Gender Differences in Low-Molecular-Mass-Induced Acute Lung Inflammation in Mice
Abstract
1. Introduction
2. Results
2.1. Plasma Estradiol Concentration
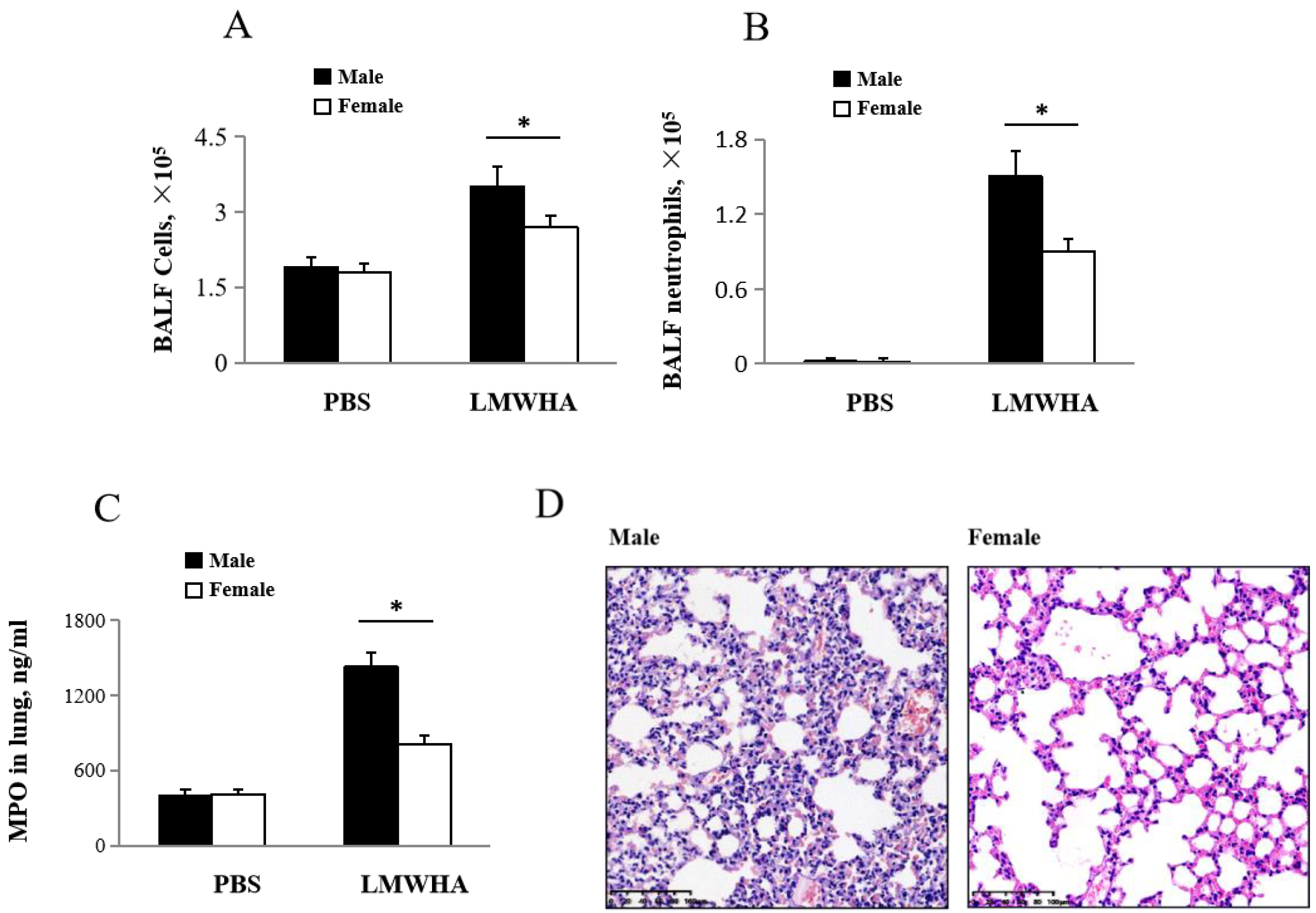
2.2. Gender Differences after LMMHA Induces Lung Inflammation after Intratracheal Administration
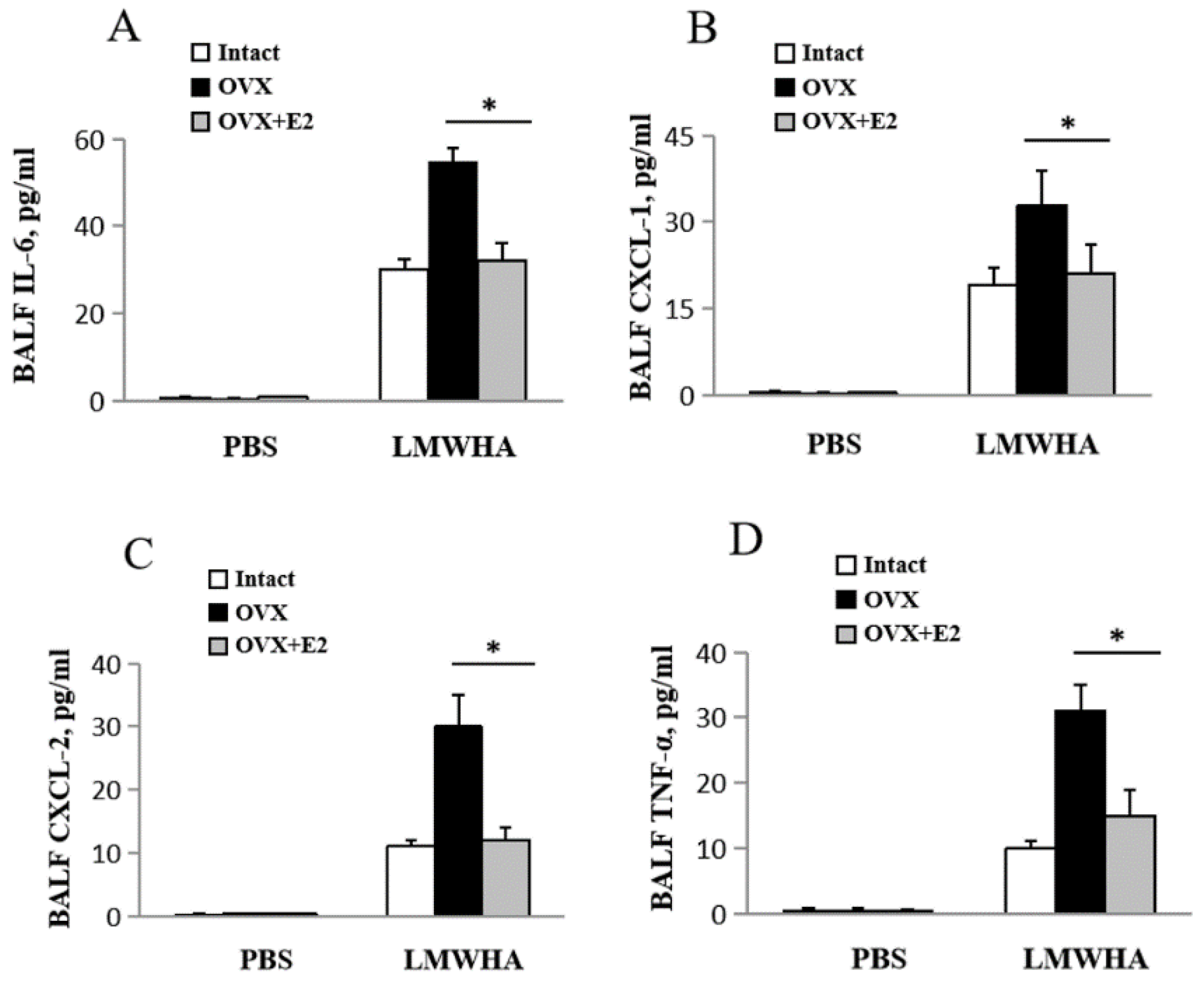
2.3. Differentially Expressed Cytokine Levels in Mice Lungs Induced by LWMHA
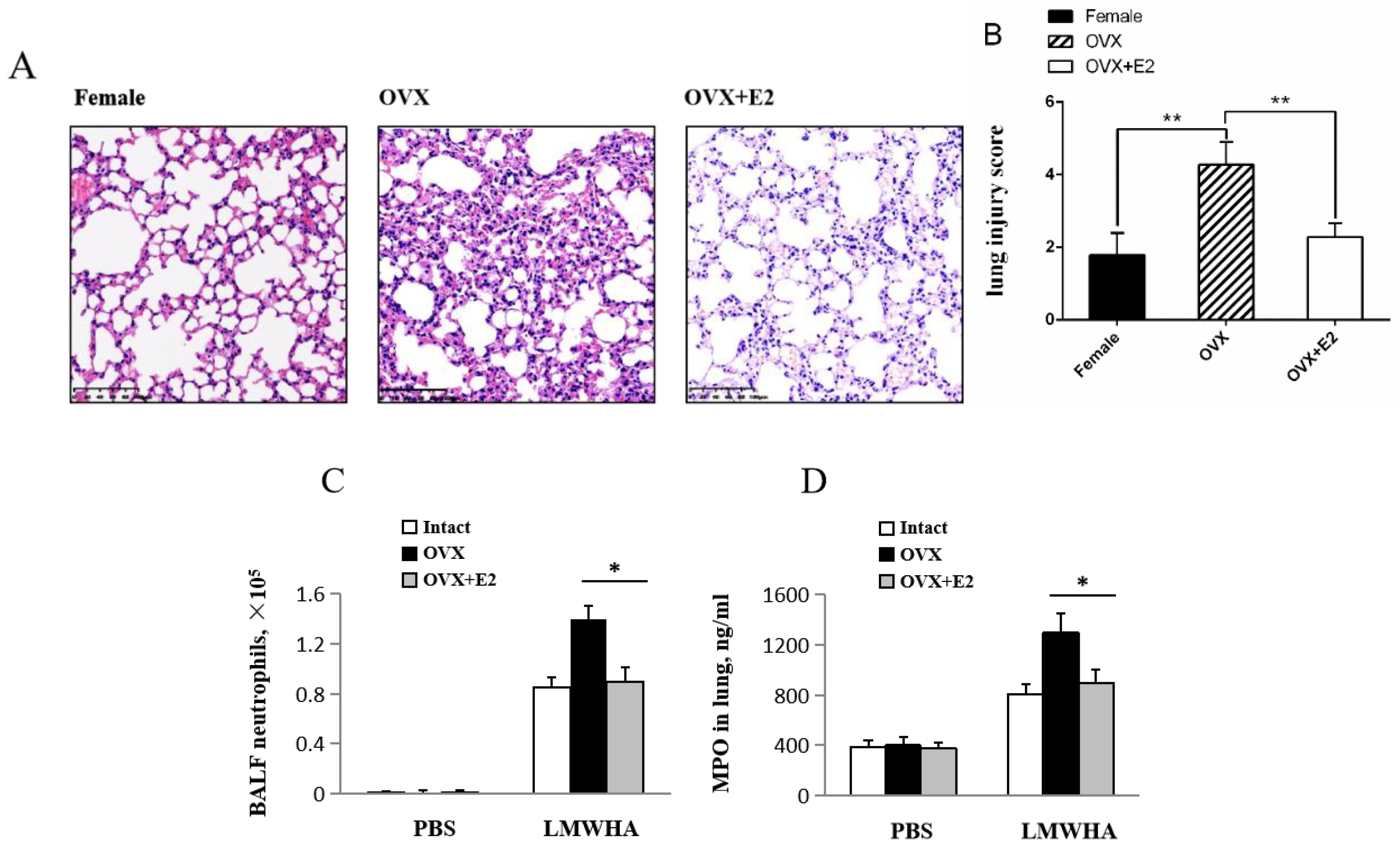
2.4. Estradiol Effects on LMMHA-Induced Lung Injury
3. Discussion
4. Materials and Methods
4.1. Mice and Model Description
4.2. Ovariectomized (OVX)
4.3. Bronchoalveolar Lavage Fluid (BALF)
4.4. ELISA
4.5. Myeloperoxidase Assay
4.6. Lung Injury Score
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LMMHA | Low molecular mass hyaluronan |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor necrosis factor α |
| CXCL-1 | Chemokine (C-X-C motif) ligand 1 |
| CXCL-2 | Chemokine (C-X-C motif) ligand 2 |
| OVX | Ovariectomized |
| ALI | Acute lung injury |
| HA | Hyaluronan |
| BALF | Bronchoalveolar Lavage Fluid |
| MPO | Myeloperoxidase |
| E2 | 17β-estradiol |
| ARDS | Acute Respiratory Distress Syndrome |
References
- Weissmann, B.; Meyer, K.; Sampson, P.; Linker, A. Isolation of oligosaccharides enzymati- cally produced from hyaluronic acid. J. Biol. Chem. 1954, 208, 417–429. [Google Scholar] [PubMed]
- Lauer, M.E.; Dweik, R.A.; Garantziotis, S.; Aronica, M.A. The Rise and Fall of Hyaluronan in Respiratory Diseases. Int. J. Cell Biol. 2015, 2015, 712507. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Suzuki, R.; Mizuno, T.; Abe, K.; Yamauchi, K. Therapeutic implication of genetic variants of IL13 and STAT4 in airway remodelling with bronchial asthma. Clin. Exp. Allergy 2016, 46, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Roth, M.; Klagas, I.; Karakiulakis, G.; Tamm, M.; Stolz, D. COPD Exacerbations are Associated with Proinflammatory Degradation of Hyaluronic Acid. Chest 2015, 148, 1497–1507. