Using bromoethane as the starting material, the desired yellow solid 1b was isolated (1.70 g, 75%). M.p. 115.2–117.7 °C. 1H NMR (300 MHz, DMSO-d6) δ = 8.35–8.22 (m, 3H), 7.93 (t, J = 6.0 Hz, 1H), 7.62 (t, J = 6.0 Hz, 1H), 6.98 (d, J = 6.0 Hz, 2H), 4.13 (q, J = 3.0 Hz, 2H), 1.39 (t, J = 6.0 Hz, 3H); 13C NMR (75 MHz, DMSO-d6) δ = 160.97, 152.55, 135.81, 135.25, 130.15, 128.52, 125.20, 122.41, 119.68, 113.10, 110.99, 102.31, 64.28, 14.94; HRMS (ESI) m/z calcd for C15H13O3+[M + H]+: 241.0786; found: 241.0782. Chromatographic purity: 97.6% (HPLC).
3-Isopropoxy-6H-benzo[c]chromen-6-one (1c): Using 2-bromopropane as the starting material, the desired yellow solid 1c was isolated (1.70 g, 71%). M.p. 58.7–59.1 °C. 1H NMR (300 MHz, DMSO-d6) δ = 8.68–7.33 (m, 5H), 6.96 (s, 2H), 4.77 (m, 1H), 1.32 (d, J = 6.0 Hz, 6H); 13C NMR (75 MHz, DMSO-d6) δ = 160.98, 159.92, 152.59, 135.76, 135.23, 130.13, 128.45, 125.22, 122.34, 119.65, 113.83, 110.83, 103.23, 70.42, 22.13; HRMS (ESI) m/z calcd for C16H15O3+[M + H]+: 255.0943; found: 255.0940. Chromatographic purity: 97.8% (HPLC).
3-Propoxy-6H-benzo[c]chromen-6-one (1d): Using 1-bromopropane as the starting material, the desired white solid 1d was isolated (1.99 g, 83%). M.p. 67.6–69.2 °C. 1H NMR (300 MHz, DMSO-d6) δ = 8.28 (t, J = 6.0 Hz, 1H), 8.23–8.17 (m, 2H), 7.92–7.86 (m, 1H), 7.61–7.55 (m, 1H), 6.99–6.93 (m, 2H), 4.04–3.99 (m,2H), 1.83–1.71 (m, 2H), 1.01 (t, J = 6.0 Hz, 3H); 13C NMR (75 MHz, DMSO-d6) δ = 161.07, 160.95, 152.50, 135.74, 135.21, 130.11, 128.46,125.12, 122.35,119.64, 113.06, 110.95,102.29, 70.00, 22.35, 10.80; HRMS (ESI) m/z calcd for C16H15O3+[M + H]+: 255.0943; found: 255.0940. Chromatographic purity: 98.9% (HPLC).
3-(Sec-butoxy)-6H-benzo[c]chromen-6-one (1e): Using 2-bromobutane as the starting material, the desired yellow solid 1e was isolated (1.79 g, 71%). M.p. 73.2–75.8 °C. 1H NMR (400 MHz, DMSO-d6) δ = 8.36–8.19 (m, 3H), 7.93–7.88 (m, 1H), 7.62–7.57 (m, 1H), 7.00–6.97 (m, 2H), 4.60–4.53 (m, 1H), 1.74–1.58 (m, 2H), 1.28 (d, J = 8.0 Hz, 3H), 0.95 (t, J = 4.0 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ = 160.97, 160.25, 152.58, 135.73, 135.24, 130.12, 128.41, 125.19, 122.31, 119.64, 113.83, 110.83, 103.21, 75.23, 28.89, 19.35, 9.92; HRMS (ESI) m/z calcd for C17H17O3+[M + H]+: 269.1099; found: 269.1095. Chromatographic purity: 99.2% (HPLC).
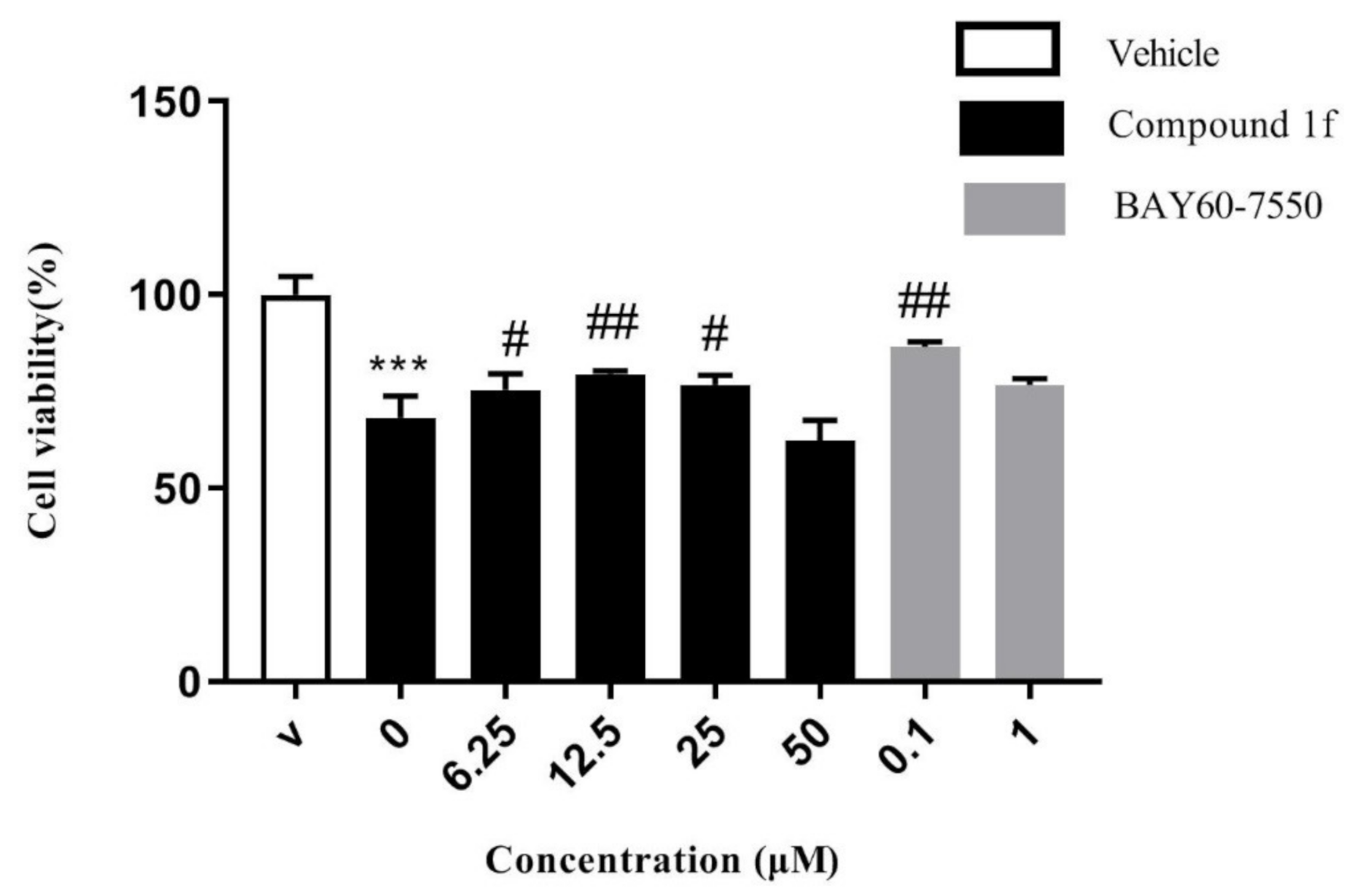
3-butoxy-6H-benzo[c]chromen-6-one (1f): Using 1-bromobutane as the starting material, the desired yellow solid 1f was isolated (1.41 g, 56%). M.p. 53.6–55.5 °C. 1H NMR (300 MHz, DMSO-d6) δ = 8.34–8.20 (m, 3H), 7.91 (t, J = 9.0 Hz, 1H), 7.60 (t, J = 9.0 Hz, 1H), 7.01–6.99 (d, J = 6.0 Hz, 2H), 4.09 (t, J = 6.0 Hz, 2H), 1.74(t, J = 9.0 Hz, 2H), 1.50–1.42 (m, 2H), 0.95 (t, J = 9.0 Hz, 3H); 13C NMR (75 MHz, DMSO-d6) δ = 161.13, 160.98, 152.54, 135.78, 135.25, 130.14, 128.49, 125.16, 122.39, 119.67, 113.13, 110.98, 102.34, 68.30, 31.03, 19.17, 14.15; HRMS (ESI) m/z calcd for C17H17O3+[M + H]+: 269.1099; found: 269.1095. Chromatographic purity: 98.7% (HPLC).
