A Nano-Emulsion Platform Functionalized with a Fully Human scFv-Fc Antibody for Atheroma Targeting: Towards a Theranostic Approach to Atherosclerosis
Abstract
1. Introduction
2. Results
2.1. PEGylated NE Formulations and Characterization
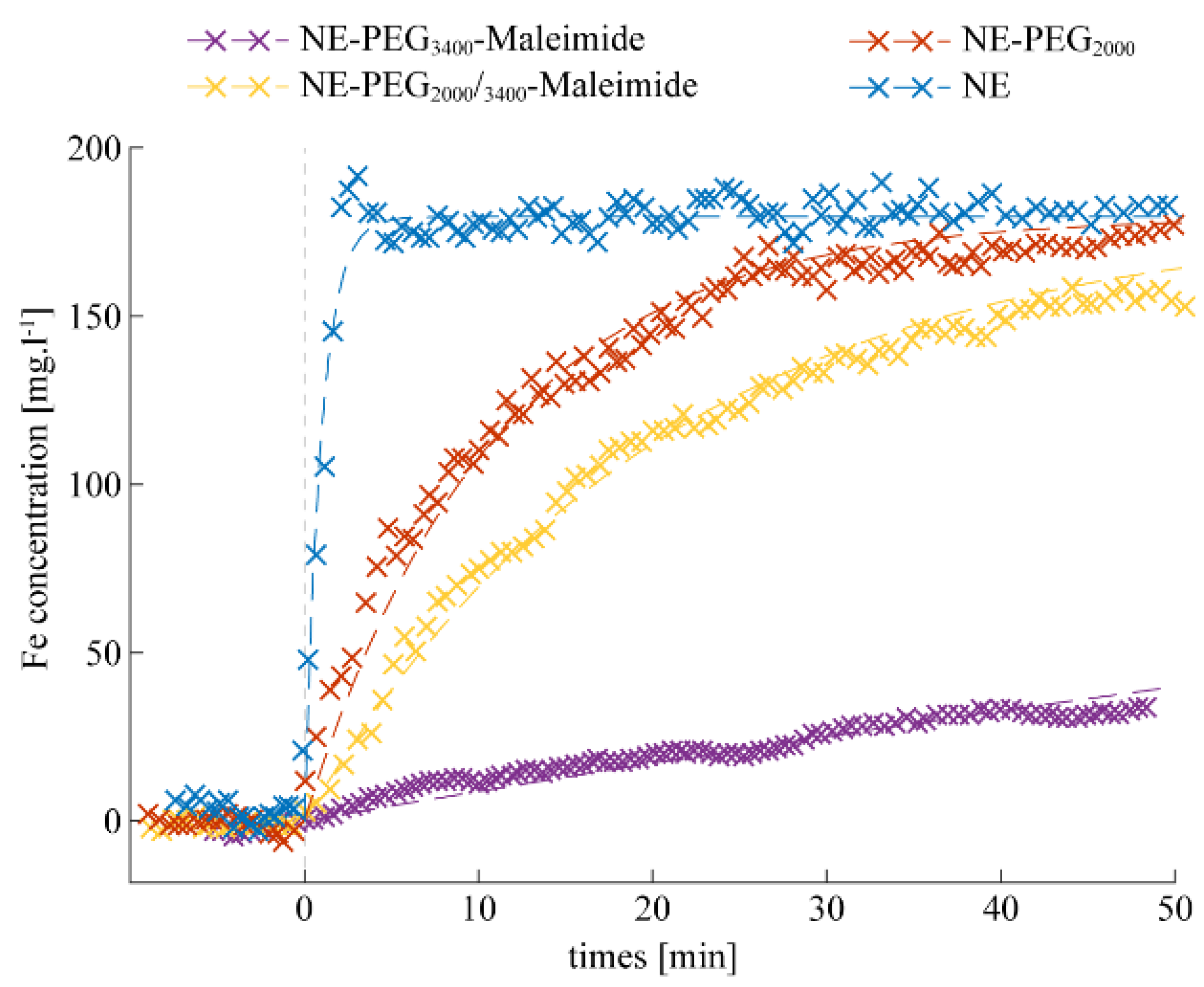
2.2. Stealthy Features of PEGylated NEs
2.3. Bio-Conjugation with P3 HuAb and In Vivo Clearance of NE-P3
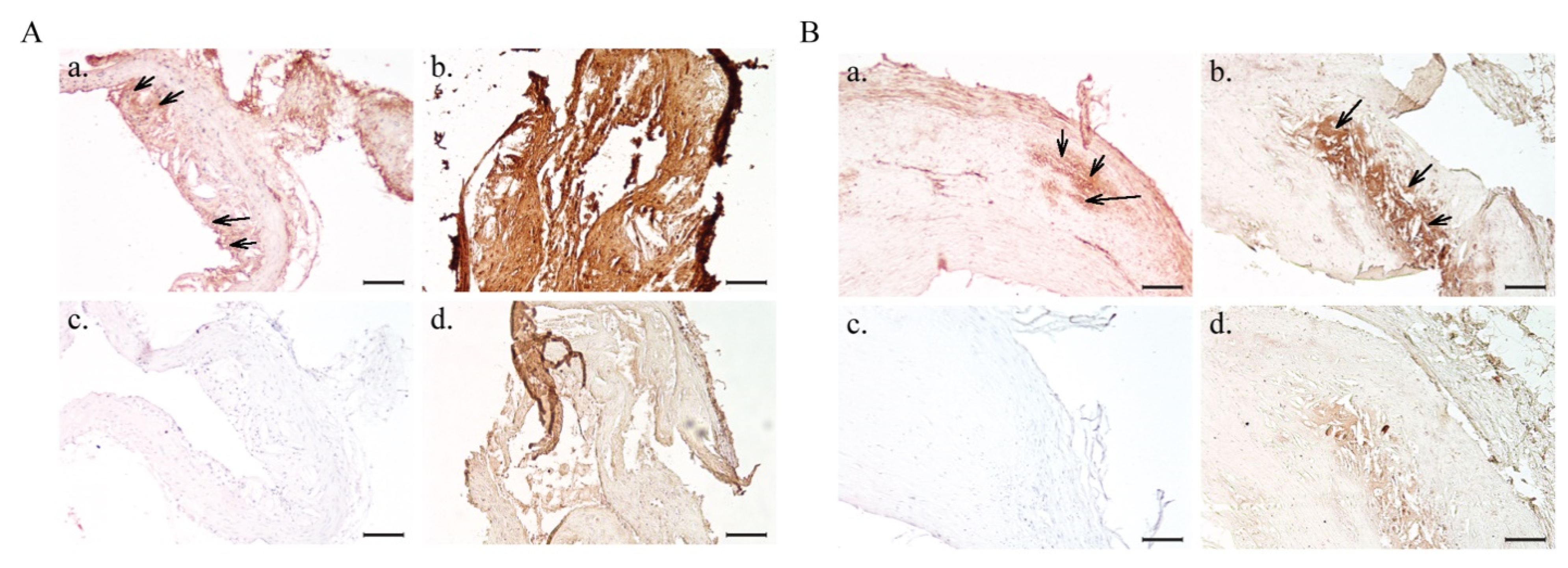
2.4. In Vitro Atheroma Targeting
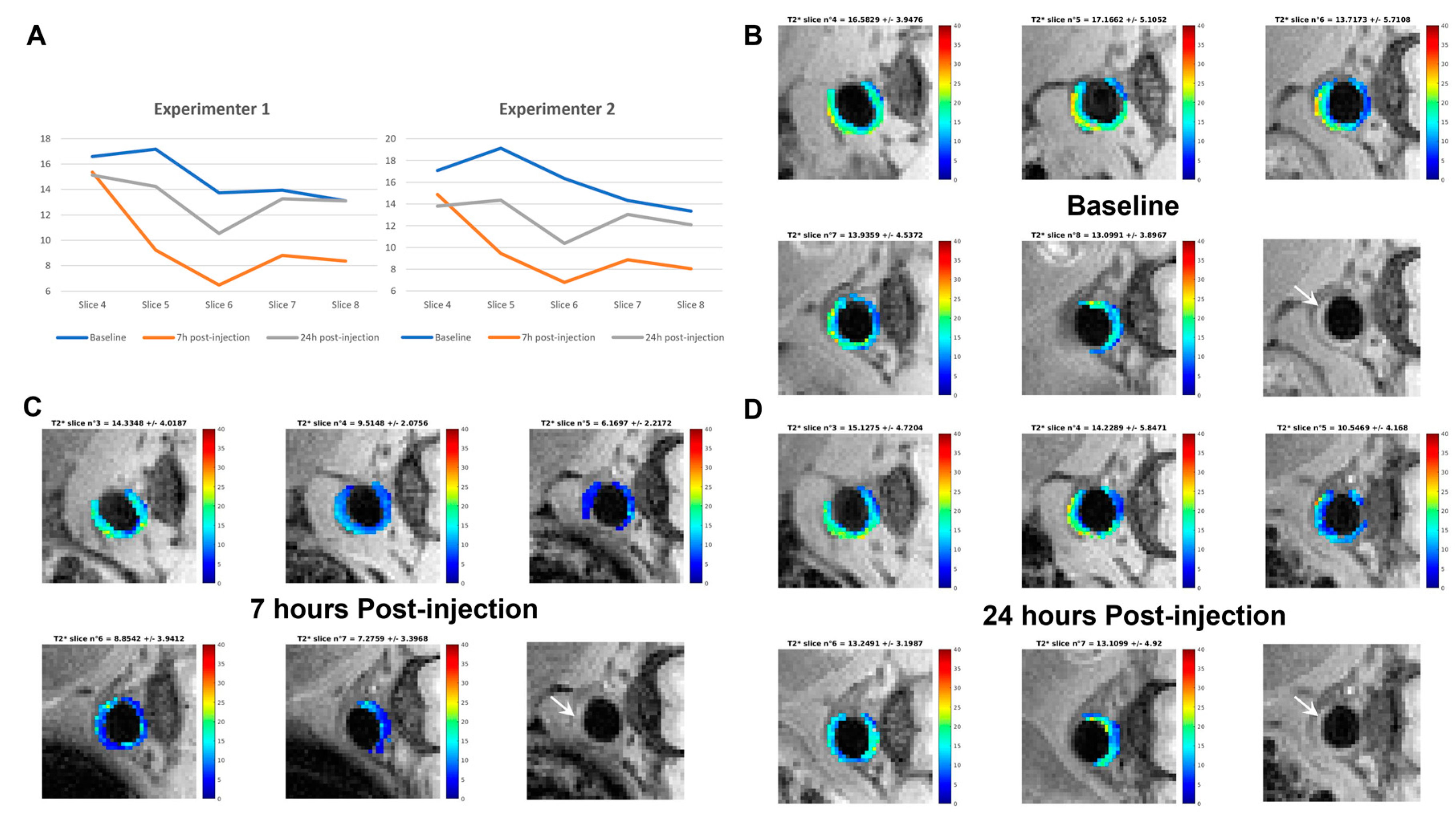
2.5. Preliminary Data on In Vivo Atheroma Targeting by NE-P3
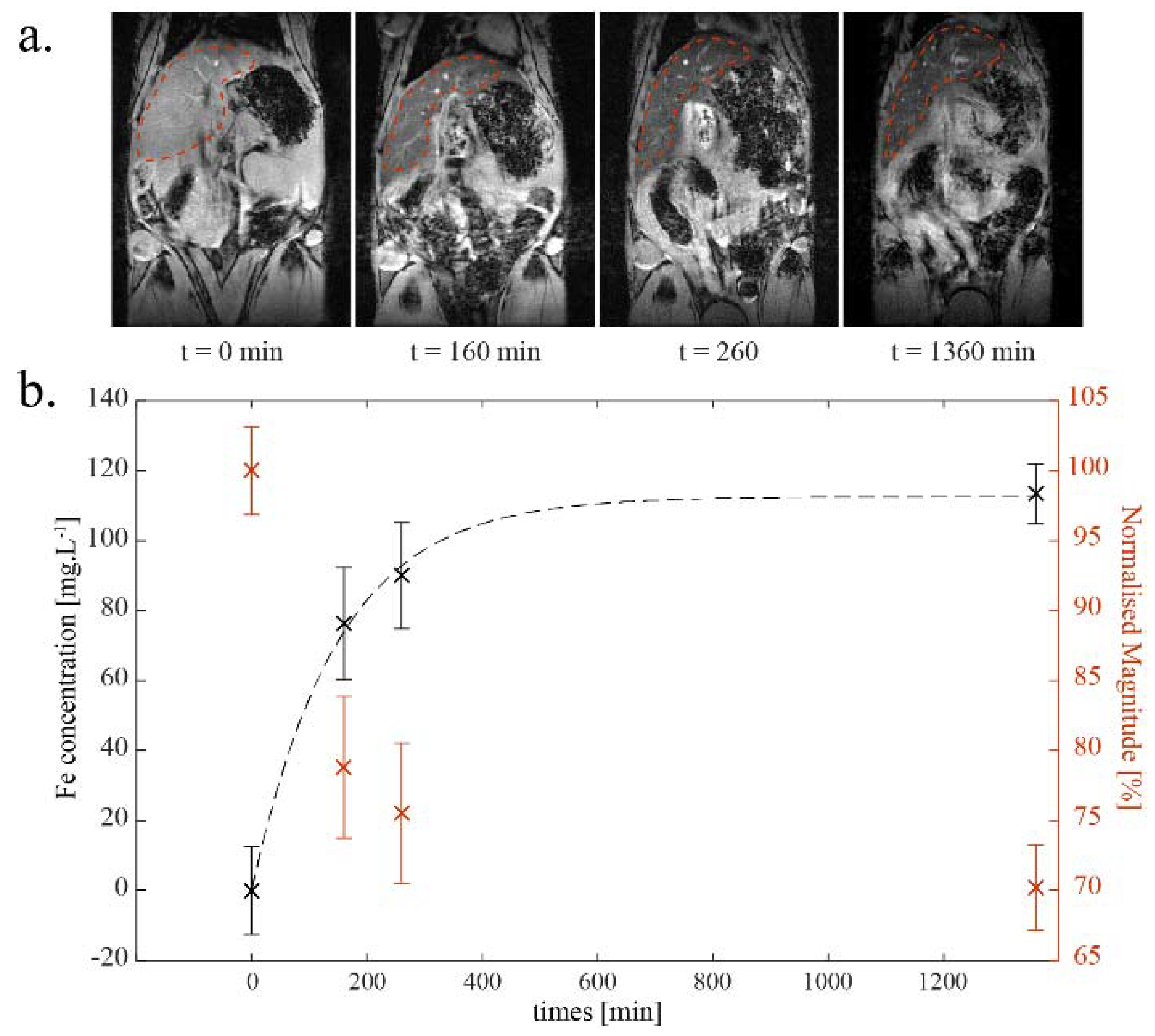
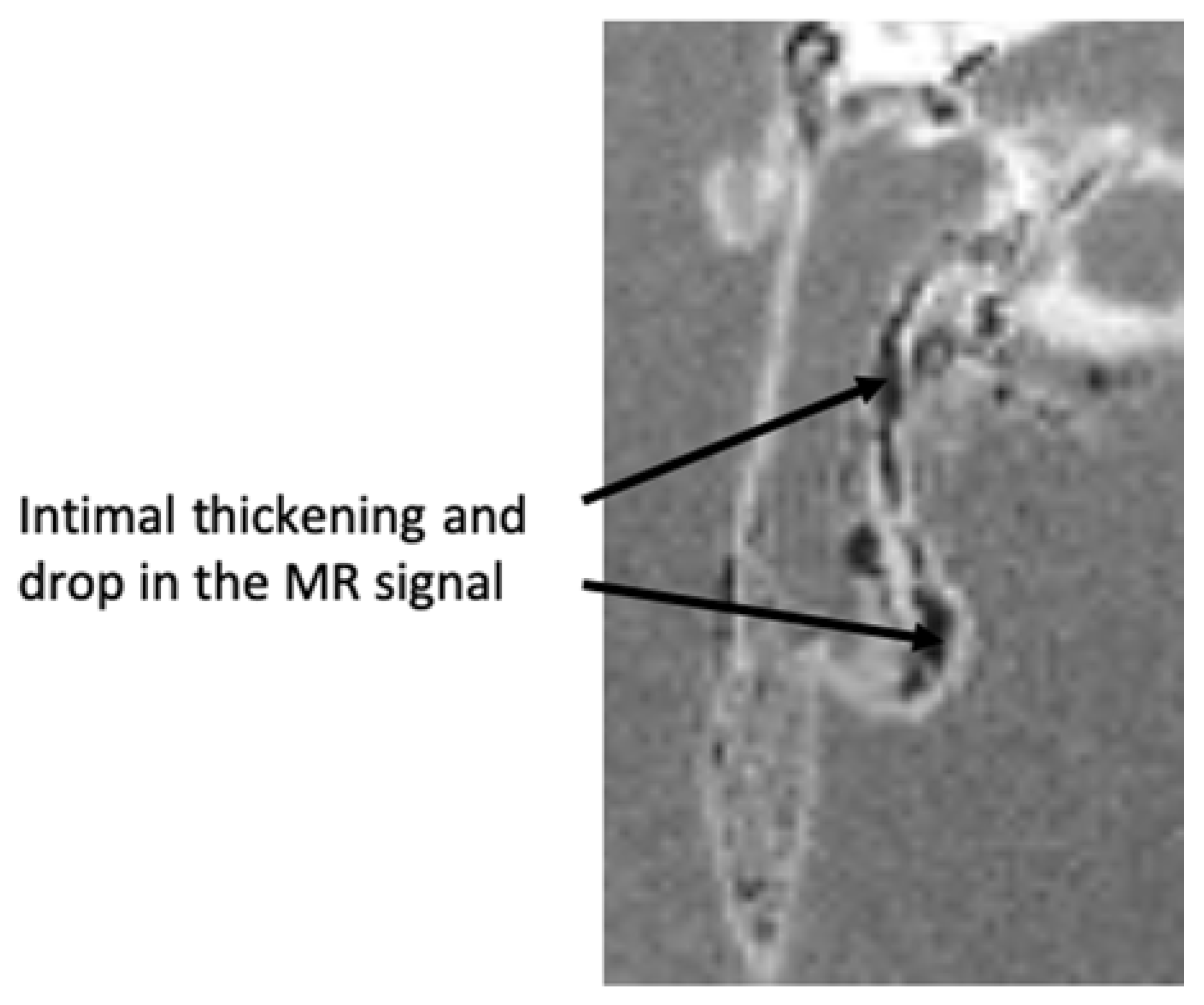
2.6. Ex Vivo Analyses of Isolated Aorta Samples
2.6.1. Ex Vivo MRI
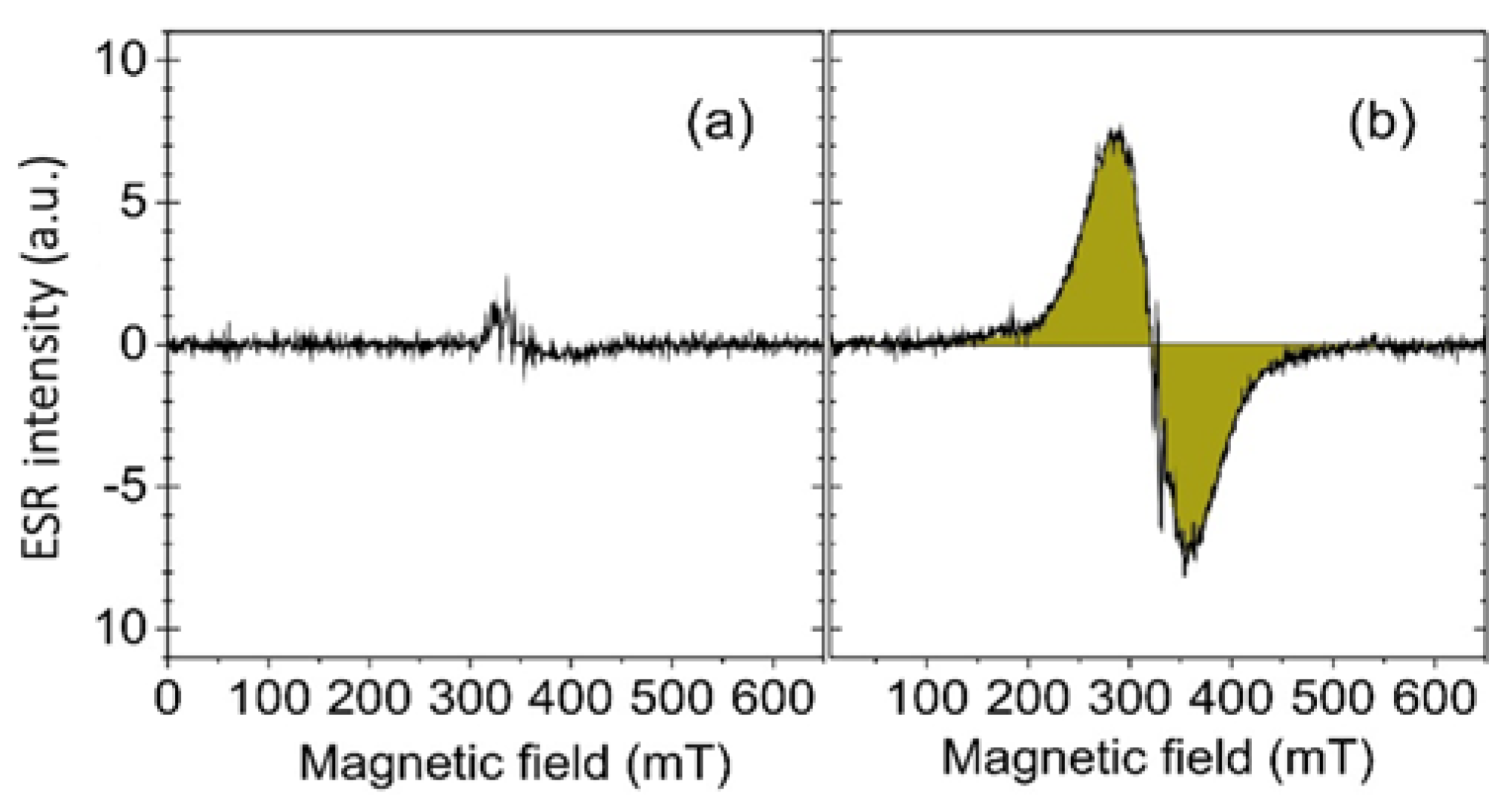
2.6.2. Iron Oxide Accumulation Assessment by ESR
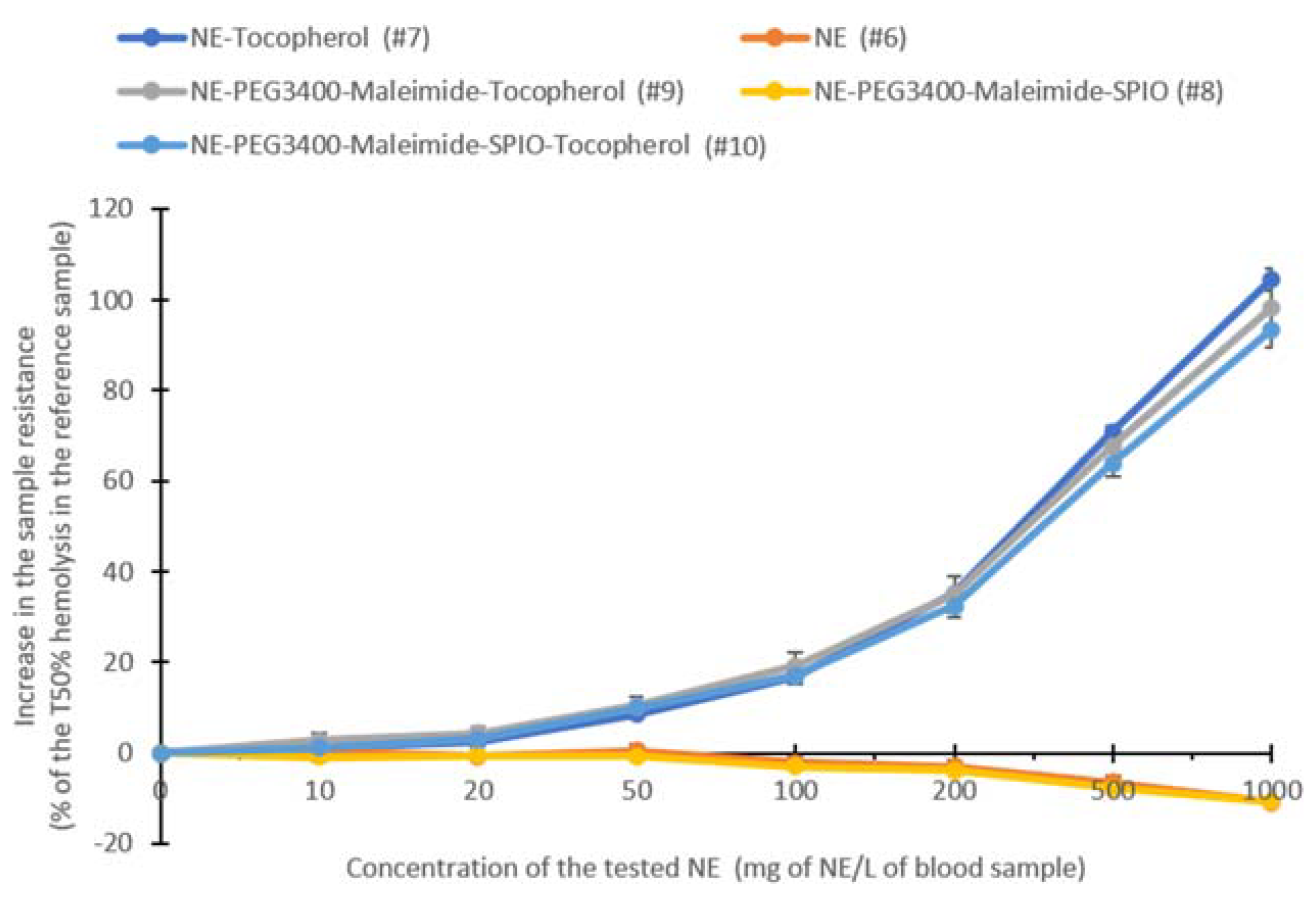
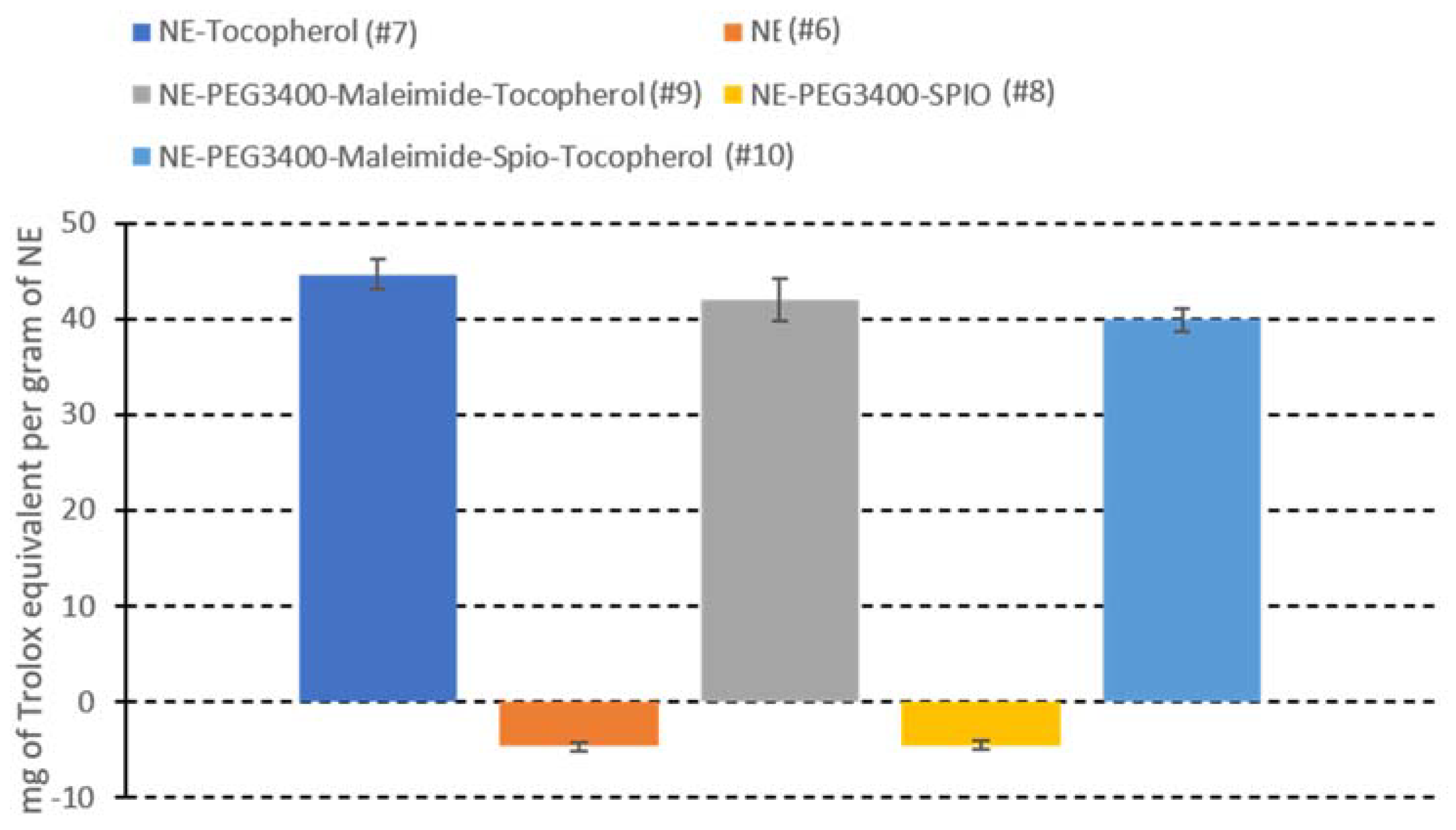
2.7. Antioxidant Alpha-Tocopherol-Containing NEs for a Theranostic Approach: Formulation, Characterization, and Activity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. NE Formulations
4.3. NE Characterization
4.4. P3 Antibody Bio-Conjugation to NEs
4.5. In Vitro Immunoreactivity Analysis by IHC
4.6. In Vivo Experimental Animal Model
4.7. Magnetic Resonance Imaging
4.7.1. In Vivo Assessment of Stealthy Features by Dynamic MRI
4.7.2. Estimation of the NE Half-Life in the Blood
4.7.3. In Vivo Dynamic MRI and T2* Mapping of Atheromatous Plaques in Apoe−/− Mice
4.8. Ex Vivo Analyses of Isolated Aorta Samples
4.8.1. MRI Analyses
4.8.2. Iron Quantification by ESR
4.9. In Vitro Antioxidant Measurement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef]
- Rudd, J.H.F.; Davies, J.R.; Weissberg, P.L. Imaging of atherosclerosis—Can we predict plaque rupture? Trends Cardiovasc. Med. 2005, 15, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Burke, A.P.; Kolodgie, F.D.; Farb, A. Vulnerable plaque: The pathology of unstable coronary lesions. J. Interv. Cardiol. 2002, 15, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, I.; Saura, M.; Castejón, B.; Martin, A.M.; Herruzo, I.; Balatsos, N.; Zamorano, J.L.; Zaragoza, C. Preclinical models of atherosclerosis. The future of Hybrid PET/MR technology for the early detection of vulnerable plaque. Expert Rev. Mol. Med. 2016, 18, e6. [Google Scholar] [CrossRef] [PubMed]
- Montiel Schneider, M.G.; Lassalle, V.L. Magnetic iron oxide nanoparticles as novel and efficient tools for atherosclerosis diagnosis. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 93, 1098–1115. [Google Scholar] [CrossRef]
- Mehta, A.; Shah, S. Unstable or High Risk Plaque: How Do We Approach It? Med. J. Armed Forces India 2006, 62, 2–7. [Google Scholar] [CrossRef][Green Version]