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, D.; Liang, J.; Meltzer, E.B.; Gray, A.; Miura, R.; Wogensen, L.; Yamaguchi, Y.; Noble, P.W. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J. Exp. Med. 2011, 208, 1459–1471. [Google Scholar] [CrossRef]
- Kredel, M.; Muellenbach, R.M.; Brock, R.W.; Wilckens, H.; Brederlau, J.; Roewer, N.; Wunder, C. Liver dysfunction after lung recruitment manoeuvres during pressure-controlled ventilation in experimental acute respiratory distress. Crit. Care 2007, 11, R13. [Google Scholar] [CrossRef]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan in tissue injury and repair. Annu. Rev. Cell Dev. Biol. 2007, 23, 435–461. [Google Scholar] [CrossRef]
- Ottonello, L.; Frumento, G.; Arduino, N.; Bertolotto, M.; Dallegri, F. Differential regulation of spontaneous and immune complex-induced neutrophil apoptosis by proinflammatory cytokines. J. Leukocyte Biol. 2002, 72, 125–132. [Google Scholar]
- Taylor, K.R.; Trowbridge, J.M.; Rudisill, J.A.; Termeer, C.C.; Simon, J.C.; Gallo, R.L. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J. Biol. Chem. 2004, 279, 17079–17084. [Google Scholar] [CrossRef]
- Leu, S.W.; Shi, L.; Xu, C.; Zhao, Y.; Liu, B.; Li, Y.; Shiedlin, A.; Xiang, C.; Shen, H.; Quinn, D.A.; et al. TLR4 through IFN-b Promotes Low Molecular Mass Hyaluronan-Induced Neutrophil Apoptosis. J. Immunol. 2011, 186, 556–562. [Google Scholar] [CrossRef]
- Zhao, H.; Leu, S.W.; Shi, L.; Shi, L.; Dedaj, R.; Zhao, G.; Garg, H.; Shen, L.; Lien, E.; Fitzgerald, K.A.; et al. TLR4 is a negative regulator in noninfectious lung inflammation. J. Immunol. 2010, 184, 5308–5314. