Osteogenesis of 3D-Printed PCL/TCP/bdECM Scaffold Using Adipose-Derived Stem Cells Aggregates; An Experimental Study in the Canine Mandible
Abstract
1. Introduction
2. Results
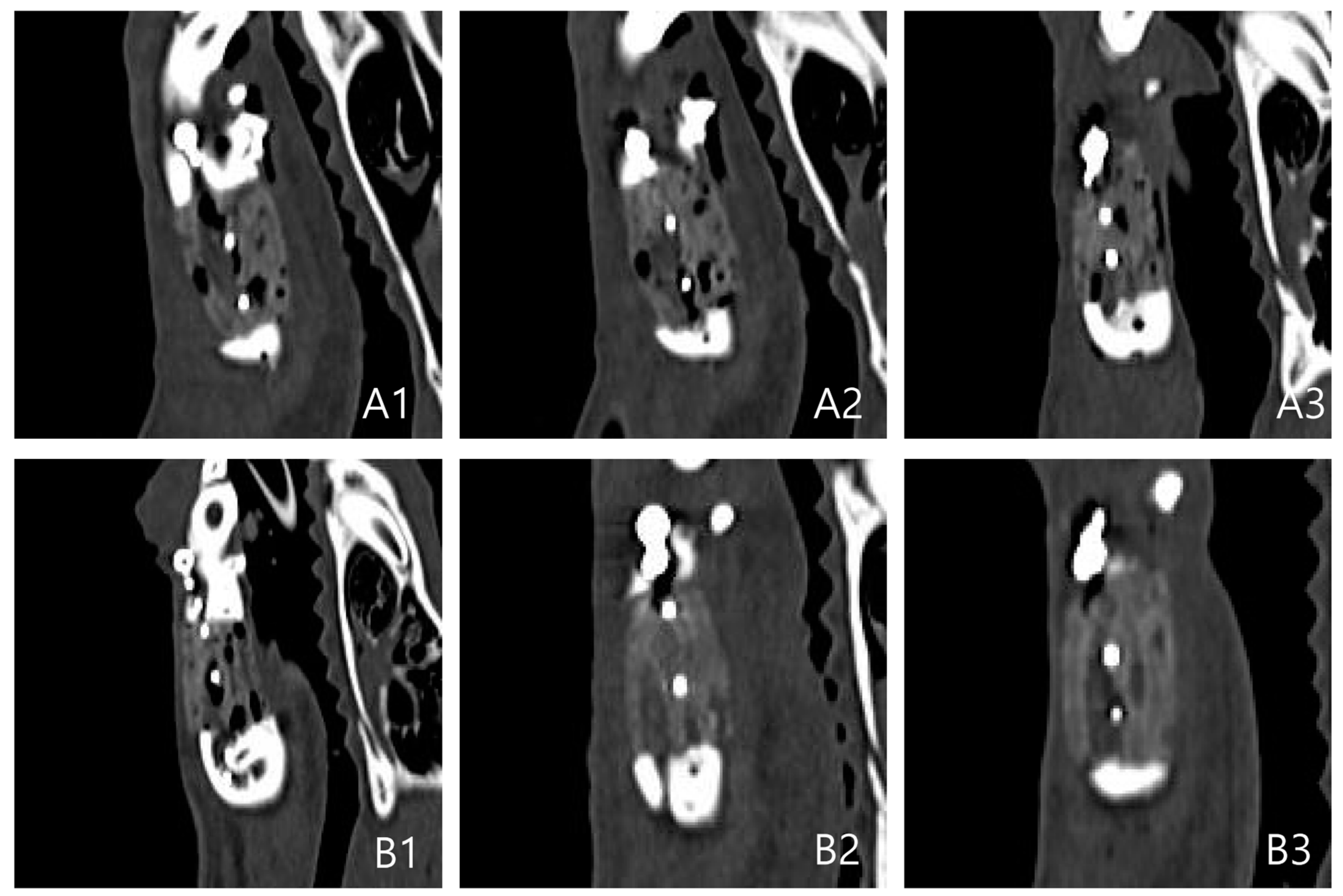
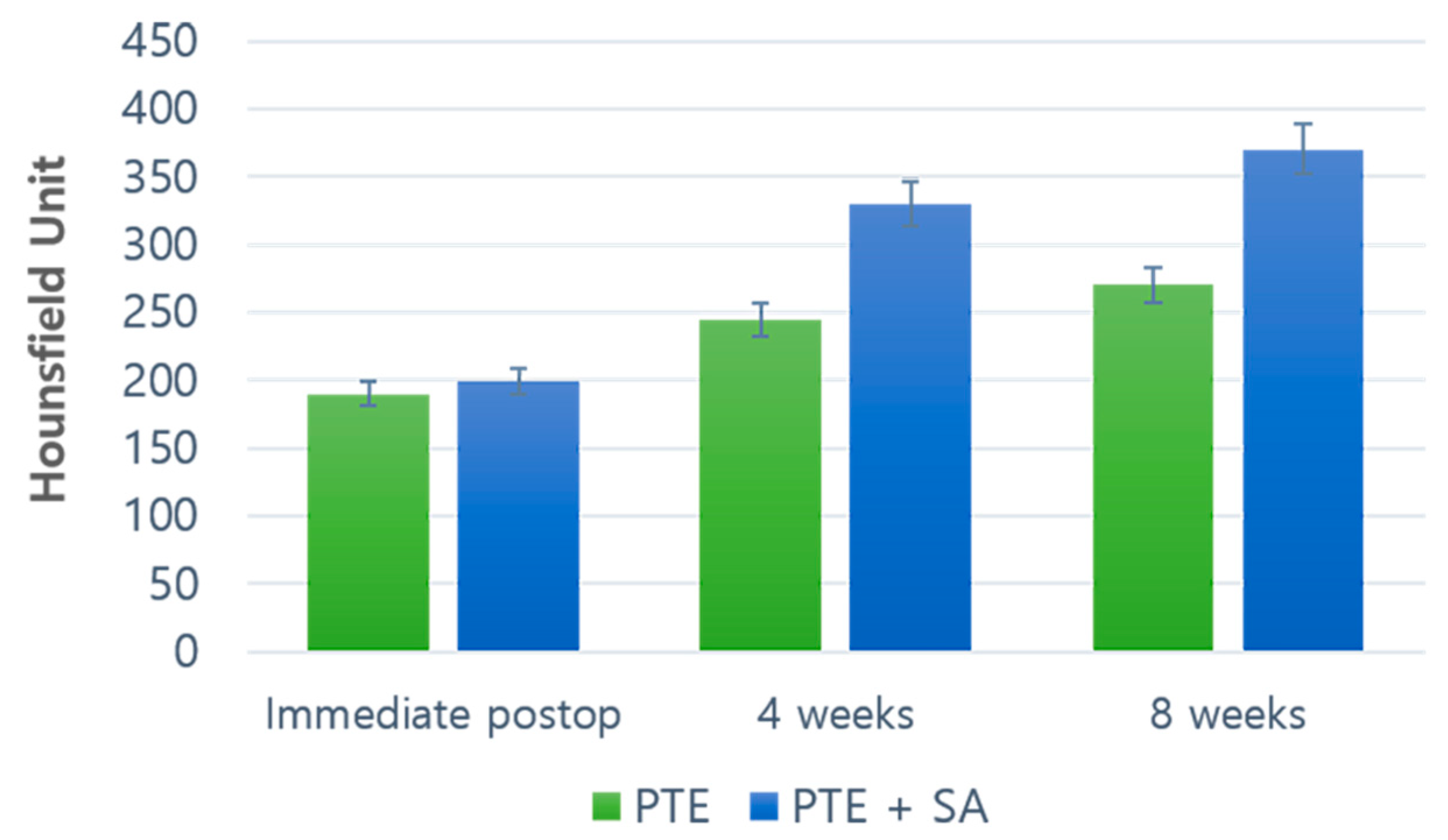
2.1. Outcomes of the Evaluation Using CT
CT Results: Coronal, Axial, and Sagittal Views
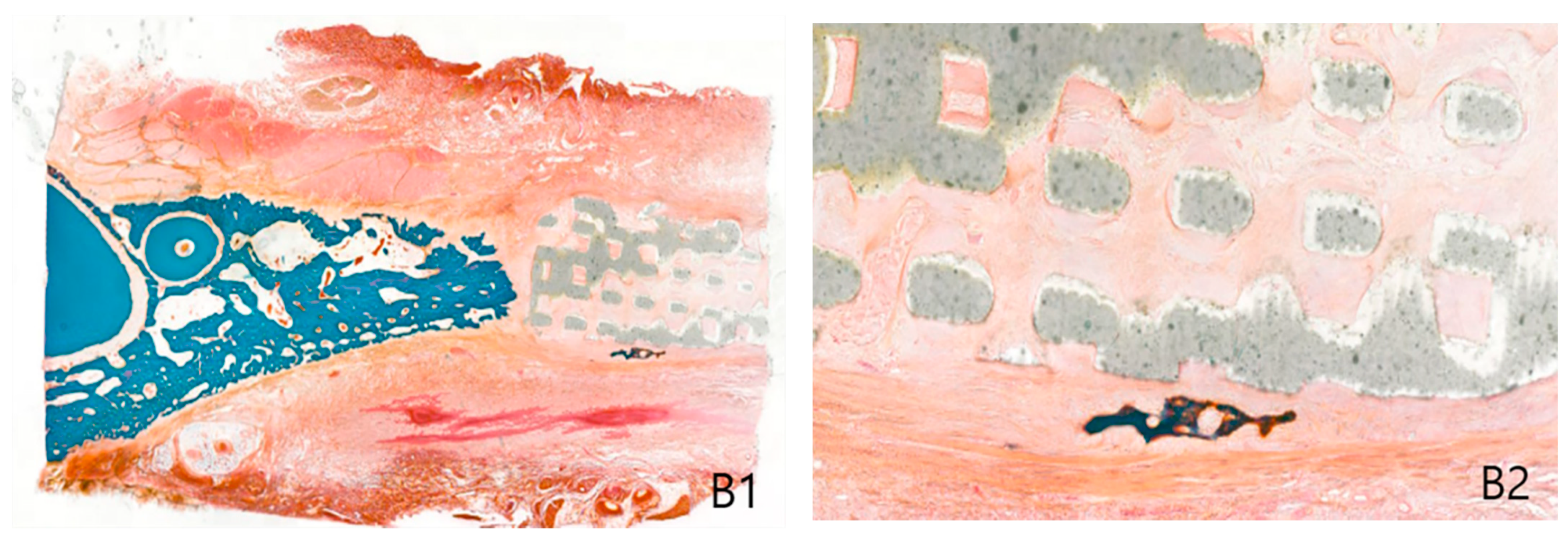
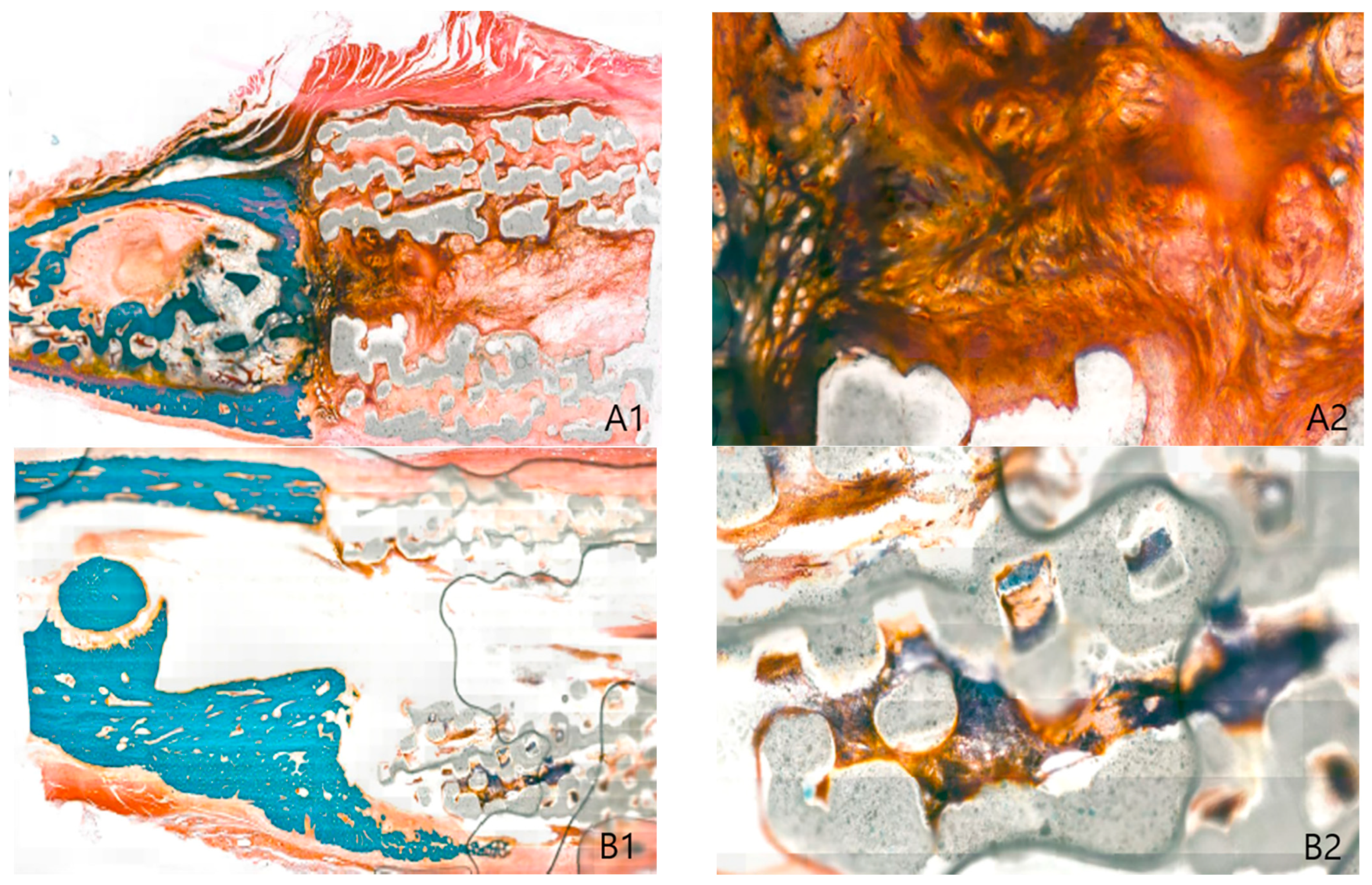
2.2. Histological Findings
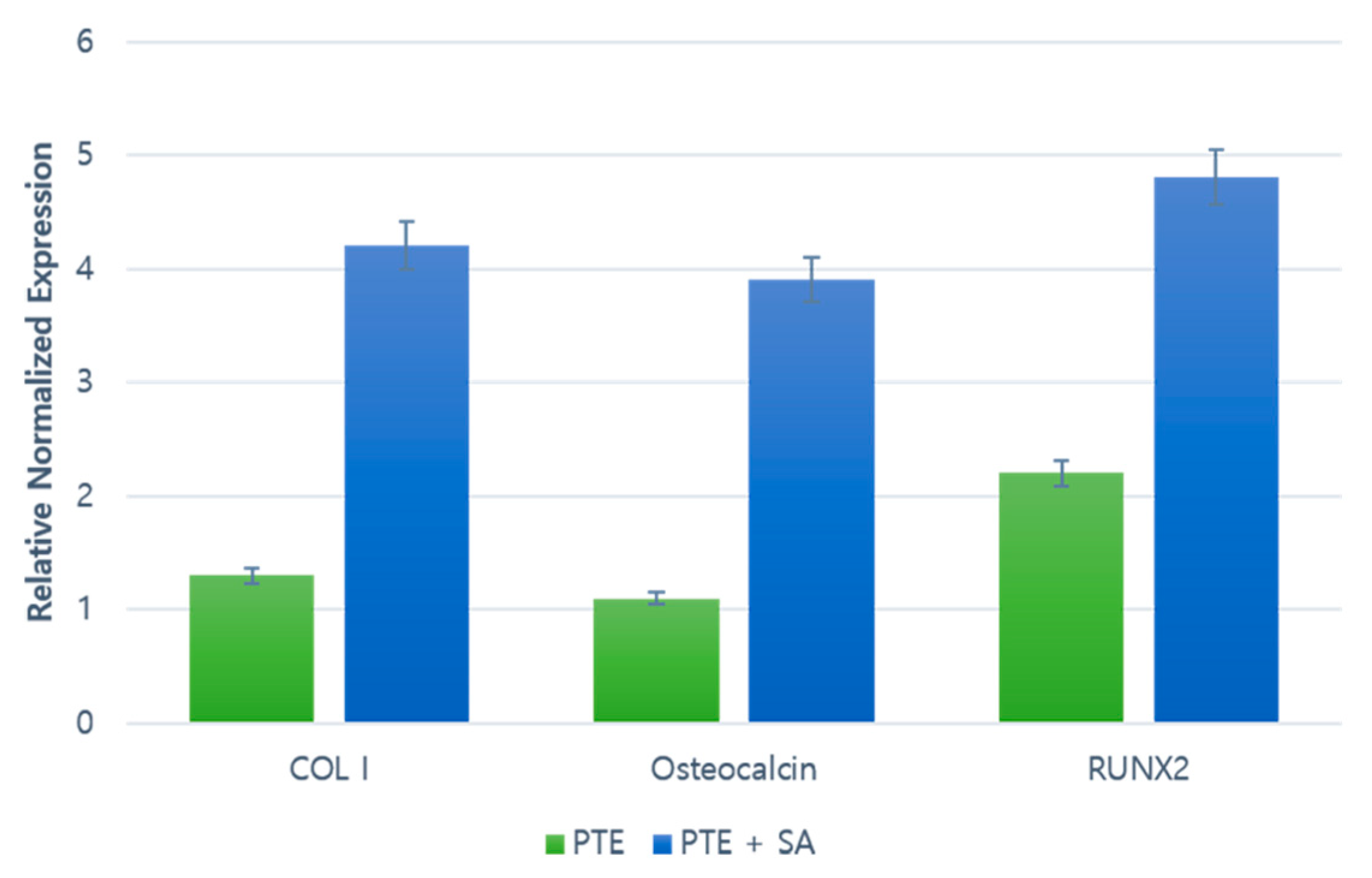
2.3. RT–PCR
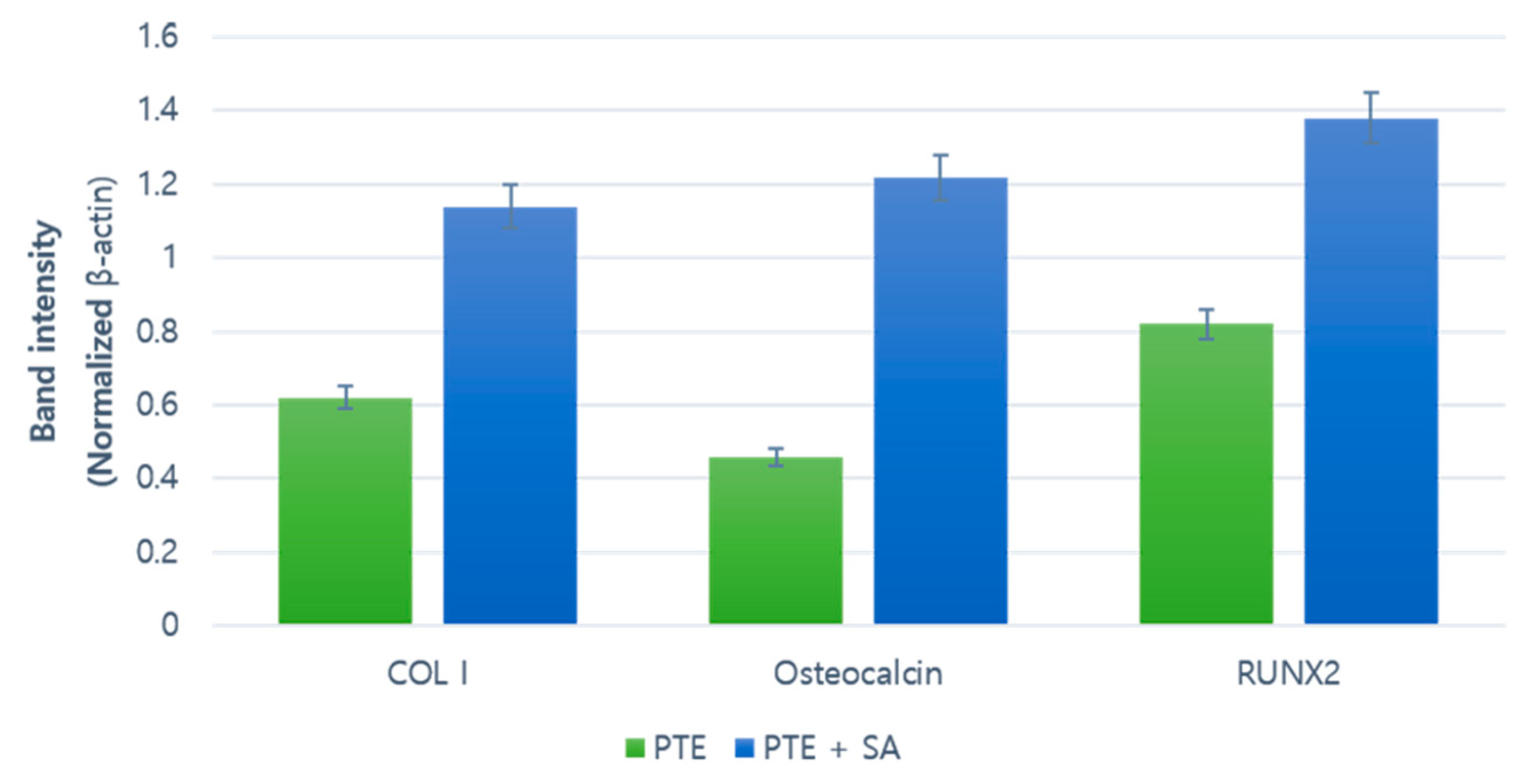
2.4. Western Blot
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. 3D Printing Scaffold Manufacturing of PCL/TCP/bdECM
4.1.2. Identification of ADSCs and Aggregates Formation
4.1.3. Experimental Animals and Groups
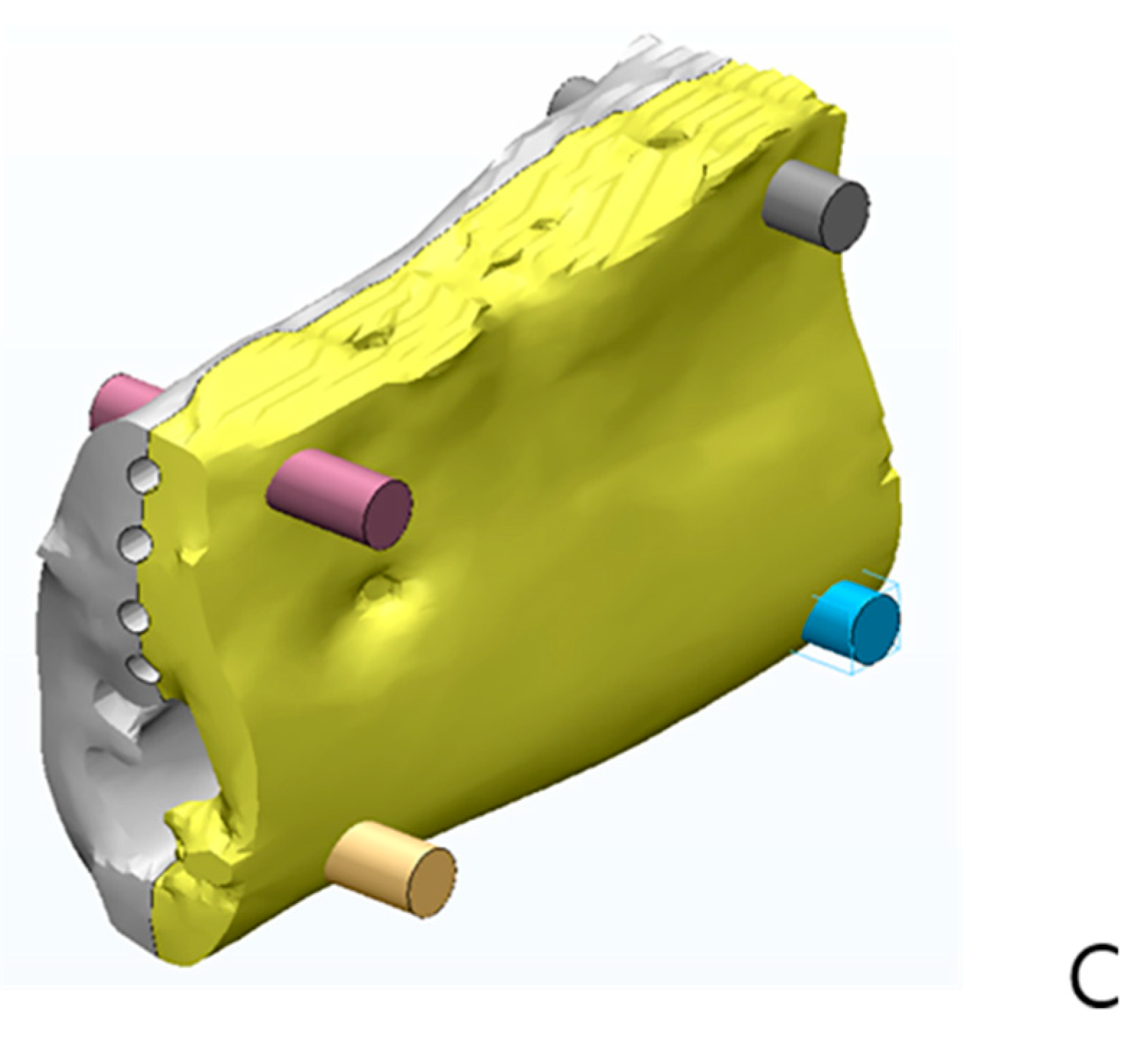
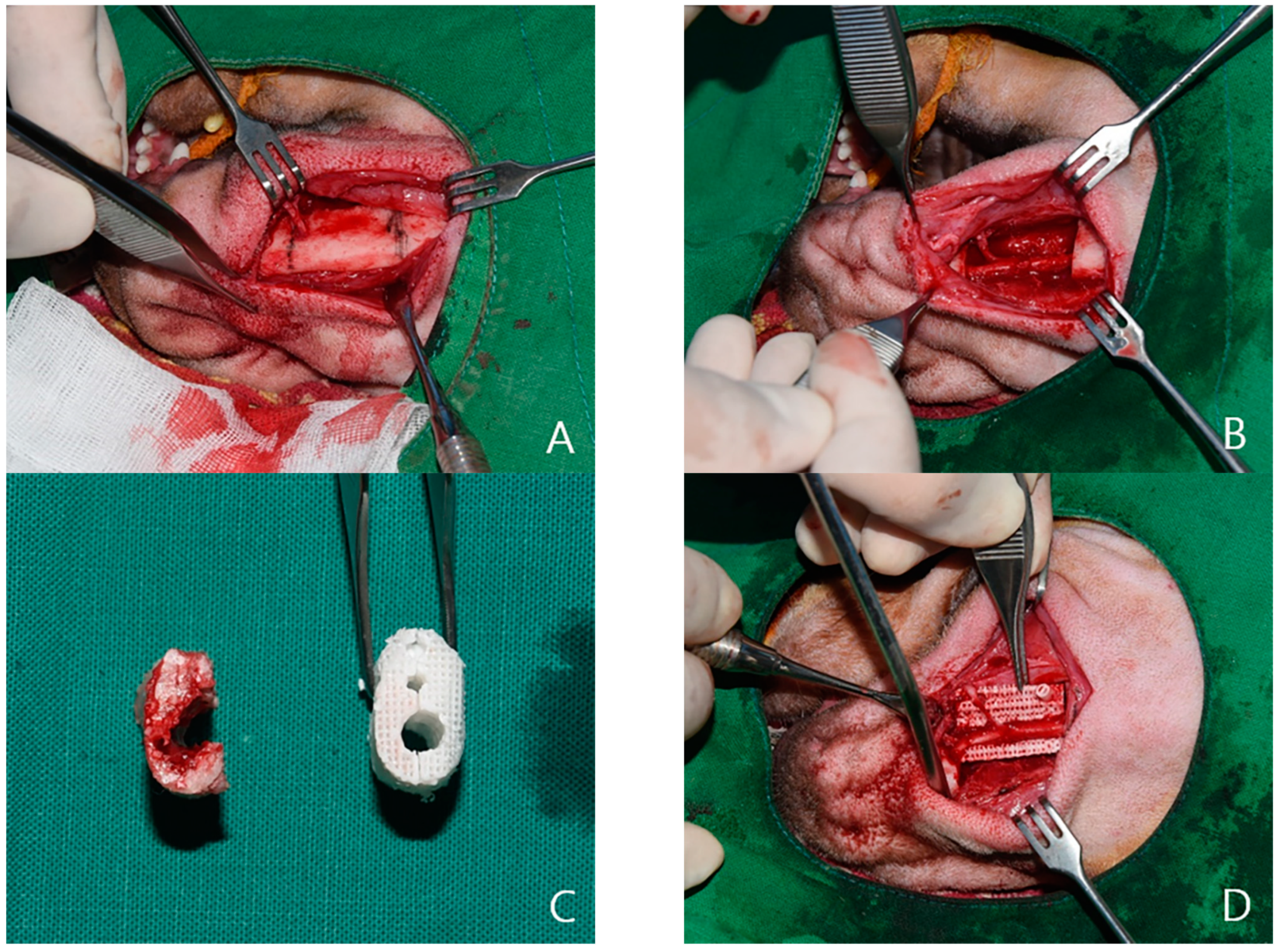
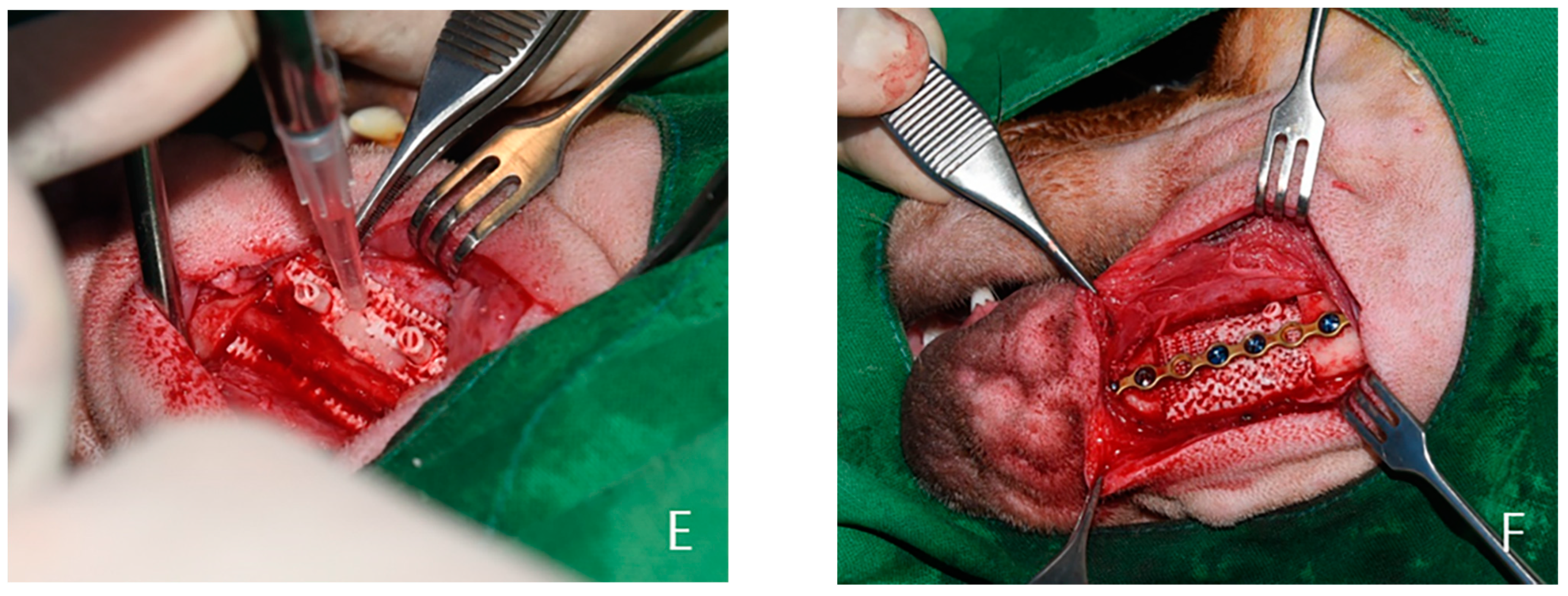
4.2. Experimental Methods
4.3. Outcome Evaluation
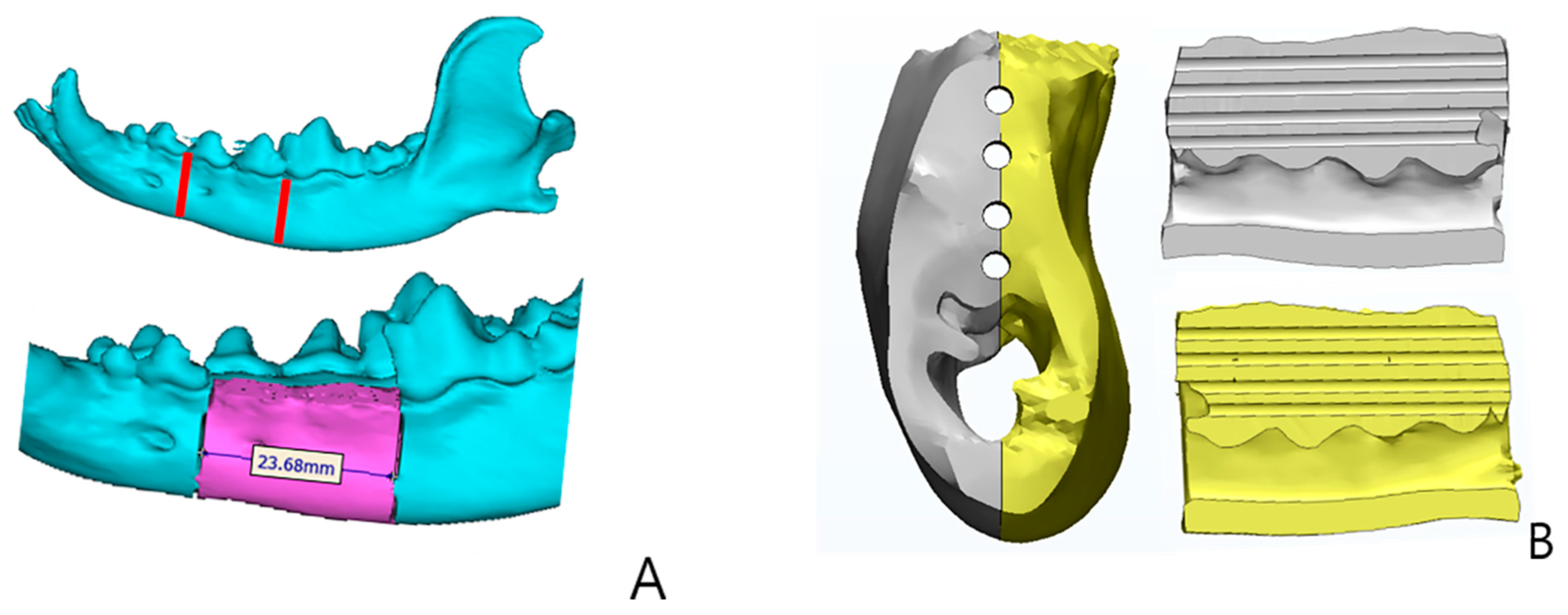
4.3.1. Evaluation Using CT
4.3.2. Histological Evaluation
4.3.3. RT–PCR
4.3.4. Western Blot
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fullerton, J.N.; Frodsham, G.C.; Day, R.M. 3D Printing for the Many, Not the Few. Nat. Biotechnol. 2014, 32, 1086–1087. [Google Scholar] [CrossRef]
- Hoy, M.B. 3D Printing: Making Things at the Library. Med. Ref. Serv. Q. 2013, 32, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D Bioprinting of Tissues and Organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Sachlos, E.; Czernuszka, J.T. Making Tissue Engineering Scaffolds Work. Review: On the Application of Solid Freeform Fabrication Technology to the Production of Tissue Engineering Scaffolds. Eur. Cells Mater. 2003, 5, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, M.; Köster, G.; Krause, T.; Merten, H.A.; Schmid, A. Degradation of and Intraosseous Reactions to Biodegradable Poly-L-Lactide Screws: A Study in Minipigs. Arch. Orthop. Trauma. Surg. 1998, 118, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Päivärinta, U.; Böstman, O.; Majola, A.; Toivonen, T.; Törmälä, P.; Rokkanen, P. Intraosseous Cellular Response to Biodegradable Fracture Fixation Screws Made of Polyglycolide or Polylactide. Arch. Orthop. Trauma. Surg. 1993, 112, 71–74. [Google Scholar] [CrossRef]