- Kashyap, V.S.; Pavkov, M.L.; Bishop, P.D.; Nassoiy, S.P.; Eagleton, M.J.; Clair, D.G.; Ouriel, K. Angiography Underestimates Peripheral Atherosclerosis: Lumenography Revisited. J. Endovasc. Ther. 2008, 15, 117–125. [Google Scholar] [CrossRef]
- Little, W.C.; Constantinescu, M.; Applegate, R.J.; Kutcher, M.A.; Burrows, M.T.; Kahl, F.R.; Santamore, W.P. Can coronary angiography predict the site of a subsequent myocardial infarction in patients with mild-to-moderate coronary artery disease? Circulation 1988, 78, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.M.; Davies, M.J. Vulnerable plaque. Relation of characteristics to degree of stenosis in human coronary arteries. Circulation 1996, 94, 928–931. [Google Scholar] [CrossRef]
- Osborn, E.A.; Jaffer, F.A. Imaging atherosclerosis and risk of plaque rupture. Curr. Atheroscler. Rep. 2013, 15, 359. [Google Scholar] [CrossRef]
- Rathod, K.S.; Hamshere, S.M.; Jones, D.A.; Mathur, A. Intravascular ultrasound versus optical coherence tomography for Coronary artery imaging—Apples and oranges? Interv. Cardiol. Lond. Engl. 2015, 10, 8–15. [Google Scholar] [CrossRef]
- Tarkin, J.M.; Dweck, M.R.; Evans, N.R.; Takx, R.A.P.; Brown, A.J.; Tawakol, A.; Fayad, Z.A.; Rudd, J.H.F. Imaging Atherosclerosis. Circ. Res. 2016, 118, 750–769. [Google Scholar] [CrossRef]
- Tearney, G.J.; Yabushita, H.; Houser, S.L.; Aretz, H.T.; Jang, I.-K.; Schlendorf, K.H.; Kauffman, C.R.; Shishkov, M.; Halpern, E.F.; Bouma, B.E. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation 2003, 107, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Mulder, W.J.M.; Jaffer, F.A.; Fayad, Z.A.; Nahrendorf, M. Imaging and nanomedicine in inflammatory atherosclerosis. Sci. Transl. Med. 2014, 6, 239sr1. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, J.; Huang, J.-W.; Song, J.-C.; Ma, Z.-L.; Shi, H.-B. In vivo MRI detection of atherosclerosis in ApoE-deficient mice by using tenascin-C-targeted USPIO. Acta Radiol. Stockh. Swed. 1987 2018, 59, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Corti, R.; Fuster, V. Imaging of atherosclerosis: Magnetic resonance imaging. Eur. Heart J. 2011, 32, 1709–1719. [Google Scholar] [CrossRef]
- Poon, C.; Gallo, J.; Joo, J.; Chang, T.; Bañobre-López, M.; Chung, E.J. Hybrid, metal oxide-peptide amphiphile micelles for molecular magnetic resonance imaging of atherosclerosis. J. Nanobiotechnol. 2018, 16, 92. [Google Scholar] [CrossRef]
- Pellico, J.; Ruiz-Cabello, J.; Herranz, F. Microwave-driven Synthesis of Iron Oxide Nanoparticles for Fast Detection of Atherosclerosis. J. Vis. Exp. JoVE 2016. [Google Scholar] [CrossRef]
- Herranz, F.; Salinas, B.; Groult, H.; Pellico, J.; Lechuga-Vieco, A.; Bhavesh, R.; Ruiz-Cabello, J. Superparamagnetic Nanoparticles for Atherosclerosis Imaging. Nanomaterials 2014, 4, 408–438. [Google Scholar] [CrossRef] [PubMed]