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ma, Y.; Zhang, L. Low-molecular-mass hyaluronan induces pulmonary inflammation by up-regulation of Mcl-1 to inhibit neutrophil apoptosis via PI3K/Akt1 pathway. Immunology 2018, 155, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Phua, J.; Badia, J.R.; Adhikari, N.K.; Friedrich, J.O.; Fowler, R.A.; Singh, J.M.; Scales, D.C.; Stather, D.R.; Li, A.; Jones, A.; et al. Has mortality from acute respiratory distress syndrome decreased over time?: A systematic review. Am. J. Respir. Crit. Care Med. 2009, 179, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Aggarwal, A.N.; Gupta, D.; Behera, D.; Jindal, S.K. Etiology and outcomes of pulmonary and extrapulmonary acute lung injury/ARDS in a respiratory ICU in North India. Chest 2006, 130, 724–729. [Google Scholar] [CrossRef]
- Moss, M.; Mannino, D.M. Race and gender differences in acute respiratory distress syndrome deaths in the United States An analysis of multiple-cause mortality data (1979–1996). Crit. Care Med. 2002, 30, 1679–1685. [Google Scholar] [CrossRef]
- Thais Fantozzi, E.; Rodrigues-Garbin, S.; Yamamoto Ricardo-da-Silva, F.; Martins Oliveira-Filho, R.; Spina, D.; Tavares-de-Lima, W.; Riffo-Vasquez, Y. Acute lung injury induced by intestinal ischemia and reperfusion is altered in obese female mice. Pulm. Pharmacol. Ther. 2018, 49, 54–59. [Google Scholar] [CrossRef]
- Speyer, C.L.; Rancilio, N.J.; McClintock, S.D.; Crawford, J.D.; Ward, P.A. Regulatory effects of estrogen on acute lung inflammation in mice. Am. J. Physiol. Cell Physiol. 2005, 288, C881–C890. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, Y.; Chu, Z.; Yan, B.; Xu, L. Protective effect of cryptotanshinone on lipopolysaccharide-induced acute lung injury in mice. Eur. J. Pharmacol. 2014, 723, 494–500. [Google Scholar] [CrossRef]
- Tsujimoto, H.; Ono, S.; Mochizuki, H.; Aosasa, S.; Matsumoto, A. Role of Macrophage Inflammatory Protein 2 in Acute Lung Injury in Murine Peritonitis. J. Surg. Res. 2002, 103, 61–67. [Google Scholar] [CrossRef]
