Generation of GCaMP6s-Expressing Zebrafish to Monitor Spatiotemporal Dynamics of Calcium Signaling Elicited by Heat Stress
Abstract
1. Introduction
2. Results
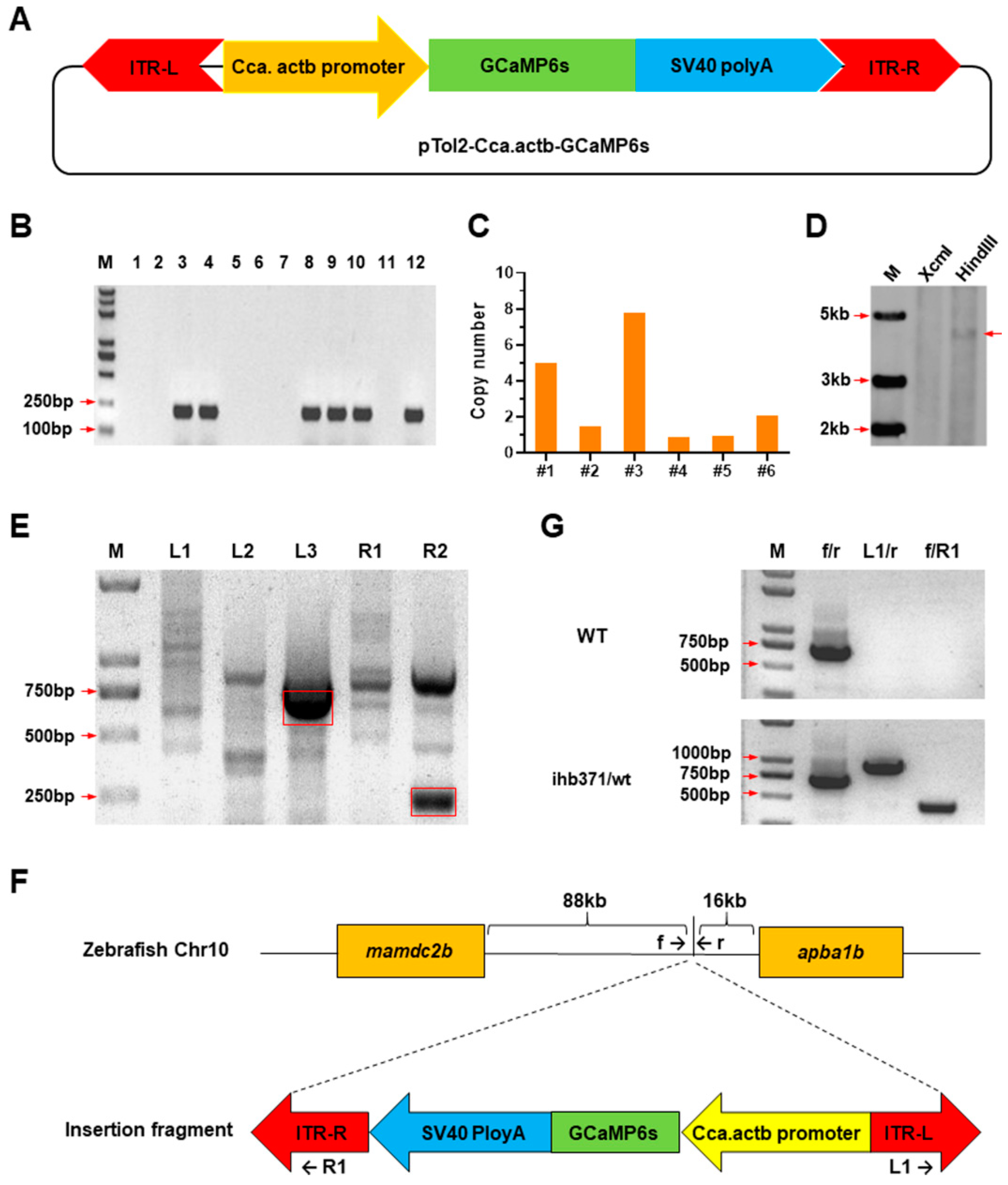
2.1. Generation and Characterization of GCaMP6s-Expressing Zebrafish
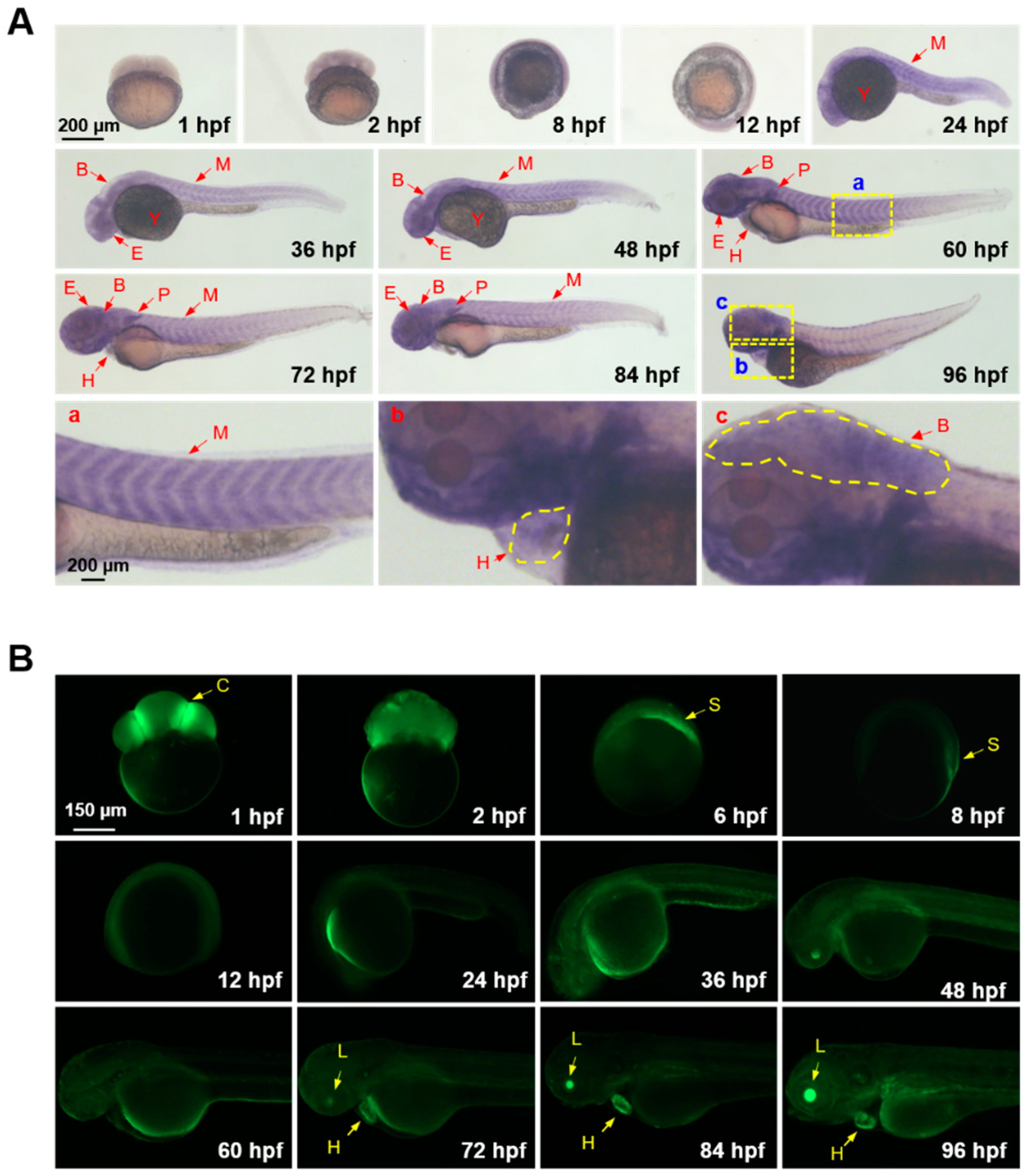
2.2. GCaMP6s Expression and Ca2+ Events in Embryos and Larvae of the Transgenic Zebrafish
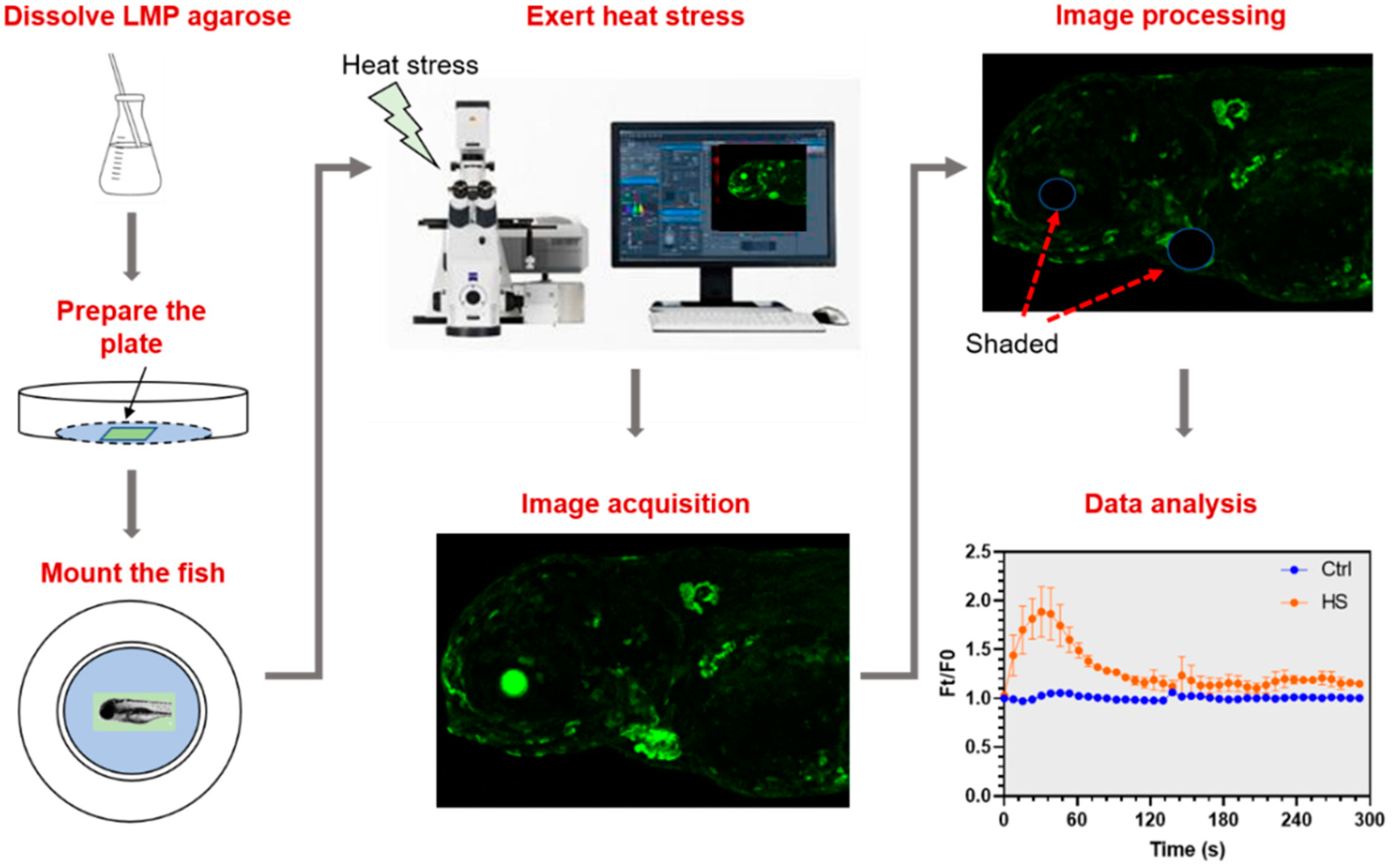
2.3. Establishment of a Protocol to Analyze In Vivo Activities of Calcium Signaling Triggered by Heat Stress
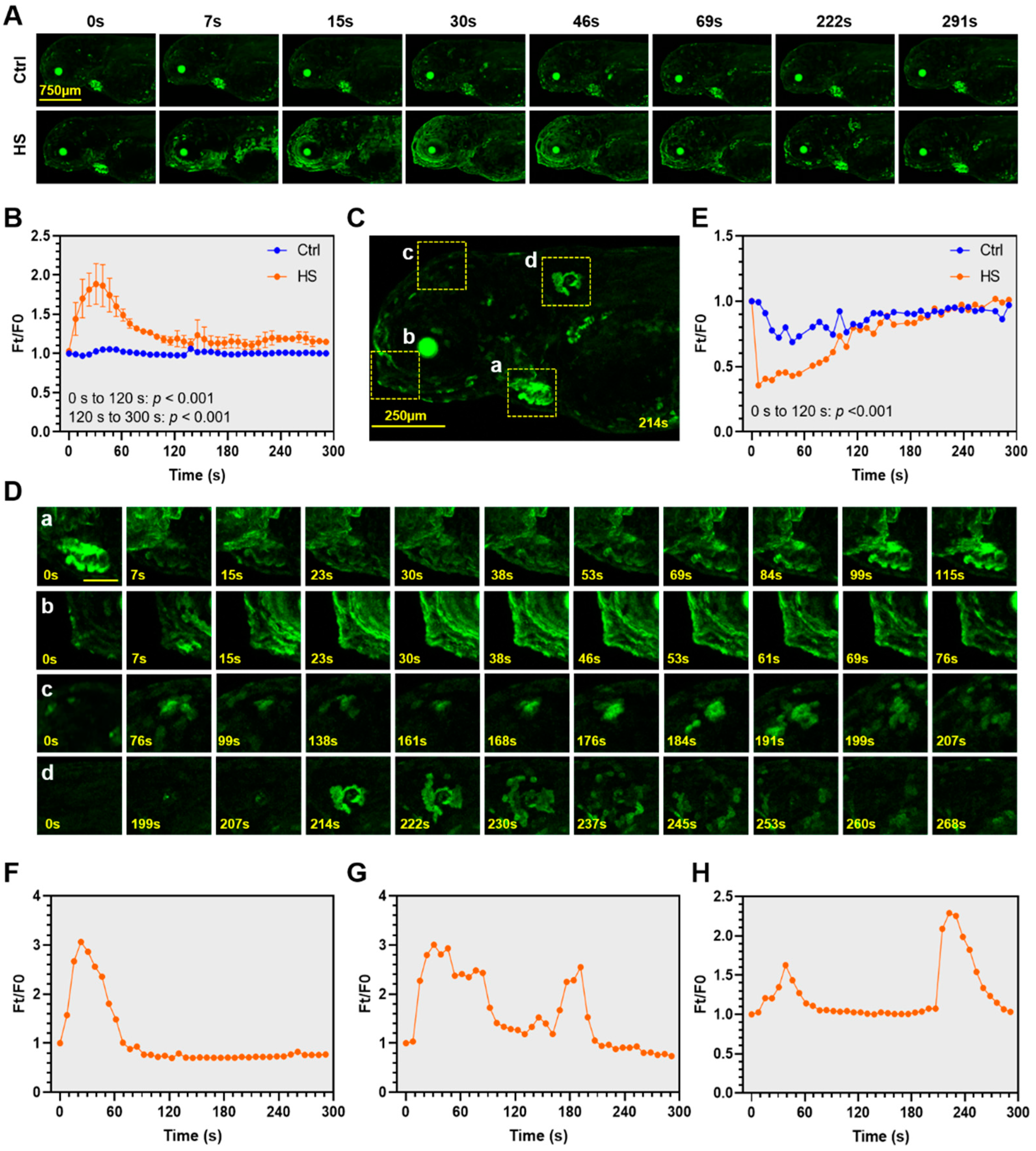
2.4. Spatiotemporal Dynamics of Heat Stress-Elicited Calcium Signaling in Zebrafish Larvae
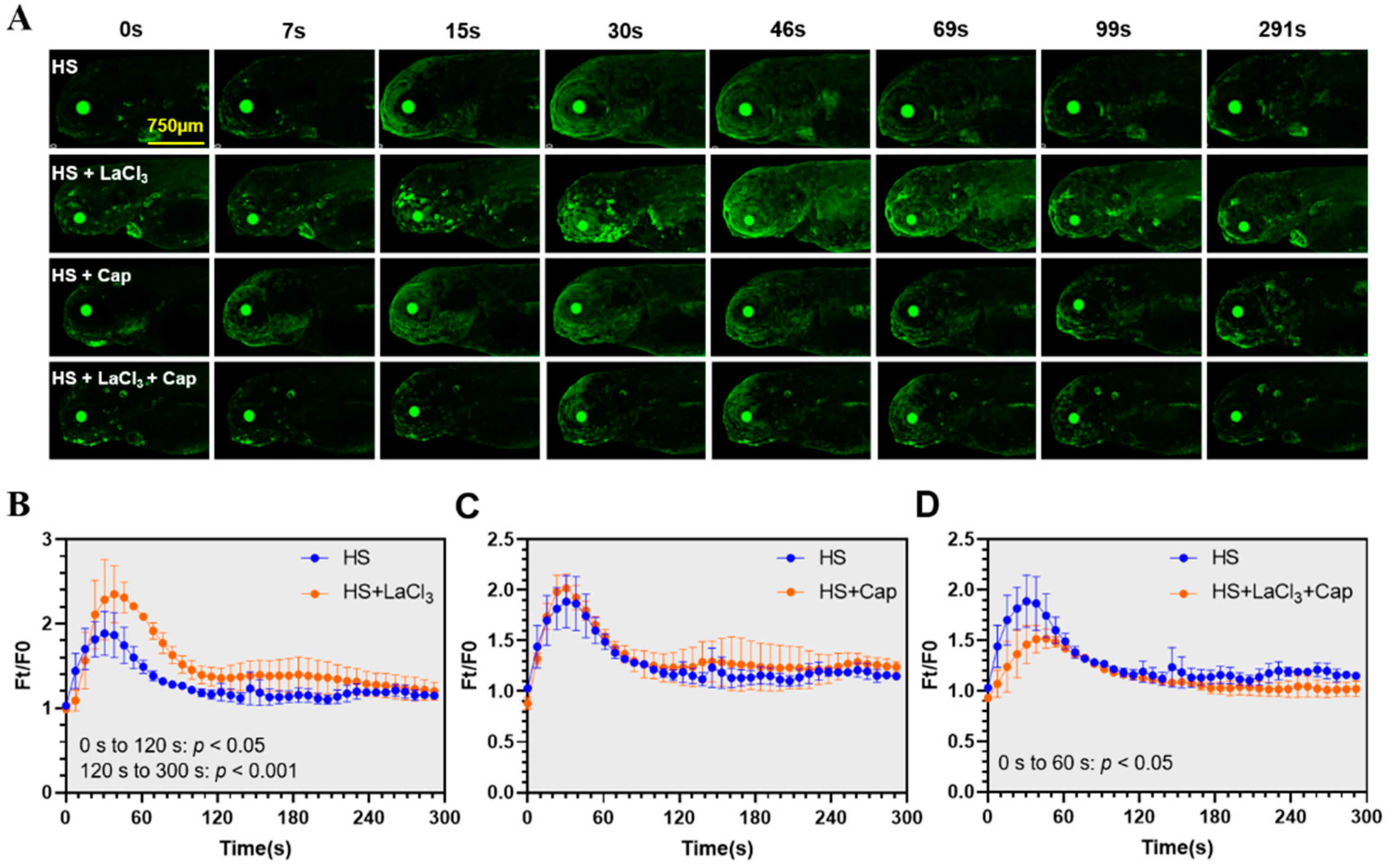
2.5. Combination of Lanthanum Chloride (LaCl3) and Capsazepine Attenuated the Heat-Elicited Ca2+ Events
2.6. Functions of the Ca2+-CaMKII-HSF1 Pathway in Regulating the HSR of Zebrafish Larvae
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Maintenance of Zebrafish
4.3. Generation of GCaMP6s-Expressing Transgenic Construct
4.4. Preparation of Tol2 Transposase Capped mRNA and Microinjection
4.5. Transgenic Fish Screening
4.6. Quantitative Real-Time PCR
4.7. Southern Blotting
4.8. Genome Walking
4.9. Whole Mount In Situ Hybridization
4.10. Fluorescence Microscopy
4.11. Fluorescence Intensity Measurement and Analysis
4.12. Heat and Chemical Treatments
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ROS | reactive oxygen species |
| HSR | heat shock response |
| HSP | heat shock protein |
| HSF | heat shock transcription factor |
| HSE | heat shock element |
| PTM | post translational modification |
| CaMKII | calcium/calmodulin-dependent protein kinase II |
| TRP | transient receptor potential |
| GECI | genetically encoded calcium indicator |
| GCaMP | green fluorescent protein/calmodulin protein |
| qPCR | real-time quantitative PCR |
| WT | wild type |
| TAIL–PCR | thermal asymmetric interlaced PCR |
| LaCl3 | lanthanum chloride |
References
- Brett, J.R. Energetic Responses of Salmon to Temperature—Study of Some Thermal Relations in Physiology and Fresh water Ecology of Sockeye Salmon (Oncorhynchus-Nerka). Am. Zool. 1971, 11, 99–113. [Google Scholar] [CrossRef]
