Do Autism Spectrum and Autoimmune Disorders Share Predisposition Gene Signature Due to mTOR Signaling Pathway Controlling Expression?
Abstract
:1. Introduction
2. Results
2.1. SFARI Gene Database and AID Genes Comparative Gene-Set and Pathway Analysis
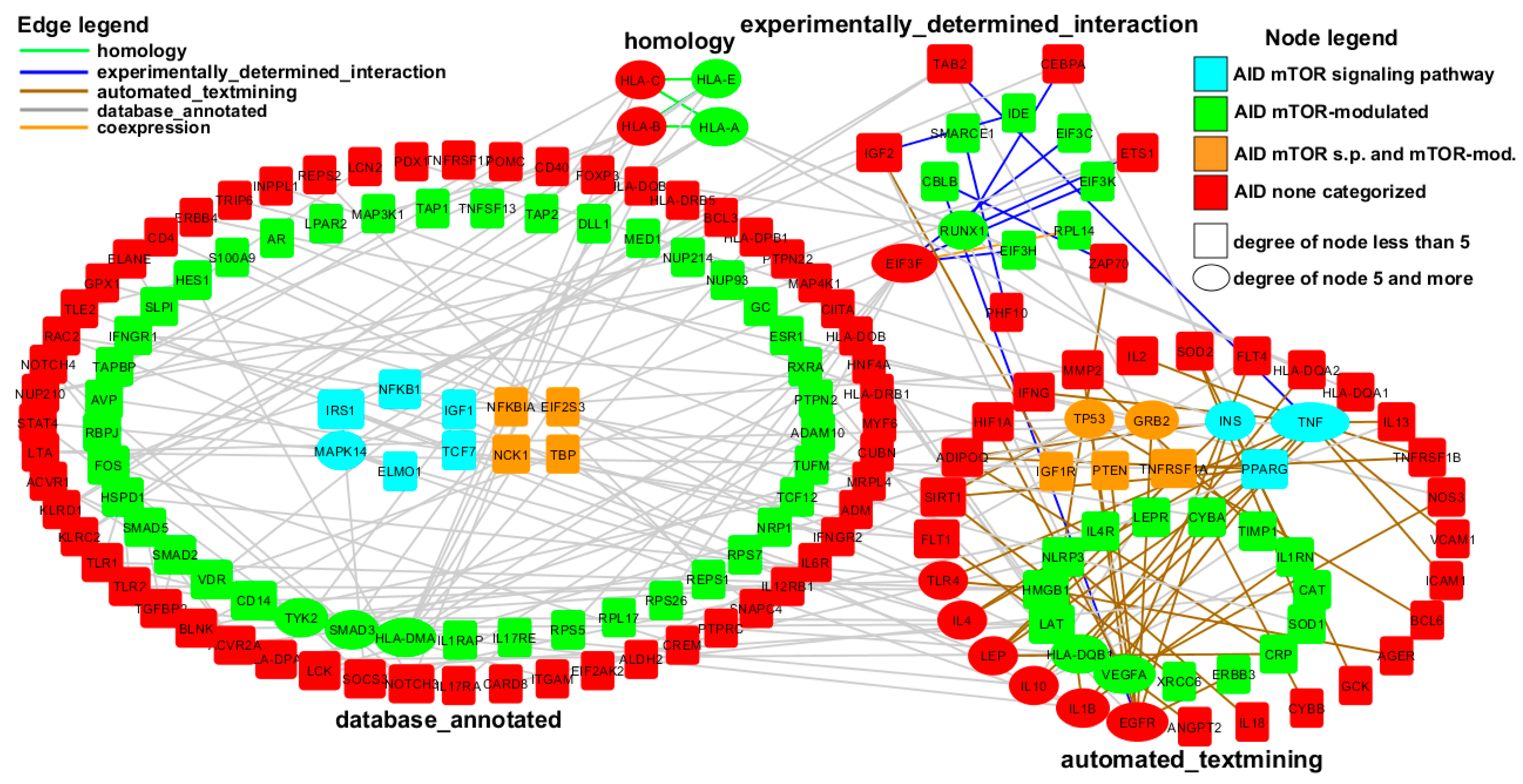
2.2. AID Genes Network Analysis
3. Discussion
4. Materials and Methods
4.1. Extracting Genes from Diverse Data Sources
- Genes implicated in autism susceptibility (from SFARI Gene database released 12-11-2020 (Supplementary Table S1))—992 genes;
- mTOR-sensitive genes (mTOR-sensitive 5UTR.xlsx [46])—6543 genes;
4.2. Assignment of Genes to Categories and Pathway Analysis
4.3. Network Construction
- The initial set of 729 genes (27 AID mTOR signaling pathway genes, 271 AID mTOR-modulated genes, 431 AID non-categorized genes) was submitted to the STRING database, the cutoff for interactions was 0.7 (high confidence). At the first stage, we found 3124 interactions between 528 genes;
- Based on the found interactions, the network was reconstructed in the Cytoscape software package. Since we were mainly interested in the links between AID mTOR-dependent genes and AID non-categorized genes, the links within these groups were excluded, which allowed us to reduce the number of genes to 421, and the number of interactions to 1124;
- We increased the confidence cutoff from 0.7 to 0.9 and then to 0.95. The network for each of the above cutoffs is available in Supplementary Figure S1 (network.cys).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASD | autism spectrum disorder |
| AID | autoimmune disorders |
| SFARI | Simon’s Foundation Autism Research Initiative |
| mTOR | mechanistic target of rapamycin |
| FMRP | fragile X mental retardation protein |
References
- Abrahams, B.S.; Arking, D.E.; Campbell, D.B.; Meord, H.C.; Morrow, E.M.; Weiss, L.A.; Menashe, I.; Wadkins, T.; Banerjee-Basu, S.; Packer, A. SFARI Gene 2.0: A community-driven knowledgebase for the autism spectrum disorders (ASDs). Mol. Autism 2013, 4, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoghbi, H.Y.; Bear, M.F. Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities. Cold Spring Harb. Perspect. Biol. 2012, 4, a009886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onore, C.; Yang, H.; van de Water, J.; Ashwood, P. Dynamic Akt/mTOR Signaling in Children with Autism Spectrum Disorder. Front. Pediatr. 2017, 5, 43. [Google Scholar] [CrossRef] [Green Version]
- Tylee, D.S.; Hess, J.L.; Quinn, T.P.; Barve, R.; Huang, H.; Zhang-James, Y.; Chang, J.; Stamova, B.S.; Sharp, F.R.; Hertz-Picciotto, I.; et al. Blood transcriptomic comparison of individuals with and without autism spectrum disorder: A combined-samples mega-analysis. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2017, 174, 181–201. [Google Scholar] [CrossRef] [Green Version]