- Closa, D. Activation of alveolar macrophages in lung injury associated with experimental acute pancreatitis is mediated by the liver. Ann. Surg. 1999, 229, 230–236. [Google Scholar] [CrossRef]
- Lentsch, A.B.; Yoshidome, H.; Cheadle, W.G.; Miller, F.N.; Edwards, M. Chemokine involvement in hepatic ischemia/reperfusion injury in mice: Roles for macrophage inflammatory protein-2 and KC. Hepatology 1998, 27, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Jordana, M.; Kirpalani, H.; Driscoll, K.E.; Schall, T.J.; Gauldie, J. Cytokine expression by neutrophils and macrophages in-vivo-endotoxin induces tumor-necrosis-factor-alpha, macrophage inflammatory protein-2, interleukin-1-beta, and interleukin-6 but not rantes or transforming growth factor-beta(1) messenger-rna expression in acute lung inflammation. Am. J. Respir. Cell Mol. Biol. 1994, 10, 148–153. [Google Scholar] [PubMed]
- Greenberger, M.J.; Strieter, R.M.; Kunkel, S.L.; Danforth, J.M.; Laichalk, L.L.; Mcgillicuddy, D.C.; Standiford, T.J. Neutralization of macrophage inflammatory protein-2 attenuates neutrophil recruitment and bacterial clearance in murine Klebsiella pneumonia. Infect. Immun. 1996, 173, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Angele, M.K.; Knferl, M.W.; Schwacha, M.G.; Ayala, A.; Chaudry, I.H. Hemorrhage decreases macrophage inflammatory protein 2 and interleukin-6 release A possible mechanism for increased wound infection. Ann. Surg. 1999, 229, 651–660. [Google Scholar] [CrossRef]
- Schmal, H.; Shanley, T.P.; Jones, M.L.; Friedl, H.P.; Ward, P.A. Role for macrophage inflammatory protein-2 in lipopolysaccharide-induced lung injury in rats. J. Immunol. 1996, 156, 1963–1972. [Google Scholar]
- Anadkat, J.S.; Kuzniewicz, M.W.; Chaudhari, B.P.; Cole, F.S.; Hamvas, A. Increased risk for respiratory distress among white, male, late preterm and term infants. J. Perinatol. 2012, 32, 780–785. [Google Scholar] [CrossRef]
