Tear Proteomics Study of Dry Eye Disease: Which Eye Do You Adopt as the Representative Eye for the Study?
Abstract
1. Introduction
2. Results
2.1. Binocular Clinical Performance
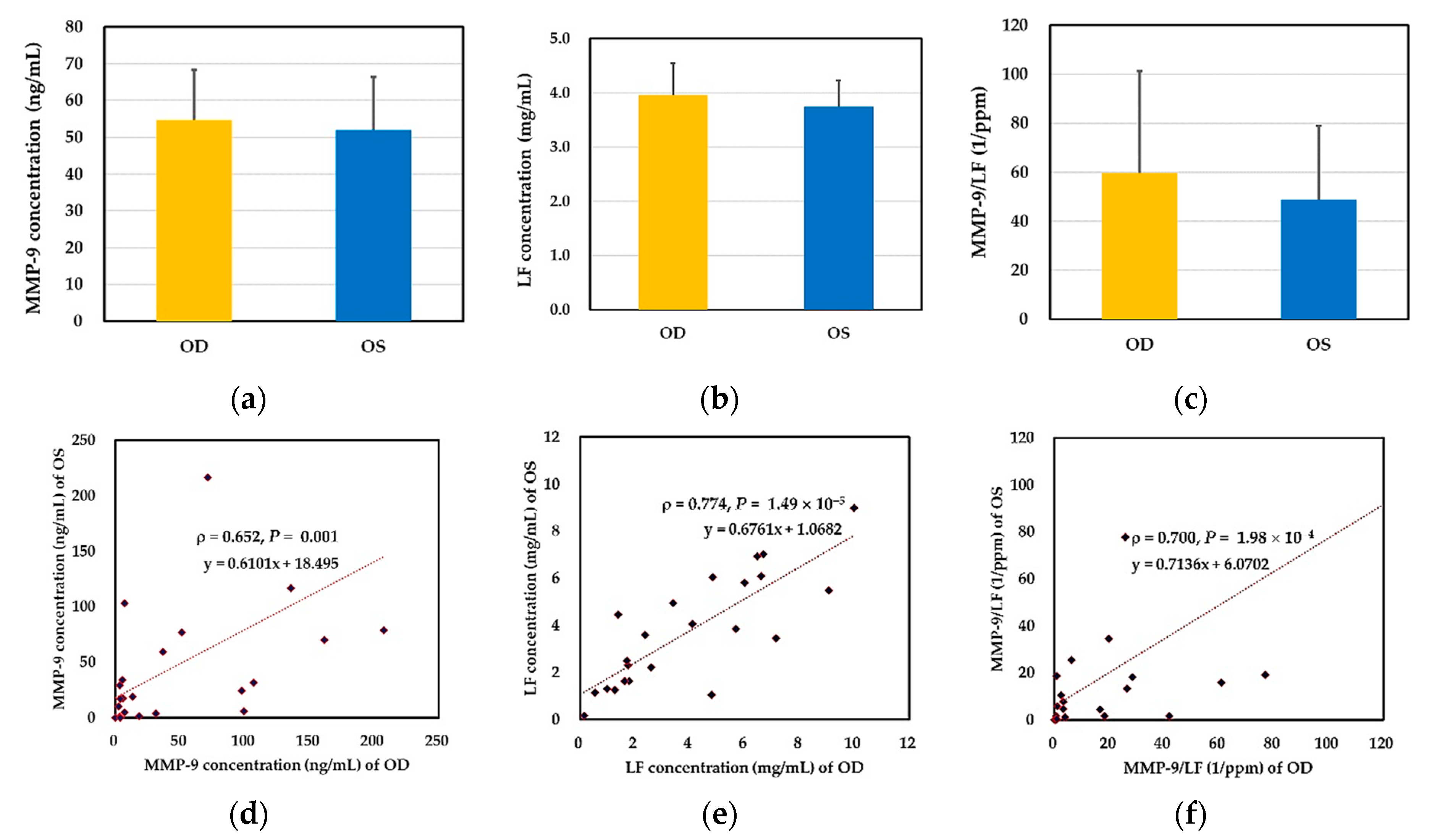
2.2. Binocular Tear Biochemical Markers
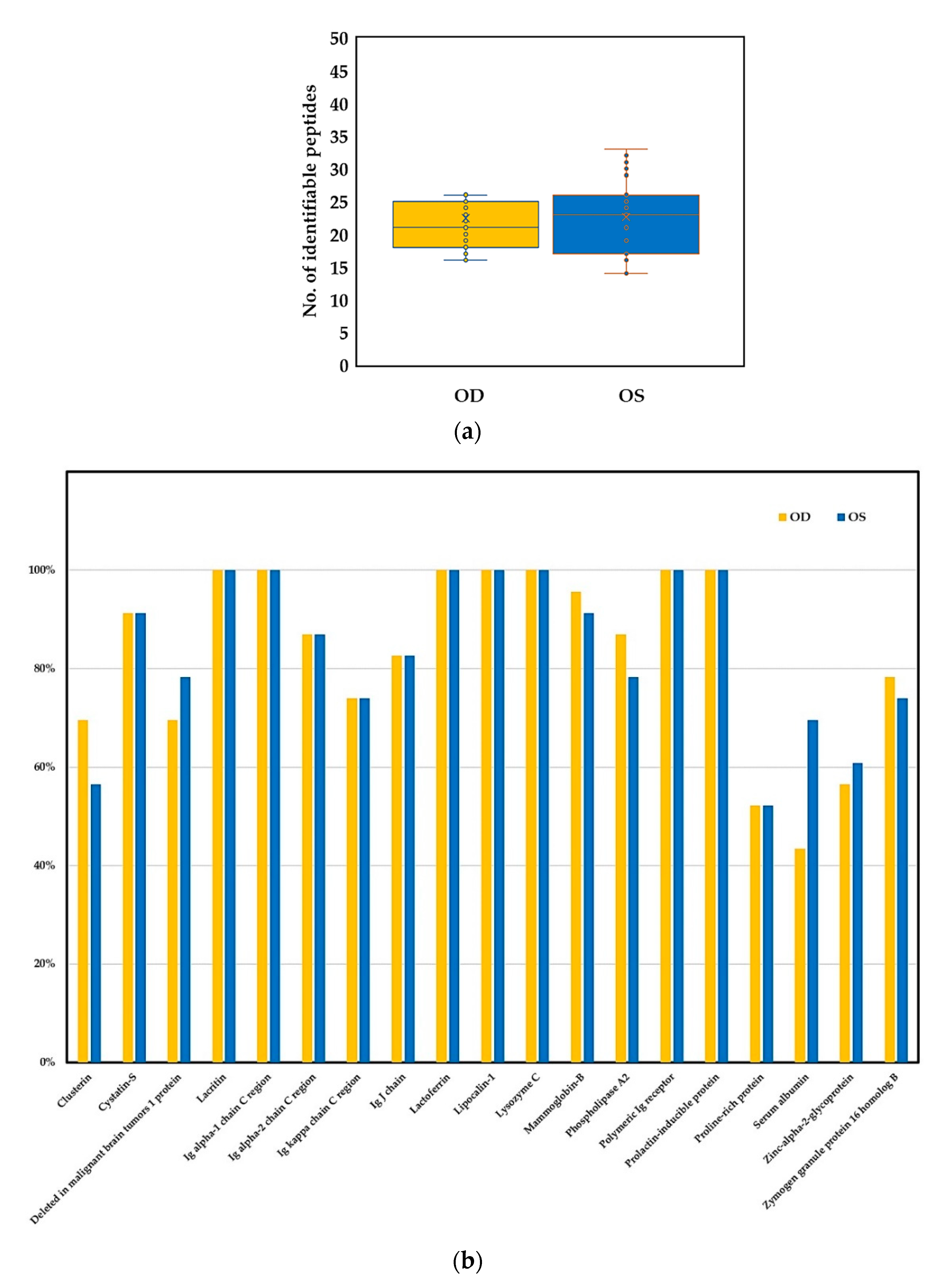
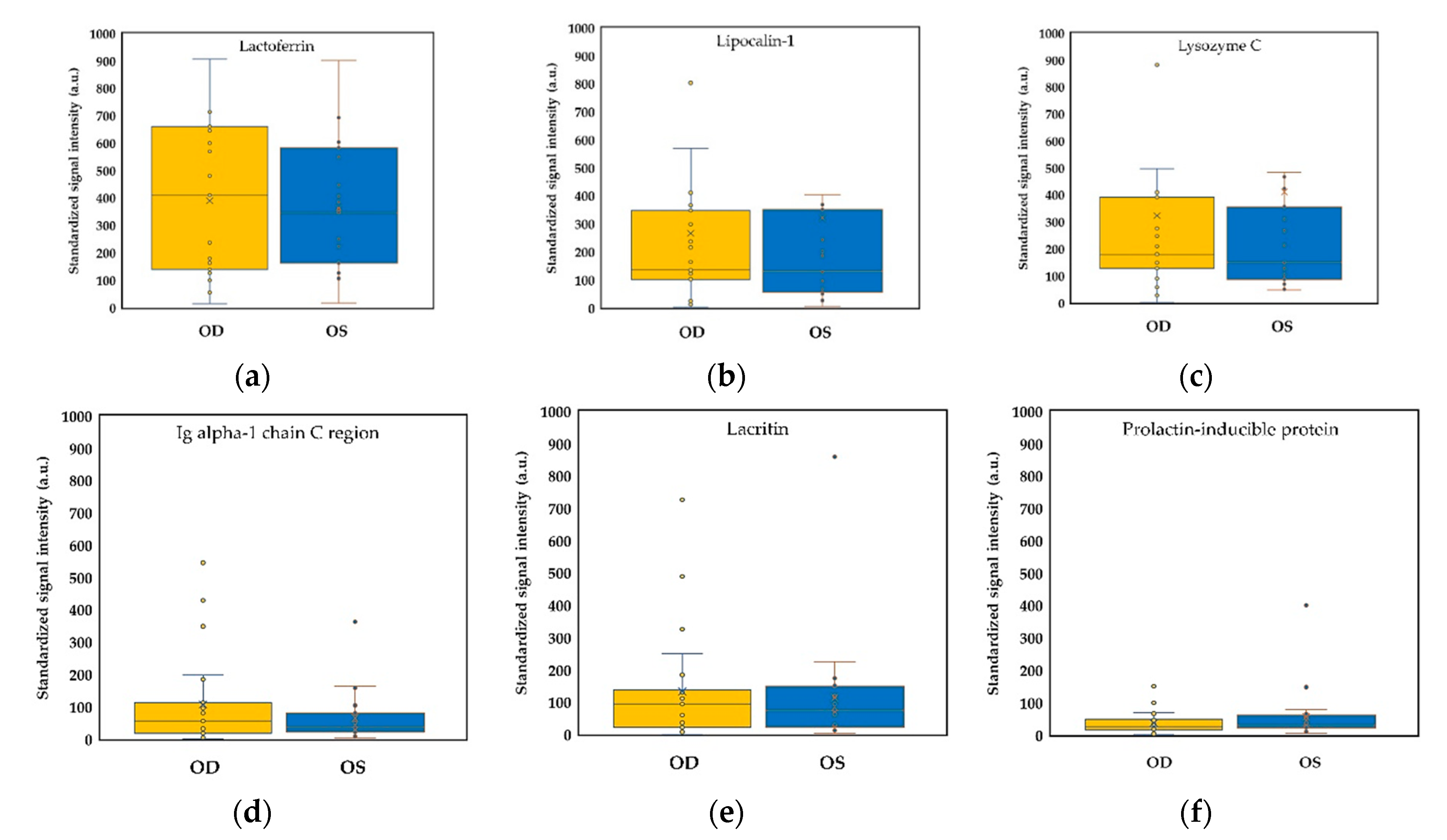
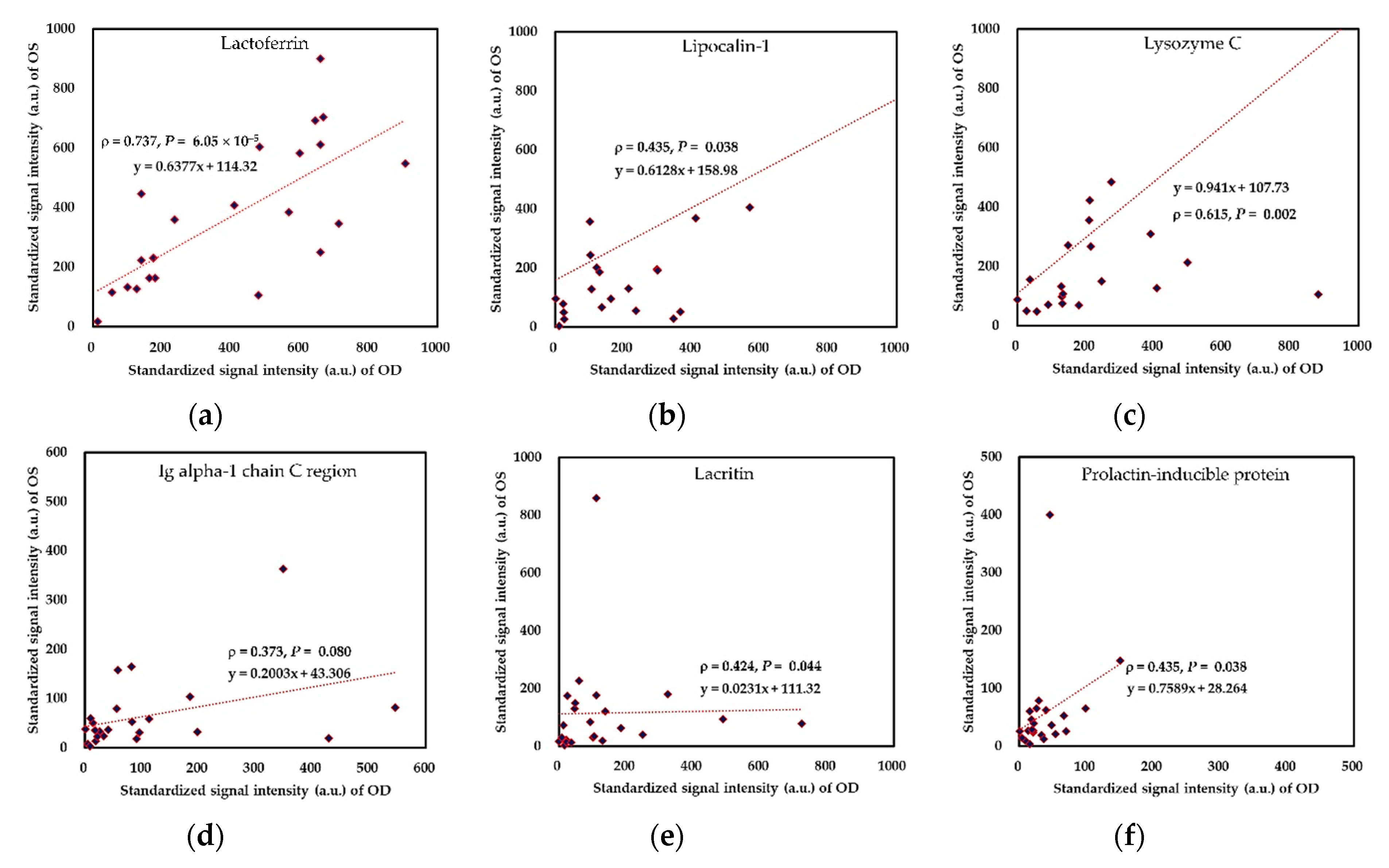
2.3. Binocular Tear Proteomic Spectra
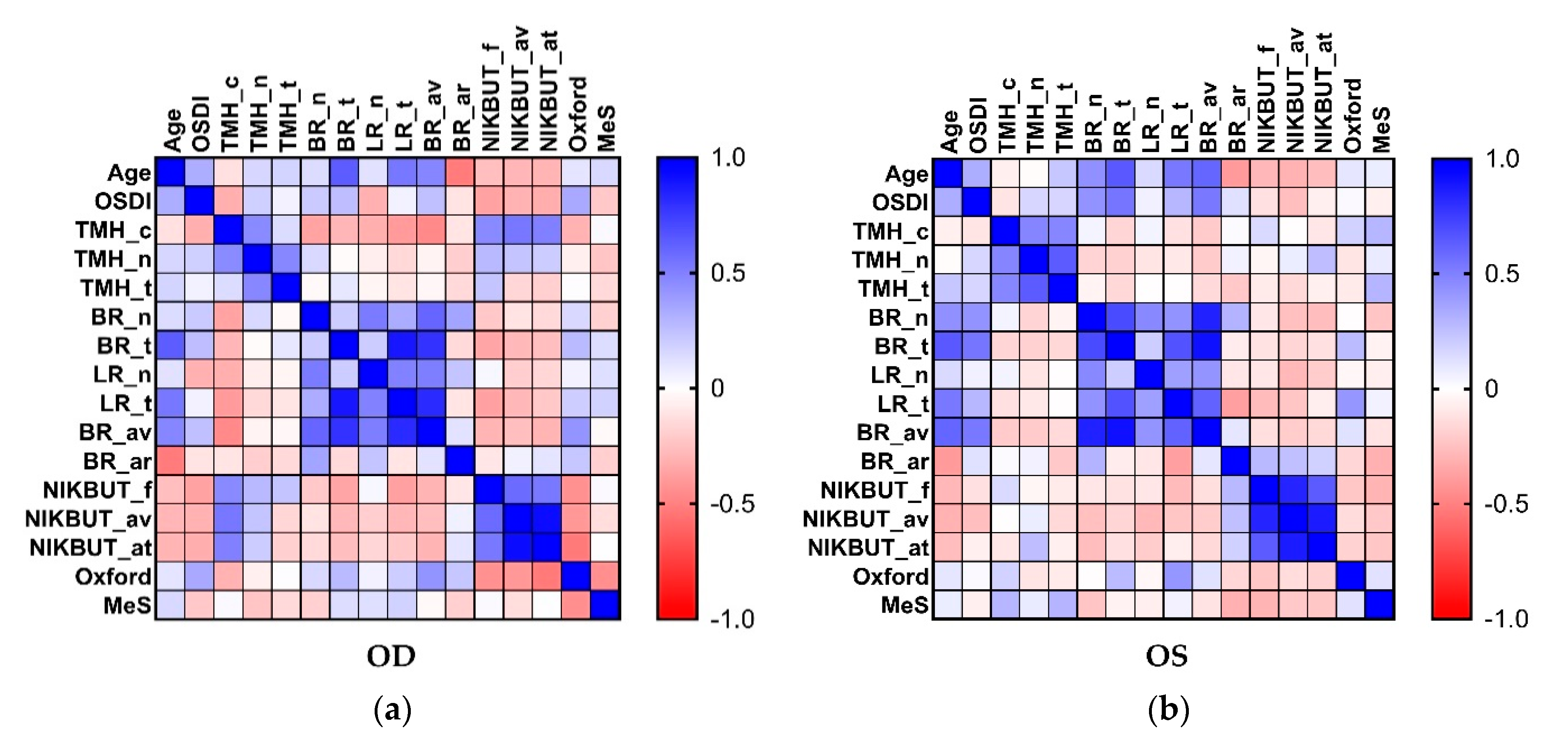
2.4. Association between Clinical Parameters in the Ipsilateral Eye
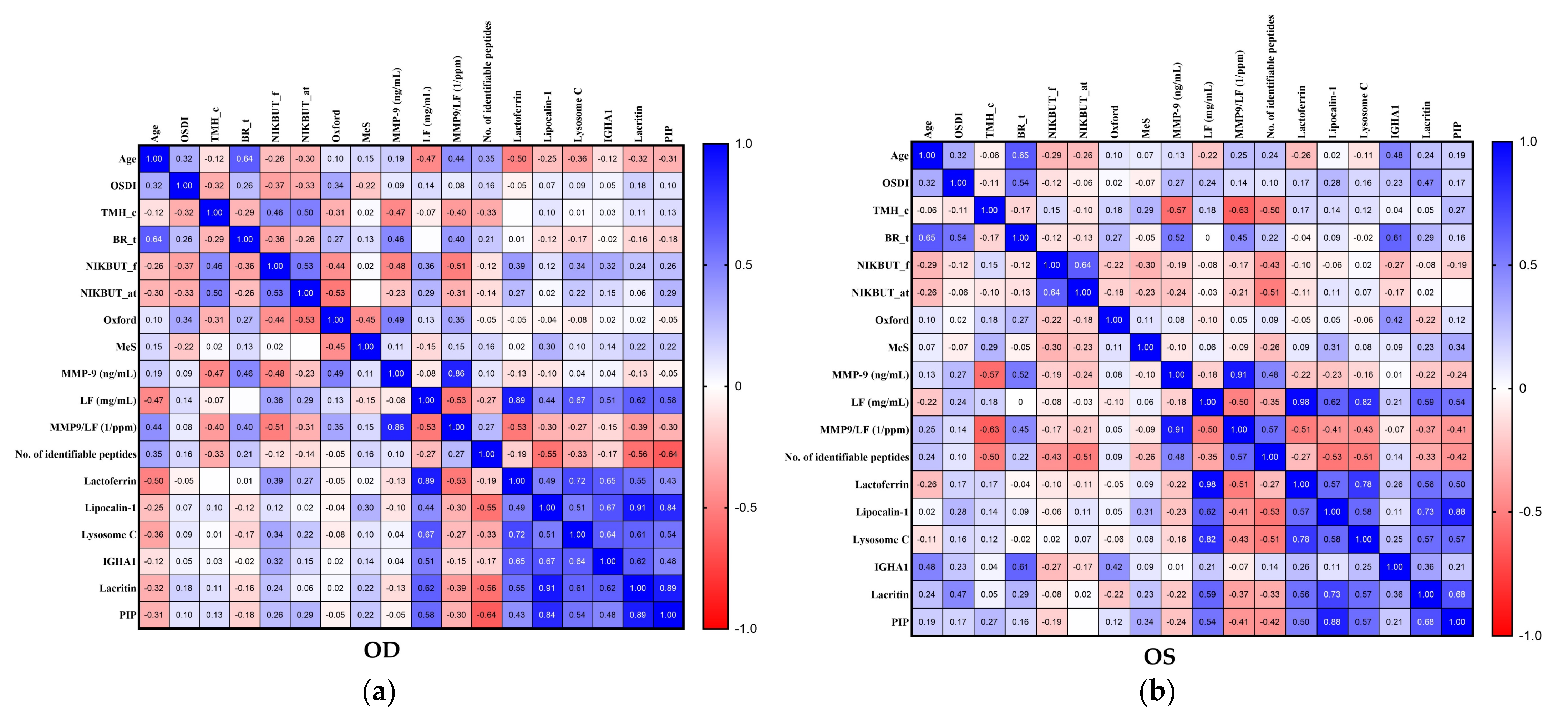
2.5. Association between Selected Clinical Parameters and Tear Biomarkers in the Ipsilateral Eye
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Assessment Protocol and Tear Sampling
4.3. Ocular Surface Homeostatic Markers of DED
4.3.1. Quantification of Tear Secretion
4.3.2. Determination of Ocular Surface Inflammation
4.3.3. Assessment of Tear Film Stability
4.3.4. Evaluation of Meibomian Gland Dropout
4.4. Tear Biochemical Markers of DED
4.4.1. Measurement of the Lactoferrin Concentration of Tear Fluid
4.4.2. Measurement of the MMP-9 Concentration of Tear Fluid
4.5. Analysis of Tear Proteome
4.5.1. In-Solution Digestion of Tear Proteins
4.5.2. LC-MS/MS Analysis
4.6. Determination of Sample Size
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CGMH | Chang Gung Memorial Hospital |
| DED | dry eye disease |
| ELISA | enzyme-linked immunosorbent assay |
| LC-MS/MS | liquid chromatography coupled tandem mass spectrometry |
| LF | lactoferrin |
| MMP-9 | matrix metallopeptidase 9 |
| NIKBUT | non-invasive keratographic break-up time |
| NIKBUT_av | non-invasive keratographic average break-up time |
| NIKBUT_f | non-invasive keratographic first break-up time |
| OSDI | ocular surface disease index |
| R-scan | ocular surface redness scan |
| TMH | tear meniscus height |
References
