IKBKB siRNA-Encapsulated Poly (Lactic-co-Glycolic Acid) Nanoparticles Diminish Neuropathic Pain by Inhibiting Microglial Activation
Abstract
1. Introduction
2. Results
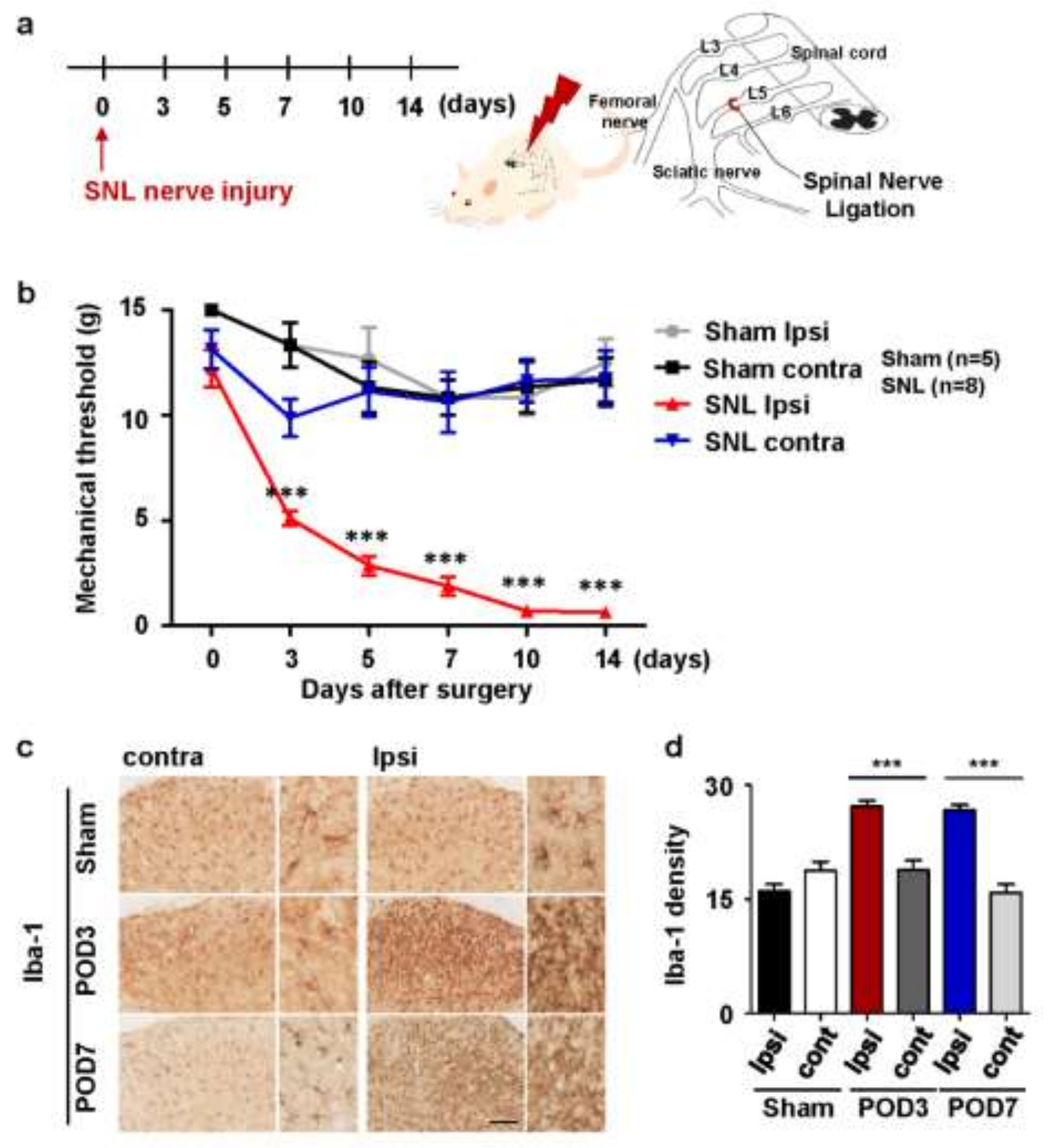
2.1. Upregulation of Microglial Activation in Rats with Spinal Nerve Ligation-Induced Neuropathic Pain
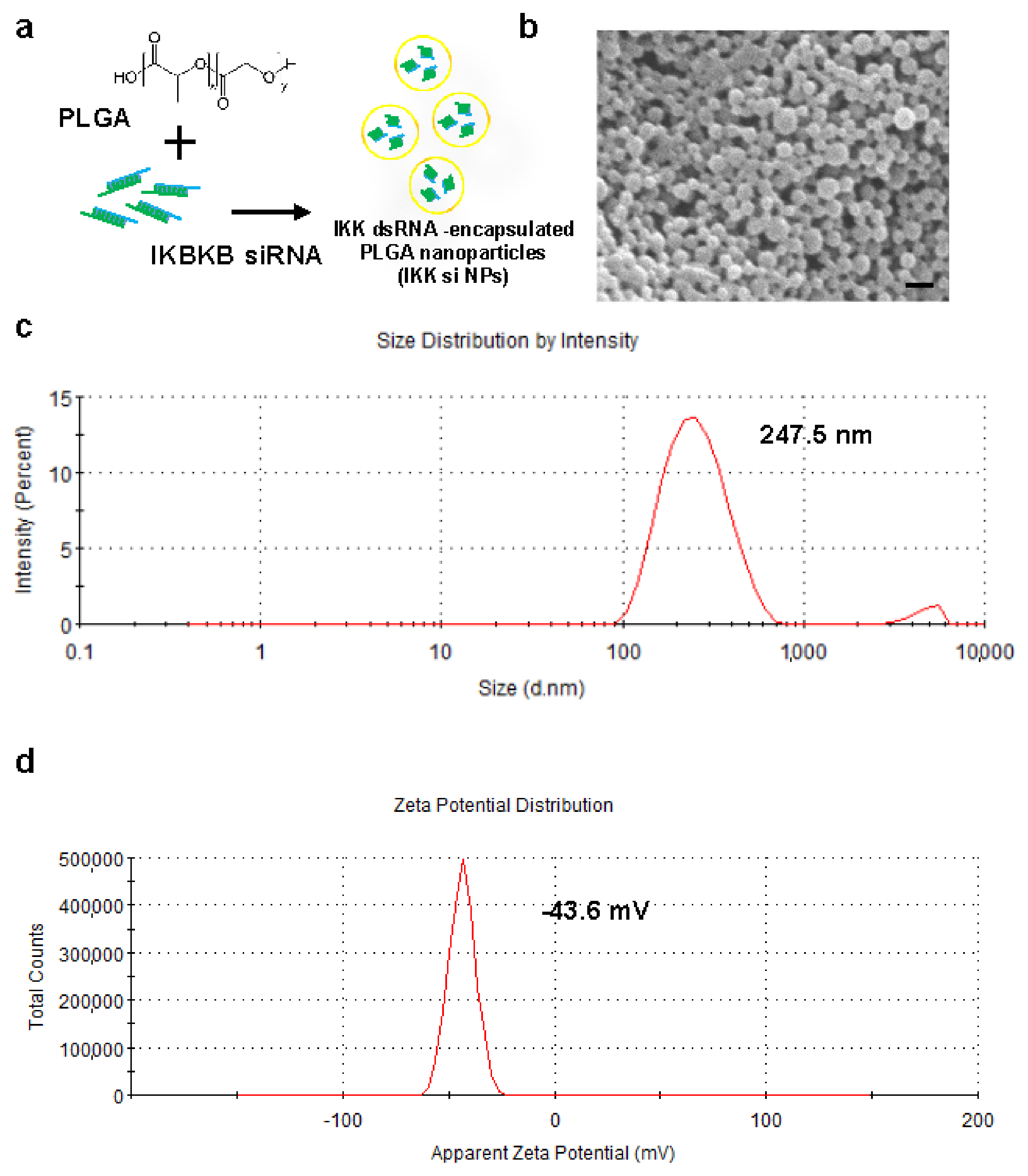
2.2. Preparation and Characterization of IKBKB siRNA-Encapsulated PLGA Nanoparticles
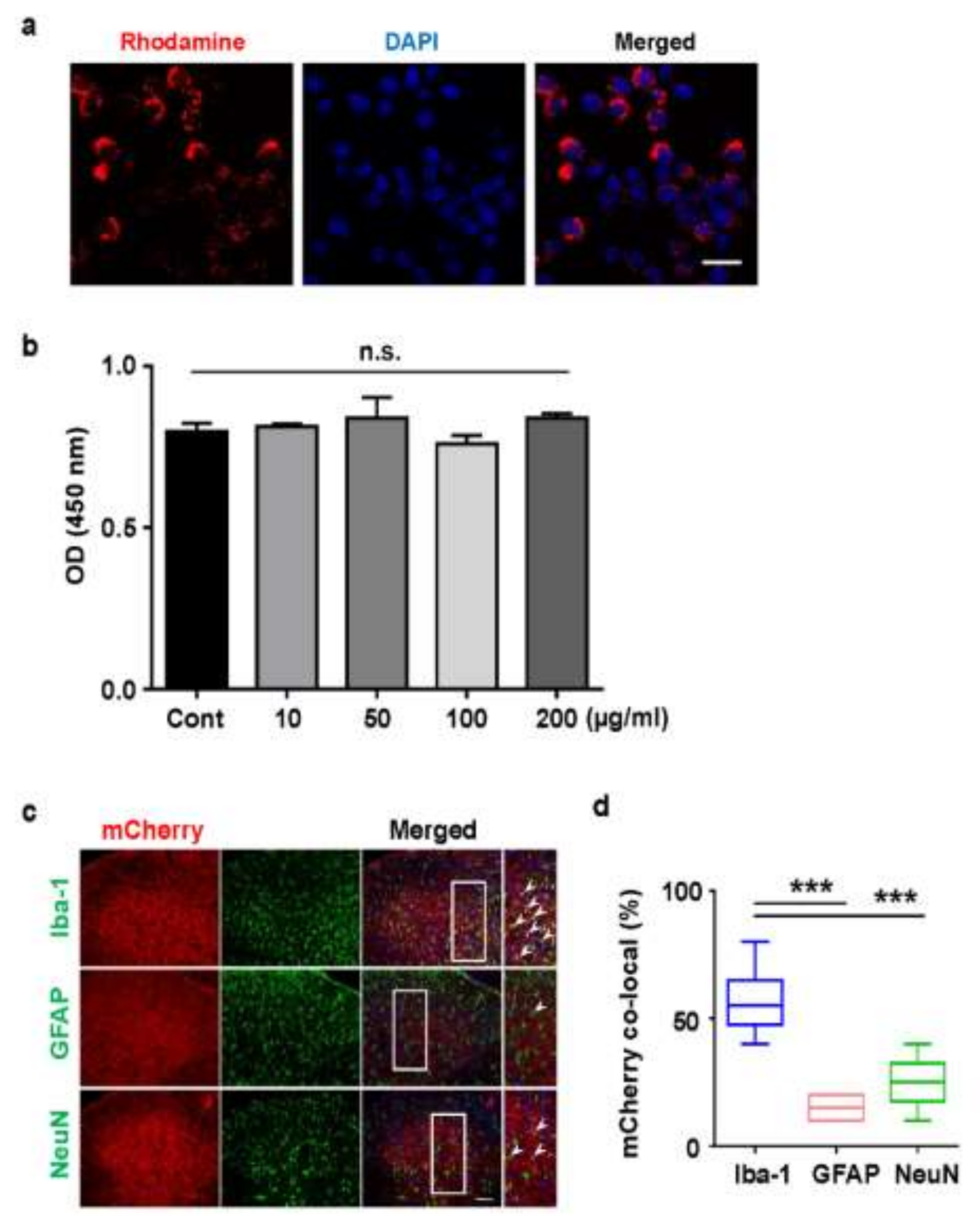
2.3. Cellular Uptake of Rhodamine Conjugated PLGA Nanoparticles and AAV-EF1α-mCherry Vector-Encapsulated PLGA Nanoparticles
2.4. Intrathecal Injection of IKBKB siRNA-Encapsulated PLGA Nanoparticles Diminishes Mechanical Hypersensitivity in SNL-Induced Rats
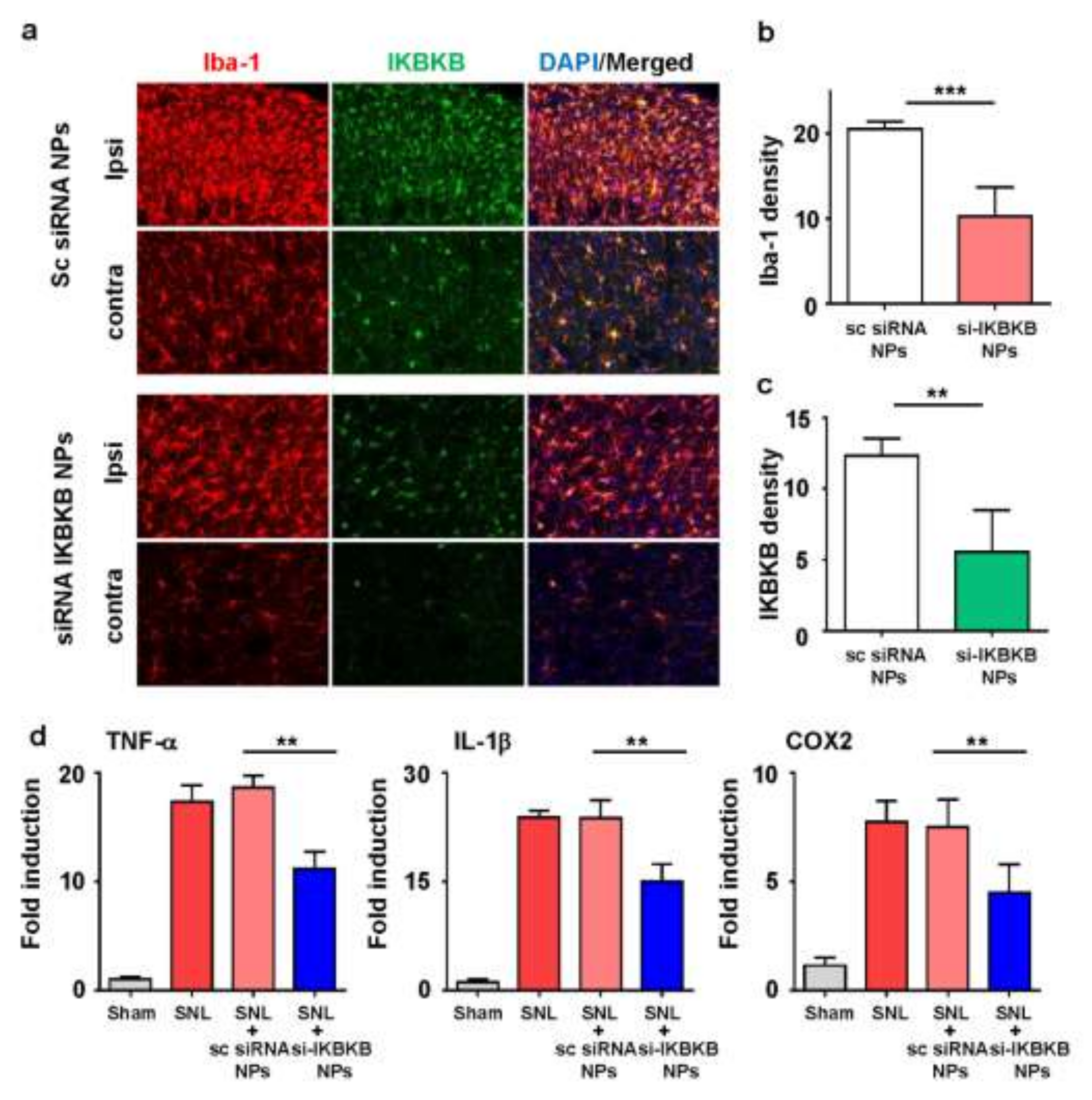
2.5. IKBKB siRNA-Encapsulated PLGA Nanoparticles Reduce Microglial Activation and Modulate the Expression of Neuropathic Pain-Related Genes in the Dorsal Horns of SNL-Induced Rat Spinal Cords
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Induction of Neuropathic Pain with L5 SNL
4.3. Intrathecal Injection
4.4. Pain Behavior Test with Von Frey Filaments
4.5. Quantitative Reverse Transcription-Polymerase Chain Reaction
4.6. PLGA Nanoparticles Preparation
4.7. Histological Analysis and Immunohistochemistry
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woolf, C.J.; Mannion, R.J. Neuropathic pain: Aetiology, symptoms, mechanisms, and management. Lancet 1999, 353, 1959–1964. [Google Scholar] [CrossRef]