- Lahm, T.; Crisostomo, P.R.; Markel, T.A.; Wang, M.; Weil, B.R.; Novotny, N.M.; Meldrum, D.R. The effects of estrogen on pulmonary artery vasoreactivity and hypoxic pulmonary vasoconstriction: Potential new clinical implications for an old hormone. Crit. Care Med. 2008, 36, 2174–2183. [Google Scholar] [CrossRef]
- Frink, M.; Thobe, B.M.; Hsieh, Y.C.; Choudhry, M.A.; Schwacha, M.G.; Bland, K.I.; Chaudry, I.H. 17-Estradiol inhibits keratinocytederived chemokine production following trauma-hemorrhage. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 292, 585–591. [Google Scholar] [CrossRef]
- Hsieh, Y.C.; Frink, M.; Hsieh, C.H.; Choudhry, M.A.; Schwacha, M.G.; Bland, K.I.; Chaudry, I.H. Downregulation of migration inhibitory factor is critical for estrogen-mediated attenuation of lung tissue damage following trauma-hemorrhage. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 292, 1227–1232. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Santagati, S.; Sautebin, L.; Mazzon, E.; Calabro, G.; Serraino, I.; Caputi, A.P.; Maggi, A. 17-Estradiol antiinflammatory activity in carrageenan-induced pleurisy. Endocrinology 2000, 141, 1455–1463. [Google Scholar] [CrossRef]
- De Oliveira, A.P.; Peron, J.P.; Damazo, A.S.; Franco, A.L.; Domingos, H.V.; Oliani, S.M.; Oliveira-Filho, R.M.; Vargaftig, B.B.; Tavares-de-Lima, W. Female sex hormones mediate the allergic lung reaction by regulating the release of inflammatory mediators and the expression of lung E-selectin in rats. Respir. Res. 2010, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.S.; Yabroff, K.R.; Mandel, D.M.; Johnson, K.J.; Ward, P.A. Role of O2 in neutrophil recruitment into sites of dermal and pulmonary vasculitis. Free Radic. Biol. Med. 1990, 8, 163–172. [Google Scholar] [CrossRef]