- Hofmann, G.E.; Todgham, A.E. Living in the Now: Physiological Mechanisms to Tolerate a Rapidly Changing Environment. Annu. Rev. Physiol. 2010, 72, 127–145. [Google Scholar] [CrossRef]
- Richter, K.; Haslbeck, M.; Buchner, J. The Heat Shock Response: Life on the Verge of Death. Mol. Cell 2010, 40, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Logan, C.A.; Buckley, B.A. Transcriptomic responses to environmental temperature in eurythermal and stenothermal fishes. J. Exp. Biol. 2015, 218, 1915–1924. [Google Scholar] [CrossRef]
- Somero, G.N. The cellular stress response and temperature: Function, regulation, and evolution. J. Exp. Zool. Part A 2020, 333, 379–397. [Google Scholar] [CrossRef]
- Petitjean, Q.; Jean, S.; Gandar, A.; Cote, J.; Laffaille, P.; Jacquin, L. Stress responses in fish: From molecular to evolutionary processes. Sci. Total Environ. 2019, 684, 371–380. [Google Scholar] [CrossRef]
- Bosch, T.C.G.; Krylow, S.M.; Bode, H.R.; Steele, R.E. Thermotolerance and Synthesis of Heat-Shock Proteins—These Responses Are Present in Hydra-Attenuata but Absent in Hydra-Oligactis. Proc. Natl. Acad. Sci. USA 1988, 85, 7927–7931. [Google Scholar] [CrossRef]
- Parker, C.S.; Topol, J. A Drosophila RNA Polymerase-II Transcription Factor Binds to the Regulatory Site of an Hsp 70 Gene. Cell 1984, 37, 273–283. [Google Scholar] [CrossRef]
- Mahat, D.B.; Salamanca, H.H.; Duarte, F.M.; Danko, C.G.; Lis, J.T. Mammalian Heat Shock Response and Mechanisms Underlying Its Genome-wide Transcriptional Regulation. Mol. Cell 2016, 62, 63–78. [Google Scholar] [CrossRef]
- Pirkkala, L.; Nykanen, P.; Sistonen, L. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 2001, 15, 1118–1131. [Google Scholar] [CrossRef]
- Perisic, O.; Xiao, H.; Lis, J.T. Stable Binding of Drosophila Heat-Shock Factor to Head-to-Head and Tail-to-Tail Repeats of a Conserved 5-Bp Recognition Unit. Cell 1989, 59, 797–806. [Google Scholar] [CrossRef]