- Szczudlik, A.; Dobrogowski, J.; Wordliczek, J.; Stepien, A.; Krajnik, M.; Leppert, W.; Woron, J.; Przeklasa-Muszynska, A.; Kocot-Kepska, M.; Zajaczkowska, R.; et al. Diagnosis and management of neuropathic pain: Review of literature and recommendations of the Polish Association for the Study of Pain and the Polish Neurological Society—Part Two. Neurol. Neurochir. Pol. 2014, 48, 423–435. [Google Scholar] [CrossRef]
- Austin, P.J.; Moalem-Taylor, G. The neuro-immune balance in neuropathic pain: Involvement of inflammatory immune cells, immune-like glial cells and cytokines. J. Neuroimmunol. 2010, 229, 26–50. [Google Scholar] [CrossRef]
- Vilhardt, F. Microglia: Phagocyte and glia cell. Int. J. Biochem. Cell Biol. 2005, 37, 17–21. [Google Scholar] [CrossRef]
- Piehl, F.; Lidman, O. Neuroinflammation in the rat—CNS cells and their role in the regulation of immune reactions. Immunol. Rev. 2001, 184, 212–225. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.B.; Cafferty, W.B.; Marchand, F. Immune and glial cell factors as pain mediators and modulators. Exp. Neurol. 2005, 192, 444–462. [Google Scholar] [CrossRef] [PubMed]
- Terayama, R.; Omura, S.; Fujisawa, N.; Yamaai, T.; Ichikawa, H.; Sugimoto, T. Activation of microglia and p38 mitogen-activated protein kinase in the dorsal column nucleus contributes to tactile allodynia following peripheral nerve injury. Neuroscience 2008, 153, 1245–1255. [Google Scholar] [CrossRef]