- Savill, J.; Dransfield, I.; Gregory, C.; Haslett, C. A blast from the past: Clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2002, 2, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Matute-Bello, G.; Liles, W.C.; Radella II, F.; Steinberg, K.P.; Ruzinski, J.T.; Jonas, M.; Chi, E.Y.; Hudson, L.D.; Martin, T.R. Neutrophil apoptosis in the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1997, 156, 1969–1977. [Google Scholar] [CrossRef] [PubMed]
- Hutchens, M.P.; Dunlap, J.; Hurn, P.D.; Jarnberg, P.O. Renal ischemia: Does sex matter? Anesth. Anal. 2008, 107, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.T.; McCullough, L.D. Pathways to ischemic neuronal cell death: Are sex differences relevant? J. Transl. Med. 2008, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Dunlay, S.M.; Roger, V.L. Gender Differences in the Pathophysiology, Clinical Presentation, and Outcomes of Ischemic Heart Failure. Curr. Heart. Fail Rep. 2012, 9, 267–276. [Google Scholar] [CrossRef]
- Laura, M.; Margarita, G.; Joaguin, D.; Luis, R.; Santos, C.; Antonio, L. Expression of estrogen receptor subtypes and neuronal nitric oxide synthase in neutrophils from women and men: Regulation by estrogen. Cardiovasc. Res. 2002, 56, 43–51. [Google Scholar]
- Garcia-Duran, M.; de Frutos, T.; Diaz-Recasens, J.; Garcia-Galvez, G.; Jimenez, A.; Monton, M.; Farre, J.; de Miguel, L.S.; Gonzalez-Fernandez, F.; Arriero, M.D.; et al. Estrogen stimulates neuronal nitric oxide synthase protein expression in human neutrophils. Circ. Res. 1999, 85, 1020–1026. [Google Scholar] [CrossRef]
- Dubal, D.B.; Zhu, H.; Yu, J.; Rau, S.W.; Shughrue, P.J.; Merchenthaler, I.; Kindy, M.S.; Wise, P.M. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc. Natl. Acad. Sci. USA 2001, 98, 1952–1957. [Google Scholar] [CrossRef]
- Vegeto, E.; Belcredito, S.; Etteri, S.; Ghisletti, S.; Brusadelli, A.; Meda, C.; Krust, A.; Dupont, S.; Ciana, P.; Chambon, P.; et al. Estrogen receptor-mediates the brain antiinflammatory activity of estradiol. Proc. Natl. Acad. Sci. USA 2003, 100, 9614–9619. [Google Scholar] [CrossRef] [PubMed]
