C60 Fullerene Reduces 3-Nitropropionic Acid-Induced Oxidative Stress Disorders and Mitochondrial Dysfunction in Rats by Modulation of p53, Bcl-2 and Nrf2 Targeted Proteins
Abstract
1. Introduction
2. Results
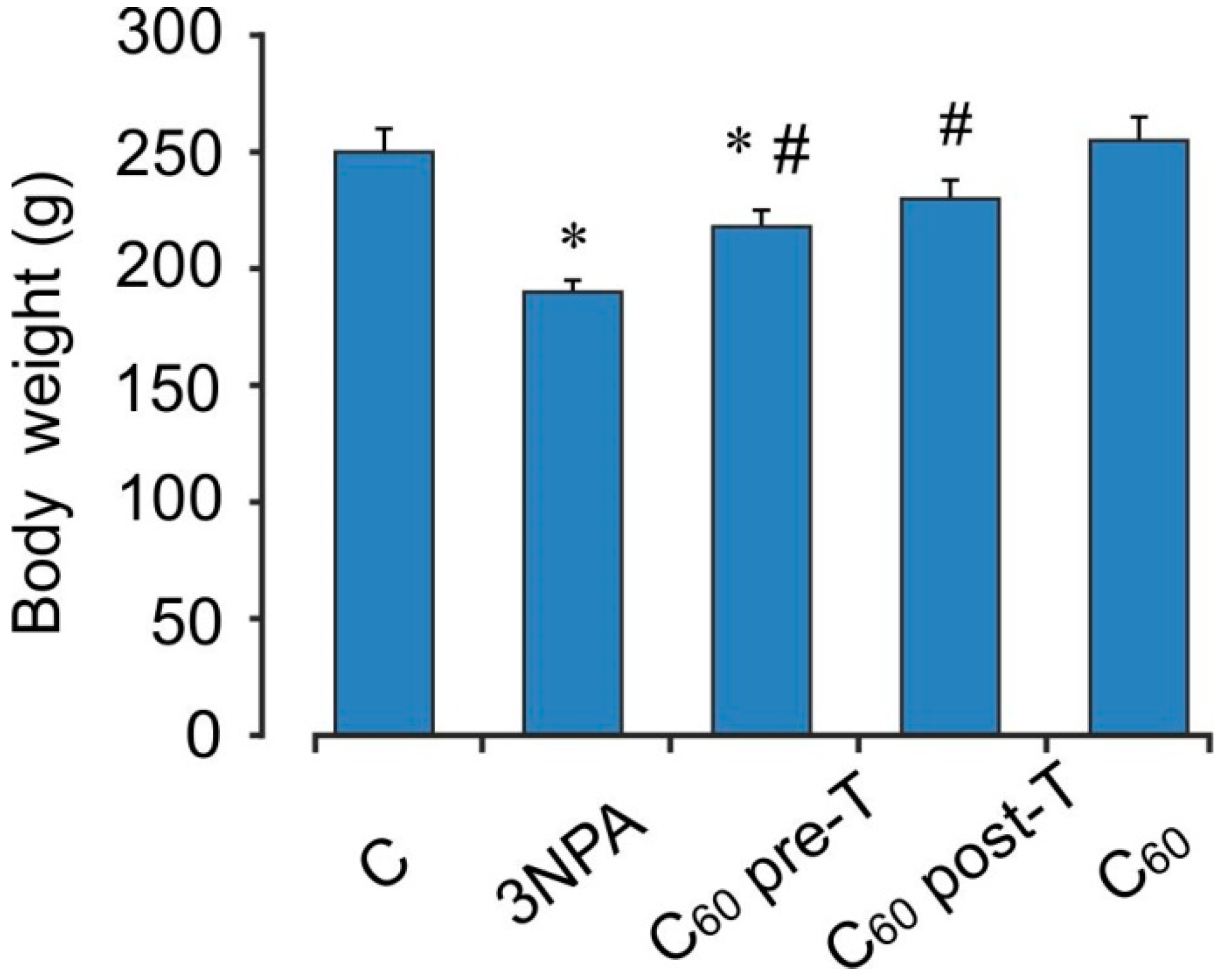
2.1. Body Weight Changes
2.2. Neurological Scoring
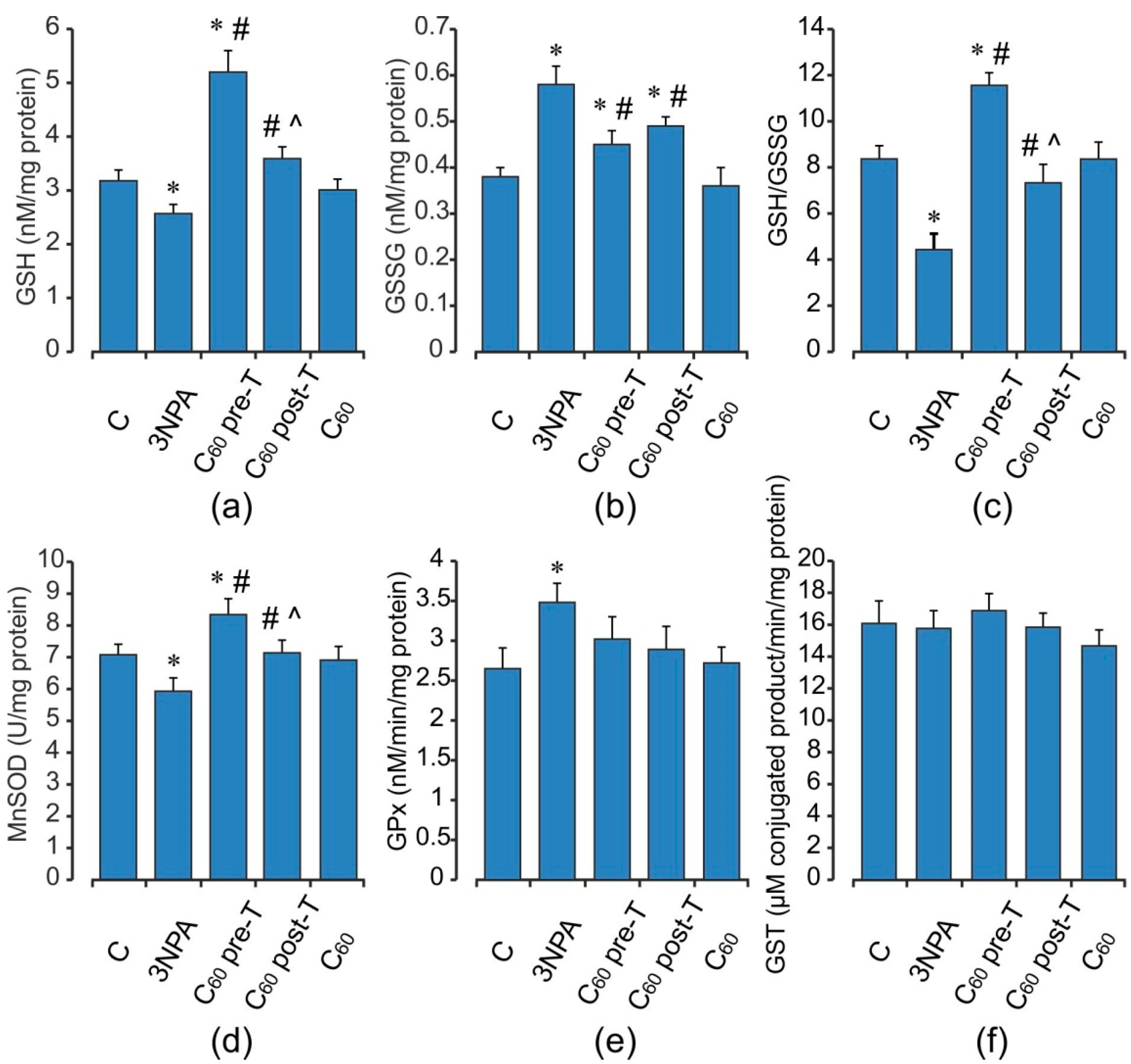
2.3. Oxidative Status of the Brain Mitochondria after Acute 3-NPA Exposure and C60 Supplementation
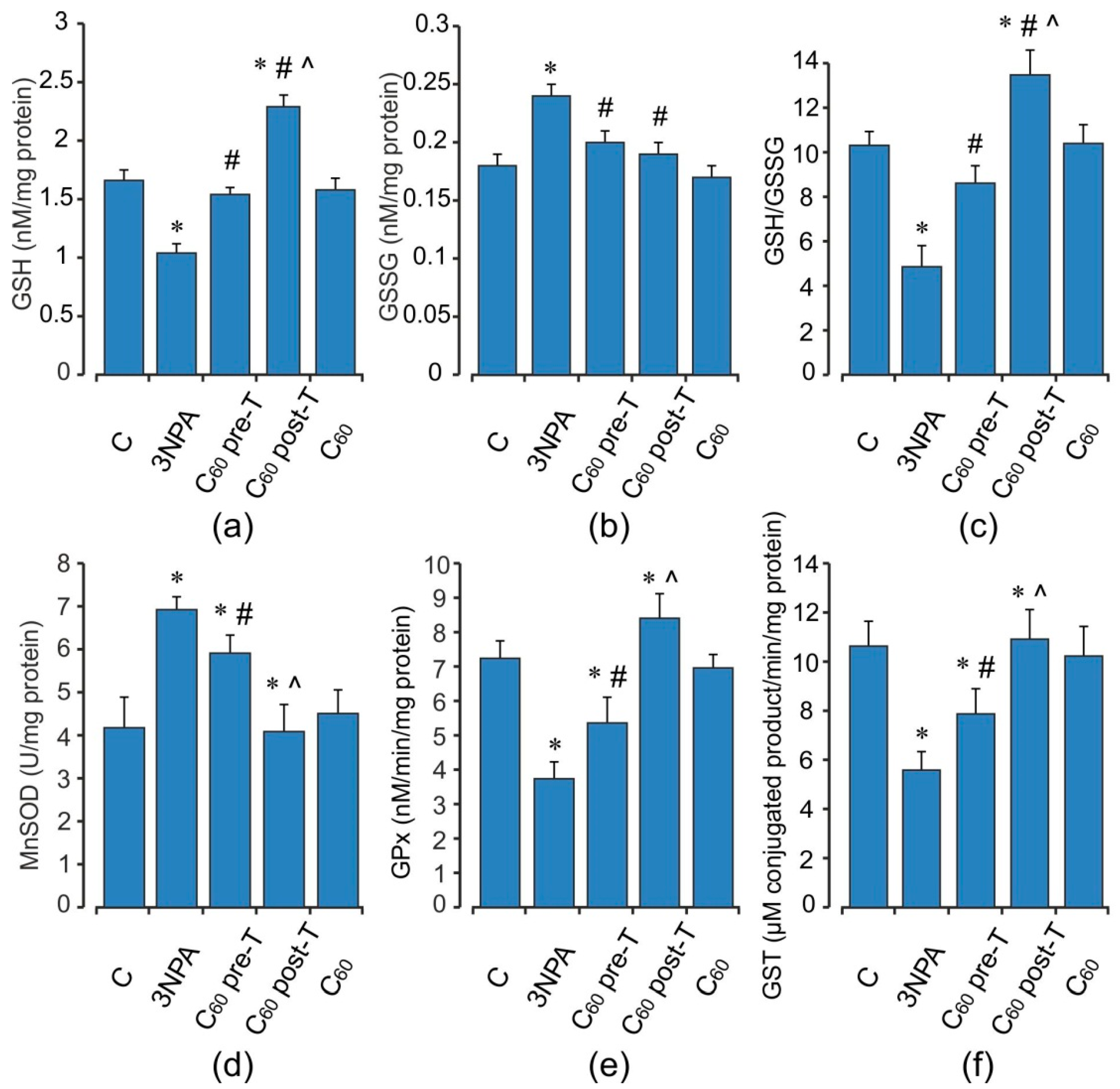
2.4. Oxidative Status of the Skeletal Muscle Mitochondria after Acute 3-NPA Exposure and C60 Supplementation
2.5. Activity of Electron Transport Chain Enzymes in Brain and Skeletal Muscle Mitochondria after Acute 3-NPA Exposure and C60 Supplementation
2.6. Effect of 3-NPA Exposure and C60 Supplementation on Pro- and Anti-Apoptotic Markers
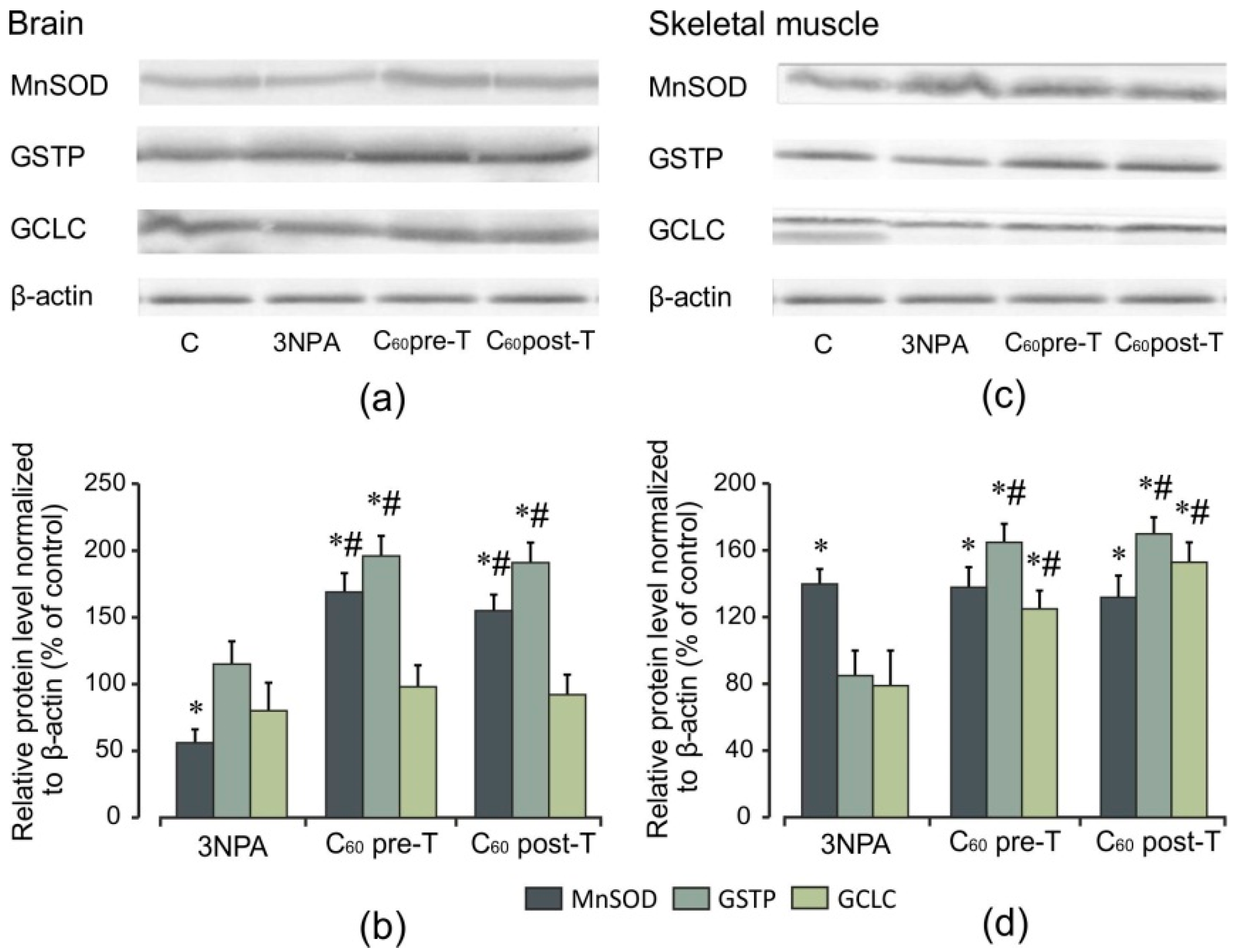
2.7. Effect of 3-NPA Exposure and C60 Supplementation on Protein Expression of Nrf2 and Nrf2 Target Proteins
2.8. Effect of C60 Supplementation Alone on Oxidative Stress Markers and Indices of Antioxidant Defense
3. Discussion
4. Materials and Methods
4.1. Material Preparation and Characterization
4.2. Experimental Design and Sample Collection
4.3. Neurological Scoring (Movement Analysis)
4.4. Sample Collection and Mitochondria Isolation
4.4.1. Isolation of Rat Brain Mitochondria
4.4.2. Isolation of Rat Skeletal Muscle Mitochondria
4.5. Evaluation of Oxidative Stress Markers
4.5.1. Measurement of Reactive Oxygen Species (ROS) Formation
4.5.2. Measurement of Superoxide Radical Production
4.5.3. Measurement of Hydroperoxide
4.5.4. Lipid Peroxidation Assay
4.6. Evaluation of Biochemical Parameters
4.6.1. Mitochondrial Respiratory Chain Enzymes
4.6.2. Enzyme Activity Assay
4.6.3. Measurement of the Reduced and Oxidized Glutathione Contents
4.7. Western Blot Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jezek, P.; Hlavata, L. Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. Int. J. Biochem. Cell. Biol. 2005, 37, 2478–2503. [Google Scholar] [CrossRef]
- Ross, C.A.; Tabrizi, S.J. Huntington’s disease: From molecular pathogenesis to clinical treatment. Lancet Neurol. 2011, 10, 83–98. [Google Scholar] [CrossRef]
- Zuccato, C.; Valenza, M.; Cattaneo, E. Molecular mechanisms and potential therapeutical targets in Huntington’s disease. Physiol. Rev. 2010, 90, 905–981. [Google Scholar] [CrossRef]
- Mehrotra, A.; Sandhir, R. Mitochondrial cofactors in experimental Huntington’s disease: Behavioral, biochemical and histological evaluation. Behav. Brain Res. Rev. 2014, 261, 345–355. [Google Scholar] [CrossRef]