- Tanga, F.Y.; Raghavendra, V.; DeLeo, J.A. Quantitative real-time RT-PCR assessment of spinal microglial and astrocytic activation markers in a rat model of neuropathic pain. Neurochem. Int. 2004, 45, 397–407. [Google Scholar] [CrossRef]
- Clark, A.K.; Gentry, C.; Bradbury, E.J.; McMahon, S.B.; Malcangio, M. Role of spinal microglia in rat models of peripheral nerve injury and inflammation. Eur. J. Pain 2007, 11, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Ledeboer, A.; Sloane, E.M.; Milligan, E.D.; Frank, M.G.; Mahony, J.H.; Maier, S.F.; Watkins, L.R. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain 2005, 115, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Marchand, F.; Tsantoulas, C.; Singh, D.; Grist, J.; Clark, A.K.; Bradbury, E.J.; McMahon, S.B. Effects of Etanercept and Minocycline in a rat model of spinal cord injury. Eur. J. Pain 2009, 13, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Hartung, J.E.; Eskew, O.; Wong, T.; Tchivileva, I.E.; Oladosu, F.A.; O’Buckley, S.C.; Nackley, A.G. Nuclear factor-kappa B regulates pain and COMT expression in a rodent model of inflammation. Brain Behav. Immun. 2015, 50, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Kaltschmidt, C.; Kaltschmidt, B.; Baeuerle, P.A. Brain synapses contain inducible forms of the transcription factor NF-kappa B. Mech. Dev. 1993, 43, 135–147. [Google