- Bockaert, J.; Marin, P. mTOR in Brain Physiology and Pathologies. Physiol. Rev. 2015, 95, 1157–1187. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, D.; Liu, X. mTOR Signaling in T Cell Immunity and Autoimmunity. Int. Rev. Immunol. 2015, 34, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Warner, L.M.; Adams, L.M.; Sehgal, S.N. Rapamycin prolongs survival and arrests pathophysiologic changes inmurine systemic lupus erythematosus. Arthritis Rheum. 1994, 37, 289–297. [Google Scholar] [CrossRef]
- Fernandez, D.R.; Telarico, T.; Bonilla, E.; Li, Q.; Banerjee, S.; Middleton, F.A.; Phillips, P.E.; Crow, M.K.; Oess, S.; Muller-Esterl, W.; et al. Activation of mammalian target of rapamycin controls the loss of TCRzeta in lupus T cells through HRES-1/Rab4-regulated lysosomal degradation. J. Immunol. 2009, 182, 2063–2073. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wang, Z.; Dai, L.; Cao, L.; Su, J.; Zhu, M.; Yu, Z.; Bai, X.; Ruan, C. Effects of rapamycin combined with low dose prednisone in patients with chronic immune thrombocytopenia. Clin. Dev. Immunol. 2013, 2013, 548085. [Google Scholar] [CrossRef] [Green Version]
- Monti, P.; Scirpoli, M.; Maffi, P.; Piemonti, L.; Secchi, A.; Bonifacio, E.; Roncarolo, M.G.; Battaglia, M. Rapamycin monotherapy in patients with type 1 diabetes modifies CD4+CD25+FOXP3+ regulatory T-cells. Diabetes 2008, 57, 2341–2347. [Google Scholar] [CrossRef] [Green Version]
- Esposito, M.; Ruffini, F.; Bellone, M.; Gagliani, N.; Battaglia, M.; Martino, G.; Furlan, R. Rapamycin inhibits relapsing experimental autoimmune encephalomyelitis by both effector and regulatory T cells modulation. J. Neuroimmun. 2010, 220, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Massey, D.C.; Bredin, F.; Parkes, M. Use of sirolimus (rapamycin) to treat refractory Crohn’s disease. Gut 2008, 57, 1294–1296. [Google Scholar] [CrossRef] [PubMed]
- Suto, T.; Karonitsch, T. The immunobiology of mTOR in autoimmunity. J. Autoimmun. 2020, 110, 102373. [Google Scholar] [CrossRef]
- Taylor, M.J.; Rosenqvist, M.A.; Larsson, H.; Gillberg, C.; D’Onofrio, B.M.; Lichtenstein, P.; Lundström, S. Etiology of Autism Spectrum Disorders and Autistic Traits Over Time. JAMA Psychiatry 2020, 77, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Karlsson, H.; Dalman, C.; Widman, L.; Rai, D.; Gardner, R.M.; Magnusson, C.; Sandin, S.; Tabb, L.P.; Newschaffer, C.J.; et al. The Familial Risk of Autism Spectrum Disorder with and without Intellectual Disability. Autism Res. 2020, 13, 2242–2250. [Google Scholar] [CrossRef] [PubMed]
