1. Introduction
Chondrosarcomas are the second-most common primary malignant bone tumors after osteosarcomas [
1,
2]. They mainly affect adults between 30 and 70 years old. Chondrosarcomas are an heterogeneous group of tumors characterized by the production of an extracellular matrix with cartilaginous characteristics [
3]. They are classified into different histological grades, from low to high, related to their metastatic potential and associated survival rates. Chondrosarcomas are resistant to conventional radiotherapy and chemotherapy. Several mechanisms are involved in their resistance [
4]. For instance, they are poorly vascularized, produce an abundant cartilaginous extracellular matrix and are composed of a limited number of proliferating cells which, as whole, hinder drug efficacy. The hypoxic microenvironment of chondrosarcomas is also a major contributor to their radio-resistance, because it prevents the formation of antineoplastic reactive oxygen species (ROS) after irradiation [
4]. Therefore, lethality varies between 10% and 50%. Consequently, the only effective treatment for chondrosarcomas is extensive resection of the tumor [
4]. However, surgery is not always feasible, especially for tumors located, for example, at the base of the skull. Therefore, there is an urgent need for new therapeutic strategies to treat chondrosarcomas and/or overcome their resistance to conventional therapies.
MicroRNAs (miRNAs) are small non-coding RNAs of about 20–25 nucleotides, which act as post-transcriptional regulators of mRNA, generally through base-pairing and a subsequent translation blockade or mRNA decay [
5]. A single miRNA can regulate several hundred different mRNAs. Additionally, several miRNAs can share the same mRNA as a target, thereby allowing miRNAs to be key regulators of complex networks of targets [
6,
7]. MiRNAs play an essential role in many physiological processes, but also in numerous pathological conditions, particularly in cancer progression, including chondrosarcomas. Several studies have reported an aberrant expression of miRNAs in chondrosarcomas [
8,
9]. For example, miR-100, already identified as a tumor suppressor in many cancers, is downregulated in chondrosarcomas compared with normal articular chondrocytes [
8]. Another study found that miR-100 is able to re-sensitize resistant chondrosarcoma cells to cisplatin through the direct targeting of mammalian target of rapamycin kinase (mTOR) [
10]. Consequently, miRNAs can be used as diagnostic and prognostic biomarkers, but may also allow the discovery of novel therapeutic targets in chondrosarcomas [
9,
11,
12]. Various pathways, regulated by miRNAs, that influence proliferation, progression, invasion, angiogenesis and chemosensitivity have been identified in chondrosarcomas. However, to the best of our knowledge, no study has explored the effect of miRNAs that directly target anti-apoptotic molecules such as B-cell lymphoma-2 (Bcl-2), Bcl-2 lymphoma-extra large (Bcl-xL) and Myeloid cell leukemia-1 (McL-1) in chondrosarcomas. Only two studies using siRNA designed to target anti-apoptotic molecules Bcl-2, Bcl-xL, X-linked inhibitor of Apoptosis Protein (XIAP) or survivin have demonstrated an increase in sensitivity to doxorubicin or irradiation [
13,
14]. The restoration of chemosensitivity to doxorubicin and cisplatin has also been obtained with the BH3 mimetic ABT-737 in various chondrosarcoma cell lines [
15,
16].
In the present study, using functional high-throughput miRNA screening and miRNA target prediction, we selected five miRNAs that can induce apoptosis in the SW1353 chondrosarcoma cell line by targeting
BCL2,
BCL2L1 or
MCL1 mRNAs. Thereafter, we performed functional studies with the individual miRNAs: miR-149-5p, miR-342-5p, miR-491-5p, miR-541-5p and miR-625-5p. The potential cytotoxic and chemosensitizing effects of the miRNAs were studied under both normoxia and physioxia (hypoxia), the latter being more representative of the in situ physiopathological microenvironment of chondrosarcomas [
17]. We validated the apoptotic effects of miR-491-5p and miR-342-5p in three chondrosarcoma cell lines in both oxic conditions and identified key signaling pathways involved in their activity. Using a luciferase assay, we demonstrated that miR-342-5p directly inhibits both anti-apoptotic
BCL2L1 and
BCL2 mRNAs post-transcriptionally, and miR-491-5p directly inhibits
BCL2L1 mRNA post-transcriptionally. Considering the importance of autophagy in cancer biology and the growing number of autophagy-related miRNAs [
18], we also evaluated autophagy, and found that miR-342-5p can activate this process. Finally, we demonstrated for the first time the tumor suppressive effect of miR-342-5p in a 3D organoid grown under hypoxia, a culture model more representative of the physiopathology of chondrosarcomas than 2D cell cultures.
3. Discussion
The resistance of chondrosarcomas to conventional radiotherapy and chemotherapy calls for the development of new treatments. The use of therapeutic miRNAs offers the advantage of a multi-target approach and can help to discover new therapeutic targets for clinical applications [
9,
11]. In previous studies, the functions of about 20 miRNAs have been reported in chondrosarcomas [
9,
24]. Based on high-throughput screening of a human library of 1200 miRNAs, we found numerous miRNAs able to decrease cell proliferation in the SW1353 chondrosarcoma cell line. Among these, we identified five miRNAs with putative pro-apoptotic activity because they potentially target anti-apoptotic proteins such as Bcl-xL and Bcl-2, which are thought to play a critical role in the chemoresistance of chondrosarcoma chondrocytes [
15,
16,
25]. Moreover, two miRNAs (miR-342-5p and miR-541-5p) also seemed to exert chemosensitizing effects when combined with CDDP. Real-time cell analyses and endpoint morphological analyses are frequently performed in glass culture plates under normoxia, but chondrosarcoma cells live in a hypoxic environment. Therefore, we carried out functional analyses in standard plastic culture plates to validate our approach and to unambiguously identify miRNAs with realistic cytotoxic and chemosensitizing effects. Considering the role of hypoxia in the resistance of tumor cells to conventional treatments [
4,
26], we performed functional analyses under both normoxia (21% O
2) and hypoxia (3% O
2). We validated the tumor-suppressive effects of miR-491-5p and miR-342-5p on three chondrosarcoma cell lines cultured in monolayer. Their effects have already been described in other cancers, but to date, our work is the first to identify miRNAs acting as tumor suppressors in a hypoxic microenvironment. Moreover, because the traditional 2D cell culture does not reflect the in situ physiopathological microenvironment, we used for the first time a 3D organoid culture model to further confirm the effects of miRNAs on SW1353 cells.