- Prévot, G.; Kauss, T.; Lorenzato, C.; Gaubert, A.; Larivière, M.; Baillet, J.; Laroche-Traineau, J.; Jacobin-Valat, M.J.; Adumeau, L.; Mornet, S.; et al. Iron oxide core oil-in-water nanoemulsion as tracer for atherosclerosis MPI and MRI imaging. Int. J. Pharm. 2017, 532, 669–676. [Google Scholar] [CrossRef]
- Wallyn, J.; Anton, N.; Mertz, D.; Begin-Colin, S.; Perton, F.; Serra, C.A.; Franconi, F.; Lemaire, L.; Chiper, M.; Libouban, H.; et al. Magnetite- and Iodine-Containing Nanoemulsion as a Dual Modal Contrast Agent for X-ray/Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2019, 11, 403–416. [Google Scholar] [CrossRef]
- Zernecke, A.; Winkels, H.; Cochain, C.; Williams, J.W.; Wolf, D.; Soehnlein, O.; Robbins, C.S.; Monaco, C.; Park, I.; McNamara, C.A.; et al. Meta-analysis of leukocyte diversity in atherosclerotic mouse aortas. Circ. Res. 2020, 127, 402–426. [Google Scholar] [CrossRef]
- Cochain, C.; Vafadarnejad, E.; Arampatzi, P.; Pelisek, J.; Winkels, H.; Ley, K.; Wolf, D.; Saliba, A.-E.; Zernecke, A. Single-Cell RNA-Seq Reveals the Transcriptional Landscape and Heterogeneity of Aortic Macrophages in Murine Atherosclerosis. Circ. Res. 2018, 122, 1661–1674. [Google Scholar] [CrossRef] [PubMed]
- Amoozgar, Z.; Yeo, Y. Recent advances in stealth coating of nanoparticle drug delivery systems. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 219–233. [Google Scholar] [CrossRef]
- Ryan, S.M.; Mantovani, G.; Wang, X.; Haddleton, D.M.; Brayden, D.J. Advances in PEGylation of important biotech molecules: Delivery aspects. Expert Opin. Drug Deliv. 2008, 5, 371–383. [Google Scholar] [CrossRef]
- Owens, D.E.; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. PEG-based micelles as carriers of contrast agents for different imaging modalities. Adv. Drug Deliv. Rev. 2002, 54, 235–252. [Google Scholar] [CrossRef]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Hak, S.; Garaiova, Z.; Olsen, L.T.; Nilsen, A.M.; de Lange Davies, C. The effects of oil-in-water nanoemulsion polyethylene glycol surface density on intracellular stability, pharmacokinetics, and biodistribution in tumor bearing mice. Pharm. Res. 2015, 32, 1475–1485. [Google Scholar] [CrossRef]
- Prévot, G.; Duonor-Cérutti, M.; Larivière, M.; Laroche-Traineau, J.; Jacobin-Valat, M.J.; Barthélémy, P.; Clofent-Sanchez, G.; Crauste-Manciet, S. Data on atherosclerosis specific antibody conjugation to nanoemulsions. Data Brief 2017, 15, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, M.; Wallert, M.; Lorkowski, S.; Peter, K. Cardiovascular and Metabolic Protection by Vitamin E: A Matter of Treatment Strategy? Antioxidants 2020, 9, 935. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Ibelles, P.; Mas-Oliva, J. Antioxidants in the Fight Against Atherosclerosis: Is This a Dead End? Curr. Atheroscler. Rep. 2018, 20, 36. [Google Scholar] [CrossRef]