- Generali, E.; Ceribelli, A.; Stazi, M.A.; Selmi, C. Lessons learned from twins in autoimmune and chronic inflammatory diseases. J. Autoimmun. 2017, 83, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Sur, S. In silico analysis reveals interrelation of enriched pathways and genes in type 1 diabetes. Immunogenetics 2020, 72, 399–412. [Google Scholar] [CrossRef]
- Tuller, T.; Atar, S.; Ruppin, E.; Gurevich, M.; Achiron, A. Common and specific signatures of gene expression and protein-protein interactions in autoimmune diseases. Genes Immun. 2013, 14, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Trifonova, E.A.; Klimenko, A.I.; Mustafin, Z.S.; Lashin, S.A.; Kochetov, A.V. The mTOR Signaling Pathway Activity and Vitamin D Availability Control the Expression of Most Autism Predisposition Genes. Int. J. Mol. Sci. 2019, 20, 6332. [Google Scholar] [CrossRef] [Green Version]
- Hughes, H.K.; Mills Ko, E.; Rose, D.; Ashwood, P. Immune Dysfunction and Autoimmunity as Pathological Mechanisms in Autism Spectrum Disorders. Front. Cell. Neurosci. 2018, 12, 405. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Ding, Y.; Wu, F.; Li, R.; Xie, G.; Hou, J.; Mao, P. Family history of autoimmune diseases is associated with an increased risk of autism in children: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2015, 55, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Ashwood, P.; van de Water, J. Is autism an autoimmune disease? Autoimmun. Rev. 2004, 3, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Edmiston, E.; Ashwood, P.; van de Water, J. Autoimmunity, Autoantibodies, and Autism Spectrum Disorder. Biol. Psychiatry 2017, 81, 383–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiStasio, M.M.; Nagakura, I.; Nadler, M.J.; Anderson, M.P. T lymphocytes and cytotoxic astrocyte blebs correlate across autism brains. Ann. Neurol. 2019, 86, 885–898. [Google Scholar] [CrossRef] [PubMed]
- Jaini, R.; Loya, M.G.; King, A.T.; Thacker, S.; Sarn, N.B.; Yu, Q.; Stark, G.R.; Eng, C. Germline PTEN mutations are associated with a skewed peripheral immune repertoire in humans and mice. Hum. Mol. Genet. 2020, 29, 2353–2364. [Google Scholar] [CrossRef]
- Vlaskamp, D.R.M.; Shaw, B.J.; Burgess, R.; Mei, D.; Montomoli, M.; Xie, H.; Myers, C.T.; Bennett, M.F.; Xiangwei, W.; Williams, D.; et al. SYNGAP1 encephalopathy: A distinctive generalized developmental and epileptic encephalopathy. Neurology 2019, 92, e96–e107. [Google Scholar] [CrossRef] [Green Version]
- Currais, A.; Farrokhi, C.; Dargusch, R.; Goujon-Svrzic, M.; Maher, P. Dietary glycemic index modulates the behavioral and biochemical abnormalities associated with autism spectrum disorder. Mol. Psychiatry 2016, 21, 426–436. [Google Scholar] [CrossRef]