- Sassone, J.; Colciago, C.; Cislaghi, G.; Silani, V.; Ciammola, A. Huntington’s disease: The current state of research with peripheral tissues. Exp. Neurol. 2009, 219, 385–397. [Google Scholar] [CrossRef]
- Bozzi, M.; Sciandra, F. Molecular Mechanisms Underlying Muscle Wasting in Huntington’s Disease. Int. J. Mol. Sci. 2020, 21, 8314. [Google Scholar] [CrossRef]
- Tunez, I.; Tasset, I.; Perez-De La Cruz, V.; Santamaria, A. 3-Nitropropionic acid as a tool to study the mechanisms involved in Huntington’s disease: Past, present and future. Molecules 2010, 15, 878–916. [Google Scholar] [CrossRef]
- Damiano, M.L.; Galvan, N.; Deglon, N.; Brouillet, E. Mitochondria in Huntington’s disease. Biochim. Biophys. Acta 2010, 1802, 52–61. [Google Scholar] [CrossRef]
- Mandavilli, B.S.; Boldogh, I.; Van Houten, B. 3-Nitropropionic acid-induced hydrogen peroxide, mitochondrial DNA damage, and cell death are attenuated by Bcl-2 overexpression in PC12 cell. Brain Res. Mol. Brain Res. 2005, 18, 215–223. [Google Scholar] [CrossRef]
- Ludolph, A.C.; He, F.; Spencer, P.S.; Hammerstad, J.; Sabri, M. 3-Nitropropionic acid-exogenous animal neurotoxin and possible human striatal toxin. Can. J. Neurol. Sci. 1991, 18, 492–498. [Google Scholar] [CrossRef]
- Fão, L.; Rego, A.C. Mitochondrial and redox-based therapeutic strategies in Huntington’s disease. Antioxid. Redox. Signal. 2021, 10, 650–653. [Google Scholar] [CrossRef]
- Johnson, W.M.; Wilson-Delfosse, A.L.; Mieyal, J.J. Dysregulation of glutathione homeostasis in neurodegenerative diseases. Nutrients 2012, 9, 1399–1440. [Google Scholar] [CrossRef]
- Hayes, J.D.; McLellan, L.I. Glutathione and glutathione dependent enzymes represent a coordinately regulated defence against oxidative stress. Free Radic. Res. 2009, 31, 273–300. [Google Scholar] [CrossRef]
- Gonchar, O.; Mankovskaya, I. Antioxidant system in adaptation to intermittent hypoxia. J. Biol. Sci. 2010, 10, 545–554. [Google Scholar] [CrossRef][Green Version]
- Cooper, J.; Kristal, B.S. Multiple roles of glutathione in the central nervous system. Biol. Chem. 1997, 378, 793–802. [Google Scholar]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 15, 13291–13295. [Google Scholar] [CrossRef]
- Ma, Q. Transcriptional responses to oxidative stress: Pathological and toxicological implications. Pharmacol. Ther. 2010, 125, 376–393. [Google Scholar] [CrossRef]
- Wild, A.C.; Moinova, H.R.; Mulcahy, R.T. Regulation of gamma-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J. Biol. Chem. 1999, 274, 33627–33636. [Google Scholar] [CrossRef]