- Abbina, S.; Parambath, A. 14—PEGylation and its alternatives: A summary. In Engineering of Biomaterials for Drug Delivery Systems; Parambath, A., Ed.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Cambridge, UK, 2018; pp. 363–376. ISBN 978-0-08-101750-0. [Google Scholar]
- Schöttler, S.; Becker, G.; Winzen, S.; Steinbach, T.; Mohr, K.; Landfester, K.; Mailänder, V.; Wurm, F.R. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat. Nanotechnol. 2016, 11, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Hörmann, K.; Zimmer, A. Drug delivery and drug targeting with parenteral lipid nanoemulsions—A review. J. Control. Release Off. J. Control. Release Soc. 2016, 223, 85–98. [Google Scholar] [CrossRef]
- Prévot, G.; Soria, F.N.; Thiolat, M.-L.; Daniel, J.; Verlhac, J.B.; Blanchard-Desce, M.; Bezard, E.; Barthélémy, P.; Crauste-Manciet, S.; Dehay, B. Harnessing Lysosomal pH through PLGA Nanoemulsion as a Treatment of Lysosomal-Related Neurodegenerative Diseases. Bioconjug. Chem. 2018, 29, 4083–4089. [Google Scholar] [CrossRef] [PubMed]
- Lovelyn, C.; Attama, A.A. Current State of Nanoemulsions in Drug Delivery. J. Biomater. Nanobiotechnol. 2011, 2, 626–639. [Google Scholar] [CrossRef]
- Jarzyna, P.A.; Skajaa, T.; Gianella, A.; Cormode, D.P.; Samber, D.D.; Dickson, S.D.; Chen, W.; Griffioen, A.W.; Fayad, Z.A.; Mulder, W.J.M. Iron oxide core oil-in-water emulsions as a multifunctional nanoparticle platform for tumor targeting and imaging. Biomaterials 2009, 30, 6947–6954. [Google Scholar] [CrossRef]
- Gianella, A.; Jarzyna, P.A.; Mani, V.; Ramachandran, S.; Calcagno, C.; Tang, J.; Kann, B.; Dijk, W.J.R.; Thijssen, V.L.; Griffioen, A.W.; et al. Multifunctional Nanoemulsion Platform for Imaging Guided Therapy Evaluated in Experimental Cancer. ACS Nano 2011, 5, 4422–4433. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.-L.; Yoshida, T.; Kathuria-Prakash, N.; Zaki, I.H.; Varallyay, C.G.; Semple, S.I.; Saouaf, R.; Rigsby, C.K.; Stoumpos, S.; Whitehead, K.K.; et al. Multicenter Safety and Practice for Off-Label Diagnostic Use of Ferumoxytol in MRI. Radiology 2019, 293, 554–564. [Google Scholar] [CrossRef]
- Bangalore, S.; Maron David, J.; Stone Gregg, W.; Hochman Judith, S. Routine Revascularization Versus Initial Medical Therapy for Stable Ischemic Heart Disease. Circulation 2020, 142, 841–857. [Google Scholar] [CrossRef] [PubMed]
- Arbab-Zadeh, A.; Fuster, V. The Myth of “The Vulnerable Plaque”: Transitioning from a Focus on Individual Lesions to Atherosclerotic Disease Burden for Coronary Artery Disease Risk Assessment. J. Am. Coll. Cardiol. 2015, 65, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Arbab-Zadeh, A.; Fuster, V. From Detecting the Vulnerable Plaque to Managing the Vulnerable Patient: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 1582–1593. [Google Scholar] [CrossRef]