Scholar] [CrossRef]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Lagana, A.; Veneziano, D.; Russo, F.; Pulvirenti, A.; Giugno, R.; Croce, C.M.; Ferro, A. Computational design of artificial RNA molecules for gene regulation. Methods Mol. Biol. 2015, 1269, 393–412. [Google Scholar] [CrossRef]
- Pantazis, P.; Dimas, K.; Wyche, J.H.; Anant, S.; Houchen, C.W.; Panyam, J.; Ramanujam, R.P. Preparation of siRNA-encapsulated PLGA nanoparticles for sustained release of siRNA and evaluation of encapsulation efficiency. Methods Mol. Biol. 2012, 906, 311–319. [Google Scholar] [CrossRef]
- Menon, J.U.; Kuriakose, A.; Iyer, R.; Hernandez, E.; Gandee, L.; Zhang, S.; Takahashi, M.; Zhang, Z.; Saha, D.; Nguyen, K.T. Dual-Drug Containing Core-Shell Nanoparticles for Lung Cancer Therapy. Sci. Rep. 2017, 7, 13249. [Google Scholar] [CrossRef]
- Campolongo, M.J.; Luo, D. Drug delivery: Old polymer learns new tracts. Nat. Mater. 2009, 8, 447–448. [Google Scholar] [CrossRef] [PubMed]
- Phạm, T.L.; Kim, D.W. Poly (lactic-co-glycolic acid) nanomaterial-based treatment options for pain management: A review. Nanomedicine 2020, 15, 1897–1913. [Google Scholar] [CrossRef]
- Karin, M. How NF-kappaB is activated: The role of the IkappaB kinase (IKK) complex. Oncogene 1999, 18, 6867–6874. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Karin, M. NF-kappaB in cancer: A marked target. Semin. Cancer Biol. 2003, 13, 107–114. [Google Scholar] [CrossRef]