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Dana, R.; Bradley, J.L.; Guerin, A.; Pivneva, I.; Stillman, I.Ö.; Evans, A.M.; Schaumberg, D.A. Estimated Prevalence and Incidence of Dry Eye Disease Based on Coding Analysis of a Large, All-age United States Health Care System. Am. J. Ophthalmol. 2019, 202, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K.; Yokoi, N.; Shimazaki, J.; Watanabe, H.; Dogru, M.; Yamada, M.; Kinoshita, S.; Kim, H.M.; Tchah, H.W.; Hyon, J.Y.; et al. New Perspectives on Dry Eye Definition and Diagnosis: A Consensus Report by the Asia Dry Eye Society. Ocul. Surf. 2017, 15, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Dogru, M.; Kawashima, M.; Nakamura, S.; Tsubota, K. Advances in the diagnosis and treatment of dry eye. Prog. Retin. Eye Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Okumura, Y.; Inomata, T.; Iwata, N.; Sung, J.; Fujimoto, K.; Fujio, K.; Midorikawa-Inomata, A.; Miura, M.; Akasaki, Y.; Murakami, A. A Review of Dry Eye Questionnaires: Measuring Patient-Reported Outcomes and Health-Related Quality of Life. Diagnostics 2020, 10, 559. [Google Scholar] [CrossRef]
- Vu, C.H.V.; Kawashima, M.; Yamada, M.; Suwaki, K.; Uchino, M.; Shigeyasu, C.; Hiratsuka, Y.; Yokoi, N.; Tsubota, K. Influence of Meibomian Gland Dysfunction and Friction-Related Disease on the Severity of Dry Eye. Ophthalmology 2018, 125, 1181–1188. [Google Scholar] [CrossRef]
- Cho, W.H.; Fang, P.C.; Yu, H.J.; Lin, P.W.; Huang, H.M.; Kuo, M.T. Analysis of tear film spatial instability for pediatric myopia under treatment. Sci. Rep. 2020, 10, 14789. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.Y.; Seo, K.Y.; Kim, T.I.; Chin, H.S.; Jung, J.W. Location and pattern of non-invasive keratographic tear film break-up according to dry eye disease subtypes. Acta Ophthalmol. 2019, 97, e1089–e1097. [Google Scholar] [CrossRef]
- Baek, J.; Doh, S.H.; Chung, S.K. Comparison of Tear Meniscus Height Measurements Obtained With the Keratograph and Fourier Domain Optical Coherence Tomography in Dry Eye. Cornea 2015, 34, 1209–1213. [Google Scholar] [CrossRef]