- Gonzales, J.; Kossatz, S.; Roberts, S.; Pirovano, G.; Brand, C.; Pérez-Medina, C.; Donabedian, P.; de la Cruz, M.J.; Mulder, W.J.M.; Reiner, T. Nanoemulsion-Based Delivery of Fluorescent PARP Inhibitors in Mouse Models of Small Cell Lung Cancer. Bioconjug. Chem. 2018, 29, 3776–3782. [Google Scholar] [CrossRef]
- Calcagno, V.; Vecchione, R.; Quagliariello, V.; Marzola, P.; Busato, A.; Giustetto, P.; Profeta, M.; Gargiulo, S.; Cicco, C.D.; Yu, H.; et al. Oil Core–PEG Shell Nanocarriers for In Vivo MRI Imaging. Adv. Healthc. Mater. 2019, 1801313. [Google Scholar] [CrossRef]
- Veronese, F.M.; Mero, A. The impact of PEGylation on biological therapies. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2008, 22, 315–329. [Google Scholar] [CrossRef]
- Liu, F.; Liu, D. Long-circulating emulsions (oil-in-water) as carriers for lipophilic drugs. Pharm. Res. 1995, 12, 1060–1064. [Google Scholar] [CrossRef] [PubMed]
- Alayoubi, A.; Alqahtani, S.; Kaddoumi, A.; Nazzal, S. Effect of PEG surface conformation on anticancer activity and blood circulation of nanoemulsions loaded with tocotrienol-rich fraction of palm oil. AAPS J. 2013, 15, 1168–1179. [Google Scholar] [CrossRef]
- Ganta, S.; Sharma, P.; Paxton, J.W.; Baguley, B.C.; Garg, S. Pharmacokinetics and pharmacodynamics of chlorambucil delivered in long-circulating nanoemulsion. J. Drug Target. 2010, 18, 125–133. [Google Scholar] [CrossRef]
- Devalapally, H.; Silchenko, S.; Zhou, F.; McDade, J.; Goloverda, G.; Owen, A.; Hidalgo, I.J. Evaluation of a nanoemulsion formulation strategy for oral bioavailability enhancement of danazol in rats and dogs. J. Pharm. Sci. 2013, 102, 3808–3815. [Google Scholar] [CrossRef][Green Version]
- Cheng, Y.; Liu, M.; Hu, H.; Liu, D.; Zhou, S. Development, Optimization, and Characterization of PEGylated Nanoemulsion of Prostaglandin E1 for Long Circulation. AAPS PharmSciTech 2015, 17, 409–417. [Google Scholar] [CrossRef]
- Hak, S.; Helgesen, E.; Hektoen, H.H.; Huuse, E.M.; Jarzyna, P.A.; Mulder, W.J.M.; Haraldseth, O.; de Lange Davies, C. The effect of nanoparticle polyethylene glycol surface density on ligand-directed tumor targeting studied in vivo by dual modality imaging. ACS Nano 2012, 6, 5648–5658. [Google Scholar] [CrossRef]
- Li, S.-D.; Huang, L. Stealth nanoparticles: High density but sheddable PEG is a key for tumor targeting. J. Control. Release Off. J. Control. Release Soc. 2010, 145, 178–181. [Google Scholar] [CrossRef]
- Falcone, C.; Lucibello, S.; Mazzucchelli, I.; Bozzini, S.; D’Angelo, A.; Schirinzi, S.; Totaro, R.; Falcone, R.; Bondesan, M.; Pelissero, G. Galectin-3 plasma levels and coronary artery disease: A new possible biomarker of acute coronary syndrome. Int. J. Immunopathol. Pharmacol. 2011, 24, 905–913. [Google Scholar] [CrossRef]