3-(pentyloxy)-6H-benzo[c]chromen-6-one (1g): Using 1-bromopentane as the starting material, the desired yellow solid 1g was isolated (1.49 g, 56%). M.p. 69.4–71.8 °C. 1H NMR (400 MHz, DMSO-d6) δ = 8.34–8.20 (m, 3H), 7.91 (t, J = 8.0 Hz, 1H), 7.60 (t, J = 8.0 Hz, 1H), 7.01–6.98 (m, 2H), 4.08 (t, J = 6.0 Hz, 2H), 1.79–1.72 (m, 2H), 1.46–1.32 (m, 4H), 0.91 (t, J = 8.0 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ = 161.10, 160.97, 153.65, 135.77, 135.23, 130.13, 128.47, 125.14, 122.37, 119.66, 113.10, 110.95, 103.11, 68.56, 29.42, 28.12, 22.36, 17.18; HRMS (ESI) m/z calcd for C18H19O3+[M + H]+: 283.1256; found: 283.1252. Chromatographic purity: 99.2% (HPLC).
ethyl 2-((6-oxo-6H-benzo[c]chromen-3-yl)oxy)acetate (1h): Using ethyl 2-bromoacetate as the starting material, the desired yellow solid 1h was isolated (1.40 g, 50%). M.p. 124.7–125.8 °C. 1H NMR (300 MHz, DMSO-d6) δ = 8.36–8.21 (m, 3H), 7.93 (t, J = 7.5 Hz, 1H), 7.62 (t, J = 7.5 Hz, 1H), 7.04 (dt, J = 9.0, 3.0 Hz, 2H), 4.94 (s, 2H), 4.20 (q, J = 7.0 Hz, 2H), 1.24 (t, J = 7.5Hz, 3H); 13C NMR (75 MHz, DMSO-d6) δ = 168.79, 160.88, 159.94, 152.37, 136.32, 135.03, 130.17, 128.74, 125.29, 122.52, 120.20, 113.06, 111.79, 103.34, 66.14, 62.31, 15.96; HRMS (ESI) m/z calcd for C17H15O5+[M + H]+: 299.0841; found: 299.0837. Chromatographic purity: 98.3% (HPLC).
3-((tetrahydro-2H-pyran-4-yl)methoxy)-6H-benzo[c]chromen-6-one (1i): Using 4-(bromomethyl)tetrahydro-2H-pyran as the starting material, the desired yellow solid 1i was isolated (1.60 g, 55%). M.p. 133.0–134.8 °C. 1H NMR (300 MHz, CDCl3) δ = 8.34 (d, J = 9.0 Hz, 1H), 7.96 (dd, J = 18.0, 9.0 Hz, 2H), 7.78 (t, J = 7.5 Hz, 1H), 7.50 (t, J = 7.5 Hz, 1H), 6.91–6.82 (m, 2H), 4.04 (dd, J = 12.0, 9.0 Hz, 2H), 3.87 (t, J = 6.0Hz, 2H), 3.46 (t, J = 12.0 Hz, 2H), 2.17–2.06 (m, 1H), 1.80 (d, J = 3.0 Hz, 2H), 1.57–4.13 (m, 2H); 13C NMR (75 MHz, DMSO-d6) δ = 161.09, 160.97, 152.53, 135.79, 135.22, 130.15, 128.53, 125.19, 122.41, 119.69, 113.13, 111.08, 102.44, 73.00, 67.06, 34.77, 29.60; HRMS (ESI) m/z calcd for C19H19O4+[M + H]+: 311.1205; found: 311.1201. Chromatographic purity: 97.3% (HPLC).
3-(Benzyloxy)-6H-benzo[c]chromen-6-one (1j): Using (bromomethyl)benzene as the starting material, the desired yellow solid 1j was isolated (2.81 g, 99%). M.p. 113.2–115.3 °C. 1H NMR (300 MHz, DMSO-d6) δ = 8.31 (dd, J = 15.0, 9.0 Hz, 2H), 8.21 (dd, J = 9.0, 1.2 Hz, 1H), 7.94–7.89 (m, 1H), 7.61–7.58 (m, 1H), 7.52–7.48 (m, 2H), 7.45–7.35 (m, 3H), 7.12–7.07 (m, 2H), 5.24 (s, 2H); 13C NMR (75 MHz, DMSO-d6) δ = 160.95, 160.70, 152.48, 136.92, 135.81, 135.17, 130.16, 128.99, 128.61, 128.53, 128.37, 125.26, 122.47, 119.76, 113.44, 111.34, 102.91, 70.25; HRMS (ESI) m/z calcd for C20H15O3+ [M + H]+: 303.0943; found: 303.0940. Chromatographic purity: 96.8% (HPLC).