- Yin, J.J.; Lao, F.; Fu, P.P.; Wamer, W.G.; Zhao, Y.; Wang, P.C.; Qiu, Y.; Sun, B.; Xing, G.; Dong, J.; et al. The scavenging of reactive oxygen species and the potential for cell protection by functionalized fullerene materials. Biomaterials 2009, 30, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Hardt, J.I.; Quick, K.L.; Kim-Han, J.S.; Erlanger, B.F.; Huang, T.T.; Epstein, C.J.; Dugan, L.L. A biologically effective fullerene (C60) derivative with superoxide dismutase mimetic properties. Free Radic. Biol. Med. 2004, 37, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Hao, J.; Zhang, X.; Yu, B.; Ren, J.; Luo, C.; Li, Q.; Huang, Q.; Shi, X.; Li, W.; et al. The polyhydroxylated fullerene derivative C60(OH)24 protects mice from ionizing-radiation-induced immune and mitochondrial dysfunction. Toxicol. Appl. Pharmacol. 2010, 15, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Chirico, F.; Fumelli, C.; Marconi, A.; Tinari, A.; Straface, E.; Malorni, W.; Pellicciari, R.; Pincelli, C. Carboxyfullerenes localize within mitochondria and prevent the UVB-induced intrinsic apoptotic pathway. Exp. Dermatol. 2007, 16, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Gonchar, O.O.; Maznychenko, A.V.; Bulgakova, N.V.; Vereshchaka, V.V.; Tomiak, T.; Ritter, U.; Prylutskyy, Y.I.; Mankovska, I.M.; Kostyukov, A.I. C60 Fullerene Prevents Restraint Stress-Induced Oxidative Disorders in Rat Tissues: Possible Involvement of the Nrf2/ARE-Antioxidant Pathway. Oxid. Med. Cell. Longev. 2018, 2018, 2518676. [Google Scholar] [CrossRef] [PubMed]
- Prylutskyy, Y.I.; Vereshchaka, V.V.; Maznychenko, A.V.; Bulgakova, N.V.; Gonchar, O.O.; Kyzyma, O.A.; Ritter, U.; Scharff, P.; Tomiak, T.; Nozdrenko, D.M.; et al. C60 fullerene as promising therapeutic agent for correcting and preventing skeletal muscle fatigue. J. Nanobiotechnol. 2017, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Vereshchaka, I.V.; Bulgakova, N.V.; Maznychenko, A.V.; Gonchar, O.O.; Prylutskyy, Y.I.; Ritter, U.; Moska, W.; Tomiak, T.; Nozdrenko, D.M.; Mishchenko, I.V.; et al. C60 fullerenes diminish muscle fatigue in rats comparable to N-acetylcysteine or β-alanine. Front. Physiol. 2018, 9, 517. [Google Scholar] [CrossRef] [PubMed]
- Bakry, R.; Vallant, R.M.; Najam-ul-Haq, M.; Rainer, M.; Szabo, Z.; Huck, C.W.; Bonn, G.K. Medicinal applications of fullerenes. Int. J. Nanomed. 2007, 2, 639–649. [Google Scholar]
- Soni, S.; Ruhela, R.K.; Medhi, B. Nanomedicine in Central Nervous System (CNS) Disorders: A Present and Future Prospective. Adv. Pharm. Bull. 2016, 6, 319–335. [Google Scholar] [CrossRef]
- Ye, S.; Zhou, T.; Cheng, K.; Chen, M.; Wang, Y.; Jiang, Y.; Yang, P. Carboxylic Acid Fullerene (C60) Derivatives Attenuated Neuroinflammatory Responses by Modulating Mitochondrial Dynamics. Nanoscale Res. Lett. 2015, 10, 953. [Google Scholar] [CrossRef]