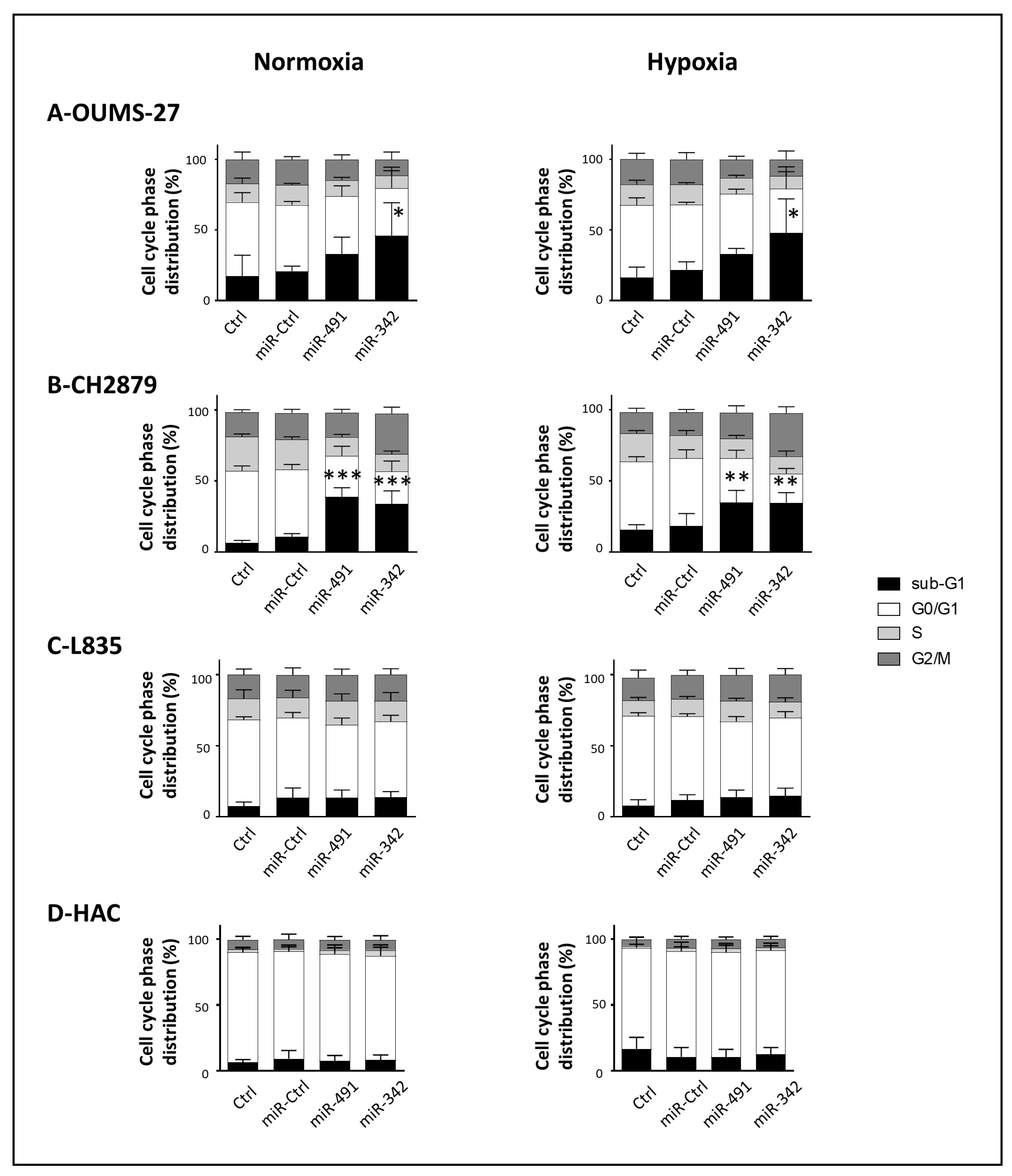
We used four cell lines derived from central conventional chondrosarcomas of grade II (SW1353) and grade III (OUMS-27, CH2879 and L835). They are all resistant to CDDP: CH2879 and OUMS-27 are the least resistant (inhibitory concentration to reach 50% reduction in cell viability (IC
50), 40 µM and 50 µM, respectively), L835 has an IC
50 value of at least 200 µM and SW1353 is the most resistant with an IC
50 of at least 400 µM [
15]. Mutation analysis performed on TP53 and on isocitrate dehydrogenase (IDH) 1 or 2 has revealed distinct characteristics: SW1353 harbors both IDH2 and TP53 mutations, OUMS-27 harbors a TP53 mutation, CH2879 is wild type for IDH and TP53 mutations and L835 harbors an IDH1 mutation [
27,
28]. Only the L835 cell line did not respond to miR-491-5p and miR-342-5p, and harbors an IDH1 mutation. Gain-of-function mutations of IDH1/2 are found in 38–70% of primary central chondrosarcomas and only lead to DNA hypermethylation. Moreover, direct inhibition of the IDH1 mutant enzyme does not change the tumorigenic properties of chondrosarcoma cell lines in vitro [
29]. The four cell lines express Bcl-2 and Bcl-xL proteins at different levels [
15,
25]. These latter two studies showed that L835 cells express higher levels of Bcl-xL protein than Bcl-2 protein. Moreover, inhibition of Bcl-xL and Bcl-2 with the ABT-737 drug reduces cell viability and induces apoptosis of L835 cells after 72 h of incubation [
15]. Inhibition of Bcl-xL by both miR-491-5p and miR-342-5p should therefore have resulted in cell death by apoptosis. Complementary experiments would be necessary to verify inhibition of Bcl-xL by both miR-491-5p and miR-342-5p on L835 cells.
Unlike miR-541-3p [
30,
31], miR-541-5p has never been studied in cancers. MiR-625-5p and miR-149-5p have been previously reported to have tumor suppressive roles in various cancers, alone or combined with various chemotherapeutic agents [
32,
33,
34,
35,
36]. Even if miR-149-5p seems to play a dual role in cancer [
37], both miR-625-5p and miR-149-5p can induce apoptosis. Our screening showed that these three miRNAs likely have antiproliferative effects, but we could not confirm any significant antimetabolic, cytotoxic or killer effects on the SW1353 chondrosarcoma cell line. Furthermore, we did not validate the chemosensitizing effect of miR-541-5p 72 h post-transfection. Because cells growing on glass are more sensitive than cells growing on plastic, it is possible that killer effects require a longer incubation period in standard cell culture conditions, possibly more than 96 h post-transfection.
MiR-491-5p has already been described as a tumor suppressor in various cancers and several of its direct targets have been identified and include Bcl-xL in colorectal cancer [
21], Bcl-xL and EGFR in ovarian cancer [
19], Bcl-xL and TP53 in pancreatic cancer [
20], Bcl-xL, EGFR and CDK6 in glioblastoma [
22], Pyruvate Kinase M2 (PKM2) [
38] and Forkhead box Protein P4 (FOXP4) in osteosarcoma [
39], the most common type of bone cancers. In our study, miR-491-5p was first used as a positive control of cytotoxicity for the high-throughput screening. Given its high therapeutic potential, we next wanted to confirm its tumor suppressive effect on chondrosarcoma cell line, which has never been studied. We found it was able to induce cell death in SW1353, OUMS-27 and CH2879 chondrosarcoma cell lines cultured in monolayer under normoxia and hypoxia.
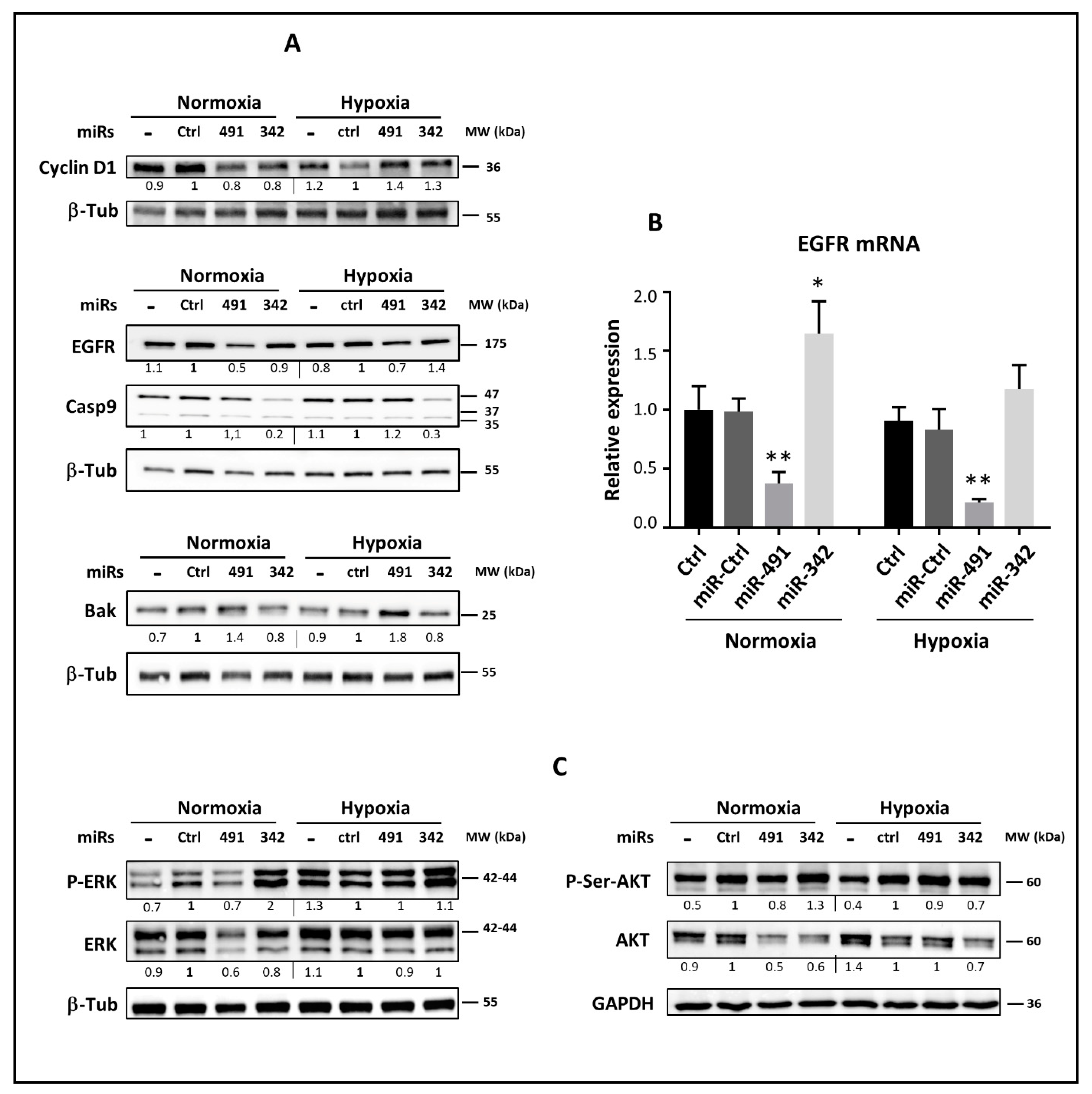
EGFR is constitutively activated in high-grade chondrosarcoma tumors [
40]. Regardless of the oxygen level, miR-491-5p inhibited EGFR at the protein and mRNA levels. This inhibition suggests a possible downstream inhibition of the AKT and MAPK signaling pathways, leading to a decrease in chondrosarcoma cell proliferation and migration, as shown in other studies investigating the inhibition or silencing of EGFR in chondrosarcomas [
40]. Previous studies have shown that the downstream inhibition of MAPK and AKT activity by miR-491-5p depends on the type of cancer or the cell line. In the SW1990 pancreatic cell line, miR-491-5p inhibits PI3K/AKT, but not RAS/MAPK [
20]. This miRNA also inhibits AKT and MAPK signaling pathways in IGROV1-R10 ovarian cancer cells, whereas SKOV3, another ovarian cell line, maintains its AKT and MAPK activity [
19]. In the present study, the effect of miR-491-5p on AKT and MAPK signaling pathways was heterogeneous among the chondrosarcoma cell lines and also depended on the oxic conditions.