- Wei, A.; Le, Q.; Hong, J.; Wang, W.; Wang, F.; Xu, J. Assessment of Lower Tear Meniscus. Optom Vis. Sci. 2016, 93, 1420–1425. [Google Scholar] [CrossRef]
- Downie, L.E.; Keller, P.R.; Vingrys, A.J. Assessing ocular bulbar redness: A comparison of methods. Ophthalmic Physiol. Opt. 2016, 36, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Pérez Bartolomé, F.; Martínez de la Casa, J.M.; Arriola Villalobos, P.; Fernández Pérez, C.; Polo, V.; Sánchez Jean, R.; García Feijoó, J. Ocular Redness Measured with the Keratograph 5M in Patients Using Anti-Glaucoma Eye Drops. Semin. Ophthalmol. 2018, 33, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Vigo, L.; Taroni, L.; Bernabei, F.; Pellegrini, M.; Sebastiani, S.; Mercanti, A.; Di Stefano, N.; Scorcia, V.; Carones, F.; Giannaccare, G. Ocular Surface Workup in Patients with Meibomian Gland Dysfunction Treated with Intense Regulated Pulsed Light. Diagnostics 2019, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Robin, M.; Liang, H.; Rabut, G.; Augstburger, E.; Baudouin, C.; Labbé, A. The Role of Meibography in the Diagnosis of Meibomian Gland Dysfunction in Ocular Surface Diseases. Transl. Vis. Sci. Technol. 2019, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C.; Aragona, P.; Van Setten, G.; Rolando, M.; Irkeç, M.; Benítez del Castillo, J.; Geerling, G.; Labetoulle, M.; Bonini, S. Diagnosing the severity of dry eye: A clear and practical algorithm. Br. J. Ophthalmol. 2014, 98, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.K.; Lin, I.C.; Chien, L.N.; Lin, T.Y.; How, Y.T.; Chen, K.H.; Dusting, G.J.; Tseng, C.L. Dry Eye Disease: A Review of Epidemiology in Taiwan, and its Clinical Treatment and Merits. J. Clin. Med. 2019, 8, 1227. [Google Scholar] [CrossRef] [PubMed]
- Arita, R.; Mizoguchi, T.; Kawashima, M.; Fukuoka, S.; Koh, S.; Shirakawa, R.; Suzuki, T.; Morishige, N. Meibomian Gland Dysfunction and Dry Eye Are Similar but Different Based on a Population-Based Study: The Hirado-Takushima Study in Japan. Am. J. Ophthalmol. 2019, 207, 410–418. [Google Scholar] [CrossRef]