- Peltoniemi, H.H.; Hallikainen, D.; Toivonen, T.; Helevirta, P.; Waris, T. SR-PLLA and SR-PGA Miniscrews: Biodegradation and Tissue Reactions in the Calvarium and Dura Mater. J. Craniomaxillofac. Surg. 1999, 27, 42–50. [Google Scholar] [CrossRef]
- Bergsma, E.J.; Rozema, F.R.; Bos, R.R.; de Bruijn, W.C. Foreign Body Reactions to Resorbable Poly(l-Lactide) Bone Plates and Screws Used for the Fixation of Unstable Zygomatic Fractures. J. Oral Maxillofac. Surg. 1993, 51, 666–670. [Google Scholar] [CrossRef]
- D’Urso, P.S.; Earwaker, W.J.; Barker, T.M.; Redmond, M.J.; Thompson, R.G.; Effeney, D.J.; Tomlinson, F.H. Custom Cranioplasty Using Stereolithography and Acrylic. Br. J. Plast. Surg. 2000, 53, 200–204. [Google Scholar] [CrossRef]
- Faber, J.; Berto, P.M.; Quaresma, M. Rapid Prototyping as a Tool for Diagnosis and Treatment Planning for Maxillary Canine Impaction. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 583–589. [Google Scholar] [CrossRef]
- Müller, A.; Krishnan, K.G.; Uhl, E.; Mast, G. The Application of Rapid Prototyping Techniques in Cranial Reconstruction and Preoperative Planning in Neurosurgery. J. Craniofac. Surg. 2003, 14, 899–914. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Kim, N. Clinical Application of Three-Dimensional Printing Technology in Craniofacial Plastic Surgery. Arch. Plast. Surg. 2015, 42, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Mannoor, M.S.; Jiang, Z.; James, T.; Kong, Y.L.; Malatesta, K.A.; Soboyejo, W.O.; Verma, N.; Gracias, D.H.; McAlpine, M.C. 3D Printed Bionic Ears. Nano Lett. 2013, 13, 2634–2639. [Google Scholar] [CrossRef] [PubMed]