- Dugan, L.L.; Lovett, E.G.; Quick, K.L.; Lotharius, J.; Lin, T.T.; O’Malley, K.L. Fullerene-based antioxidants and neurodegenerative disorders. Parkinsonism Relat. Disord. 2001, 7, 243–246. [Google Scholar] [CrossRef]
- Ye, S.; Chen, M.; Jiang, Y.; Chen, M.; Zhou, T.; Wang, Y.; Hou, Z.; Ren, L. Polyhydroxylated fullerene attenuates oxidative stress-induced apoptosis via a fortifying Nrf2-regulated cellular antioxidant defence system. Int. J. Nanomed. 2014, 9, 2073–2087. [Google Scholar] [CrossRef]
- Cai, X.; Jia, H.; Liu, Z.; Hou, B.; Luo, C.; Feng, Z.; Li, W.; Liu, J. Polyhydroxylated Fullerene derivative C60(OH)24 prevents mitochondrial dysfunction and oxidative damage in an MPP1-induced cellular model of Parkinson’s disease. J. Neurosci. Res. 2008, 86, 3622–3634. [Google Scholar] [CrossRef] [PubMed]
- Ciammola, A.; Sassone, J.; Alberti, L.; Meola, G.; Mancinelli, E.; Russo, M.A.; Squitieri, F.; Silani, V. Increased apoptosis, huntingtin inclusions and altered differentiation in muscle cell cultures from Huntington’s disease subjects. Cell. Death. Differ. 2008, 13, 2068–2078. [Google Scholar] [CrossRef] [PubMed]
- She, P.; Zhang, Z.; Marchionini, D.; Diaz, W.C.; Jetton, T.J.; Kimball, S.R.; Vary, T.C.; Lang, C.H.; Lynch, C.J. Molecular characterization of skeletal muscle atrophy in the R6/2 mouse model of Huntington’s disease. Am. J. Physiol. Endocrinol. Metab. 2011, 301, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, Y.; Clair, D. ROS and p53: A versatile partnership. Free Radic. Biol. Med. 2008, 44, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Gambino, V.; De Michele, G.; Venezia, O.; Migliaccio, P.; Dall’Olio, V.; Bernard, L.; Minardi, S.P.; Della Fazia, M.A.; Bartoli, D.; Servillo, G.; et al. Oxidative stress activates a specific p53 transcriptional response that regulates cellular senescence and aging. Aging Cell 2013, 12, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Speidel, D. Transcription-independent p53 apoptosis: An alternative route to death. Trends Cell Biol. 2010, 20, 14–24. [Google Scholar] [CrossRef]
- Borras, C.; Gomez-Cabrera, M.C.; Vina, J. The dual role of p53: DNA protection and antioxidant. Free Radic. Res. 2011, 45, 643–652. [Google Scholar] [CrossRef]
- Kim, E.J.; Jang, M.; Lee, M.J.; Choi, J.H.; Lee, S.J.; Kim, S.K.; Jang, D.S.; Cho, I.H. Schisandra chinensis Stem 3-Nitropropionic acid-induced striatal toxicity via activation of the Nrf2 pathway and inhibition of the MAPKs and NF-kB pathways. Front. Pharmacol. 2017, 8, 673. [Google Scholar] [CrossRef]