- Chappaz, S.; Law, C.W.; Dowling, M.R.; Carey, K.T.; Lane, R.M.; Ngo, L.H.; Wickramasinghe, V.O.; Smyth, G.K.; Ritchie, M.E.; Kile, B.T. Germline heterozygous mutations in Nxf1 perturb RNA metabolism and trigger thrombocytopenia and lymphopenia in mice. Blood Adv. 2020, 4, 1270–1283. [Google Scholar] [CrossRef] [Green Version]
- Fu, B.; Zhao, M.; Wang, L.; Patil, G.; Smith, J.A.; Juncadella, I.J.; Zuvela-Jelaska, L.; Dorf, M.E.; Li, S. RNAi Screen and Proteomics Reveal NXF1 as a Novel Regulator of IRF5 Signaling. Sci. Rep. 2017, 7, 2683. [Google Scholar] [CrossRef] [Green Version]
- Grillo, L.; Reitano, S.; Belfiore, G.; Spalletta, A.; Amata, S.; Bottitta, M.; Barone, C.; Falco, M.; Fichera, M.; Romano, C. Familial 1.1 Mb deletion in chromosome Xq22.1 associated with mental retardation and behavioural disorders in female patients. Eur. J. Med. Genet. 2010, 53, 113–116. [Google Scholar] [CrossRef]
- Wyman, B.; Perl, A. Metabolic pathways mediate pathogenesis and offer targets for treatment in rheumatic diseases. Curr. Opin. Rheumatol. 2020, 32, 184–191. [Google Scholar] [CrossRef]
- Murdaca, G.; Tonacci, A.; Negrini, S.; Greco, M.; Borro, M.; Puppo, F.; Gangemi, S. Emerging role of vitamin D in autoimmune diseases: An update on evidence and therapeutic implications. Autoimmun. Rev. 2019, 18, 102350. [Google Scholar] [CrossRef]
- Jia, F.; Wang, B.; Shan, L.; Xu, Z.; Staal, W.G.; Du, L. Core Symptoms of Autism Improved After Vitamin D Supplementation. Pediatrics 2015, 135, e196–e198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, F.; Shan, L.; Wang, B.; Li, H.; Feng, J.; Xu, Z.; Saad, K. Fluctuations in clinical symptoms with changes in serum 25(OH) vitamin D levels in autistic children: Three cases report. Nutr. Neurosci. 2019, 22, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, M.L.; Pagano, M.T.; Pierdominici, M.; Ortona, E. The role of vitamin D in autoimmune diseases: Could sex make the difference? Biol. Sex Differ. 2021, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Miclea, A.; Bagnoud, M.; Chan, A.; Hoepner, R. A Brief Review of the Effects of Vitamin D on Multiple Sclerosis. Front. Immunol. 2020, 11, 781. [Google Scholar] [CrossRef]
- Mocci, G.; Marzo, M.; Papa, A.; Armuzzi, A.; Guidi, L. Dermatological adverse reactions during anti-TNF treatments: Focus on inflammatory bowel disease. J. Crohns Colitis 2013, 7, 769–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treasure Island (FL): StatPearls Publishing. 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545252/ (accessed on 11 August 2020).