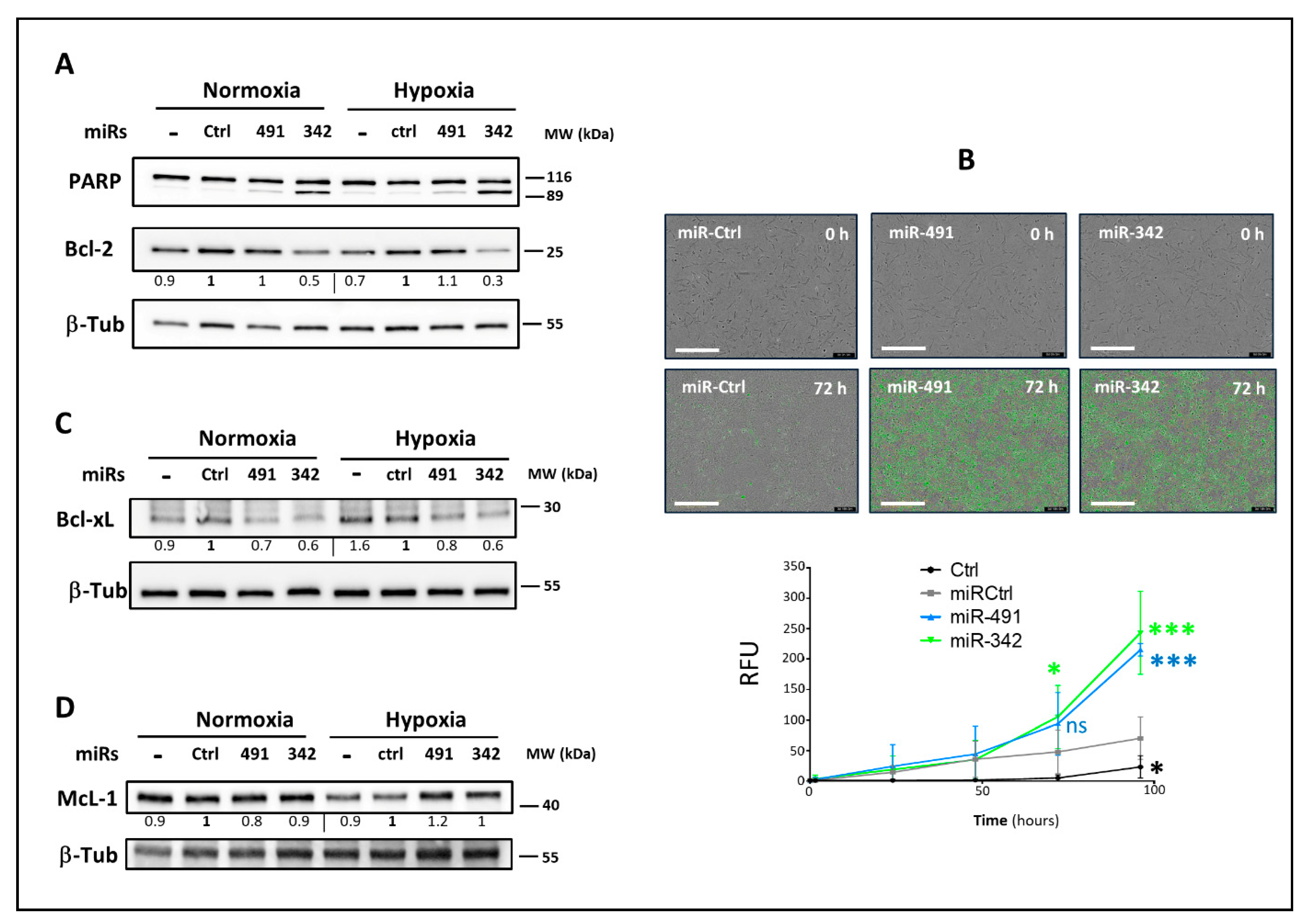
MiR-491-5p did not influence the expression of the Bcl-2 anti-apoptotic protein in any of the three investigated chondrosarcoma cell lines sensitive to miR-491-5p. Similarly, although the anti-apoptotic McL-1 may be a potential target of miR-491-5p, this miRNA did not influence McL-1 protein expression in SW1353 and OUMS-27 cell lines, and rather tended to increase McL-1 expression in CH2879 cells, as observed in the IGROV1-R10 ovarian cell line [
19]. Like others, we found that miR-491-5p directly targets
BCL2L1 mRNA [
19,
20,
22]. Consequently, miR-491-5p decreased the expression of the anti-apoptotic protein Bcl-xL in the three chondrosarcoma cell lines responding to miR-491-5p under both oxic conditions. It also increased the expression of the Bak pro-apoptotic protein. Both these events may therefore contribute to its apoptotic activity in chondrosarcoma cells.
The miR-342-5p mechanism of action has been less investigated than that of miR-342-3p and, to our knowledge, never in chondrosarcomas. It has been identified as a tumor suppressor in neuroblastomas [
30]. It inhibits colon cancer tumorigenesis through direct targeting of the N-a-acetyl transferase 10 protein (NAA10) and also promotes apoptosis [
41]. In osteosarcomas, miR-342-5p inhibits cell growth, migration and invasion, and restores sensitivity to doxorubicin through direct targeting of Wnt member 7B (WnT7b) [
42]. In the present study, high-throughput screening revealed that miR-342-5p has some antiproliferative effects on SW1353 cells. However, miR-342-5p did not significantly affect AKT and ERK mitogenic signaling pathways as previously reported for breast cancer cells [
43], or
AKT mRNA in inflammatory macrophages [
44]. We observed AKT inhibition at the protein level in two out of the three cell lines (SW1353, CH2879), but at the same time the phosphorylation of AKT increased in CH2879 cells. There is evidence in the literature that miR-342-5p increases the level of phosphorylated forms of AKT via targeting a phosphatase on cardiomyocytes [
23]. Others have shown that miR-342-5p attenuates the protein level of total AKT, whereas its phosphorylated protein level is stable in neural stem cells [
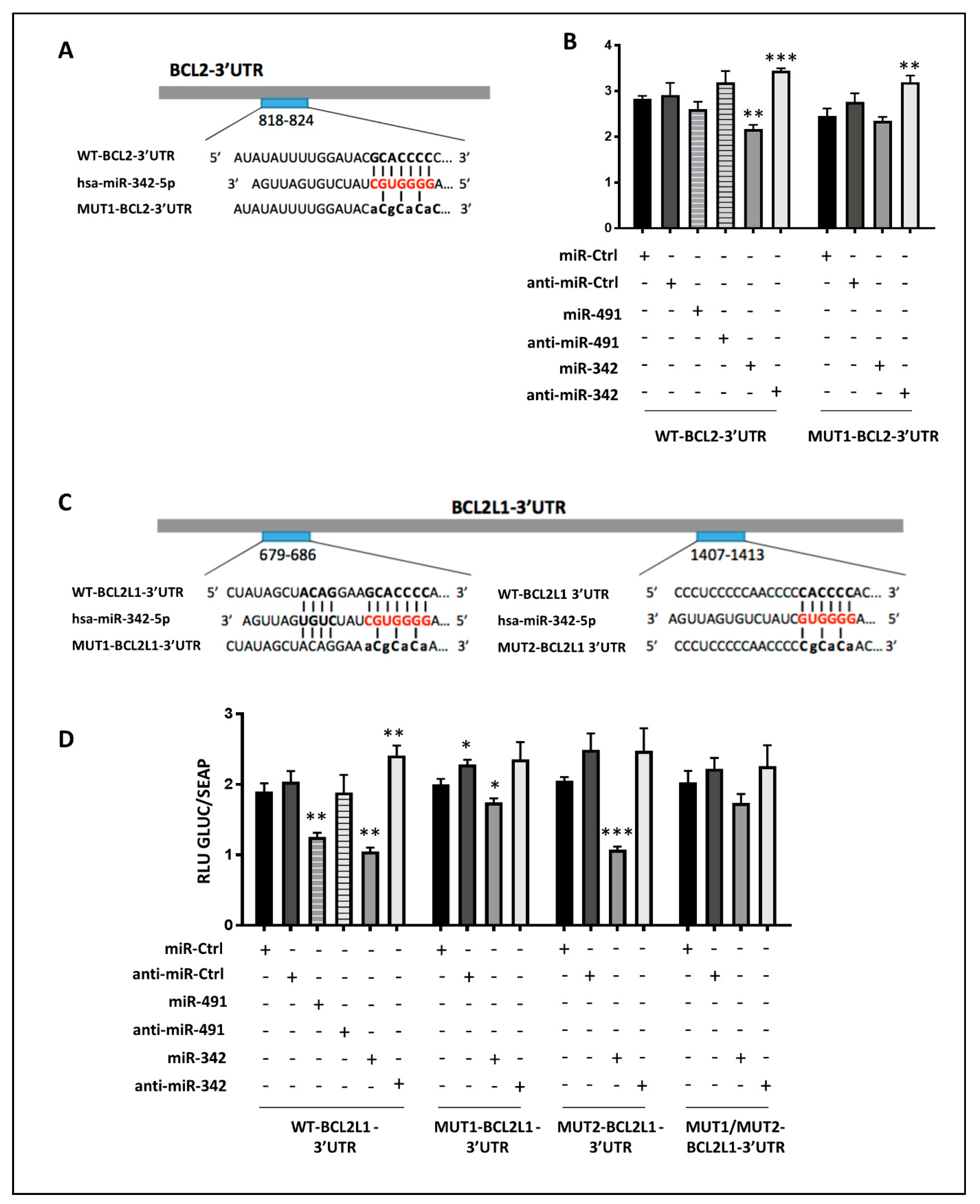
45]. In view of our results, we cannot assume that the antiproliferative effects of miR-342-5p are linked to AKT and ERK pathways, but are likely linked to its pro-apoptotic effects. Indeed, miR-342-5p induced cell death and apoptosis in SW1353, OUMS-27 and CH2879 cells, independently of the oxygen level. In the three chondrosarcoma cell lines, miR-342-5p inhibited the expression of the anti-apoptotic proteins Bcl-2 and Bcl-xL, but not that of McL-1, with a more sustained effect under hypoxia. We also reported for the first time that miR-342-5p downregulates Bcl-2 protein expression by directly binding to sequence 818–824 of BCL2-3′UTR. Like Soriano et al. [
30], we found that miR-342-5p directly targets the
BCL2L1 gene and in addition, we identified its binding site at position 679–686 of BCL2L1-3′UTR. Soriano et al. also identified the
CCND1 gene as a direct target of miR-342-5p in neuroblastomas, but in our experiments on chondrosarcoma cells lines, cyclin D1 protein expression was not affected by miR-342-5p.