- Hemadou, A.; Laroche-Traineau, J.; Antoine, S.; Mondon, P.; Fontayne, A.; Le Priol, Y.; Claverol, S.; Sanchez, S.; Cerutti, M.; Ottones, F.; et al. An innovative flow cytometry method to screen human scFv-phages selected by in vivo phage-display in an animal model of atherosclerosis. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Hemadou, A.; Giudicelli, V.; Smith, M.L.; Lefranc, M.-P.; Duroux, P.; Kossida, S.; Heiner, C.; Hepler, N.L.; Kuijpers, J.; Groppi, A.; et al. Pacific Biosciences Sequencing and IMGT/HighV-QUEST Analysis of Full-Length Single Chain Fragment Variable from an In Vivo Selected Phage-Display Combinatorial Library. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef]
- Ding, J.; Wang, Y.; Ma, M.; Zhang, Y.; Lu, S.; Jiang, Y.; Qi, C.; Luo, S.; Dong, G.; Wen, S.; et al. CT/fluorescence dual-modal nanoemulsion platform for investigating atherosclerotic plaques. Biomaterials 2013, 34, 209–216. [Google Scholar] [CrossRef]
- Hyafil, F.; Cornily, J.-C.; Feig, J.E.; Gordon, R.; Vucic, E.; Amirbekian, V.; Fisher, E.A.; Fuster, V.; Feldman, L.J.; Fayad, Z.A. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nat. Med. 2007, 13, 636–641. [Google Scholar] [CrossRef]
- Briley-Saebo, K.C.; Amirbekian, V.; Mani, V.; Aguinaldo, J.G.S.; Vucic, E.; Carpenter, D.; Amirbekian, S.; Fayad, Z.A. Gadolinium mixed-micelles: Effect of the amphiphile on in vitro and in vivo efficacy in apolipoprotein E knockout mouse models of atherosclerosis. Magn. Reson. Med. 2006, 56, 1336–1346. [Google Scholar] [CrossRef]
- Senders, M.L.; Hernot, S.; Carlucci, G.; van de Voort, J.C.; Fay, F.; Calcagno, C.; Tang, J.; Alaarg, A.; Zhao, Y.; Ishino, S.; et al. Nanobody-Facilitated Multiparametric PET/MRI Phenotyping of Atherosclerosis. JACC Cardiovasc. Imaging 2019, 12, 2015–2026. [Google Scholar] [CrossRef]
- Broisat, A.; Hernot, S.; Toczek, J.; De Vos, J.; Riou, L.M.; Martin, S.; Ahmadi, M.; Thielens, N.; Wernery, U.; Caveliers, V.; et al. Nanobodies targeting mouse/human VCAM1 for the nuclear imaging of atherosclerotic lesions. Circ. Res. 2012, 110, 927–937. [Google Scholar] [CrossRef]
- Jacobin-Valat, M.-J.; Laroche-Traineau, J.; Larivière, M.; Mornet, S.; Sanchez, S.; Biran, M.; Lebaron, C.; Boudon, J.; Lacomme, S.; Cérutti, M.; et al. Nanoparticles functionalised with an anti-platelet human antibody for in vivo detection of atherosclerotic plaque by magnetic resonance imaging. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 927–937. [Google Scholar] [CrossRef]