- Lim, H.; Lee, H.; Noh, K.; Lee, S.J. IKK/NF-kappaB-dependent satellite glia activation induces spinal cord microglia activation and neuropathic pain after nerve injury. Pain 2017, 158, 1666–1677. [Google Scholar] [CrossRef] [PubMed]
- Schwab, J.M.; Frei, E.; Klusman, I.; Schnell, L.; Schwab, M.E.; Schluesener, H.J. AIF-1 expression defines a proliferating and alert microglial/macrophage phenotype following spinal cord injury in rats. J. Neuroimmunol. 2001, 119, 214–222. [Google Scholar] [CrossRef]
- Noh, C.; Shin, H.J.; Lee, S.; Kim, S.I.; Kim, Y.H.; Lee, W.H.; Kim, D.W.; Lee, S.Y.; Ko, Y.K. CX3CR1-Targeted PLGA Nanoparticles Reduce Microglia Activation and Pain Behavior in Rats with Spinal Nerve Ligation. Int. J. Mol. Sci. 2020, 21, 3469. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Chung, J.M. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 1992, 50, 355–363. [Google Scholar] [CrossRef]
- Hylden, J.L.; Wilcox, G.L. Intrathecal morphine in mice: A new technique. Eur. J. Pharmacol. 1980, 67, 313–316. [Google Scholar] [CrossRef]
- Kanngiesser, M.; Haussler, A.; Myrczek, T.; Kusener, N.; Lim, H.Y.; Geisslinger, G.; Niederberger, E.; Tegeder, I. Inhibitor kappa B kinase beta dependent cytokine upregulation in nociceptive neurons contributes to nociceptive hypersensitivity after sciatic nerve injury. J. Pain 2012, 13, 485–497. [Google Scholar] [CrossRef]