- Xu, D.M.; Zalmas, L.P.; La Thangue, N.B. A transcription cofactor required for the heat-shock response. EMBO Rep. 2008, 9, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Takii, R.; Fujimoto, M.; Matsumoto, M.; Srivastava, P.; Katiyar, A.; Nakayama, K.I.; Nakai, A. The pericentromeric protein shugoshin 2 cooperates with HSF1 in heat shock response and RNA Pol II recruitment. EMBO J. 2019, 38, e102566. [Google Scholar] [CrossRef] [PubMed]
- Akerfelt, M.; Morimoto, R.I.; Sistonen, L. Heat shock factors: Integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 2010, 11, 545–555. [Google Scholar] [CrossRef]
- Gomez-Pastor, R.; Burchfiel, E.T.; Thiele, D.J. Regulation of heat shock transcription factors and their roles in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 4–19. [Google Scholar] [CrossRef]
- Holmberg, C.I.; Hietakangas, V.; Mikhailov, A.; Rantanen, J.O.; Kallio, M.; Meinander, A.; Hellman, J.; Morrice, N.; MacKintosh, C.; Morimoto, R.I.; et al. Phosphorylation of serine 230 promotes inducible transcriptional activity of heat shock factor 1. EMBO J. 2001, 20, 3800–3810. [Google Scholar] [CrossRef]
- Saidi, Y.; Finka, A.; Muriset, M.; Bromberg, Z.; Weiss, Y.G.; Maathuis, F.J.M.; Goloubinoff, P. The Heat Shock Response in Moss Plants Is Regulated by Specific Calcium-Permeable Channels in the Plasma Membrane. Plant Cell 2009, 21, 2829–2843. [Google Scholar] [CrossRef]
- Saito, S.; Tominaga, M. Evolutionary tuning of TRPA1 and TRPV1 thermal and chemical sensitivity in vertebrates. Temperature 2017, 4, 141–152. [Google Scholar] [CrossRef]
- Patapoutian, A.; Peier, A.M.; Story, G.M.; Viswanath, V. Thermotrp channels and beyond: Mechanisms of temperature sensation. Nat. Rev. Neurosci. 2003, 4, 529–539. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Gau, P.; Poon, J.; Ufret-Vincenty, C.; Snelson, C.D.; Gordon, S.E.; Raible, D.W.; Dhaka, A. The Zebrafish Ortholog of TRPV1 Is Required for Heat-Induced Locomotion. J. Neurosci. 2013, 33, 5249–5260. [Google Scholar] [CrossRef]
- Li, W.; Lee, Y.; Kim, J.; Kang, S.; Kim, S.; Kim, K.; Park, C.; Chung, J. Transient receptor potential vanilloid-1 mediates heat shock-induced matrix metalloproteinase-1 expression in human epidermal keratinocytes. J. Investig. Dermatol. 2007, 127, S137. [Google Scholar] [CrossRef]