3-(2-(1H-pyrazol-1-yl)ethoxy)-6H-benzo[c]chromen-6-one (1k): Using 1-(2-bromoethyl)-1H-pyrazole as the starting material, the desired white solid 1k was isolated (1.24 g, 43%). M.p. 164.3–166.6 °C. 1H NMR (300 MHz, DMSO-d6) δ = 8.32 (d, J = 9.0 Hz, 1H), 8.27–8.19 (m, 2H), 7.93–7.88 (m, 1H), 7.82 (d, J = 2.1 Hz, 1H), 7.63–7.57 (m, 1H), 7.48 (d, J = 3.0 Hz, 1H), 7.01–6.95 (m, 2H), 6.27 (t, J = 3.0 Hz, 1H), 4.55 (m, 2H), 4.46 (m, 2H); 13C NMR (75 MHz, DMSO-d6) δ = 160.94, 160.43, 152.48, 139.39, 135.84, 135.12, 131.08, 130.17, 128.67, 125.29, 122.49, 119.77, 113.16, 111.46, 105.72, 102.63, 67.60, 50.93; HRMS (ESI) m/z calcd for C18H15N2O3+[M + H]+: 307.1004; found: 307.1001. Chromatographic purity: 96.5% (HPLC).
3-(Pyrimidin-2-yloxy)-6H-benzo[c]chromen-6-one (1l): Using 2-bromopyrimidine as the starting material, the desired yellow solid 1l was isolated (1.83 g, 67%). M.p. 174.0–176.6 °C. 1H NMR (400 MHz, DMSO-d6) δ = 8.70 (d, J = 8.0 Hz, 2H), 8.45(d, J = 8.0 Hz, 2H), 8.28 (d, J = 12.0 Hz, 1H), 7.98 (t, J = 10.0 Hz, 1H), 7.70 (t, J = 10.0 Hz, 1H), 7.41–7.29 (m, 3H); 13C NMR (100 MHz, DMSO-d6) δ = 164.80, 160.66, 154.69, 151.92, 135.92, 134.55, 130.26, 129.58, 125.31, 123.09, 120.57, 119.02, 117.93, 115.50, 111.00; HRMS (ESI) m/z calcd for C17H11N2O3+[M + H]+: 291.0691; found: 291.0686. Chromatographic purity: 98.2% (HPLC).
3-(2-Hydroxyethoxy)-6H-benzo[c]chromen-6-one (1m): Using 2-bromoethanol as the starting material, the desired white solid 1m was isolated (2.34 g, 98%). M.p. 125.7–126.4 °C. 1H NMR (400 MHz, DMSO-d6) δ = 8.32 (d, J = 8.0 Hz, 1H), 8.27–8.24 (m, 1H), 8.21 (dd, J = 8.0, 4.0 Hz, 1H), 7.94–7.90 (m, 1H), 7.61 (t, J = 8.0 Hz, 1H), 7.03–7.00 (m, 2H), 5.01 (t, J = 6.0 Hz, 1H), 4.11 (t, J = 4.0 Hz, 2H), 3.76 (q, J = 4.0 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ = 161.16, 161.07, 152.52, 135.90, 135.23, 130.19, 128.61, 125.23, 122.42, 119.67, 113.25, 111.05, 102.43, 70.65, 59.87; HRMS (ESI) m/z calcd for C15H13O4+[M + H]+: 257.0736; found: 257.0732. Chromatographic purity: 98.3% (HPLC).
3-((Tetrahydrofuran-2-yl)methoxy)-6H-benzo[c]chromen-6-one (1n): Using 2-(bromomethyl)tetrahydrofuran as the starting material, the desired yellow solid 1n was isolated (1.31 g, 47%). M.p. 82.8–84.1 °C. 1H NMR (400 MHz, CDCl3) δ = 8.36 (d, J = 8.0 Hz, 1H), 8.00 (d, J = 8.0 Hz, 1H), 7.94 (d, J = 8.0 Hz, 1H), 7.79 (t, J = 8.0 Hz, 1H), 7.51 (t, J = 8.0 Hz, 1H), 6.96 (dd, J = 8.0, 4.0 Hz, 1H), 6.89 (d, J = 4.0 Hz, 1H), 4.37–4.30 (m, 1H), 4.08–3.95 (m, 3H), 3.91–3.85 (q, J = 8.0 Hz, 1H), 2.18–2.09 (m, 1H), 2.06–1.94 (m, 2H), 1.85–1.76 (m, 1H); 13C NMR (100 MHz, CDCl3) δ = 161.55, 160.74, 152.49, 135.15, 134.88, 130.54, 127.76, 123.77, 121.11, 119.97, 112.90, 111.28, 102.27, 76.85, 70.86, 68.72, 28.19, 25.76; HRMS (ESI) m/z calcd for C18H17O4+[M + H]+: 297.1049; found: 297.1045. Chromatographic purity: 99.1% (HPLC).
3-((1,3-Dioxolan-2-yl)methoxy)-6H-benzo[c]chromen-6-one (1o): Using 2-(bromomethyl)-1,3-dioxolane as the starting material, the desired white solid 1o was isolated 1.93 g, 69%). M.p. 135.6–137.8 °C. 1H NMR (400 MHz, CDCl3) δ = 8.35 (d, J = 8.0 Hz, 1H), 8.00 (d, J = 8.0 Hz, 1H), 7.94 (d, J = 8.0 Hz, 1H), 7.81–7.77 (m, 1H), 7.51 (t, J = 8.0 Hz, 1H), 6.96 (dd, J = 12.0, 4.0 Hz, 1H), 6.90 (d, J = 4.0 Hz, 1H), 5.34 (t, J = 4.0 Hz, 1H), 4.14–3.96 (m, 6H); 13C NMR (100 MHz, CDCl3) δ = 161.45, 160.29, 152.43, 135.02, 134.90, 130.54, 127.86, 123.84, 121.14, 120.01, 112.74, 111.61, 102.49, 101.66, 69.01, 65.41; HRMS (ESI) m/z calcd for C17H15O5+[M + H]+: 299.0841; found: 299.0839. Chromatographic purity: 98.6% (HPLC).
3-((3,5-Dimethoxybenzyl)oxy)-6H-benzo[c]chromen-6-one (1p): Using 1-(bromomethyl)-3,5-dimethoxybenzene as the starting material, the desired white solid 1p was isolated (3.37 g, 99%). M.p. 133.8–135.0 °C. 1H NMR (300 MHz, DMSO-d6) δ = 8.23–8.11 (m, 3H), 7.82 (t, J = 9.0 Hz, 1H), 7.52 (t, J = 7.5 Hz, 1H), 7.00– 6.97 (m, 2H), 6.58 (d, J = 3.0 Hz, 2H), 6.40 (t, J = 3.0 Hz, 1H), 5.09 (s, 2H), 3.68 (s, 6H); 13C NMR (75 MHz, DMSO-d6) δ = 161.03, 160.94, 160.56, 152.43, 139.24, 135.76, 135.13, 130.13, 128.58, 125.22, 122.43, 119.73, 113.39, 111.34, 106.01, 102.87, 99.98, 70.04, 55.65; HRMS (ESI) m/z calcd for C22H19O5+[M + H]+: 363.1154; found: 363.1151. Chromatographic purity: 98.4% (HPLC).