- Gerstle, T.L.; Ibrahim, A.M.S.; Kim, P.S.; Lee, B.T.; Lin, S.J. A Plastic Surgery Application in Evolution: Three-Dimensional Printing. Plast. Reconstr. Surg. 2014, 133, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Benders, K.E.; van Weeren, P.R.; Badylak, S.F.; Saris, D.B.; Dhert, W.J.; Malda, J. Extracellular Matrix Scaffolds for Cartilage and Bone Regeneration. Trends Biotechnol. 2013, 31, 169–176. [Google Scholar] [CrossRef]
- Lomas, R.J.; Gillan, H.L.; Matthews, J.B.; Ingham, E.; Kearney, J.N. An Evaluation of the Capacity of Differently Prepared Demineralised Bone Matrices (DBM) and Toxic Residuals of Ethylene Oxide (EtOx) to Provoke an Inflammatory Response in Vitro. Biomaterials 2001, 22, 913–921. [Google Scholar] [CrossRef]
- Markel, D.C.; Guthrie, S.T.; Wu, B.; Song, Z.; Wooley, P.H. Characterization of the Inflammatory Response to Four Commercial Bone Graft Substitutes Using a Murine Biocompatibility Model. J. Inflam. Res. 2012, 5, 13–18. [Google Scholar]
- Sawkins, M.J.; Bowen, W.; Dhadda, P.; Markides, H.; Sidney, L.E.; Taylor, A.J.; Rose, F.R.; Badylak, S.F.; Shakesheff, K.M.; White, L.J. Hydrogels Derived from Demineralized and Decellularized Bone Extracellular Matrix. Acta Biomater. 2013, 9, 7865–7873. [Google Scholar] [CrossRef]
- Pati, F.; Jang, J.; Ha, D.H.; Won Kim, S.; Rhie, J.W.; Shim, J.H.; Kim, D.H.; Cho, D.W. Printing Three-Dimensional Tissue Analogues with Decellularized Extracellular Matrix Bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef]
- La, W.-G.; Jang, J.; Kim, B.S.; Lee, M.S.; Cho, D.-W.; Yang, H.S. Systemically Replicated Organic and Inorganic Bony Microenvironment Fornewboneformationgenerated bya3D Printing Technology. RSC Adv. 2016, 6, 11546–11553. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Keely, P.J. Mechanical Signaling Through the Cytoskeleton Regulates Cell Proliferation by Coordinated Focal Adhesion and Rho GTPase Signaling. J. Cell Sci. 2011, 124, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Dragoo, J.L.; Choi, J.Y.; Lieberman, J.R.; Huang, J.; Zuk, P.A.; Zhang, J.; Hedrick, M.H.; Benhaim, P. Bone Induction by BMP-2 Transduced Stem Cells Derived from Human Fat. J. Orthop. Res. 2003, 21, 622–629. [Google Scholar] [CrossRef]