- Silva, M.C.; Amaral, M.D.; Morimoto, R.I. Neuronal Reprograming of Protein Homeostasis by Calcium-Dependent Regulation of the Heat Shock Response. PLoS Genet 2013, 9, e1003711. [Google Scholar] [CrossRef] [PubMed]
- Virdi, A.S.; Pareek, A.; Singh, P. Evidence for the possible involvement of calmodulin in regulation of steady state levels of Hsp90 family members (Hsp87 and Hsp85) in response to heat shock in sorghum. Plant Signal. Behav. 2011, 6, 393–399. [Google Scholar] [CrossRef]
- Liu, H.T.; Li, B.; Shang, Z.L.; Li, X.Z.; Mu, R.L.; Sun, D.Y.; Zhou, R.G. Calmodulin is involved in heat shock signal transduction in wheat. Plant Physiol. 2003, 132, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Gao, F.; Li, G.L.; Han, J.L.; Liu, D.L.; Sun, D.Y.; Zhou, R.G. The calmodulin-binding protein kinase 3 is part of heat-shock signal transduction in Arabidopsis thaliana. Plant J. 2008, 55, 760–773. [Google Scholar] [CrossRef]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef]
- Bootman, M.D.; Berridge, M.J.; Roderick, H.L. Calcium signalling: More messengers, more channels, more complexity. Curr. Biol. 2002, 12, R563–R565. [Google Scholar] [CrossRef]
- Petersen, O.H.; Michalak, M.; Verkhratsky, A. Calcium signalling: Past, present and future. Cell Calcium 2005, 38, 161–169. [Google Scholar] [CrossRef]
- Akerboom, J.; Chen, T.W.; Wardill, T.J.; Tian, L.; Marvin, J.S.; Mutlu, S.; Calderon, N.C.; Esposti, F.; Borghuis, B.G.; Sun, X.R.; et al. Optimization of a GCaMP Calcium Indicator for Neural Activity Imaging. J. Neurosci. 2012, 32, 13819–13840. [Google Scholar] [CrossRef]
- Long, Y.; Li, L.C.; Li, Q.; He, X.Z.; Cui, Z.B. Transcriptomic Characterization of Temperature Stress Responses in Larval Zebrafish. PLoS ONE 2012, 7, e37209. [Google Scholar]
- Chen, J.K.; Xia, L.; Bruchas, M.R.; Solnica-Krezel, L. Imaging early embryonic calcium activity with GCaMP6s transgenic zebrafish. Dev. Biol. 2017, 430, 385–396. [Google Scholar] [CrossRef]