Surprisingly, in the three chondrosarcoma cell lines, miR-342-5p also had anti-apoptotic effects, because it tended to decrease (by 1.2-fold) the protein expression of the Bak pro-apoptotic protein, and that of the pro-caspase-9 (at least 3-fold). Our data are in line with those of Hou et al., who identified exosomal miR-342-5p as a key cardioprotective molecule inhibiting apoptotic signaling via direct targeting of caspase-9 and Jun Kinase 2 (JNK2) in cardiomyocytes [
23]. Caspase-9 activation is stimulated by dimerization instead of cleavage within the apoptosome. Dimerization facilitates autocatalytic cleavage, which results in the stabilization of the dimer [
46]. Caspase-9 showed complete activity in its uncleaved form [
47]. This caspase initiates apoptosis by cleaving and thereby activating executioner caspases-3, -6 and -7. In our study, cleaved caspase-9 was not clearly observed in all chondrosarcoma cell lines, even with miR-491-5p, whose activation of the intrinsic apoptosis pathway has already been demonstrated [
19,
20]. Despite anti-apoptotic potential, miR-342-5p clearly induced apoptosis as demonstrated by the induction of cleaved PARP and caspase-3/7 activity.
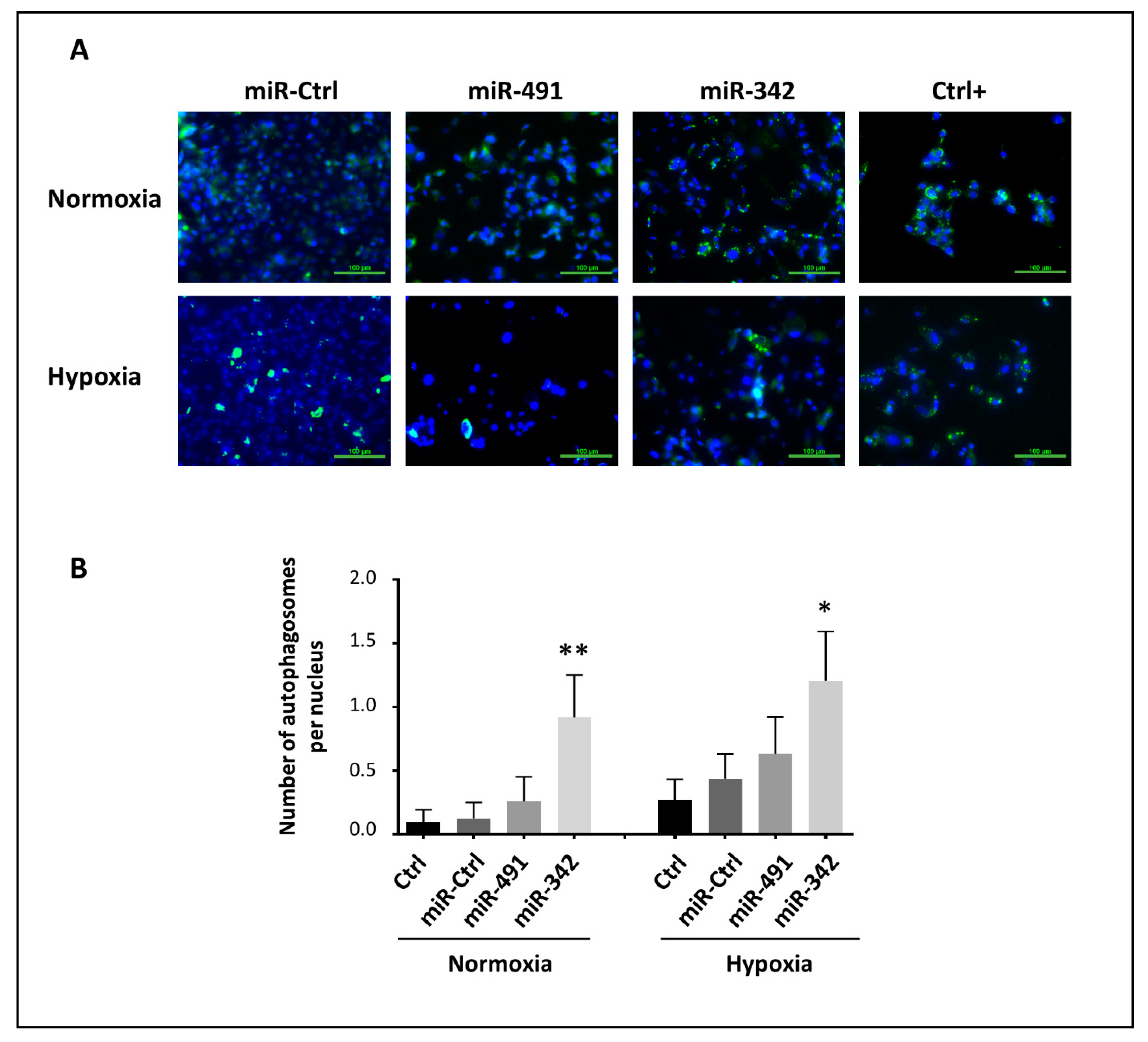
Autophagy is a lysosomal degradation pathway that protects cells from deleterious cytoplasmic components. Autophagy can also be associated with cell death [
48,
49]. Here, we found that miR-342-5p induced autophagy in SW1353 cells. The effect was less significant in hypoxia, probably because autophagy was already activated in a hypoxic environment. We also report the inhibition of Bcl-2 and Bcl-xL by miR-342-5p, which may contribute to the activation of autophagy. Indeed, Bcl-2 family proteins can inhibit autophagy by targeting and inhibiting Atg6/Beclin 1, which has an essential role in the formation of autophagosomes [
50,
51,
52]. Inhibition of Bcl-xL by miR-491-5p did not, however, appear to be enough to induce autophagy. We cannot rule out the possibility that miR-342-5p can directly target autophagy-related components, but we identified, for the first time, miR-342-5p as an autophagy-regulating miRNA.
In a previous study on SW1353, OUMS-27, CH2879 and L835 cells, inhibition of Bcl-xL and Bcl-2 with ABT-737 restored the chemosensitivity to doxorubicin and CDDP (5 µM), with the more marked inhibition in cell viability after 72 h [
15]. This study also used 10 µM CDDP in combination with WEHI-539, a selective inhibitor of Bcl-xL, to induce apoptosis of chondrosarcoma cells after 72 h of treatment [
25]. In contrast, in our study, miR-491-5p, which inhibits Bcl-xL, and miR-342-5p, which downregulates both Bcl-2 and Bcl-xL, did not chemosensitize chondrosarcoma cells to CDDP. We used sublethal doses of CDDP to induce cell cycle stalling at the S and/or G2/M phases (0.33 µM for OUMS-27, 1.65 µM for L835 and CH2879 and 3.3 µM for SW1353). We performed functional analysis 24 h after CDDP treatment. Therefore, the chosen kinetics and these sub-lethal doses of CDDP may not be adequate to reveal any chemosensitizing effect of these miRNAs. Other experiments are required to explore this hypothesis.