- Perumal, N.; Funke, S.; Pfeiffer, N.; Grus, F.H. Proteomics analysis of human tears from aqueous-deficient and evaporative dry eye patients. Sci. Rep. 2016, 6, 29629. [Google Scholar] [CrossRef]
- Nebbioso, M.; Del Regno, P.; Gharbiya, M.; Sacchetti, M.; Plateroti, R.; Lambiase, A. Analysis of the Pathogenic Factors and Management of Dry Eye in Ocular Surface Disorders. Int. J. Mol. Sci. 2017, 18, 1764. [Google Scholar] [CrossRef]
- Koh, S.; Ikeda, C.; Watanabe, S.; Oie, Y.; Soma, T.; Watanabe, H.; Maeda, N.; Nishida, K. Effect of non-invasive tear stability assessment on tear meniscus height. Acta Ophthalmol. 2015, 93, e135–e139. [Google Scholar] [CrossRef]
- Kuo, M.-T.; Fang, P.-C.; Chao, T.-L.; Chen, A.; Lai, Y.-H.; Huang, Y.-T.; Tseng, C.-Y. Tear Proteomics Approach to Monitoring Sjögren Syndrome or Dry Eye Disease. Int. J. Mol. Sci. 2019, 20, 1932. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Beuerman, R.W.; Chan, C.M.; Zhao, S.Z.; Li, X.R.; Yang, H.; Tong, L.; Liu, S.; Stern, M.E.; Tan, D. Identification of tear fluid biomarkers in dry eye syndrome using iTRAQ quantitative proteomics. J. Proteome Res. 2009, 8, 4889–4905. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Thangavelu, M.; Zhang, L.; Green, K.B.; Nichols, K.K. iTRAQ Quantitative Proteomics in the Analysis of Tears in Dry Eye Patients. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5052. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Sheng, M.; Li, J.; Yan, G.; Lin, A.; Li, M.; Wang, W.; Chen, Y. Tear proteomic analysis of Sjögren syndrome patients with dry eye syndrome by two-dimensional-nano-liquid chromatography coupled with tandem mass spectrometry. Sci. Rep. 2014, 4, 5772. [Google Scholar] [CrossRef]
- Jung, J.H.; Ji, Y.W.; Hwang, H.S.; Oh, J.W.; Kim, H.C.; Lee, H.K.; Kim, K.P. Proteomic analysis of human lacrimal and tear fluid in dry eye disease. Sci. Rep. 2017, 7, 13363. [Google Scholar] [CrossRef]