- Huang, J.I.; Beanes, S.R.; Zhu, M.; Lorenz, H.P.; Hedrick, M.H.; Benhaim, P.H. Rat Extramedullary Adipose Tissue as a Source of Osteochondrogenic Progenitor Cells. Plast. Reconstr. Surg. 2002, 109, 1033–1041. [Google Scholar] [CrossRef]
- Ogawa, R.; Mizuno, H.; Hyakusoku, H.; Watanabe, A.; Migita, M.; Shimada, T. Chondrogenic and Osteogenic Differentiation of Adipose-Derived Stem Cells Isolated from GFP Transgenic Mice. J. Nippon Med. Sch. 2004, 71, 240–241. [Google Scholar] [CrossRef]
- Lee, J.W.; Chu, S.G.; Kim, H.T.; Choi, K.Y.; Oh, E.J.; Shim, J.H.; Yun, W.S.; Huh, J.B.; Moon, S.H.; Kang, S.S.; et al. Osteogenesis of Adipose-Derived and Bone Marrow Stem Cells with Polycaprolactone/Tricalcium Phosphate and Three-Dimensional Printing Technology in a Dog Model of Maxillary Bone Defects. Polymers 2017, 9, 450. [Google Scholar] [CrossRef] [PubMed]
- Gross, B.C.; Erkal, J.L.; Lockwood, S.Y.; Chen, C.; Spence, D.M. Evaluation of 3D Printing and Its Potential Impact on Biotechnology and the Chemical Sciences. Anal. Chem. 2014, 86, 3240–3253. [Google Scholar] [CrossRef]
- Bartlett, S. Printing Organs on Demand. Lancet Respir. Med. 2013, 1, 684. [Google Scholar] [CrossRef]
- Science and Society. Experts Warn Against Bans on 3D Printing. Science 2013, 342, 439. [Google Scholar]
- Lipson, H. New World of 3-D Printing Offers “Completely New Ways of Thinking”: Q & A with Author, Engineer, and 3-D Printing Expert Hod Lipson. IEEE Pulse 2013, 4, 12–14. [Google Scholar] [PubMed]
- Cui, X.; Boland, T.; D’Lima, D.D.; Lotz, M.K. Thermal Inkjet Printing in Tissue Engineering and Regenerative Medicine. Recent Pat. Drug Deliv. Formul. 2012, 6, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Schubert, C.; van Langeveld, M.C.; Donoso, L.A. Innovations in 3D Printing: A 3D Overview from Optics to Organs. Br. J. Ophthalmol. 