- Oumzil, K.; Ramin, M.A.; Lorenzato, C.; Hémadou, A.; Laroche, J.; Jacobin-Valat, M.J.; Mornet, S.; Roy, C.-E.; Kauss, T.; Gaudin, K.; et al. Solid Lipid Nanoparticles for Image-Guided Therapy of Atherosclerosis. Bioconjug. Chem. 2016, 27, 569–575. [Google Scholar] [CrossRef]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Prévot, G.; Mornet, S.; Lorenzato, C.; Kauss, T.; Adumeau, L.; Gaubert, A.; Baillet, J.; Barthélémy, P.; Clofent-Sanchez, G.; Crauste-Manciet, S. Data on iron oxide core oil-in-water nanoemulsions for atherosclerosis imaging. Data Brief 2017, 15, 876–881. [Google Scholar] [CrossRef]
- van Ewijk, G.A.; Vroege, G.J.; Philipse, A.P. Convenient preparation methods for magnetic colloids. J. Magn. Magn. Mater. 1999, 201, 31–33. [Google Scholar] [CrossRef]
- Zachiu, C.; Denis de Senneville, B.; Moonen, C.; Ries, M. A framework for the correction of slow physiological drifts during MR-guided HIFU therapies: Proof of concept. Med. Phys. 2015, 42, 4137–4148. [Google Scholar] [CrossRef]
- Zachiu, C.; Papadakis, N.; Ries, M.; Moonen, C.; Denis De Senneville, B. An improved optical flow tracking technique for real-time MR-guided beam therapies in moving organs. Phys. Med. Biol. 2015, 60, 9003. [Google Scholar] [CrossRef]
- Dahnke, H.; Schaeffter, T. Limits of detection of SPIO at 3.0 T using T2 relaxometry. Magn. Reson. Med. 2005, 53, 1202–1206. [Google Scholar] [CrossRef]
- Caspar-Bauguil, S.; Maestre, N.; Segafredo, C.; Galinier, A.; Garcia, J.; Prost, M.; Périquet, B.; Pénicaud, L.; Salvayre, R.; Casteilla, L. Evaluation of whole antioxidant defenses of human mononuclear cells by a new in vitro biological test: Lack of correlation between erythrocyte and mononuclear cell resistance to oxidative stress. Clin. Biochem. 2009, 42, 510–514. [Google Scholar] [CrossRef]
| NE | NE–PEG2000 | NE–PEG3400–Maleimide | NE–PEG2000/3400–Maleimide | |
|---|---|---|---|---|
| Formulation Number | (#1) | (#2) | (#3) | (#4) |
| Mean diameter (nm) ± SD (n = 3) | 175.8 ± 1.0 | 190.9 ± 2.2 | 197.2 ± 4.6 | 191.0 ± 2.4 |
| Polydispersity index | 0.108 | 0.134 | 0.115 | 0.097 |
| NE | NE–PEG2000 | NE–PEG3400–Maleimide | NE–PEG2000/3400–Maleimide | NE–PEG3400–Maleimide-P3 | |
|---|---|---|---|---|---|
| Formulation Number | (#1) | (#2) | (#3) | (#4) | (#5) |
| Blood half-life (min) ± SD (n = 3) * (n = 1) | <1 | 7.2 ± 3.4 | 14.1 ± 2.5 | 138.7 ± 11.2 | 103 * |
| NE | NE–Tocopherol | NE–PEG3400–Maleimide–SPIO | NE–PEG3400–Maleimide–Tocopherol | NE–PEG3400–Maleimide–SPIO–Tocopherol | |
|---|---|---|---|---|---|
| Formulation Number | (#6) | (#7) | (#8) | (#9) | (#10) |
| Miglyol 840 (%) | 20 | 15 | 20 | 15 | 15 |
| Alpha-tocopherol (%) | - | 5 | - | 5 | 5 |
| Tween 80 (%) | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Lipoid 80 (%) | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 |
| Milli-Q water (%) | qs 100 | qs 100 | qs 100 | qs 100 | qs 100 |
| SPIO (µmol/mL) | - | - | 12.5 | - | 12.5 |
| Lipid–PEG3400–maleimide (µmol/mL) | - | - | 4.4 | 4.4 | 4.4 |