- Suganya, S.N.; Sumathi, T. Effect of rutin against a mitochondrial toxin, 3-nitropropionic acid induced biochemical, behavioral and histological alterations—A pilot study on Huntington’s disease model in rats. Metab. Brain Dis. 2017, 32, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Yamakura, F.; Taka, H.; Fujimura, T.; Murayama, K. Inactivation of human manganese-superoxide dismutase by peroxynitrite is caused by exclusive nitration of tyrosine 34 to 3-nitrotyrosine. J. Biol. Chem. 1998, 273, 14085–14089. [Google Scholar] [CrossRef]
- Sandhir, R.; Mehrotra, A.; Kamboj, S.S. Lycopene prevents 3-nitropropionic acid-induced mitochondrial oxidative stress and dysfunctions in nervous system. Neurochem. Int. 2010, 57, 579–587. [Google Scholar] [CrossRef]
- Binawade, Y.; Jagtap, A. Neuroprotective effect of lutein against 3-Nitropropionic acid–induced Huntington’s Disease-Like symptoms: Possible behavioral, biochemical, and cellular alterations. J. Med. Food 2013, 16, 934–943. [Google Scholar] [CrossRef]
- Sorolla, M.A.; Reverter-Branchat, G.; Tamarit, J.; Ferrer, I.; Ros, J.; Cabiscol, E. Proteomic and oxidative stress analysis in human brain samples of Huntington disease. Free Radic. Biol. Med. 2008, 45, 667–678. [Google Scholar] [CrossRef]
- Gonchar, O.; Mankovska, I. Hypoxia/reoxygenation modulates oxidative stress level and antioxidative potential in lung mitochondria: Possible participation of p53 and NF-kB target proteins. Arch. Pulm. Respir. Care 2017, 3, 35–43. [Google Scholar] [CrossRef]
- Keller, J.N.; Kindy, M.S.; Holtsberg, F.W.; St Clair, D.K.; Yen, H.C.; Germeyer, A.; Steiner, S.M.; Bruce-Keller, A.J.; Hutchins, J.B.; Mattson, M.P. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduce ischemic brain injury: Suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J. Neurosci. 1998, 18, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Yamakura, F.; Kawasaki, H. Post-translational modifications of superoxide dismutase. Biochim. Biophys. Acta 2010, 1804, 318–325. [Google Scholar] [CrossRef]
- Lee, W.T.; Chang, C. Magnetic resonance imaging and spectroscopy in assessing 3-nitropropionic acid-induced brain lesions: An animal model of Huntington’s disease. Prog. Neurobiol. 2004, 72, 87–110. [Google Scholar] [CrossRef] [PubMed]
- Maznychenko, A.V.; Mankivska, O.P.; Sokolowska, I.V.; Kopyak, B.S.; Tomiak, T.; Bulgakova, N.V.; Gonchar, O.O.; Prylutskyy, Y.I.; Ritter, U.; Mishchenko, I.V.; et al. C60 fullerenes increase the intensity of rotational movements in non anesthetized hemiparkinsonic rats. Acta Neurobiol. Exp. 2020, 80, 32–37. [Google Scholar] [CrossRef]