- Long, Y.; Song, G.L.; Yan, J.J.; He, X.Z.; Li, Q.; Cui, Z.B. Transcriptomic characterization of cold acclimation in larval zebrafish. BMC Genom. 2013, 14, 612. [Google Scholar] [CrossRef]
- Muto, A.; Ohkura, M.; Kotani, T.; Higashijima, S.; Nakai, J.; Kawakami, K. Genetic visualization with an improved GCaMP calcium indicator reveals spatiotemporal activation of the spinal motor neurons in zebrafish. Proc. Natl. Acad. Sci. USA 2011, 108, 5425–5430. [Google Scholar] [CrossRef]
- Turrini, L.; Fornetto, C.; Marchetto, G.; Mullenbroich, M.C.; Tiso, N.; Vettori, A.; Resta, F.; Masi, A.; Mannaioni, G.; Pavone, F.S.; et al. Optical mapping of neuronal activity during seizures in zebrafish. Sci. Rep. 2017, 7, 3025. [Google Scholar] [CrossRef]
- Kim, C.K.; Miri, A.; Leung, L.C.; Berndt, A.; Mourrain, P.; Tank, D.W.; Burdine, R.D. Prolonged, brain-wide expression of nuclear-localized GCaMP3 for functional circuit mapping. Front. Neural Circuits 2014, 8, 138. [Google Scholar] [CrossRef]
- Chi, N.C.; Shaw, R.M.; Jungblut, B.; Huisken, J.; Ferrer, T.; Arnaout, R.; Scott, I.; Beis, D.; Xiao, T.; Baier, H.; et al. Genetic and physiologic dissection of the vertebrate cardiac conduction system. PLoS Biol. 2008, 6, e109. [Google Scholar] [CrossRef]
- Liu, Z.J.; Zhu, Z.Y.; Roberg, K.; Faras, A.; Guise, K.; Kapuscinski, A.R.; Hackett, P.B. Isolation and characterization of beta-actin gene of carp (Cyprinus carpio). DNA Seq. 1990, 1, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Knight, H.; Trewavas, A.J.; Knight, M.R. Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J. 1997, 12, 1067–1078. [Google Scholar] [CrossRef]
- Dai, W.; Bai, Y.; Hebda, L.; Zhong, X.; Liu, J.; Kao, J.; Duan, C. Calcium deficiency-induced and TRP channel-regulated IGF1R-PI3K-Akt signaling regulates abnormal epithelial cell proliferation. Cell Death Differ. 2014, 21, 568–581. [Google Scholar] [CrossRef]
- Yoon, Y.J.; Kim, J.A.; Shin, K.D.; Shin, D.S.; Han, Y.M.; Lee, Y.J.; Lee, J.S.; Kwon, B.M.; Han, D.C. KRIBB11 Inhibits HSP70 Synthesis through Inhibition of Heat Shock Factor 1 Function by Impairing the Recruitment of Positive Transcription Elongation Factor b to the hsp70 Promoter. J. Biol. Chem. 2011, 286, 1737–1747. [Google Scholar] [CrossRef]