- Chung, J.M.; Kim, H.K.; Chung, K. Segmental spinal nerve ligation model of neuropathic pain. Methods Mol. Med. 2004, 99, 35–45. [Google Scholar] [CrossRef]
- Zhang, E.; Yi, M.H.; Ko, Y.; Kim, H.W.; Seo, J.H.; Lee, Y.H.; Lee, W.; Kim, D.W. Expression of LC3 and Beclin 1 in the spinal dorsal horn following spinal nerve ligation-induced neuropathic pain. Brain Res. 2013, 1519, 31–39. [Google Scholar] [CrossRef]
- Ma, Z.; Han, Q.; Wang, X.; Ai, Z.; Zheng, Y. Galectin-3 Inhibition Is Associated with Neuropathic Pain Attenuation after Peripheral Nerve Injury. PLoS ONE 2016, 11, e0148792. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Park, H.; Shin, N.; Kwon, H.H.; Yin, Y.; Hwang, J.A.; Kim, S.I.; Kim, S.R.; Kim, S.; Joo, Y.; et al. p47phox siRNA-Loaded PLGA Nanoparticles Suppress ROS/Oxidative Stress-Induced Chondrocyte Damage in Osteoarthritis. Polymers 2020, 12, 443. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Yin, Y.; Park, H.; Park, S.; Triantafillu, U.L.; Kim, Y.; Kim, S.R.; Lee, S.Y.; Kim, D.K.; Hong, J.; et al. p38 siRNA-encapsulated PLGA nanoparticles alleviate neuropathic pain behavior in rats by inhibiting microglia activation. Nanomedicine 2018, 13, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Park, H.; Shin, N.; Kwon, H.H.; Yin, Y.; Hwang, J.A.; Song, H.J.; Kim, J.; Kim, D.W.; Beom, J. Pink1-Mediated Chondrocytic Mitophagy Contributes to Cartilage Degeneration in Osteoarthritis. J. Clin. Med. 2019, 8, 1849. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Shin, H.