- Budanov, A.V. The role of tumor suppressor p53 in the antioxidant defense and metabolism. Subcell. Biochem. 2014, 85, 337–358. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chaiswing, L.; Velez, J.M.; Batinic-Haberle, I.; Colburn, N.H.; Oberley, T.D.; St. Clair, D.K. p53 translocation to mitochondria precedes its nuclear translocation and targets mitochondrial oxidative defense protein-manganese superoxide dismutase. Cancer Res. 2005, 65, 3745–3750. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, X.; Bhalla, K.; Kim, C.N.; Ibrado, A.M.; Cai, J.; Peng, T.I.; Jones, D.P.; Wang, X. Prevention of apoptosis by Bcl-2: Release of cytochrome c from mitochondria blocked. Science 1997, 275, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Voehringer, D.W. BCL-2 and glutathione: Alterations in cellular redox state that regulate apoptosis sensitivity. Free Radic. Biol. Med. 1999, 27, 945–950. [Google Scholar] [CrossRef]
- Gopinath, K.; Prakash, D.; Sudhandiran, G. Neuroprotective effect of naringin, a dietary flavonoid against 3-nitropropionic acid-induced neuronal apoptosis. Neurochem. Int. 2011, 59, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Drane, P.; Bravard, A.; Bouvard, V.; May, E. Reciprocal down-regulation of p53 and SOD2 gene expression implication in p53 mediated apoptosis. Oncogene 2001, 20, 430–439. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, J.; Drori, S.; Uldry, M.; Silvaggi, J.M.; Rhee, J.; Jäger, S.; Handschin, C.; Zheng, K.; Lin, J.; Yang, W.; et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 2006, 127, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Kowaltowski, A.J.; Vercesi, A.E.; Fiskum, G. Bcl-2 prevents mitochondrial permeability transition and cytochrome c release via maintenance of reduced pyridine nucleotides. Cell Death Differ. 2000, 7, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, T.A.; Kostov, R.V.; Kazantsev, A.G. The role of Nrf2 signaling in counteracting neurodegenerative diseases. FEBS J. 2018, 285, 3576–3590. [Google Scholar] [CrossRef] [PubMed]
- Calkins, M.J.; Johnson, D.A.; Townsend, J.A.; Vargas, M.R.; Dowell, J.A.; Williamson, T.P.; Kraft, A.D.; Lee, J.M.; Li, J.; Johnson, J.A. The Nrf2/ARE Pathway as a potential therapeutic target in neurodegenerative disease. Antioxid. Redox Signal. 2009, 11, 497–508. [Google Scholar] [CrossRef]
- Brandes, M.S.; Gray, N.E. NRF2 as a therapeutic target in neurodegenerative diseases. ASN Neuro 2020, 12, 1–23. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kang, M.I.; Okawa, H.; Ohtsuji, M.; Zenke, Y.; Chiba, T.; Igarashi, K.; Yamamoto, M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 2004, 24, 7130–7139. [Google Scholar] [CrossRef]