2014, 98, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Banks, J. Adding Value in Additive Manufacturing: Researchers in the United Kingdom and Europe Look to 3D Printing for Customization. IEEE Pulse 2013, 4, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, K.; Sharma, A.; Giraddi, G.; Shahi, A.K. Versatility of Titanium 3D Plate in Comparison with Conventional Titanium Miniplate Fixation for the Management of Mandibular Fracture. J. Maxillofac. Oral Surg. 2012, 11, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Zinser, M.J.; Mischkowski, R.A.; Sailer, H.F.; Zöller, J.E. Computer-assisted Orthognathic Surgery: Feasibility Study Using Multiple CAD/CAM Surgical Splints. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ping, F.Y.; Chen, J.; Yan, F.G.; Mao, H.Q.; Shi, Y.H.; Zhao, Z.Y. Application of CAD/CAM Techniques in Mandible Large-Scale Defect and Reconstruction with Vascularized Fibular Bone Graft. Zhejiang Da Xue Xue Bao Yi Xue Ban 2007, 36, 498–502. [Google Scholar]
- Zhang, T.; Zhang, Y.; Li, Y.S.; Gui, L.; Mao, C.; Chen, Y.N.; Zhao, J.Z. Application of CTA and CAD\CAM Techniques in Mandible Reconstruction with Free Fibula Flap. Zhonghua Zheng Xing Wai Ke Za Zhi 2006, 22, 325–327. [Google Scholar]
- Dankowski, R.; Baszko, A.; Sutherland, M.; Firek, L.; Kałmucki, P.; Wróblewska, K.; Szyszka, A.; Groothuis, A.; Siminiak, T. 3D Heart Model Printing for Preparation of Percutaneous Structural Interventions: Description of the Technology and Case Report. Kardiol. Pol. 2014, 72, 546–551. [Google Scholar] [CrossRef]
- Rengier, F.; Mehndiratta, A.; von Tengg-Kobligk, H.; Zechmann, C.M.; Unterhinninghofen, R.; Kauczor, H.U.; Giesel, F.L. 3D Printing Based on Imaging Data: Review of Medical applications. Int. J. Comput. Assist. Radiol. Surg. 2010, 5, 335–341. [Google Scholar] [CrossRef]
- McGowan, J. 3D Printing Technology Speeds Development. Health Estate 2013, 67, 100–102. [Google Scholar] [PubMed]
- Lee, H.; Fang, N.X. Micro 3D Printing Using a Digital Projector and Its Application in the Study of Soft Materials Mechanics. J. Vis. Exp. 2012, 69, e4457. [Google Scholar] [CrossRef] [PubMed]