- Soria, J.; Acera, A.; Merayo-Lloves, J.; Durán, J.A.; González, N.; Rodriguez, S.; Bistolas, N.; Schumacher, S.; Bier, F.F.; Peter, H.; et al. Tear proteome analysis in ocular surface diseases using label-free LC-MS/MS and multiplexed-microarray biomarker validation. Sci. Rep. 2017, 7, 17478. [Google Scholar] [CrossRef]
- Markoulli, M.; Papas, E.; Petznick, A.; Holden, B. Validation of the Flush Method as an Alternative to Basal or Reflex Tear Collection. Curr. Eye Res. 2011, 36, 198–207. [Google Scholar] [CrossRef]
- Bron, A.J.; Evans, V.E.; Smith, J.A. Grading Of Corneal and Conjunctival Staining in the Context of Other Dry Eye Tests. Cornea 2003, 22, 640–650. [Google Scholar] [CrossRef]
- Schiffman, R.M. Reliability and Validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615. [Google Scholar] [CrossRef]
- Wu, S.; Hong, J.; Tian, L.; Cui, X.; Sun, X.; Xu, J. Assessment of Bulbar Redness with a Newly Developed Keratograph. Optom. Vis. Sci. 2015, 92, 892–899. [Google Scholar] [CrossRef]
- Mousavi, M.; Jesus, D.A.; Garaszczuk, I.K.; Szczesna-Iskander, D.H.; Iskander, D.R. The utility of measuring tear film break-up time for prescribing contact lenses. Contact Lens Anterior Eye 2018, 41, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Pult, H.; Riede-Pult, B.H. Non-contact meibography: Keep it simple but effective. Contact Lens Anterior Eye 2012, 35, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Sonobe, H.; Ogawa, Y.; Yamada, K.; Shimizu, E.; Uchino, Y.; Kamoi, M.; Saijo, Y.; Yamane, M.; Citterio, D.; Suzuki, K.; et al. A novel and innovative paper-based analytical device for assessing tear lactoferrin of dry eye patients. Ocul. Surf. 2019, 17, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Vagge, A.; Senni, C.; Bernabei, F.; Pellegrini, M.; Scorcia, V.; Traverso, C.E.; Giannaccare, G. Therapeutic Effects of Lactoferrin in Ocular Diseases: From Dry Eye Disease to Infections. Int. J. Mol. Sci. 2020, 21, 6668. [Google Scholar] [CrossRef]
- Lanza, N.L.; Valenzuela, F.; Perez, V.L.; Galor, A. The Matrix Metalloproteinase 9 Point-of-Care Test in Dry Eye. Ocul. Surf. 2016, 14, 189–195. [Google Scholar] [CrossRef]