-J.; Noh, C.; Kim, S.-I.; Ko, Y.-K.; Lee, S.-Y.; Lim, C.; Hong, B.; Yang, S.-Y.; Kim, D.-W.; et al. IKBKB siRNA-Encapsulated Poly (Lactic-co-Glycolic Acid) Nanoparticles Diminish Neuropathic Pain by Inhibiting Microglial Activation. Int. J. Mol. Sci. 2021, 22, 5657. https://doi.org/10.3390/ijms22115657
Lee S, Shin H-J, Noh C, Kim S-I, Ko Y-K, Lee S-Y, Lim C, Hong B, Yang S-Y, Kim D-W, et al. IKBKB siRNA-Encapsulated Poly (Lactic-co-Glycolic Acid) Nanoparticles Diminish Neuropathic Pain by Inhibiting Microglial Activation. International Journal of Molecular Sciences. 2021; 22(11):5657. https://doi.org/10.3390/ijms22115657
Chicago/Turabian StyleLee, Seounghun, Hyo-Jung Shin, Chan Noh, Song-I Kim, Young-Kwon Ko, Sun-Yeul Lee, Chaeseong Lim, Boohwi Hong, Sin-Young Yang, Dong-Woon Kim, and et al. 2021. "IKBKB siRNA-Encapsulated Poly (Lactic-co-Glycolic Acid) Nanoparticles Diminish Neuropathic Pain by Inhibiting Microglial Activation" International Journal of Molecular Sciences 22, no. 11: 5657. https://doi.org/10.3390/ijms22115657
APA StyleLee, S., Shin, H.-J., Noh, C., Kim, S.-I., Ko, Y.-K., Lee, S.-Y., Lim, C., Hong, B., Yang, S.-Y., Kim, D.-W., Lee, W.-H., & Kim, Y.-H. (2021). IKBKB siRNA-Encapsulated Poly (Lactic-co-Glycolic Acid) Nanoparticles Diminish Neuropathic Pain by Inhibiting Microglial Activation. International Journal of Molecular Sciences, 22(11), 5657. https://doi.org/10.3390/ijms22115657