- Won, J.-Y.; Park, C.-Y.; Bae, J.-H.; Ahn, G.; Kim, C.; Lim, D.-H.; Cho, D.-W.; Yun, W.-S.; Shim, J.-H.; Huh, J.-B. Evaluation of 3D Printed PCL/PLGA/β-TCP versus Collagen Membranes for Guided Bone Regeneration in a Beagle Implant Model. Biomed. Mater. 2016, 11, 055013. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-H.; Won, J.-Y.; Park, J.-H.; Bae, J.-H.; Ahn, G.; Kim, C.-H.; Lim, D.-H.; Cho, D.-W.; Yun, W.-S.; Bae, E.-B.; et al. Effects of 3D-Printed Polycaprolactone/β-Tricalcium Phosphate Membranes on Guided Bone Regeneration. Int. J. Mol. Sci. 2017, 18, 899. [Google Scholar] [CrossRef]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine; Elsevier Inc.: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Todo, M.; Park, S.D.; Arakawa, K.; Takenoshita, Y. Relationship Between Microstructure and Fracture Behavior of Bioabsorbable HA/PLLA Composites. Compos. Compos. Part A Appl. Sci. Manuf. 2006, 37, 2221–2225. [Google Scholar] [CrossRef]
- Mauli Agrawal, C.; Athanasiou, K.A. Technique to Control pH in Vicinity of Biodegrading PLA-PGA Implants. J. Biomed. Mater. Res. 1997, 38, 105–114. [Google Scholar] [CrossRef]
- Habibovic, P.; de Groot, K. Osteoinductive Biomaterials--Properties and Relevance in Bone Repair. J. Tissue Eng. Regen. Med. 2007, 1, 25–32. [Google Scholar] [CrossRef]
- Habibovic, P.; Gbureck, U.; Doillon, C.J.; Bassett, D.C.; van Blitterswijk, C.A.; Barralet, J.E. Osteoconduction and Osteoinduction of Low-Temperature 3D Printed Bioceramic Implants. Biomaterials 2008, 29, 944–953. [Google Scholar] [CrossRef]
- Sellaro, T.L.; Ranade, A.; Faulk, D.M.; McCabe, G.P.; Dorko, K.; Badylak, S.F.; Strom, S.C. Maintenance of Human Hepatocyte Function In Vitro by Liver-Derived Extracellular Matrix Gels. Tissue Eng. A 2010, 16, 1075–1082. [Google Scholar] [CrossRef]
- Einhorn, T.A.; Lane, J.M.; Burstein, A.H.; Kopman, C.R.; Vigorita, V.J. The Healing of Segmental Bone Defects Induced by Demineralized Bone Matrix. A Radiographic and Biomechanical Study. J. Bone Jt. Surg. Am. 1984, 66, 274–279. [Google Scholar] [CrossRef]