- Melamed, I.R.; Heffron, M.; Testori, A.; Lipe, K. A pilot study of high-dose intravenous immunoglobulin 5% for autism: Impact on autism spectrum and markers of neuroinflammation. Autism Res. 2018, 11, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.D.; Yim, Y.S.; Wimmer, R.D.; Kim, H.; Ryu, C.; Welch, G.M.; Andina, M.; King, H.O.; Waisman, A.; Halassa, M.M.; et al. IL-17a promotes sociability in mouse models of neurodevelopmental disorders. Nature 2020, 577, 249–253. [Google Scholar] [CrossRef]
- Malek, M.; Ashraf-Ganjouei, A.; Moradi, K.; Bagheri, S.; Mohammadi, M.R.; Akhondzadeh, S. Prednisolone as Adjunctive Treatment to Risperidone in Children with Regressive Type of Autism Spectrum Disorder: A Randomized, Placebo-Controlled Trial. Clin. Neuropharmacol. 2020, 43, 39–45. [Google Scholar] [CrossRef]
- Pape, K.; Tamouza, R.; Leboyer, M.; Zipp, F. Immunoneuropsychiatry—Novel perspectives on brain disorders. Nat. Rev. Neurol. 2019, 15, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, A.; Dieleman, G.C.; Smit, A.B.; Verhage, M.; Verhulst, F.C.; Polderman, T.J.C.; Posthuma, D. Gene-set analysis shows association between FMRP targets and autism spectrum disorder. Eur. J. Hum. Genet. 2017, 25, 863–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darnell, J.C.; van Driesche, S.J.; Zhang, C.; Hung, K.Y.S.; Mele, A.; Fraser, C.E.; Stone, E.F.; Chen, C.; Fak, J.J.; Chi, S.W.; et al. FMRP Stalls Ribosomal Translocation on mRNAs Linked to Synaptic Function and Autism. Cell 2011, 146, 247–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandin, V.; Masvidal, L.; Hulea, L.; Gravel, S.-P.; Cargnello, M.; McLaughlan, S.; Cai, Y.; Balanathan, P.; Morita, M.; Rajakumar, A.; et al. nanoCAGE reveals 50 UTR features that define specific modes of translation of functionally related MTOR-sensitive mRNAs. Genome Res. 2016, 26, 636–648. [Google Scholar] [CrossRef] [Green Version]
- Caron, E.; Ghosh, S.; Matsuoka, Y.; Ashton-Beaucage, D.; Therrien, M.; Lemieux, S.; Perreault, C.; Roux, P.P.; Kitano, H. A comprehensive map of the mTOR signaling network. Mol. Syst. Biol. 2010, 6, 453. [Google Scholar] [CrossRef]
- Wang, T.-T.; Tavera-Mendoza, L.E.; Laperriere, D.; Libby, E.; Burton MacLeod, N.; Nagai, Y.; Bourdeau, V.; Konstorum, A.; Lallemant, B.; Zhang, R.; et al. Large-Scale in Silico and Microarray-Based Identification of Direct 1,25-Dihydroxyvitamin D3 Target Genes. Mol. Endocrinol. 2005, 19, 2685–2695. [Google Scholar] [CrossRef] [Green Version]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353. [Google Scholar] [CrossRef] [Green Version]