- Kulasekaran, G.; Ganapasam, S. Neuroprotective efficacy of naringin on 3-nitropropionic acid induced mitochondrial dysfunction through the modulation of Nrf2 signaling pathway in PC12 cells. Mol. Cell. Biochem. 2015, 409, 199–211. [Google Scholar] [CrossRef]
- Maznychenko, A.V.; Bulgakova, N.V.; Sokolowska, I.V.; Butowska, K.; Borowik, A.; Mankivska, O.P.; Piosik, J.; Tomiak, T.; Gonchar, O.O.; Maisky, V.O.; et al. Fatigue-induced Fos immunoreactivity within the lumbar cord and amygdale decreases after C60 fullerene pretreatment. Sci. Rep. 2020, 10, 9826. [Google Scholar] [CrossRef] [PubMed]
- Courtes, A.A.; Arantes, L.P.; Barcelos, R.P.; da Silva, I.K.; Boligon, A.A.; Athayde, M.L.; Puntel, R.L.; Soares, F.A. Protective effects of aqueous extract of luehea divaricata against behavioral and oxidative changes induced by 3-nitropropionic acid in rats. Evid. Based Complement. Altern. Med. 2015, 2015, 723431. [Google Scholar] [CrossRef]
- Sims, N.R. Rapid isolation of metabolically active mitochondria from rat brain and subregions using Percoll density gradient centrifugation. J. Neurochem. 1990, 55, 698–707. [Google Scholar] [CrossRef]
- Graham, J.M. Isolation of mitochondria by differential centrifugation. Curr. Protoc. Cell Biol. 1999. [Google Scholar] [CrossRef]
- Reiniers, M.J.; van Golen, R.F.; Bonnet, S.; Broekgaarden, M.; van Gulik, T.M.; Egmond, M.R.; Heger, M. Preparation and practical applications of 2′,7′-dichlorodihydrofluorescein in redox assays. Anal. Chem. 2017, 89, 3853–3857. [Google Scholar] [CrossRef] [PubMed]
- Boveris, A. Determination of the production of superoxide radicals and hydrogen peroxide in mitochondria. Methods Enzymol. 1984, 105, 429–435. [Google Scholar] [CrossRef]
- Wolff, S.P. Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol. 1994, 233, 182–189. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- King, T.E.; Howard, R.L. Preparation and properties of soluble NADH dehydrogenase from cardiac muscle. Methods Enzymol. 1967, 10, 322–331. [Google Scholar] [CrossRef]
- Ackrell, B.; Kearney, E.; Singer, T. Mammalian succinate dehydrogenase. Methods Enzymol. 1978, 53, 466–470. [Google Scholar] [CrossRef]
- Wharton, D.; Tzagoloff, A. Cytochrome Oxidase from Beef Heart Mitochondria. Methods Enzymol. 1967, 10, 245–248. [Google Scholar] [CrossRef]
- Misra, H.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Flohé, L.; Gunzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Warholm, M.; Guthenberg, C.; von Bahr, C.; Mannervik, B. Glutathione transferases from human liver. Methods Enzymol. 1985, 113, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985, 113, 548–555. [Google Scholar] [CrossRef]