- Bolander, M.E.; Balian, G. The Use of Demineralized Bone Matrix in the Repair of Segmental Defects. Augmentation with Extracted Matrix Proteins and a Comparison with Autologous Grafts. J. Bone Jt. Surg. Am. 1986, 68, 1264–1274. [Google Scholar] [CrossRef]
- Gruskin, E.; Doll, B.A.; Futrell, F.W.; Schmitz, J.P.; Hollinger, J.O. Demineralized Bone Matrix in Bone Repair: History and Use. Adv. Drug Deliv. Rev. 2012, 64, 1063–1077. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.H.; Moon, T.S.; Yun, M.J.; Jeon, Y.C.; Jeong, C.M.; Cho, D.W.; Huh, J.B. Stimulation of Healing Within a Rabbit Calvarial Defect by a PCL/PLGA Scaffold Blended with TCP Using Solid Freeform Fabrication Technology. J. Mater. Sci. Mater. Med. 2012, 23, 2993–3002. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, A.A.; Song, S.J.; Susanto, E.; Chuan, P.; Lam, C.X.; Woodruff, M.A.; Hutmacher, D.W.; Cool, S.M. The Stimulation of Healing Within a Rat Calvarial Defect by mPCL-TCP/Collagen Scaffolds Loaded with rhBMP-2. Biomaterials 2009, 30, 2479–2488. [Google Scholar] [CrossRef] [PubMed]
- La, W.G.; Jin, M.; Park, S.; Yoon, H.H.; Jeong, G.J.; Bhang, S.H.; Park, H.; Char, K.; Kim, B.S. Delivery of Bone Morphogenetic protein-2 and Substance P Using Graphene Oxide for Bone Regeneration. Int. J. Nanomed. 2014, 9 (Suppl. 1), 107–116. [Google Scholar]
- Bae, J.C.; Lee, J.J.; Shim, J.H.; Park, K.H.; Lee, J.S.; Bae, E.B.; Choi, J.W.; Huh, J.B. Development and Assessment of a 3D-Printed Scaffold with rhBMP-2 for an Implant Surgical Guide Stent and Bone Graft Material: A Pilot Animal Study. Materials 2017, 10, 1434. [Google Scholar] [CrossRef]
- Jingushi, S.; Urabe, K.; Okazaki, K.; Hirata, G.; Sakai, A.; Ikenoue, T.; Iwamoto, Y. Intramuscular Bone Induction by Human Recombinant Bone Morphogenetic protein-2 with Beta-Tricalcium Phosphate as a Carrier: In Vivo Bone Banking for Muscle-Pedicle Autograft. J. Orthop. Sci. 2002, 7, 490–494. [Google Scholar] [CrossRef]