- Johnson, C.N.; Pattanayek, R.; Potet, F.; Rebbeck, R.T.; Blackwell, D.J.; Nikolaienko, R.; Sequeira, V.; Le Meur, R.; Radwanski, P.B.; Davis, J.P.; et al. The CaMKII inhibitor KN93-calmodulin interaction and implications for calmodulin tuning of Na(V)1.5 and RyR2 function. Cell Calcium 2019, 82, 102063. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Guan, Q.M.; Wu, J.M.; Yue, X.L.; Zhang, Y.Y.; Zhu, J.H. A Nuclear Calcium-Sensing Pathway Is Critical for Gene Regulation and Salt Stress Tolerance in Arabidopsis. PLoS Genet. 2013, 9, e1003755. [Google Scholar] [CrossRef]
- Vandewauw, I.; De Clercq, K.; Mulier, M.; Held, K.; Pinto, S.; Van Ranst, N.; Segal, A.; Voet, T.; Vennekens, R.; Zimmermann, K.; et al. A TRP channel trio mediates acute noxious heat sensing. Nature 2018, 555, 662–666. [Google Scholar] [CrossRef]
- Teets, N.M.; Yi, S.X.; Lee, R.E.; Denlinger, D.L. Calcium signaling mediates cold sensing in insect tissues. Proc. Natl. Acad. Sci. USA 2013, 110, 9154–9159. [Google Scholar] [CrossRef]
- Jayakumar, S.; Hasan, G. Neuronal Calcium Signaling in Metabolic Regulation and Adaptation to Nutrient Stress. Front. Neural Circuits 2018, 12, 25. [Google Scholar] [CrossRef]
- Fiol, D.F.; Kultz, D. Osmotic stress sensing and signaling in fishes. FEBS J. 2007, 274, 5790–5798. [Google Scholar] [CrossRef]
- Seale, A.P.; Richman, N.H.; Hirano, T.; Cooke, I.; Grau, E.G. Evidence that signal transduction for osmoreception is mediated by stretch-activated ion channels in tilapia. Am. J. Physiol. Cell Physiol. 2003, 284, C1290–C1296. [Google Scholar] [CrossRef][Green Version]
- Seta, K.A.; Yuan, Y.; Spicer, Z.; Lu, G.; Bedard, J.; Ferguson, T.K.; Pathrose, P.; Cole-Strauss, A.; Kaufhold, A.; Millhorn, D.E. The role of calcium in hypoxia-induced signal transduction and gene expression. Cell Calcium 2004, 36, 331–340. [Google Scholar] [CrossRef]
- Christou, M.; Fraser, T.W.K.; Berg, V.; Ropstad, E.; Kamstra, J.H. Calcium signaling as a possible mechanism behind increased locomotor response in zebrafish larvae exposed to a human relevant persistent organic pollutant mixture or PFOS. Environ. Res. 2020, 187, 109702. [Google Scholar] [CrossRef]