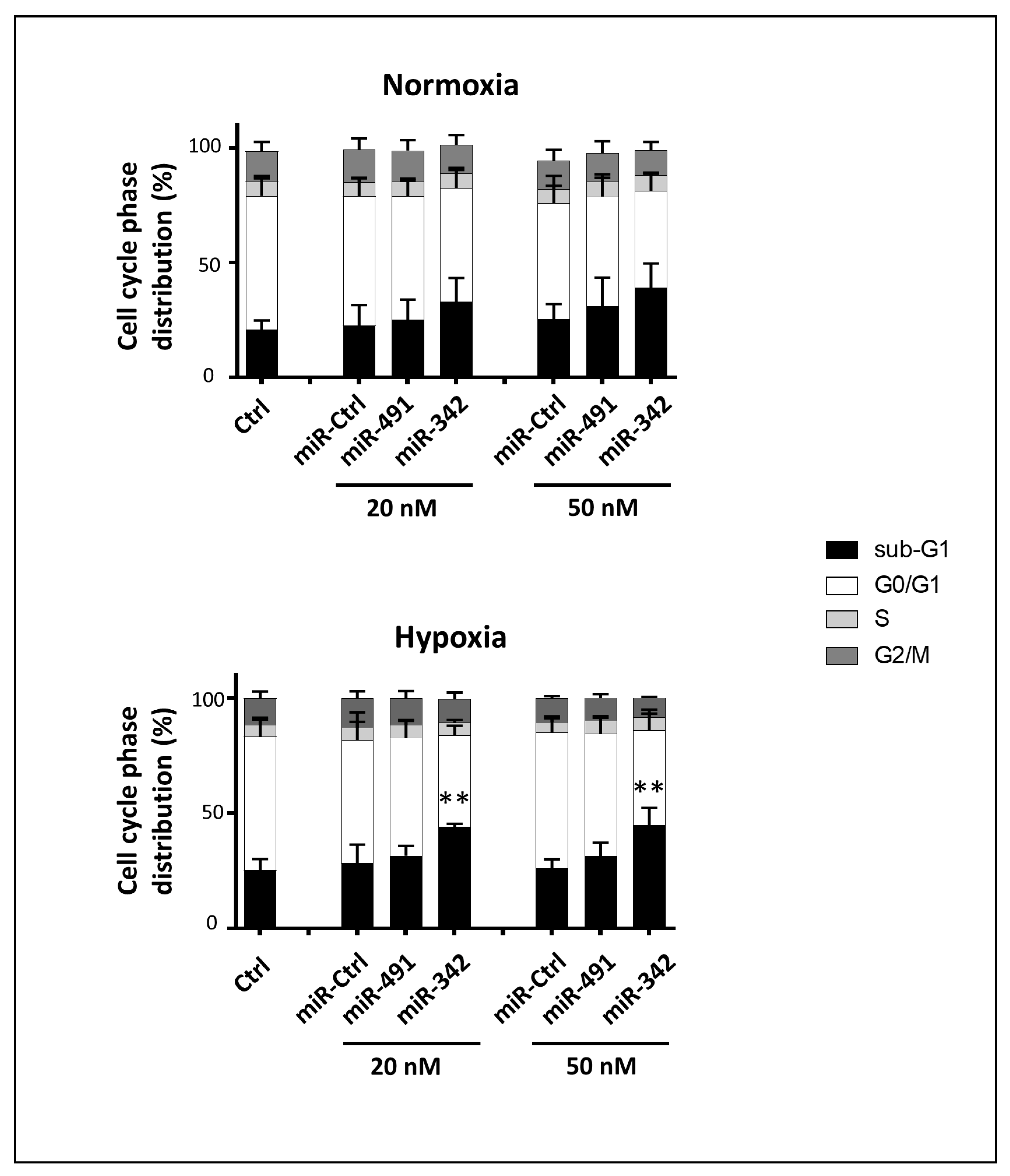
We showed that miR-491-5p and miR-342-5p do not affect the healthy primary HAC cell cycle, nor do they cause any cell death, indicating their biosafety on non-cancerous chondrocytes. Finally, we investigated their effects on a 3D organoid chondrosarcoma model that mimics the in vivo microenvironment, rather than using a classic xenograft model. We previously used collagen sponge scaffolds to successfully re-differentiate dedifferentiated HACs, or to differentiate mesenchymal stem cells into chondrocytes, with the combination of growth factors/siRNAs/hypoxia [
53,
54,
55]. These collagen sponges scaffolds have also been used to study the impact of irradiations in a 3D chondrosarcoma model [
56]. As previously described [
56], this 3D organoid chondrosarcoma model mimics an intermediated grade chondrosarcoma tissue cellularity with a homogeneous distribution of SW1353 cells into the 3D scaffold. A higher Ki67 proliferation index (33% ± 4%), measured by immunochemistry staining, was also determined after 7 days of culture of SW1353 cells into the collagen scaffold [
56]. Compared with the conventional subcutaneous xenograft implantation in
nude mice, our 3D culture model makes it possible to control oxygen tension during experimentations. The effects of the miRNAs were thus studied under hypoxia to closely mimic the in situ microenvironment of chondrosarcomas. In this model, we found that miR-491-5p was not able to induce cell death, whereas miR-342-5p provoked cell death in normoxia and hypoxia, with a higher level of significance in the more biologically relevant hypoxia. This miRNA would therefore be the most effective if considering miRNAs as a therapeutic avenue for the treatment of chondrosarcomas.
4. Materials and Methods
4.1. Cell Culture
The chondrosarcoma cell line SW1353 (ATCC® HTB-94) was grown and treated in high glucose-Dulbecco’s modified Eagle’s medium (HG-DMEM, Biowest, Nuaillé, France) supplemented with 10% fetal calf serum (FCS) (Eurobio Scientific, Courtaboeuf, France), 3 µg/mL ciprofloxacin (Sigma-Aldrich, Saint-Louis, MO, USA) and 0.5 µg/mL amphotericin (Eurobio Scientific, Courtaboeuf, France). The chondrosarcoma cell lines OUMS-27, L835 and CH2879 were kindly provided by Y. Saintigny (LARIA, Caen, France) and they initially originated from J.V.M.G Bovée’s laboratory (Department of Pathology, Leiden, the Netherlands). They were grown and treated in RPMI 1640 medium (Eurobio Scientific, Courtaboeuf, France) with 10% FCS and a mixture of penicillin (100 IU/mL) and streptomycin (100 µg/mL) (Eurobio Scientific, Courtaboeuf, France).
Human articular chondrocytes (HACs), obtained with appropriate ethical approval, were prepared from macroscopically healthy zones of femoral heads obtained from patients undergoing joint arthroplasty, as previously described [
57]. The study was performed in full accordance with local ethics committee guidelines and all the cartilage samples were collected after written and informed consent of the donors according to French legislation. All the experimental protocols were approved by the French Ministry of Higher Education and Research (Ethics Committee for Research on Human Samples CODECOH: DC 2014–2325). Chondrocytes were seeded at 4 × 10
4 cells/cm
2 in plastic dishes, with a medium consisting of HG-DMEM supplemented with 10% FCS and a mixture of 100 IU/mL penicillin, 100 mg/mL erythromycin and 0.25 mg/mL fungizone (Eurobio Scientific, Courtaboeuf, France).
All cells were certified mycoplasma-free with PCR analysis. They were maintained in a humidified atmosphere containing 5% CO
2 at 37 °C. Treatments were performed under normoxia (21% O
2) and hypoxia (3% O
2). Hypoxic cultures, including any handling, were exclusively performed in a sealed chamber, with a controlled rate of oxygenation [
57].
For 3D experiments, SW1353 cells were grown in collagen scaffolds manufactured by Symatèse Biomatériaux (Chaponost, France), as described previously for the redifferentiation of HACs [
53] or to study the impact of radiation in a 3D chondrosarcoma model [
56] with some modifications. The collagen sponge scaffolds were composed of native type I collagen (90–95%) and type III collagen (5–10%) from calf skin. These sponges, 2 mm thick and 5 mm in diameter, were cross-linked with glutaraldehyde to increase their stability and sterilized using β-radiation. Briefly, SW1353 cells were seeded at 2x10
5 cells/sponge in 96-well culture plates and incubated at 37 °C and 5% CO
2 in HG-DMEM supplemented with 10% FCS and antibiotics. After 1 h, each cell construct was transferred to 24-well plates and incubated in the same medium pre-equilibrated with 3% O
2 by bubbling for culture under hypoxia or without O
2 pre-equilibration for culture under normoxia.