3-((1,3-Dimethyl-1H-pyrazol-5-yl)methoxy)-6H-benzo[c]chromen-6-one (1q): Using 5-(bromomethyl)-1,3-dimethyl-1H-pyrazole as the starting material, the desired whitesolid 1q was isolated (2.92 g, 97%). M.p. 165.1–167.6 °C. 1H NMR (300 MHz, CDCl3) δ = 8.34 (dd, J = 9.0, 1.5 Hz, 1H), 8.00–7.93 (m, 2H), 7.82–7.76 (m, 1H), 7.54–7.49 (m, 1H), 6.95 (m, 2H), 6.16 (s, 1H), 5.07 (s, 2H), 3.87 (s, 3H), 2.26 (s, 3H); 13C NMR (75 MHz, CDCl3) δ = 161.39, 159.70, 152.41, 147.46, 136.99, 134.98, 134.89, 130.57, 128.02, 124.00, 121.17, 120.01, 112.96, 111.84, 107.14, 102.50, 60.81, 36.53, 13.44; HRMS (ESI) m/z calcd for C19H17N2O3+[M + H]+: 321.1161; found: 321.1158. Chromatographic purity: 97.1% (HPLC).
3-(Pyridin-3-ylmethoxy)-6H-benzo[c]chromen-6-one (1r): Using 3-(bromomethyl)pyridine as the starting material, the desired yellow solid 1r was isolated (1.42 g, 50%). M.p. 143.6–145.3 °C. 1H NMR (300 MHz, CDCl3) δ = 8.69 (d, J = 30.0 Hz, 2H), 8.35 (dd, J = 9.0, 3.0 Hz, 1H), 7.98 (dd, J = 12.0,6.0 Hz, 2H), 7.853–7.76 (m, 2H), 7.51 (t, J = 7.5 Hz, 1H), 7.37 (dd, J = 9.0, 3.0 Hz, 1H), 6.98 (dd, J = 9.0, 3.0 Hz, 1H), 6.97 (d, J = 3.0 Hz,1H), 5.15 (s, 2H); 13C NMR (75 MHz, CDCl3) δ = 161.39, 160.06, 152.50, 149.79, 149.07, 135.42, 134.95, 134.10, 131.74, 130.58, 127.97, 124.00, 123.65, 121.17, 120.03, 112.92, 111.75, 102.64, 67.94; HRMS (ESI) m/z calcd for C19H14NO3+[M + H]+: 304.0895; found: 304.0891. Chromatographic purity: 96.2% (HPLC).
3-(Cyclopentylmethoxy)-6H-benzo[c]chromen-6-one (1s): Using (bromomethyl)cyclopentane as the starting material, the desired yellow solid 1s was isolated (2.13 g, 77%). M.p. 90.6–91.2 °C. 1H NMR (300 MHz, CDCl3) δ = 8.34 (dd, J = 9.0, 1.5 Hz, 1H), 7.98 (dd, J = 6.0, 1.2 Hz, 1H), 7.91 (d, J = 9.0 Hz, 1H), 7.79–7.74 (m, 1H), 7.51–7.45 (m, 1H), 6.90 (dd, J = 9.0, 3.0 Hz, 1H), 6.83 (d, J = 3.0 Hz, 1H), 3.89 (d, J = 6.0 Hz, 2H), 2.47 (m, 1H), 1.96–1.79 (m, 2H), 1.752–1.55 (m, 4H), 1.44–1.32 (m, 2H); 13C NMR (75 MHz, CDCl3) δ = 161.61, 161.19, 152.56, 135.25, 134.85, 130.52, 127.61, 123.67, 121.05, 119.89, 112.87, 110.87, 102.12, 72.67, 38.90, 29.48, 25.45; HRMS (ESI) m/z calcd for C19H19O3+[M + H]+: 295.1256; found: 295.1251. Chromatographic purity: 98.4% (HPLC).
3-((4-[Hydroxymethyl]benzyl)oxy)-6H-benzo[c]chromen-6-one (1t): Using (4-(bromomethyl)phenyl)methanol as the starting material, the desired white solid 1t was isolated (2.40 g, 77%). M.p. 147.0–149.2 °C. 1H NMR (300 MHz, DMSO-d6) δ = 8.33 (d, J = 9.0 Hz, 1H), 8.29–8.21 (m, 2H), 7.96–7.90 (m, 1H), 7.65–7.60 (m, 1H), 7.49 (d, J = 9.0 Hz, 2H), 7.40 (d, J = 9.0 Hz, 2H), 7.12–7.07 (m, 2H), 5.29 (t, J = 9.0 Hz, 1H), 5.24 (s, 2H), 4.55 (d, J = 6.0 Hz, 2H); 13C NMR (75 MHz, DMSO-d6) δ = 160.95, 160.68, 152.45, 142.94, 135.79, 135.17, 130.15, 128.58, 128.24, 127.02, 125.21, 122.44, 119.72, 113.45, 111.27, 102.87, 70.14, 63.10; HRMS (ESI) m/z calcd for C21H17O4+[M + H]+: 333.1049; found: 333.1044.Chromatographic purity: 97.2% (HPLC).
3-(2-Morpholinoethoxy)-6H-benzo[c]chromen-6-one (1u): Using 4-(2-bromoethyl)morpholine as the starting material, the desired yellow solid 1u was isolated (1.22 g, 40%). M.p. 100.8–102.8 °C. 1H NMR (300 MHz, CDCl3) δ = 8.35 (dd, J = 9.0, 1.2 Hz, 1H), 7.96 (dd, J = 18.0, 6.0 Hz, 2H), 7.78 (m, 1H), 7.50 (t, J = 7.5 Hz, 1H), 6.94–6.90 (dd, J = 9.0, 3.0 Hz, 2H), 4.18 (t, J = 6.0 Hz, 2H), 3.76 (t, J = 7.5 Hz, 4H), 2.85 (t, J = 4.5 Hz, 2H), 2.61 (t, J = 4.5 Hz, 4H); 13C NMR (75 MHz, CDCl3) δ = 161.50, 160.55, 152.52, 135.09, 134.91, 130.55, 127.80, 123.80, 121.10, 119.96, 112.88, 111.29, 102.25, 66.91, 66.25, 57.43, 54.13; HRMS (ESI) m/z calcd for C19H20NO4+[M + H]+: 326.1314; found: 326.1310. Chromatographic purity: 99.2% (HPLC).
3.1.4. General Procedures for Synthesis of Compounds 2a–w
The anhydrous DMF (30 mL) was added to a 250 mL round bottom flask, compound II (9.4 mmol, 2.28 g), anhydrous K2CO3 (12.2 mmol, 1.7 g) and halides (12.2 mmol) were added, and the temperature was controlled at 80~120 °C, the reaction was stirred for 24 h. The reaction was monitored by TLC (PE:EA = 3:1). When the reaction was over, the reaction solution was to the ice-water mixture to obtain brown crude solid. After suction filtration and drying, the brown crude solid was purified by column chromatography to obtain compounds 2a–w.