- Alam, I.; Asahina, I.; Ohmamiuda, K.; Enomoto, S. Comparative Study of Biphasic Calcium Phosphate Ceramics Impregnated with rhBMP-2 as Bone Substitutes. J. Biomed. Mater. Res. 2001, 54, 129–138. [Google Scholar] [CrossRef]

| Gene Name | Sequence (5′-3′) |
|---|---|
| COL1-dog-F | CTCGTCACAGTTGGGGTTGA |
| COL1-dog-R | GGTGCAAGTATGAAGCGGGA |
| OCN-dog-F | AATTGCGCTCGAGCATCTCT |
| OCN-dog-R | ATTGCCACGGTTGCTACTGA |
| RUNX2-dog-F | GGCGGCTATAACTCTTCCCA |
| RUNX2-dog-R | ACGCAGCGGCTTTTTATTTCA |
| GAPDH-F | GTCGGAGTCAACGGATTTGG |
| GAPDH-R | GGGTGGAATCAATTGGAACAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.S.; Park, T.H.; Ryu, J.Y.; Kim, D.K.; Oh, E.J.; Kim, H.M.; Shim, J.-H.; Yun, W.-S.; Huh, J.B.; Moon, S.H.; et al. Osteogenesis of 3D-Printed PCL/TCP/bdECM Scaffold Using Adipose-Derived Stem Cells Aggregates; An Experimental Study in the Canine Mandible. Int. J. Mol. Sci. 2021, 22, 5409. https://doi.org/10.3390/ijms22115409
Lee JS, Park TH, Ryu JY, Kim DK, Oh EJ, Kim HM, Shim J-H, Yun W-S, Huh JB, Moon SH, et al. Osteogenesis of 3D-Printed PCL/TCP/bdECM Scaffold Using Adipose-Derived Stem Cells Aggregates; An Experimental Study in the Canine Mandible. International Journal of Molecular Sciences. 2021; 22(11):5409. https://doi.org/10.3390/ijms22115409
Chicago/Turabian StyleLee, Joon Seok, Tae Hyun Park, Jeong Yeop Ryu, Dong Kyu Kim, Eun Jung Oh, Hyun Mi Kim, Jin-Hyung Shim, Won-Soo Yun, Jung Bo Huh, Sung Hwan Moon, and et al. 2021. "Osteogenesis of 3D-Printed PCL/TCP/bdECM Scaffold Using Adipose-Derived Stem Cells Aggregates; An Experimental Study in the Canine Mandible" International Journal of Molecular Sciences 22, no. 11: 5409. https://doi.org/10.3390/ijms22115409
APA StyleLee, J. S., Park, T. H., Ryu, J. Y., Kim, D. K., Oh, E. J., Kim, H. M., Shim, J.-H., Yun, W.-S., Huh, J. B., Moon, S. H., Kang, S. S., & Chung, H. Y. (2021). Osteogenesis of 3D-Printed PCL/TCP/bdECM Scaffold Using Adipose-Derived Stem Cells Aggregates; An Experimental Study in the Canine Mandible. International Journal of Molecular Sciences, 22(11), 5409. https://doi.org/10.3390/ijms22115409