- Madden, C.J.; Morrison, S.F. Central nervous system circuits that control body temperature. Neurosci. Lett. 2019, 696, 225–232. [Google Scholar] [CrossRef]
- Gilbert, G.; Demydenko, K.; Dries, E.; Puertas, R.D.; Jin, X.; Sipido, K.; Roderick, H.L. Calcium Signaling in Cardiomyocyte Function. CSH Perspect. Biol. 2020, 12, a035428. [Google Scholar] [CrossRef]
- Wingo, J.E.; Ganio, M.S.; Cureton, K.J. Cardiovascular Drift During Heat Stress: Implications for Exercise Prescription. Exerc. Sport Sci. Rev. 2012, 40, 88–94. [Google Scholar] [CrossRef]
- Segal, J. Lanthanum Increases the Rat Thymocyte Cytoplasmic Free Calcium-Concentration by Enhancing Calcium Influx. Biochim. Biophys. Acta 1986, 886, 267–271. [Google Scholar] [CrossRef]
- Mead, R.H.; Clusin, W.T. Paradoxical Electromechanical Effect of Lanthanum Ions in Cardiac-Muscle Cells. Biophys. J. 1985, 48, 695–700. [Google Scholar] [CrossRef]
- Peng, W.; Zhang, Y.; Zheng, M.; Cheng, H.P.; Zhu, W.Z.; Cao, C.M.; Xiao, R.P. Cardioprotection by CaMKII-delta B Is Mediated by Phosphorylation of Heat Shock Factor 1 and Subsequent Expression of Inducible Heat Shock Protein 70. Circ. Res. 2010, 106, 102–110. [Google Scholar] [CrossRef]
- Long, Y.; Li, Q.; Zhong, S.; Wang, Y.H.; Cui, Z.B. Molecular characterization and functions of zebrafish ABCC2 in cellular efflux of heavy metals. Comp. Biochem. Phys. C 2011, 153, 381–391. [Google Scholar] [CrossRef]
- Lu, X.; Long, Y.; Li, X.; Zhang, L.; Li, Q.; Wen, H.; Zhong, S.; Cui, Z. Generation of Knockout and Transgenic Zebrafish to Characterize Abcc4 Functions in Detoxification and Efflux of Lead. Int. J. Mol. Sci. 2021, 22, 2504. [Google Scholar] [CrossRef]
- He, X.Z.; Li, J.; Long, Y.; Song, G.L.; Zhou, P.Y.; Liu, Q.X.; Zhu, Z.Y.; Cui, Z.B. Gene transfer and mutagenesis mediated by Sleeping Beauty transposon in Nile tilapia (Oreochromis niloticus). Transgenic Res. 2013, 22, 913–924. [Google Scholar] [CrossRef]