3,8-Dimethoxy-6H-benzo[c]chromen-6-one (2a): Using iodomethane as the starting material, the desired white solid 2a was isolated (1.23 g, 51%). M.p. 146.2–148.8 °C. 1H NMR (400 MHz, DMSO-d6) δ = 8.22 (d, J = 9.0 Hz, 1H), 8.14 (d, J = 9.0 Hz, 1H), 7.58 (d, J = 3.0 Hz, 1H), 7.47 (dd, J = 9.0, 3.0 Hz, 1H), 6.96 (s, 1H), 6.94 (t, J = 4.0 Hz, 1H), 3.89 (s, 3H), 3.84 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ = 160.85, 160.83, 159.23, 151.55, 128.55, 12.50, 124.34, 124.31, 120.79, 112.69, 111.30, 111.21, 101.79, 56.15, 56.04; HRMS (ESI) m/z calcd for C15H13O4+[M + H]+: 257.0736; found: 257.0731. Chromatographic purity: 99.2% (HPLC).
3-Ethoxy-8-methoxy-6H-benzo[c]chromen-6-one (2b): Using bromoethane as the starting material, the desired white solid 2b was isolated(1.14 g, 45%). M.p. 141.1–143.8 °C. 1H NMR (400 MHz, DMSO-d6) δ = 8.26 (d, J = 8.0 Hz, 1H), 8.17 (d, J = 8.0 Hz, 1H), 7.62 (d, J = 4.0 Hz, 1H), 7.51 (dd, J = 8.0, 4.0 Hz, 1H), 6.98 (s, 1H), 6.96 (t, J = 4.0 Hz, 1H), 4.13 (q, J = 8.0 Hz, 2H), 3.90 (s, 3H), 1.37 (t, J = 8.0 Hz, 3H); 13C NMR (75 MHz, DMSO-d6) δ = 160.89, 160.10, 159.23, 151.55, 128.61, 124.52, 124.39, 124.32, 120.78, 113.03, 111.32, 111.10, 102.20, 64.19, 56.05, 14.95; HRMS (ESI) m/z calcd for C16H15O4+[M + H]+: 271.0892; found: 271.0890. Chromatographic purity: 98.1% (HPLC).
8-Methoxy-3-propoxy-6H-benzo[c]chromen-6-one (2c): Using 1-bromopropane as the starting material, the desired white solid 2c was isolated (1.39 g, 52%). M.p. 109.3–111.6 °C. 1H NMR (400 MHz, DMSO-d6) δ = 8.18 (d, J = 8.0 Hz, 1H), 8.09 (d, J = 8.0 Hz, 1H), 7.56 (d, J = 4.0 Hz, 1H), 7.45 (dd, J = 8.0, 4.0 Hz, 1H), 6.94–6.90 (m, 2H), 3.99 (t, J = 6.0 Hz, 2H), 3.88 (s, 3H), 1.75 (m, 2H), 1.00 (t, J = 8.0 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ = 160.86, 160.23, 159.20, 151.52, 128.58, 124.46, 124.33, 124.27, 120.75, 113.00, 111.29, 111.08, 102.20, 69.95, 56.02, 22.37, 10.81; HRMS (ESI) m/z calcd for C17H17O4+[M + H]+: 285.1049; found: 285.1045. Chromatographic purity: 99.3% (HPLC).
3-(Sec-butoxy)-8-methoxy-6H-benzo[c]chromen-6-one (2d): Using 2-bromobutane as the starting material, the desired yellow solid 2d was isolated (0.95 g, 34%). M.p. 64.5–66.7 °C. 1H NMR (400 MHz, DMSO-d6) δ = 8.21 (d, J = 8.0 Hz, 1H), 8.12 (d, J = 8.0 Hz, 1H), 7.59 (d, J = 4.0 Hz, 1H), 7.47 (dd, J = 8.0, 4.0 Hz, 1H), 6.95 (s, 1H), 6.92 (d, J = 4.0 Hz, 1H), 4.56–4.49 (m, 1H), 3.89 (s, 3H), 1.73–1.57 (m, 2H), 1.27 (d, J = 6.0 Hz, 3H), 0.94 (t, J = 8.0 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ = 160.89, 159.40, 159.20, 151.62, 128.62, 124.57, 124.35, 124.27, 120.77, 113.84, 111.32, 111.01, 103.23, 75.18, 56.03, 28.90, 19.37, 9.94; HRMS (ESI) m/z calcd for C18H19O4+[M + H]+: 299.1205; found: 299.1201. Chromatographic purity: 98.7% (HPLC).
3-Butoxy-8-methoxy-6H-benzo[c]chromen-6-one (2e): Using 1-bromobutane as the starting material, the desired white solid 2e was isolated (1.82 g, 65%). M.p. 102.5–104.4 °C. 1H NMR (300 MHz, DMSO-d6) δ = 8.26 (d, J = 9.0 Hz, 1H), 8.19–8.15 (m, 1H), 7.62 (d, J = 3.0 Hz, 1H), 7.51 (dd, J = 9.0, 3.0 Hz, 1H), 6.98 (s, 1H), 6.96 (t, J = 4.5 Hz, 1H), 4.07 (t, J = 6.0 Hz, 2H), 3.90 (s, 3H), 1.78–1.68 (m, 2H), 1.52–1.3840 (m, 2H), 0.95 (t, J = 7.5 Hz, 3H); 13C NMR (75 MHz, DMSO-d6) δ = 160.84, 160.25, 159.20, 151.52, 128.58, 124.43, 124.29, 124.24, 120.75, 112.99, 111.31, 111.08, 102.21, 68.21, 56.02, 31.06, 19.17, 14.14; HRMS (ESI) m/z calcd for C18H19O4+[M + H]+: 299.1205; found: 299.1201. Chromatographic purity: 99.1% (HPLC).
8-Methoxy-3-(pentyloxy)-6H-benzo[c]chromen-6-one (2f): Using 1-bromopentane as the starting material, the desired white solid 2f was isolated (1.34 g, 46%). M.p. 73.1–75.5 °C. 1H NMR (300 MHz, DMSO-d6) δ = 8.23 (d, J = 9.0 Hz, 1H), 8.15–8.12 (m, 1H), 7.59 (d, J = 3.0 Hz, 1H), 7.49 (dd, J = 8.0, 3.0 Hz, 1H), 6.97–6.93 (m, 2H), 4.03 (t, J = 7.5 Hz, 2H), 3.89 (s, 3H), 1.78–1.69 (m, 2H), 1.44–1.34 (m, 4H), 0.93–0.88 (m, 3H); 13C NMR (75 MHz, DMSO-d6) δ = 160.89, 160.26, 159.22, 151.54, 128.62, 124.50, 124.39, 124.32, 120.77, 113.05, 111.29, 111.09, 102.21, 68.49, 56.04, 28.70, 28.13, 22.37, 14.40; HRMS (ESI) m/z calcd for C19H21O4+[M + H]+: 313.1362; found: 313.1359. Chromatographic purity: 98.5% (HPLC).