4.2. Drug and miRNAs
Cisplatin (Cis-diaminedichloroplatinum or CDDP) was purchased from Mylan (Merck Santé SAS, Lyon, France). Sublethal doses of CDDP were used during the transfection of miRNA as follows: 1 µg/mL (3.3 µM) for SW1353, 0.5 µg/mL (1.65 µM) for L835 and CH2879 cells and 0.1 µg/mL (3.3 µM) for OUMS-27 cells. All miRNA mimics were purchased from Dharmacon (Horizon Discovery, Cambridge, UK). MiRNA-Control (miR-Ctrl, MIMAT0000039), was based on a C. elegans miRNA sequence that has been confirmed to have minimal sequence identify with human miRNAs. Human miRNAs hsa-miR-491-5p (MIMAT0002807, noted miR-491), hsa-miR-342-5p (miR-342, MIMAT0004694), hsa-miR-541-5p (miR-541, MIMAT0004919), hsa-miR-625-5p (miR-625, MIMAT0003294) and hsa-miR-149-5p (miR-149, MIMAT0000450) were also used in this study. MiRNA hairpin inhibitors were purchased from Dharmacon: hsa-miRNA-491-5p-hairpin inhibitor (anti-miR-491, IH-300751-06), hsa-miRNA-342-5p-hairpin inhibitor (anti-miR-342, IH-301083-02) and miRNA hairpin inhibitor Negative Control (anti-miR-ctrl, IN-001005-01).
4.3. Real-Time Cell Analysis (xCELLigence)
MiRNA- and CDDP-mediated cytotoxicity were initially monitored using high-throughput screening with the Real-Time Cell Analyzer (RTCA) multi-plate instrument (xCELLigence System; ACEA, Ozyme, Saint-Quentin-en-Yvelines, France) under normoxia. This system monitors cellular events in real-time by measuring electrical impedance across interdigitated micro-electrodes integrated into the bottom surfaces of 96-well E-plates VIEW (Ozyme, Saint-Quentin-en-Yvelines, Trapp, France). RTCA 2.1.0 software calculates the cell index (CI) based on impedance. CI correlates with the area of cells attached to the bottom of the plate as described elsewhere [
58]. Briefly, 3.5 × 10
3 SW1353 cells/well were plated in 96-well E-plates VIEW. They were left to grow for 24 h before transfection with 20 nM miRNAs using Interferin
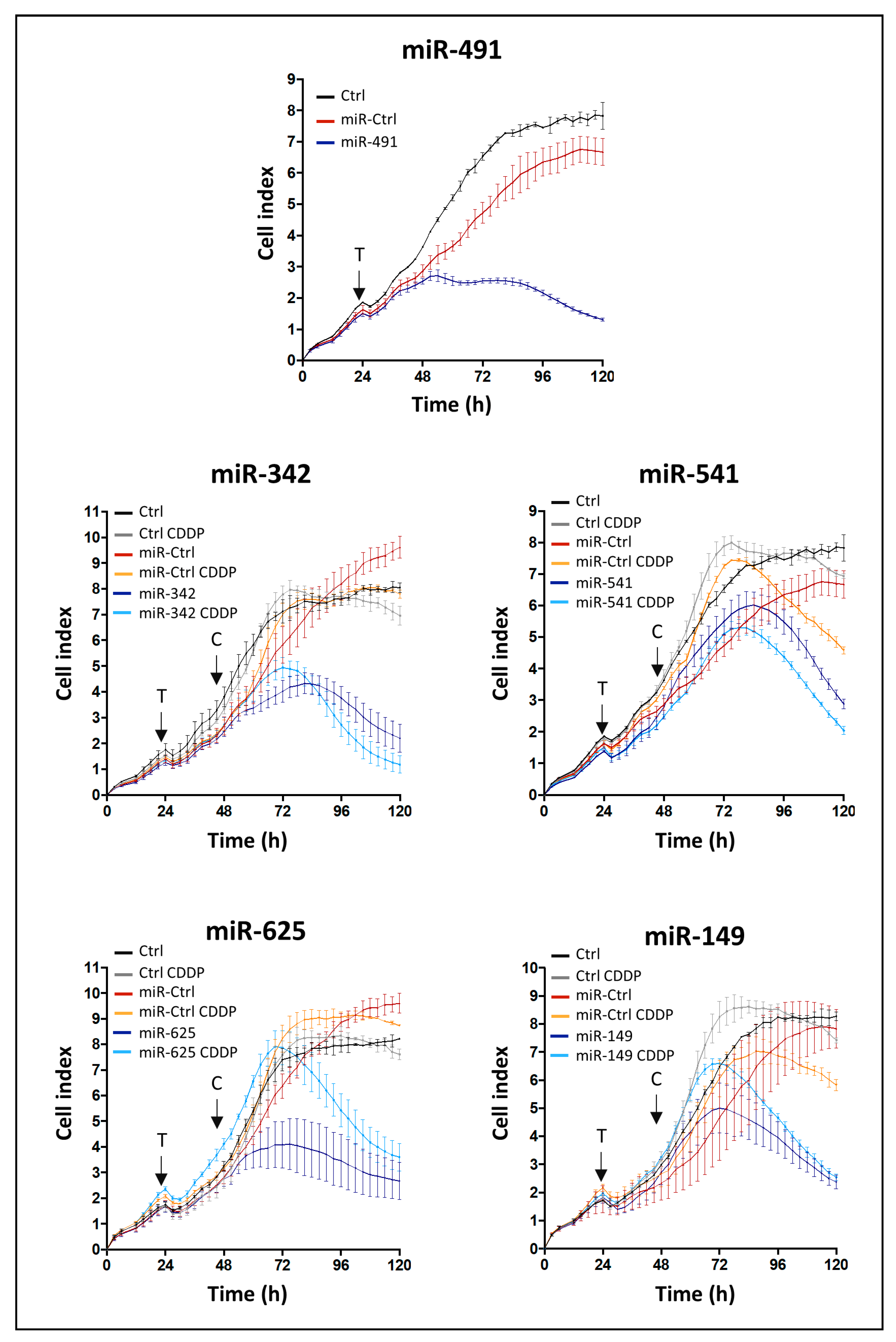
TM (Polyplus-Transfection, Strasbourg, France). In each plate, miR-Ctrl was used as a negative control of cytotoxicity. The cells were treated with or without CDDP 24 h post-transfection. Impedance was continuously measured until the end of the treatment (i.e., 96 h post-transfection and 120 h after seeding). MiRNA effect was established according to three criteria: the shape of the curve, the area under the curve (AUC) and the CI at the end of the experiment. Standard deviations of triplicates were analyzed with the RTCA software. Endpoint morphological analysis of cells was also done in a high-throughput cell imaging system (Cellavista; Roche, Basel, Switzerland) at the end of the experiment.
4.4. Transfection of miRNA and CDDP Treatment
For individual characterization and validation studies, exponentially growing cells were seeded at 7.5 × 104 cells/cm2 (for OUMS-27), 1 × 104 cells/cm2 (for SW1353 cells) and 2 × 104 cells/cm2 (for L835 and CH2879 cells). Cell lines were transfected 24 h after seeding with 20 nM of miRNA mimics using INTERFERinTM (Polyplus-Transfection, Strasbourg, France) according to the manufacturer’s instructions. An additional CDDP treatment was realized 48 h post-transfection with sublethal doses of CDDP.
For 3D experiments, SW1353 cells were transfected with 20 or 50 nM miRNA the day after seeding in the collagen sponge. HACs were transfected with 20 nM miRNA at passage 0% and at 80% confluency. In all cases, cells and media were harvested at the end of the experiment (72 h post-transfection) for further analysis.