- Jian, S.F.; Hsiao, C.C.; Chen, S.Y.; Weng, C.C.; Kuo, T.L.; Wu, D.C.; Hung, W.C.; Cheng, K.H. Utilization of Liquid Chromatography Mass Spectrometry Analyses to Identify LKB1-APC Interaction in Modulating Wnt/-Catenin Pathway of Lung Cancer Cells. Mol. Cancer Res. 2014, 12, 622–635. [Google Scholar] [CrossRef]


| OD | OS | p Value | ||
|---|---|---|---|---|
| Clinical Parameters a | OD vs. OS b | OD ∝ OS c | ||
| Number of eyes | 23 | 23 | ||
| Tear meniscus height (mm) | ||||
| Nasal meniscus | 0.36 ± 0.17 | 0.40 ± 0.21 | 0.3244 | 0.0017 |
| Central meniscus | 0.20 ± 0.08 | 0.22 ± 0.10 | 0.4349 | <0.0001 |
| Temporal meniscus | 0.30 ± 0.13 | 0.33 ± 0.13 | 0.2253 | 0.1048 |
| Ocular redness score | ||||
| Nasal bulbar redness | 1.1 ± 0.4 | 1.1 ± 0.5 | 0.9401 | 0.1542 |
| Temporal bulbar redness | 1.1 ± 0.4 | 1.2 ± 0.4 | 0.3272 | <0.0001 |
| Nasal limbal redness | 0.8 ± 0.4 | 0.7 ± 0.4 | 0.7266 | 0.1750 |
| Temporal limbal redness | 0.7 ± 0.4 | 0.9 ± 0.4 | 0.2100 | 0.0004 |
| Mean redness | 1.1 ± 0.4 | 1.1 ± 0.4 | 0.8729 | <0.0001 |
| Assessable area | 8.0 ± 2.9 | 8.5 ± 2.6 | 0.3833 | 0.0002 |
| Tear break-up time (s) | ||||
| NIKBUT_f | 6.8 ± 3.5 | 8.9 ± 5.7 | 0.3182 | 0.1296 |
| NIKBUT_av | 10.6 ± 5.1 | 12.8 ± 5.7 | 0.0233 * | <0.0001 |
| Assessable time | 15.3 ± 7.6 | 17.5 ± 7.3 | 0.0239 * | <0.0001 |
| Meiboscale | 1.3 ± 0.8 | 1.4 ± 0.8 | >0.9999 | <0.0001 |
| Oxford staining score | 0.8 ± 0.8 | 0.6 ± 0.8 | 0.5018 | 0.1226 |
| Age | OSDI | TMH_c | BR_t | NIKBUT_f | NIKBUT_at | Oxford | MeS | |
|---|---|---|---|---|---|---|---|---|
| Right Eye | ρ (p-Value) | ρ (p-Value) | ρ (p-Value) | ρ (p-Value) | ρ (p-Value) | ρ (p-Value) | ρ (p-Value) | ρ (p-Value) |
| MMP-9 (ng/mL) | 0.19 (0.3790) | 0.09 (0.6757) | −0.47 (0.0248) | 0.46 (0.0254) | −0.48 (0.0202) | −0.23 (0.2826) | 0.49 (0.0167) | 0.11 (0.6035) |
| LF (mg/mL) | −0.47 (0.0239) | 0.14 (0.5305) | −0.07 (0.7373) | 0.00 (0.9856) | 0.36 (0.0893) | 0.29 (0.1818) | 0.13 (0.5609) | −0.15 (0.5016) |