3-Isopropoxy-8-methoxy-6H-benzo[c]chromen-6-one (2g): Using 2-bromopropane as the starting material, the desired white solid 2g was isolated (0.97 g, 36%). M.p. 88.3–90.7 °C. 1H NMR (300 MHz, DMSO-d6) δ = 8.21 (d, J = 9.0 Hz, 1H), 8.14–8.11 (m, 1H), 7.58 (d, J = 3.0 Hz, 1H), 7.48 (dd, J = 9.0, 3.0 Hz, 1H), 6.95–6.91 (m, 2H), 4.75 (m, 1H), 3.89 (s, 3H), 1.32 (d, J = 6.0 Hz, 6H); 13C NMR (75 MHz, DMSO-d6) δ = 160.89, 159.19, 159.05, 151.60, 128.60, 124.55, 124.34, 124.26, 120.76, 113.81, 111.30, 110.98, 103.20, 70.33, 56.03, 22.14; HRMS (ESI) m/z calcd for C17H17O4+[M + H]+: 285.1049; found: 285.1045. Chromatographic purity: 98.6% (HPLC).
3-(2-Hydroxyethoxy)-8-methoxy-6H-benzo[c]chromen-6-one (2h): Using 2-bromoethanol as the starting material, the desired yellow solid 2h was isolated (1.51 g, 56%). M.p. 166.8–168.0 °C. 1H NMR (300 MHz, DMSO-d6) δ = 8.21 (d, J = 9.0 Hz, 1H), 8.15–8.11 (m, 1H), 7.57 (d, J = 3.0 Hz, 1H), 7.47 (dd, J = 9.0, 3.0 Hz, 1H), 6.98–6.94 (m, 2H), 4.98 (t, J = 6.0 Hz, 1H), 4.08 (dd, J = 6.0, 3.0 Hz, 2H), 3.89 (s, 3H), 3.77 (q, J = 6.0 Hz, 2H); 13C NMR (75 MHz, DMSO-d6) δ = 160.86, 160.25, 159.19, 151.50, 128.55, 124.48, 124.33, 124.26, 120.76, 113.05, 111.26, 111.14, 102.28, 70.56, 59.89, 56.02; HRMS (ESI) m/z calcd for C16H15O5+[M + H]+: 287.0841; found: 287.0837. Chromatographic purity: 98.8% (HPLC).
8-Methoxy-3-(2-(methylthio)ethoxy)-6H-benzo[c]chromen-6-one (2i): Using (2-bromoethyl)(methyl)sulfane as the starting material, the desired white solid 2i was isolated (1.18 g, 40%). M.p. 110.1–111.8 °C. 1H NMR (300 MHz, DMSO-d6) δ = 8.27 (d, J = 9 Hz, 1H), 8.19 (d, J = 9.0 Hz, 1H), 7.62 (d, J = 3.0 Hz, 1H), 7.52 (dd, J = 9.0, 3.0 Hz, 1H), 7.03–6.98 (m, 2H), 4.26 (t, J = 6.0 Hz, 2H), 3.90 (s, 3H), 2.89 (t, J = 7.5 Hz, 2H), 2.18 (s, 3H); 13C NMR (75 MHz, CDCl3) δ = 165.60, 164.53, 164.05, 156.28, 133.29, 129.33, 129.13, 125.61, 117.84, 116.16, 116.12, 107.21, 72.70, 60.82, 37.28, 20.45; HRMS (ESI) m/z calcd for C17H17O4S+[M + H]+: 317.0769; found: 317.0765. Chromatographic purity: 97.8% (HPLC).
Ethyl 2-((8-methoxy-6-oxo-6H-benzo[c]chromen-3-yl)oxy)acetate (2j): Using ethyl 2-bromoacetate as the starting material, the desired white solid 2j was isolated (1.58 g, 51%). M.p. 151.5–152.8 °C. 1H NMR (300 MHz, DMSO-d6) δ = 8.28 (d, J = 9.0 Hz, 1H), 8.21 (t, J = 6.0 Hz, 1H), 7.62 (d, J = 3.0 Hz, 1H), 7.52 (dd, J = 9.0, 3.0 Hz, 1H), 7.03– 6.99 (m, 2H), 4.92 (s, 2H), 4.20 (q, J = 6.0 Hz, 2H), 3.90 (s, 3H), 1.23 (t, J = 7.5 Hz, 3H); 13C NMR (75 MHz, DMSO-d6) δ = 168.85, 160.80, 159.42, 159.11, 151.39, 128.39, 124.63, 124.48, 124.40, 120.98, 113.01, 111.93, 111.42, 102.75, 65.41, 61.25, 56.09, 14.52; HRMS (ESI) m/z calcd for C18H17O6+[M + H]+: 329.0947; found: 329.0943. Chromatographic purity: 97.4% (HPLC).
3-(Cyclopentylmethoxy)-8-methoxy-6H-benzo[c]chromen-6-one (2k): Using (bromomethyl)cyclopentane as the starting material, the desired white solid 2k was isolated (0.90 g, 30%). M.p. 146.1–148.2 °C. 1H NMR (300 MHz, DMSO-d6) δ = 8.26 (d, J = 9.0 Hz, 1H), 8.17 (t, J = 4.5 Hz, 1H), 7.62 (d, J = 3.0 Hz, 1H), 7.51 (dd, J = 9.0, 3.0 Hz, 1H), 6.99–6.95 (m, 2H), 3.94 (d, J = 6.0 Hz, 2H), 3.90 (s, 3H), 2.38–2.28 (m, 1H), 1.82–1.74 (m, 2H), 1.65–1.51 (m, 4H), 1.38–1.27 (m, 2H); 13C NMR (75 MHz, DMSO-d6) δ = 160.92, 160.44, 159.28, 151.59, 128.66, 124.55, 124.44, 124.39, 120.83, 113.15, 111.39, 111.15, 102.35, 72.61, 56.09, 38.88, 29.44, 25.41; HRMS (ESI) m/z calcd for C20H21O4+[M + H]+: 325.1362; found: 325.1360. Chromatographic purity: 98.1% (HPLC).
8-Methoxy-3-((tetrahydrofuran-2-yl)methoxy)-6H-benzo[c]chromen-6-one (2l): Using 2-(bromomethyl)tetrahydrofuran as the starting material, the desired white solid 2l was isolated (1.71 g, 56%). M.p. 113.1–115.5 °C. 1H NMR (300 MHz, DMSO-d6) δ = 8.27 (d, J = 9.0 Hz, 1H), 8.18 (dd, J = 9.0, 3.0 Hz, 1H), 7.62 (d, J = 3.0 Hz, 1H), 7.51 (dd, J = 9.0, 3.0 Hz, 1H), 7.03–6.94 (m, 2H), 4.23–4.15 (m, 1H), 4.11–3.98 (m, 2H), 3.90 (s, 3H), 3.84–3.77 (m, 1H), 3.73–3.66 (m, 1H), 2.08–1.99 (m, 1H), 1.94–1.77 (m, 2H), 1.72–1.65 (m, 1H); 13C NMR (75 MHz, DMSO-d6) δ = 160.86, 160.14, 159.27, 151.51, 128.57, 124.52, 124.36, 120.83, 113.09, 111.35, 111.29, 102.39, 76.81, 71.08, 67.97, 56.06, 28.06, 25.67; HRMS (ESI) m/z calcd for C19H19O5+[M + H]+: 327.1154; found: 327.1150. Chromatographic purity: 99.2% (HPLC).