| Treatment | Number of Animals with Normal Behavior/Total Number of Animals Used (0) | Number of Animals with General Slowness/Total Number of Animals Used (1) | Number of Animals with Incoordination and Marked Gait Abnormalities/Total Number of Animals Used (2) | Number of Animals with Hind Limb Paralysis/Total Number of Animals Used (3) | Number of Animals with Incapacity to Move/Total Number of Animals Used (4) |
|---|---|---|---|---|---|
| Control | 10/10 | 0/10 | 0/10 | 0/10 | 0/10 |
| 3-NPA | 0/10 | 0/10 | 4/10 | 3/10 | 3/10 |
| Pre-T | 8/10 | 2/10 | 0/10 | 0/10 | 0/10 |
| Post-T | 6/10 | 3/10 | 1/10 | 0/10 | 0/10 |
| Groups | Days | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| 3-NPA (alone) | ● | ● | ● | |||||||
| C60 fullerene pre-treatment | Δ | Δ | ● Δ | ● Δ | ● Δ | |||||
| C60 fullerene post-treatment | ● | ● | ● | Δ | Δ | Δ | Δ | Δ | ||
| C60 fullerene (alone) | Δ | Δ | Δ | Δ | Δ | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonchar, O.O.; Maznychenko, A.V.; Klyuchko, O.M.; Mankovska, I.M.; Butowska, K.; Borowik, A.; Piosik, J.; Sokolowska, I. C60 Fullerene Reduces 3-Nitropropionic Acid-Induced Oxidative Stress Disorders and Mitochondrial Dysfunction in Rats by Modulation of p53, Bcl-2 and Nrf2 Targeted Proteins. Int. J. Mol. Sci. 2021, 22, 5444. https://doi.org/10.3390/ijms22115444
Gonchar OO, Maznychenko AV, Klyuchko OM, Mankovska IM, Butowska K, Borowik A, Piosik J, Sokolowska I. C60 Fullerene Reduces 3-Nitropropionic Acid-Induced Oxidative Stress Disorders and Mitochondrial Dysfunction in Rats by Modulation of p53, Bcl-2 and Nrf2 Targeted Proteins. International Journal of Molecular Sciences. 2021; 22(11):5444. https://doi.org/10.3390/ijms22115444
Chicago/Turabian StyleGonchar, Olga O., Andriy V. Maznychenko, Olena M. Klyuchko, Iryna M. Mankovska, Kamila Butowska, Agnieszka Borowik, Jacek Piosik, and Inna Sokolowska. 2021. "C60 Fullerene Reduces 3-Nitropropionic Acid-Induced Oxidative Stress Disorders and Mitochondrial Dysfunction in Rats by Modulation of p53, Bcl-2 and Nrf2 Targeted Proteins" International Journal of Molecular Sciences 22, no. 11: 5444. https://doi.org/10.3390/ijms22115444
APA StyleGonchar, O. O., Maznychenko, A. V., Klyuchko, O. M., Mankovska, I. M., Butowska, K., Borowik, A., Piosik, J., & Sokolowska, I. (2021). C60 Fullerene Reduces 3-Nitropropionic Acid-Induced Oxidative Stress Disorders and Mitochondrial Dysfunction in Rats by Modulation of p53, Bcl-2 and Nrf2 Targeted Proteins. International Journal of Molecular Sciences, 22(11), 5444. https://doi.org/10.3390/ijms22115444