| MMP9/LF (1/ppm) | 0.44 (0.0367) | 0.08 (0.7260) | −0.40 (0.0618) | 0.40 (0.0571) | −0.51 (0.0139) | −0.31 (0.1504) | 0.35 (0.1058) | 0.15 (0.5015) |
| No. of identifiable peptides | 0.35 (0.1002) | 0.16 (0.4666) | −0.33 (0.1220) | 0.21 (0.3271) | −0.12 (0.5992) | −0.14 (0.5379) | −0.05 (0.8205) | 0.16 (0.4734) |
| Lactoferrin | −0.50 (0.0157) | −0.05 (0.8036) | 0.00 (0.9955) | 0.01 (0.9613) | 0.39 (0.0635) | 0.27 (0.2153) | −0.05 (0.8313) | 0.02 (0.9312) |
| Lipocalin-1 | −0.25 (0.2475) | 0.07 (0.7486) | 0.10 (0.6489) | −0.12 (0.5768) | 0.12 (0.5960) | 0.02 (0.9105) | −0.04 (0.8550) | 0.30 (0.1652) |
| Lysosome C | −0.36 (0.0887) | 0.09 (0.6953) | 0.01 (0.9588) | −0.17 (0.4367) | 0.34 (0.1096) | 0.22 (0.3064) | −0.08 (0.7253) | 0.10 (0.6611) |
| IGHA1 | −0.12 (0.5802) | 0.05 (0.8368) | 0.03 (0.8873) | −0.02 (0.9300) | 0.32 (0.1317) | 0.15 (0.5083) | 0.02 (0.9425) | 0.14 (0.5190) |
| Lacritin | −0.32 (0.1429) | 0.18 (0.4156) | 0.11 (0.6054) | −0.16 (0.4648) | 0.24 (0.2642) | 0.06 (0.7844) | 0.02 (0.9129) | 0.22 (0.3153) |
| PIP | −0.31 (0.1517) | 0.10 (0.6366) | 0.13 (0.5434) | −0.18 (0.4157) | 0.26 (0.2390) | 0.29 (0.1819) | −0.05 (0.8080) | 0.22 (0.3075) |
| PMIGR | −0.15 (0.5066) | 0.12 (0.5749) | −0.04 (0.8579) | 0.07 (0.7589) | 0.09 (0.6901) | 0.04 (0.8702) | 0.02 (0.9176) | 0.29 (0.1809) |
| Left Eye | ||||||||
| MMP-9 (ng/mL) | 0.13 (0.5481) | 0.27 (0.2134) | −0.57 (0.0046) | 0.52 (0.0112) | −0.19 (0.3757) | −0.24 (0.2763) | 0.08 (0.7255) | −0.10 (0.6646) |
| LF (mg/mL) | −0.22 (0.3220) | 0.24 (0.2756) | 0.18 (0.4224) | 0.00 (1.0000) | −0.08 (0.7215) | −0.03 (0.8908) | −0.10 (0.6440) | 0.06 (0.7809) |
| MMP9/LF (1/ppm) | 0.25 (0.2435) | 0.14 (0.5220) | −0.63 (0.0013) | 0.45 (0.0304) | −0.17 (0.4506) | −0.21 (0.3362) | 0.05 (0.8254) | −0.09 (0.6689) |
| No. of identifiable peptides | 0.24 (0.2793) | 0.10 (0.6388) | −0.50 (0.0144) | 0.22 (0.3148) | −0.43 (0.0406) | −0.51 (0.0122) | 0.09 (0.6666) | −0.26 (0.2293) |
| Lactoferrin | −0.26 (0.2259) | 0.17 (0.4354) | 0.17 (0.4424) | −0.04 (0.8733) | −0.10 (0.6608) | −0.11 (0.6123) | −0.05 (0.8244) | 0.09 (0.6832) |