| NE | NE–Tocopherol | NE–PEG3400–Maleimide–SPIO | NE–PEG3400–Maleimide–Tocopherol | NE–PEG3400–Maleimide–SPIO–Tocopherol | |
|---|---|---|---|---|---|
| Formulation Number | (#6) | (#7) | (#8) | (#9) | (#10) |
| Mean diameter (nm) ± SD (n = 3) | 173.5 ± 0.8 | 171.7 ± 0.5 | 162.6 ± 2 | 181.3 ± 1.8 | 213.7 ± 1.2 |
| Polydispersity index | 0.140 | 0.152 | 0.123 | 0.163 | 0.255 |
| NE | NE–PEG2000 | NE–PEG3400–Maleimide | NE–PEG2000/3400–Maleimide | NE–PEG3400–Maleimide–P3 | |
|---|---|---|---|---|---|
| Formulation Number | (#1) | (#2) | (#3) | (#4) | (#5: NE-P3) |
| Miglyol 840 (%) | 20 | 20 | 20 | 20 | 20 |
| Tween 80 (%) | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Lipoid 80 (%) | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 |
| Milli-Q water (%) | qs 100 | qs 100 | qs 100 | qs 100 | qs 100 |
| SPIO (µmol/mL) | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 |
| Lipid–PEG2000 (µmol/mL) | - | 5 | - | 3.8 | - |
| Lipid–PEG3400–maleimide (µmol/mL) | - | - | 5 | 1.2 | 5 |
| P3 antibody (nmol/mL) | - | - | - | - | 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonnet, S.; Prévot, G.; Mornet, S.; Jacobin-Valat, M.-J.; Mousli, Y.; Hemadou, A.; Duttine, M.; Trotier, A.; Sanchez, S.; Duonor-Cérutti, M.; et al. A Nano-Emulsion Platform Functionalized with a Fully Human scFv-Fc Antibody for Atheroma Targeting: Towards a Theranostic Approach to Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 5188. https://doi.org/10.3390/ijms22105188
Bonnet S, Prévot G, Mornet S, Jacobin-Valat M-J, Mousli Y, Hemadou A, Duttine M, Trotier A, Sanchez S, Duonor-Cérutti M, et al. A Nano-Emulsion Platform Functionalized with a Fully Human scFv-Fc Antibody for Atheroma Targeting: Towards a Theranostic Approach to Atherosclerosis. International Journal of Molecular Sciences. 2021; 22(10):5188. https://doi.org/10.3390/ijms22105188
Chicago/Turabian StyleBonnet, Samuel, Geoffrey Prévot, Stéphane Mornet, Marie-Josée Jacobin-Valat, Yannick Mousli, Audrey Hemadou, Mathieu Duttine, Aurélien Trotier, Stéphane Sanchez, Martine Duonor-Cérutti, and et al. 2021. "A Nano-Emulsion Platform Functionalized with a Fully Human scFv-Fc Antibody for Atheroma Targeting: Towards a Theranostic Approach to Atherosclerosis" International Journal of Molecular Sciences 22, no. 10: 5188. https://doi.org/10.3390/ijms22105188
APA StyleBonnet, S., Prévot, G., Mornet, S., Jacobin-Valat, M.-J., Mousli, Y., Hemadou, A., Duttine, M., Trotier, A., Sanchez, S., Duonor-Cérutti, M., Crauste-Manciet, S., & Clofent-Sanchez, G. (2021). A Nano-Emulsion Platform Functionalized with a Fully Human scFv-Fc Antibody for Atheroma Targeting: Towards a Theranostic Approach to Atherosclerosis. International Journal of Molecular Sciences, 22(10), 5188. https://doi.org/10.3390/ijms22105188