- Sharon, G.; Cruz, N.J.; Kang, D.-W.; Gandal, M.J.; Wang, B.; Kim, Y.-M.; Zink, E.M.; Casey, C.P.; Taylor, B.C.; Lane, C.J.; et al. Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell 2019, 177, 1600–1618. [Google Scholar] [CrossRef] [Green Version]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [Green Version]
- Shannon, P.; Markiel, A.; Ozier, O. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
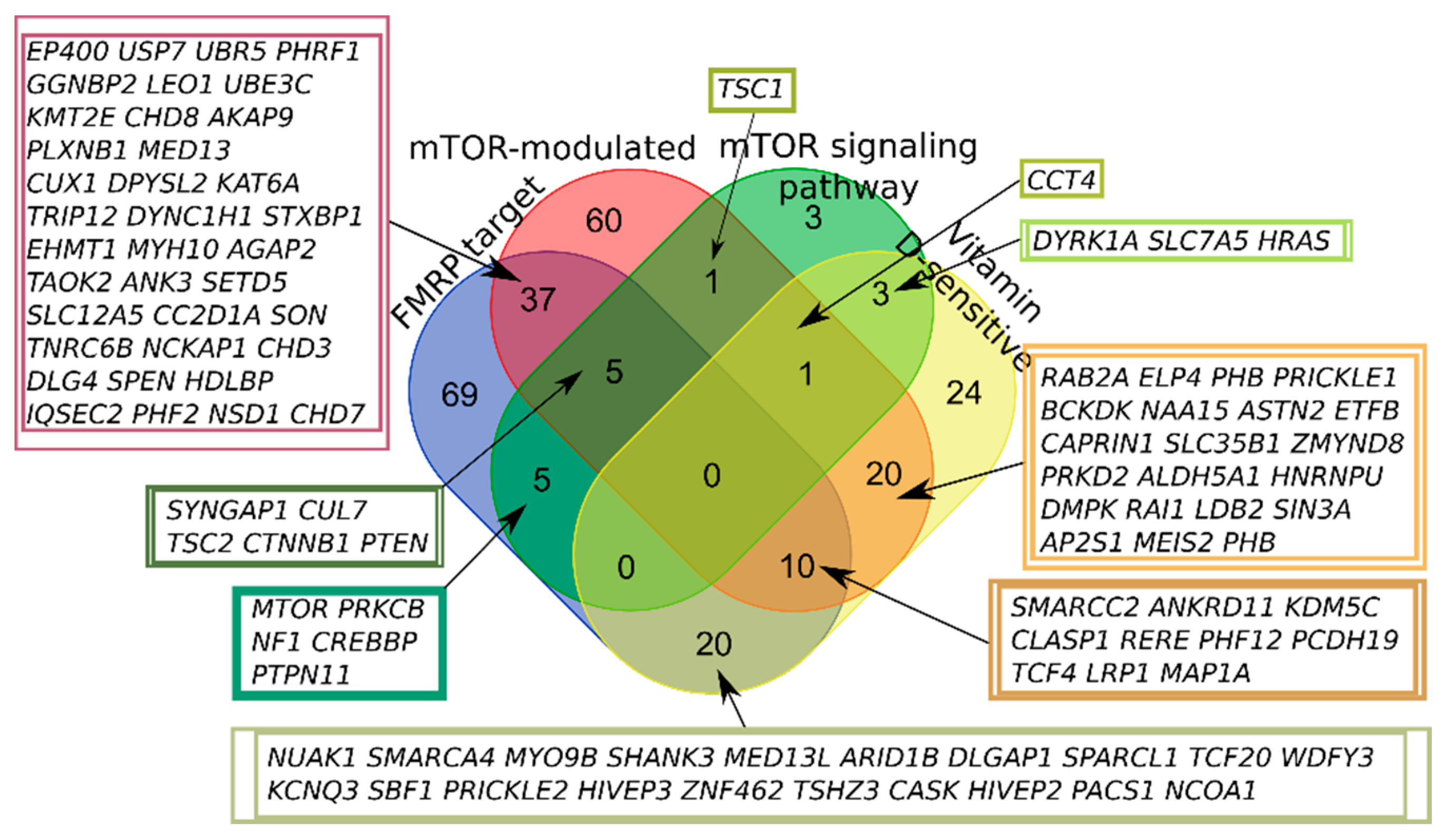


| Category of Genes | Number of Genes | ||
|---|---|---|---|
| SFARI (Whole) | Autoimmune Disorders | SFARI (High Confidence + Strong Candidate) | |
| FMRP target | 270 | 74 | 146 |
| mTOR signaling pathway | 41 | 27 | 18 |
| mTOR-modulated | 304 | 271 | 134 |
| Vitamin D-sensitive | 202 | 199 | 78 |
| None | 408 | 431 | 143 |
| Total | 992 | 871 | 401 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trifonova, E.A.; Klimenko, A.I.; Mustafin, Z.S.; Lashin, S.A.; Kochetov, A.V. Do Autism Spectrum and Autoimmune Disorders Share Predisposition Gene Signature Due to mTOR Signaling Pathway Controlling Expression? Int. J. Mol. Sci. 2021, 22, 5248. https://doi.org/10.3390/ijms22105248
Trifonova EA, Klimenko AI, Mustafin ZS, Lashin SA, Kochetov AV. Do Autism Spectrum and Autoimmune Disorders Share Predisposition Gene Signature Due to mTOR Signaling Pathway Controlling Expression? International Journal of Molecular Sciences. 2021; 22(10):5248. https://doi.org/10.3390/ijms22105248
Chicago/Turabian StyleTrifonova, Ekaterina A., Alexandra I. Klimenko, Zakhar S. Mustafin, Sergey A. Lashin, and Alex V. Kochetov. 2021. "Do Autism Spectrum and Autoimmune Disorders Share Predisposition Gene Signature Due to mTOR Signaling Pathway Controlling Expression?" International Journal of Molecular Sciences 22, no. 10: 5248. https://doi.org/10.3390/ijms22105248
APA StyleTrifonova, E. A., Klimenko, A. I., Mustafin, Z. S., Lashin, S. A., & Kochetov, A. V. (2021). Do Autism Spectrum and Autoimmune Disorders Share Predisposition Gene Signature Due to mTOR Signaling Pathway Controlling Expression? International Journal of Molecular Sciences, 22(10), 5248. https://doi.org/10.3390/ijms22105248