4.5. Metabolic Activity Analysis
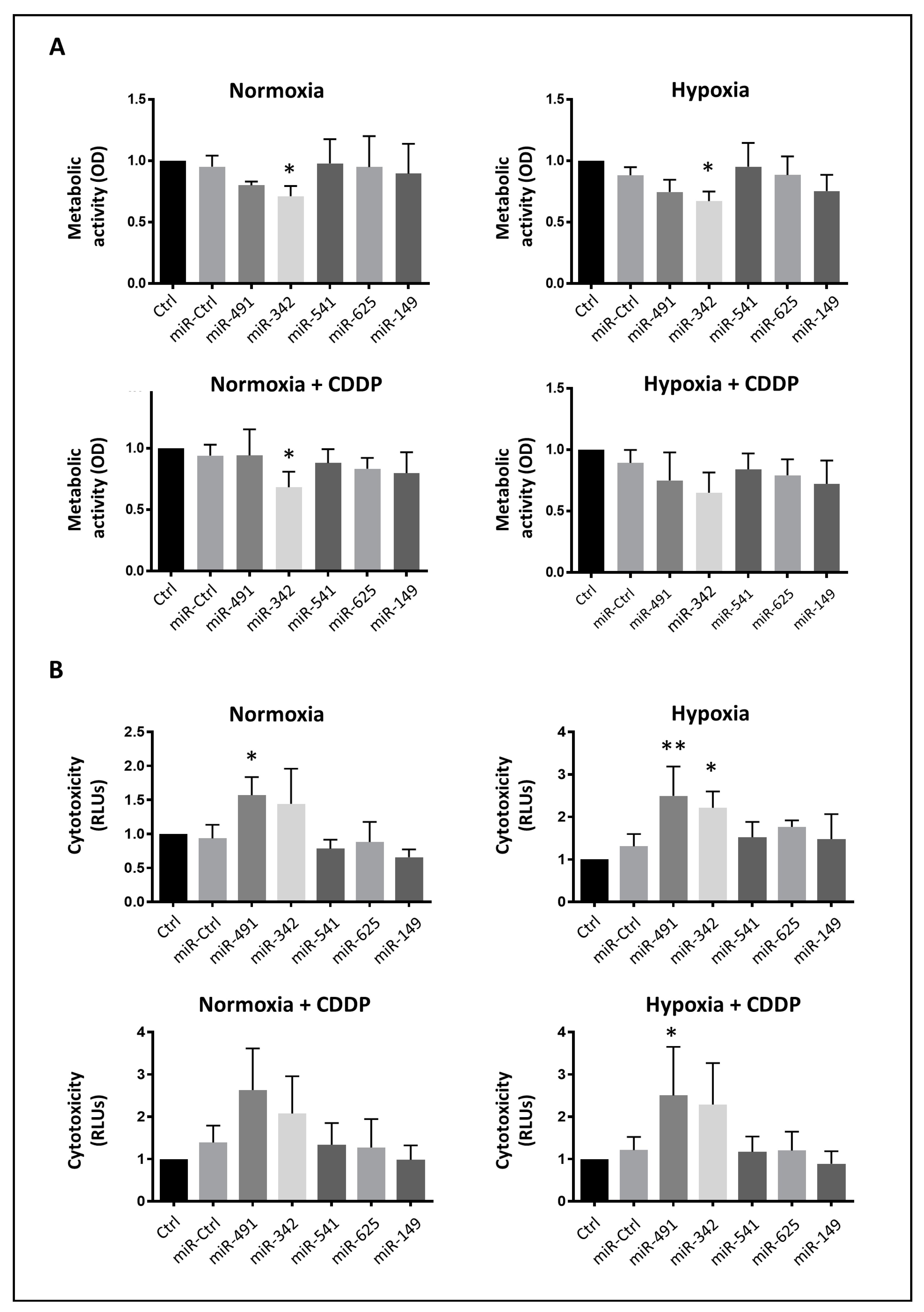
SW1353 cells were seeded onto 96-well microplates at a density of 3.5 × 103 cells/well in triplicate. Transfection of 20 nM miRNA was performed 24 h later with or without an additional CDDP treatment 48 h post-transfection, as described above. Cellular metabolic activity was estimated 72 h post-transfection using the XTT assay (Roche, Basel, Switzerland). This assay is based on the cleavage of the tetrazolium salt XTT to a soluble formazan salt only in viable cells. Therefore, the amount of formazan dye formed is directly correlated with the number of metabolically active cells in the culture. The medium was removed and the cells were incubated with XTT working solution (per well: 100 μL of culture medium, 50 μL of XTT and 1 μL of electron coupling reagent). Optical density (OD) was measured at 450 nm and 600 nm with an absorbance microplate reader (Spark 10M; Tecan, Lyon, France) after 1 h of incubation.
4.6. Cytotoxicity Assay
In parallel with the metabolic activity analysis, a bioluminescence cytotoxicity assay kit (Interchim, Montluçon, France) was used to evaluate miRNA-induced cytotoxicity 72 h post-transfection. This kit is based on the measurement of adenylate kinase (AK) rapidly released into the culture medium upon damage to the plasma membrane. AK detection was carried out in the supernatant in a simple one-step procedure involving two chemical reactions. The first reaction converts ADP to ATP by AK. The second reaction uses luciferase to catalyze the formation of the light from ATP and luciferin. The emitted light was then measured with a luminescence microplate reader (Spark 10M, Tecan Lyon, France) and expressed as relative luciferase units (RLU), reflecting the number of dead cells.
4.7. Cell Cycle Analysis
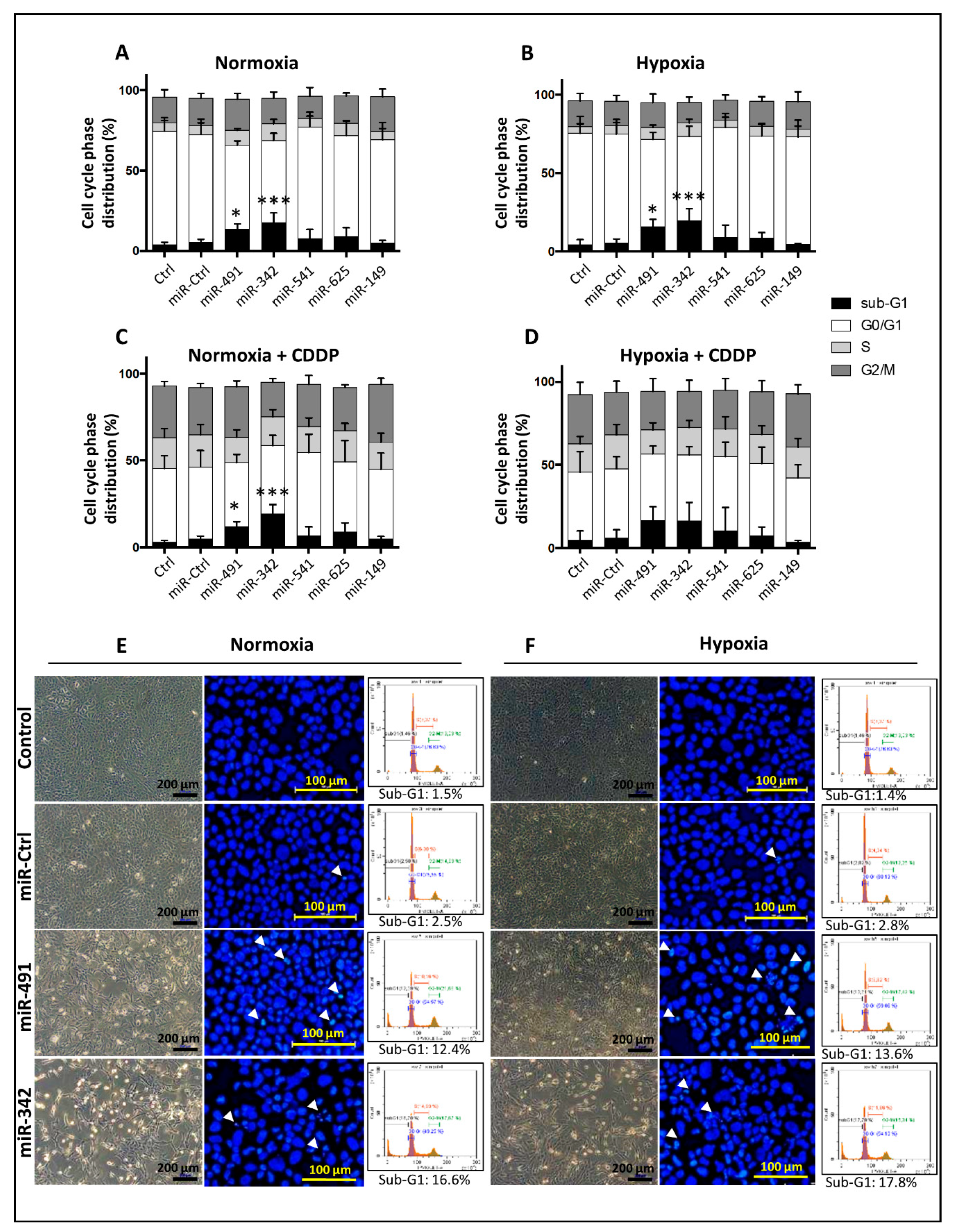
Adherent cells were harvested by trypsinization. The supernatant, containing the detached cells, and the trypsinized cells were pooled, centrifuged (1400 rpm, 10 min), rinsed with PBS, fixed in 70% ethanol, and stored at −20 °C until analysis. Fixed cells were then centrifuged (3000 rpm, 10 min) and rinsed with PBS to remove all traces of ethanol. After centrifugation (2000 rpm, 5 min), the cells were resuspended and incubated for 30 min in a PBS solution containing 20 µg/mL propidium iodide (Sigma-Aldrich, Saint-Louis, MO, USA) and 100 µg/mL RNase (Fisher Scientifics SAS, Illkirch, France). Cell cycle phase distribution was analyzed by flow cytometry using the Cytoflex S Flow Cytometer (Beckman Coulter France SAS, Paris, France). Data were computed with the Cytexpert® acquisition software (Beckman Coulter France SAS, Paris, France).