3-((1,3-Dioxolan-2-yl)methoxy)-8-methoxy-6H-benzo[c]chromen-6-one (2m): Using 2-(bromomethyl)-1,3-dioxolane as the starting material, the desired white solid 2m was isolated (0.93 g, 30%). M.p. 147.9–150.1 °C. 1H NMR (300 MHz, DMSO-d6) δ = 8.28 (d, J = 9.0 Hz, 1H), 8.19 (d, J = 9.0 Hz, 1H), 7.62 (d, J = 3.0 Hz, 1H), 7.52 (dd, J = 9.0, 3.0 Hz, 1H), 7.04–6.99 (m, 2H), 5.24 (t, J = 3.0 Hz, 1H), 4.12 (d, J = 6.0 Hz, 2H), 4.01–3.96 (m, 2H), 3.90 (s, 3H), 3.89– 3.85 (m, 2H); 13C NMR (75 MHz, DMSO-d6) δ = 160.87, 159.76, 159.38, 151.49, 128.52, 124.63, 124.48, 124.45, 120.94, 113.12, 111.62, 111.41, 102.62, 101.57, 69.02, 65.03, 56.10; HRMS (ESI) m/z calcd for C18H17O6+[M + H]+: 329.0947; found:329.0943. Chromatographic purity: 97.6% (HPLC).
3-(Cyclohexylmethoxy)-8-methoxy-6H-benzo[c]chromen-6-one (2n): Using (bromomethyl)cyclohexane as the starting material, the desired white solid 2n was isolated (1.85 g, 38%). M.p. 139.8–140.1 °C. 1H NMR (300 MHz, DMSO-d6) δ = 8.25 (d, J = 9.0 Hz, 1H), 8.18–8.14 (m, 1H), 7.61 (d, J = 3.0 Hz, 1H), 7.50 (dd, J = 9.0, 3.0 Hz, 1H), 6.99–6.95 (m, 2H), 3.90 (s, 3H), 3.87 (d, J = 6.3 Hz, 2H), 1.84–1.65 (m, 6H), 1.33–1.04 (m, 5H); 13C NMR (75 MHz, DMSO-d6) δ = 160.91, 160.43, 159.26, 151.58, 128.66, 124.54, 124.42, 124.37, 120.81, 113.10, 111.37, 111.12, 102.33, 73.67, 56.08, 37.43, 29.65, 26.49, 25.70; HRMS (ESI) m/z calcd for C21H23O4+[M + H]+: 339.1518; found: 339.1514. Chromatographic purity: 97.5% (HPLC).
3-(2-(1,3-Dioxolan-2-yl)ethoxy)-8-methoxy-6H-benzo[c]chromen-6-one (2o): Using 2-(2-bromoethyl)-1,3-dioxolane as the starting material, the desired white solid 2o was isolated (1.22 g, 38%). M.p. 151.6–153.2 °C. 1H NMR (300 MHz, DMSO-d6) δ = 8.27 (d, J = 9.0 Hz, 1H), 8.20–8.17 (m, 1H), 7.62 (d, J = 3.0 Hz, 1H), 7.51 (dd, J = 9.0, 3.0 Hz, 1H), 7.00–6.96 (m, 2H), 5.02 (t, J = 4.5 Hz, 1H), 4.18 (t, J = 6.0 Hz, 2H), 3.95–3.91 (m, 2H), 3.90 (s, 3H), 3.83–3.79 (m, 2H), 2.07 (m, 2H); 13C NMR (75 MHz, CDCl3) δ = 161.64, 159.88, 159.13, 151.57, 128.66, 124.48, 123.11, 122.82, 120.96, 112.70, 111.33, 110.92, 102.20, 101.86, 65.02, 64.08, 55.76, 33.59, 29.73; HRMS (ESI) m/z calcd for C19H19O6+[M + H]+: 343.1103; found: 343.1101. Chromatographic purity: 96.9% (HPLC).
8-Methoxy-3-((tetrahydro-2H-pyran-4-yl)methoxy)-6H-benzo[c]chromen-6-one (2p): Using 4-(bromomethyl)tetrahydro-2H-pyran as the starting material, the desired yellow solid 2p was isolated (1.79 g, 36%). M.p. 168.1–168.5 °C. 1H NMR (400 MHz, CDCl3) δ = 7.94 (d, J = 8.0 Hz, 1H), 7.87 (d, J = 8.0 Hz, 1H), 7.78 (d, J = 4.0 Hz, 1H), 7.39 (dd, J = 8.0, 4.0 Hz, 1H), 6.91 (dd, J = 8.0, 4.0 Hz, 1H), 6.86 (d, J = 4.0 Hz, 1H), 4.06 (dd, J = 12.0, 4.0 Hz, 2H), 3.94 (s, 3H), 3.88 (d, J = 8.0 Hz, 2H), 3.51–3.45 (m, 2H), 2.19–2.0 (m, 1H), 1.82–1.78 (m, 2H), 1.56–1.46 (m, 2H); 13C NMR (75 MHz, DMSO-d6) δ = 160.91, 160.29, 159.31, 151.59, 128.64, 124.60, 124.45, 124.41, 120.86, 113.14, 111.41, 111.25, 102.42, 72.99, 67.06, 56.10, 34.79, 29.62; HRMS (ESI) m/z calcd for C20H21O5+[M + H]+: 341.1311; found: 341.1307. Chromatographic purity: 97.4% (HPLC).
3-(Benzyloxy)-8-methoxy-6H-benzo[c]chromen-6-one (2q): Using (bromomethyl)benzene as the starting material, the desired white solid 2q was isolated (1.04 g, 33%). M.p. 141.7–142.5 °C. 1H NMR (300 MHz, DMSO-d6) δ = 8.27 (d, J = 9.0 Hz, 1H), 8.20 (d, J = 9.0 Hz, 1H), 7.62 (d, J = 3.0 Hz, 1H), 7.51–7.48 (m, 3H), 7.45–7.33 (m, 3H), 7.10–7.04 (m, 2H), 5.22 (s, 2H), 3.90 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δ = 162.53, 159.77, 158.99, 151.43, 136.98, 128.96, 128.33, 124.30, 121.58, 120.60, 113.25, 111.25, 102.70, 70.15, 55.98; HRMS (ESI) m/z calcd for C21H17O4+[M + H]+: 333.1049; found: 333.1045. Chromatographic purity: 98.7% (HPLC).
3-((4-[Hydroxymethyl]benzyl)oxy)-8-methoxy-6H-benzo[c]chromen-6-one (2r): Using (4-(bromomethyl)phenyl)methanol as the starting material, the desired white solid 2r was isolated (1.26 g, 37%). M.p. 192.4–194.6 °C. 1H NMR (300 MHz, DMSO-d6) δ = 8.27 (d, J = 9.0 Hz, 1H), 8.19 (d, J = 9.0 Hz, 1H), 7.62 (d, J = 3.0 Hz, 1H), 7.51 (dd, J = 9.0, 3.0 Hz, 1H), 7.44 (dd, J = 6.0, 3.0 Hz, 2H), 7.35 (d, J = 8.0 Hz, 2H), 7.08–7.03 (m, 2H), 5.20 (t, J = 6.0 Hz, 3H), 4.51 (d, J = 3.0 Hz, 2H), 3.90 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δ = 160.86, 159.87, 159.32, 151.49, 142.91, 135.27, 128.55, 128.18, 127.01, 124.57, 124.40, 120.88, 113.43, 111.44, 111.39, 102.84, 70.11, 63.11, 56.08; HRMS (ESI) m/z calcd for C22H19O5+[M + H]+: 363.1154; found: 363.1150. Chromatographic purity: 98.6% (HPLC).