| Lipocalin-1 | 0.02 (0.9144) | 0.28 (0.1922) | 0.14 (0.5176) | 0.09 (0.6900) | −0.06 (0.7820) | 0.11 (0.6308) | 0.05 (0.8332) | 0.31 (0.1517) |
| Lysosome C | −0.11 (0.6022) | 0.16 (0.4757) | 0.12 (0.6001) | −0.02 (0.9297) | 0.02 (0.9429) | 0.07 (0.7405) | −0.06 (0.7905) | 0.08 (0.7039) |
| IGHA1 | 0.48 (0.0191) | 0.23 (0.2982) | 0.04 (0.8558) | 0.61 (0.0021) | −0.27 (0.2165) | −0.17 (0.4438) | 0.42 (0.0436) | 0.09 (0.6699) |
| Lacritin | 0.24 (0.2616) | 0.47 (0.0251) | 0.05 (0.8181) | 0.29 (0.1789) | −0.08 (0.7030) | 0.02 (0.9426) | −0.22 (0.3071) | 0.23 (0.2847) |
| PIP | 0.19 (0.3748) | 0.17 (0.4350) | 0.27 (0.2072) | 0.16 (0.4794) | −0.19 (0.3940) | 0.00 (0.9982) | 0.12 (0.5789) | 0.34 (0.1151) |
| PMIGR | 0.37 (0.0804) | 0.25 (0.2512) | −0.10 (0.6443) | 0.51 (0.0127) | −0.30 (0.1609) | −0.20 (0.3625) | 0.28 (0.1932) | 0.22 (0.3168) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, M.-T.; Fang, P.-C.; Kuo, S.-F.; Chen, A.; Huang, Y.-T. Tear Proteomics Study of Dry Eye Disease: Which Eye Do You Adopt as the Representative Eye for the Study? Int. J. Mol. Sci. 2021, 22, 422. https://doi.org/10.3390/ijms22010422
Kuo M-T, Fang P-C, Kuo S-F, Chen A, Huang Y-T. Tear Proteomics Study of Dry Eye Disease: Which Eye Do You Adopt as the Representative Eye for the Study? International Journal of Molecular Sciences. 2021; 22(1):422. https://doi.org/10.3390/ijms22010422
Chicago/Turabian StyleKuo, Ming-Tse, Po-Chiung Fang, Shu-Fang Kuo, Alexander Chen, and Yu-Ting Huang. 2021. "Tear Proteomics Study of Dry Eye Disease: Which Eye Do You Adopt as the Representative Eye for the Study?" International Journal of Molecular Sciences 22, no. 1: 422. https://doi.org/10.3390/ijms22010422
APA StyleKuo, M.-T., Fang, P.-C., Kuo, S.-F., Chen, A., & Huang, Y.-T. (2021). Tear Proteomics Study of Dry Eye Disease: Which Eye Do You Adopt as the Representative Eye for the Study? International Journal of Molecular Sciences, 22(1), 422. https://doi.org/10.3390/ijms22010422