4.8. Analysis of Nuclear Morphology
Both detached and adherent cells were pooled and applied to a poly-L-lysine-coated glass slide by cyto-centrifugation at 850 rpm for 5 min (Cytospin, Fisher Scientifics SAS, Illkirch, France). They were fixed with a solution of ethanol/chloroform/acetic acid (6:3:1) before DNA staining. Slides were then treated with the UltraCruz™ Mounting Medium with DAPI (4′,6-diamidino-2-phenylindole, dilactate) (Santa Cruz Biotechnology, Dallas, TX, USA). The observations were carried out under a fluorescence microscope (Nikon Eclipse Ti; Nikon Instruments Inc., Melville, NY, USA).
4.9. Western Blotting
Both detached and adherent cells were centrifuged at 1400 rpm for 5 min, washed with PBS and stored at −20 °C until analysis. Total proteins were extracted using the RIPA-lysis buffer as previously described [
59]. Protein concentration was assessed according to the Bradford colorimetric procedure (Biorad, Hercules, CA, USA). Then, 15 µg of total proteins were separated in 10% or 12% polyacrylamide gels containing 0.1% SDS and transferred to a polyvinylidene difluoride membrane (PVDF, Biorad, Hercules, CA, USA) using the Trans Blot
®Turbo
TM Transfer system (BioRad, Hercules, CA, USA). Unspecific binding sites of the membranes were blocked with 10% non-fat milk powder in Tris-buffered saline with 0.1% Tween (TBST) for 1 h. Then, protein levels were analyzed by immunoblotting with antibodies from Cell Signaling Technology (Ozyme, Saint-Quentin-en-Yvelines, France), according to the manufacturer’s instructions: EGFR (4267), PARP (9542), Caspase-9 (9502), Bcl-xL (2764), Cyclin D1 (2878), Bak (3814), phospho-p44/42 MAPK (Thr202/Tyr204 of Erk1/2, 4370), p44/42 MAPK (Erk1/2, 9102), phospho-AKT (Ser473, 4060), AKT (9272). We also used McL-1 (S-19), GAPDH (FL-335) and β-Tubulin (D-10) antibodies from Santa Cruz Biotechnology (Dallas, TX, USA), and the Bcl-2 (M0887) antibody from DAKO. After incubation, membranes were washed in TBST, followed by an incubation with HRP-conjugated goat anti-rabbit or mouse IgG antibody (Jackson Immunoresearch, West Grove, PA, USA). Signals were visualized with a chemiluminescence imaging system (ChemiDoc
TM Touch imaging system; Biorad, Hercules, CA, USA). Each immunoblot is representative of at least three distinct experiments. Protein expression was estimated by quantifying the density of immunoblots bands adjusted to β-Tubulin or GAPDH (ImageLab
® software; Biorad, Hercules, CA, USA).
4.10. Real-Time Detection of Caspase-3/7 Mediated Apoptosis
Caspase-3/7 activity was assessed using the Incucyte® caspase-3/7 green apoptosis assay reagent (Essen BioScience, Ltd., Royston, UK). This reagent couples the activated caspase-3/7 recognition motif (DEVD) to NucView™488, a DNA intercalating dye. Thus, caspase-3/7 activity correlates with an increase in the green fluorescence in the nuclei. Briefly, 3.5 × 103 SW1353 cells/well were grown in 96-well plates and monitored in the Incucyte® S3 acquiring images (objective × 10) every 3 h in two separate regions per well after transfection of 20 nM miRNA. Each experiment was done in triplicate and analyzed using Incucyte® software.
4.11. RNA Isolation and PCR Assay
Total RNA (including miRNA) was isolated from transfected cells using the NucleoSpin® miRNA kit (Macherey-Nagel SAS, Herdt, France) according to the manufacturer’s instructions. RNA quantity and quality were assessed using the NanoDropTM 2000 spectrophotometer (ThermoFisher Scientific, Waltham, MA, USA). Reverse transcription was performed to generate cDNA from 1 µg of total RNA with the miScript II RT kit (Qiagen SAS, Courtaboeuf, France). PCR was performed on a CFX96 Touch real Time PCR (Biorad, Hercules, CA, USA). Data were analyzed using CFX manager software. For the detection of miRNAs, we used QuantiTect™ SYBR Green PCR master mix (Qiagen SAS, Courtaboeuf, France) with an universal miScript primer and specific miScript primers provided by Qiagen: Hs_miR-491-5p_1 (miR-491-5p), Hs_miR-342-5p_1 (miR-342-5p), Hs_miR-541*_1 (miR-541-5p), Hs_miR-625_3 (miR-625-5p), Hs_miR-149_1 (miR-149-5p), Hs_RNU6-2_11 (U6 small nuclear 6 or RNU-6B) and Hs_miR-15a_1 (miR-15a). The cycling conditions were 15 min for polymerase activation at 95 °C, followed by 40 cycles at 94 °C for 15 s, 55 °C for 30 s and 70 °C for 30 s. In addition, melting curves were performed to ensure specificity of PCR products. Expression values of miRNAs were normalized to RNU-6B and to miR-15a with CFX manager software. They were expressed as mean of triplicate samples ± SD.
For RNA transcript quantification, we used GoTaq®qPCR Master Mix (Promega, Charbonnières-les-Bains, France). The following primers were purchased from Eurogentec SA (Angers, France): EGFR F-5′-TGGCATCTTTAAGGGCTCCA-3′, EGFR R-5′-TGGCTAGTCGGTGTAAACGT-3′, PPIA1 F-5′-CGGATTTGATCATTTGGTG-3′, PPIA1 R-5′-CAGGGAATACGTAACCAG-3′, GAPDH F-5′-CCTGCACCACCAACTGCTTA-3′, GAPDH R-5′-GGCCAT CCACAGTCTTCTGGG-3′. GAPDH and PPIA1 were both used as endogenous reference genes to normalize EGFR expression, expressed as the mean of triplicate samples ± SD.
4.12. Luciferase miRNA Target Reporter Assay
Targetscan 7.2 2020 (
http://www.targetscan.org, accessed on 24 May 2021) was used for miRNA target prediction. Wild type (WT) and mutant (MUT) vectors of BCL2L1-3′UTR and BCL2-3′UTR were purchased from GeneCopoeia (Rockville, MD, USA). The full length BCL2L1-3’UTR (217HmiT108616-MT05; 1489 bp) and of the proximal part of BCL2-3’UTR (217HmiT016211a; 2761 bp) were inserted downstream of a Gaussia luciferase (GLUC) reporter gene in the pEZMX-MT05 vector. This vector also carries the secreted alkaline phosphatase (SEAP) reporter gene for normalization of GLUC luciferase activity, as a function of transfection efficiency. SW1353 cells were seeded at 8 × 10
4 cells/well (triplicates) in a 24-well plate the day before transfection. The cells were co-transfected with 20 nM miRNA mimic or miRNA hairpin inhibitor and with BCL2-3′UTR vector (0.25 ng/µL) using jetOPTIMUS
TM DNA transfection reagent (Polyplus-Transfection, Strasbourg, France). BCL2L1-3′UTR vector (1 ng/µL) and miRNA were co-transfected with EndoFectin transfection reagent (Genecopoeia, Rockville, MD, USA). The culture medium was collected 24 h later to measure luciferase activities with the secrete-pair dual luminescence assay kit (Genecopoeia, Rockville, MD, USA) and a luminescence microplate reader (Spark 10M, Tecan, Lyon, France). Luciferase activities were expressed as relative luciferase units (RLU) corresponding to GLUC/SEAP ratio.
4.13. Autophagy Assay
Cell MeterTM autophagy fluorescence imaging kit (AAT Bioquest®, Sunnyvale, CA, USA) was used to analyze the autophagic activity of the cells. The kit uses the Autophagy GreenTM reagent as a specific autophagosome marker. SW1353 cells were seeded onto 96-well microplates at a density of 3.5 × 103 cells/well in triplicates. They were transfected 24 h later with 20 nM miRNA as described above, and incubated for 72 h. As positive controls, cells were incubated for the last 24 h in PBS with 5% FCS. Seventy-two hours post-transfection, the medium was replaced by the Autophagy GreenTM working solution. The cells were incubated in a 37 °C, 5% CO2 incubator for 1 h. After washing with PBS, the nuclei were counterstained with DAPI (Santa Cruz Biotechnology, Dallas, TX, USA). The cells were then observed under a fluorescence microscope (Nikon Eclipse Ti; Nikon Instruments Inc, Melville, NY, USA). Autophagosomes and nuclei were counted with ImageJ software (National Institutes of Health, Bethesda, MD, USA).
4.14. Statistical Analysis
All experiments were repeated at least three times. Values are reported as means ± SD. Statistical significances of the mean of at least three independent experiments were assessed using one-way ANOVA corrected for multiple comparisons using Dunnett’s test, or two-way ANOVA corrected for multiple comparisons using Sidak’s test. Alternatively, two-tailed unpaired student’s t-test with Welch’s correction was used to analyze statistical differences within representative experiments performed in triplicate. Statistical analyses were carried out using GraphPad Prism 7 software (San Diego, CA, USA). p-values of less than 0.05 were considered significant: *** p < 0.001, ** p < 0.01, and * p < 0.05.