3-((3,5-Dimethoxybenzyl)oxy)-8-methoxy-6H-benzo[c]chromen-6-one (2s): Using 1-(bromomethyl)-3,5-dimethoxybenzene as the starting material, the desired white solid 2s was isolated (1.59 g, 43%). M.p. 153.6–155.3 °C. 1H NMR (300 MHz, CDCl3) δ = 7.89 (d, J = 9.0 Hz, 1H), 7.84 (d, J = 9.0 Hz, 1H), 7.73 (d, J = 3.0 Hz, 1H), 7.35 (dd, J = 9.0, 3.0 Hz, 1H), 6.96 (dd, J = 9.0, 3.0 Hz, 1H), 6.89 (d, J = 3.0 Hz, 1H), 6.59 (d, J = 3.0 Hz, 2H), 6.42 (t, J = 3.0 Hz, 1H), 5.05 (s, 2H), 3.91 (s, 3H), 3.80 (s, 6H); 13C NMR (75 MHz, DMSO-d6) δ = 161.04, 160.88, 160.82, 159.76, 159.33, 151.48, 139.36, 128.54, 124.62, 124.44, 120.90, 113.41, 111.51, 111.35, 106.65, 105.99, 102.85, 99.96, 70.00, 56.08, 55.67, 55.49; HRMS (ESI) m/z calcd for C23H21O6+[M + H]+: 393.1260; found: 393.1257. Chromatographic purity: 98.3% (HPLC).
3-((2-Fluorobenzyl)oxy)-8-methoxy-6H-benzo[c]chromen-6-one (2t):Using 1-(bromomethyl)-2-fluorobenzene as the starting material, the desired white solid 2t was isolated (2.47 g, 75%). M.p. 203.6–204.9 °C. 1H NMR (300 MHz, DMSO-d6) δ = 8.30 (d, J = 9.0 Hz, 1H), 8.23 (d, J = 9.0 Hz, 1H), 7.64–7.58 (m, 2H), 7.52 (dd, J = 9.0, 3.0 Hz, 1H), 7.49–7.42 (m, 1H), 7.33–7.24 (m, 2H), 7.15 (d, J = 3.0 Hz, 1H), 7.07 (dd, J = 9.0, 3.0 Hz, 1H), 5.25 (s, 2H), 3.91 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δ = 159.49, 159.27, 151.59, 130.17, 130.06, 129.80, 128.58, 124.55, 124.42, 124.37, 123.26, 122.89, 121.09, 115.70, 115.42, 112.90, 111.78, 111.00, 102.74, 64.17, 55.79; HRMS (ESI) m/z calcd for C21H16FO4+[M + H]+: 351.0954; found: 351.0950. Chromatographic purity: 97.6% (HPLC).
3-((1,3-Dimethyl-1H-pyrazol-5-yl)methoxy)-8-methoxy-6H-benzo[c]chromen-6-one (2u): Using 5-(bromomethyl)-1,3-dimethyl-1H-pyrazole as the starting material, the desired white solid 2u was isolated (1.71 g, 52%). M.p. 184.6–186.6 °C. 1H NMR (300 MHz, DMSO-d6) δ = 8.29 (d, J = 9.0 Hz, 1H), 8.21 (d, J = 9.0 Hz, 1H), 7.63 (d, J = 3.0 Hz, 1H), 7.52 (dd, J = 9.0, 3.0 Hz, 1H), 7.15 (d, J = 3.0 Hz, 1H), 7.07 (dd, J = 9.0, 3.0 Hz, 1H), 6.19 (s, 1H), 5.23 (s, 2H), 3.90 (s, 3H), 3.76 (s, 3H), 2.12 (s, 3H); 13C NMR (75 MHz, CDCl3) δ = 165.59, 164.15, 164.09, 156.20, 150.86, 142.74, 133.24, 129.37, 129.23, 129.16, 125.71, 118.13, 116.53, 116.17, 111.87, 107.73, 65.66, 60.85, 18.37; HRMS (ESI) m/z calcd for C21H19N2O4+[M + H]+: 351.1267; found: 351.1263. Chromatographic purity: 98.7% (HPLC).
8-Methoxy-3-(pyrimidin-2-yloxy)-6H-benzo[c]chromen-6-one (2v): Using 2-bromopyrimidine as the starting material, the desired white solid 2v was isolated (1.29 g, 23%). M.p. 212.3–214.0 °C. 1H NMR (300 MHz, DMSO-d6) δ = 8.69 (d, J = 6.0 Hz, 2H), 8.38 (dd, J = 9.0, 6.0 Hz, 2H), 7.68 (d, J = 3.0 Hz, 1H), 7.57 (dd, J = 9.0, 3.0 Hz, 1H), 7.38 (d, J = 3.0 Hz, 1H), 7.33 (t, J = 4.5 Hz, 1H), 7.27 (dd, J = 9.0, 3.0 Hz, 1H), 3.93 (s, 3H); 13C NMR (75 MHz, CDCl3) δ = 160.11, 158.86, 152.24, 149.99, 126.84, 123.51, 122.38, 122.22, 120.83, 117.40, 115.74, 114.80, 110.18, 109.98, 54.79; HRMS (ESI) m/z calcd for C18H13N2O4+[M + H]+: 321.0797; found: 321.0794. Chromatographic purity: 96.8% (HPLC).
3-(2-(1H-pyrazol-1-yl)ethoxy)-8-methoxy-6H-benzo[c]chromen-6-one (2w): Using 1-(2-bromoethyl)-1H-pyrazole as the starting material, the desired white solid 2w was isolated (1.93 g, 61%). M.p. 159.0–161.1 °C. 1H NMR (300 MHz, DMSO-d6) δ = 8.32 (d, J = 9.0 Hz, 1H), 8.22 (d, J = 9.0 Hz, 1H), 7.87 (d, J = 3.0 Hz, 1H), 7.67 (d, J = 3.0 Hz, 1H), 7.58–7.53 (m, 2H), 7.05–6.98 (m, 2H), 6.32 (t, J = 3.0 Hz, 1H), 4.59 (t, J = 6.0 Hz, 2H), 4.50 (t, J = 4.5 Hz, 2H), 3.95 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δ = 160.83, 159.59, 159.35, 151.49, 139.37, 131.06, 128.47, 124.61, 124.41, 120.90, 113.10, 111.60, 111.39, 105.70, 102.55, 67.55, 56.08, 50.95; HRMS (ESI) m/z calcd for C19H17N2O4+[M + H]+: 337.1110; found: 337.1106. Chromatographic purity: 98.9% (HPLC).