- Zhou, T.; Zhou, B.L.; Zhao, Y.S.; Li, Q.; Song, G.L.; Zhu, Z.Y.; Long, Y.; Cui, Z.B. Development of a Mucus Gland Bioreactor in Loach Paramisgurnus dabryanus. Int. J. Mol. Sci. 2021, 22, 687. [Google Scholar] [CrossRef]
- Yan, J.J.; Gao, Q.; Cui, Z.B.; Yang, G.L.; Long, Y. Molecular characterization of the giant freshwater prawn (Macrobrachium rosenbergii) beta-actin gene promoter. PeerJ 2018, 6, e5701. [Google Scholar] [CrossRef]
- Zhou, B.L.; Long, Y.; Song, G.L.; Li, Q.; Cui, Z.B. Molecular characterization of the lgals1 gene in large scale loach Paramisgurnus dabryanus. Gene 2016, 577, 65–74. [Google Scholar] [CrossRef]
- Liu, C.Y.; Song, G.L.; Mao, L.; Long, Y.; Li, Q.; Cui, Z.B. Generation of an Enhancer-Trapping Vector for Insertional Mutagenesis in Zebrafish. PLoS ONE 2015, 10, e0139612. [Google Scholar] [CrossRef]
- Thisse, C.; Thisse, B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 2008, 3, 59–69. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Long, Y.; Xie, J.; Ren, J.; Zhou, T.; Song, G.; Li, Q.; Cui, Z. Generation of GCaMP6s-Expressing Zebrafish to Monitor Spatiotemporal Dynamics of Calcium Signaling Elicited by Heat Stress. Int. J. Mol. Sci. 2021, 22, 5551. https://doi.org/10.3390/ijms22115551
Li F, Long Y, Xie J, Ren J, Zhou T, Song G, Li Q, Cui Z. Generation of GCaMP6s-Expressing Zebrafish to Monitor Spatiotemporal Dynamics of Calcium Signaling Elicited by Heat Stress. International Journal of Molecular Sciences. 2021; 22(11):5551. https://doi.org/10.3390/ijms22115551
Chicago/Turabian StyleLi, Fengyang, Yong Long, Juhong Xie, Jing Ren, Tong Zhou, Guili Song, Qing Li, and Zongbin Cui. 2021. "Generation of GCaMP6s-Expressing Zebrafish to Monitor Spatiotemporal Dynamics of Calcium Signaling Elicited by Heat Stress" International Journal of Molecular Sciences 22, no. 11: 5551. https://doi.org/10.3390/ijms22115551
APA StyleLi, F., Long, Y., Xie, J., Ren, J., Zhou, T., Song, G., Li, Q., & Cui, Z. (2021). Generation of GCaMP6s-Expressing Zebrafish to Monitor Spatiotemporal Dynamics of Calcium Signaling Elicited by Heat Stress. International Journal of Molecular Sciences, 22(11), 5551. https://doi.org/10.3390/ijms22115551