- Polanczyk, M.; Yellayi, S.; Zamora, A.; Subramanian, S.; Tovey, M.; Vandenbark, A.A.; Offner, H.; Zachary, J.F.; Fillmore, P.D.; Blankenhorn, E.P.; et al. Estrogen receptor-1 (Esr1) and -2 (Esr2) regulate the severity of clinical experimental allergic encephalomyelitis in male mice. Am. J. Pathol. 2004, 164, 1915–1924. [Google Scholar] [CrossRef][Green Version]
- Brouchet, L.; Krust, A.; Dupont, S.; Chambon, P.; Bayard, F.; Arnal, J.F. Estradiol accelerates reendothelialization in mouse carotid artery through estrogen receptor-αbut not estrogen receptor-β. Circulation 2001, 103, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Massaro, D.; Clerch, L.B.; Massaro, G.D. Estrogen receptor-αregulates pulmonary alveolar loss and regeneration in female mice: Morphometric and gene expression studies. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 293, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.P.; Hsieh, Y.C.; Suzuki, T.; Shimizu, T.; Choudhry, M.A.; Schwacha, M.G.; Chaudry, I.H. Salutary effects of estrogen receptor-αagonist on lung injury after trauma-hemorrhage. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 290, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Catley, M.C.; Birrell, M.A.; Hardaker, E.L.; de Alba, J.; Farrow, S.; Haj-Yahia, S.; Belvisi, M.G. Estrogen receptor α: Expression profile and possible anti-inflammatory role in disease. J. Pharmacol. Exp. Ther. 2008, 326, 83–88. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Xie, D.; Li, B.; Zhao, H. Gender Differences in Low-Molecular-Mass-Induced Acute Lung Inflammation in Mice. Int. J. Mol. Sci. 2021, 22, 419. https://doi.org/10.3390/ijms22010419
Xie Y, Xie D, Li B, Zhao H. Gender Differences in Low-Molecular-Mass-Induced Acute Lung Inflammation in Mice. International Journal of Molecular Sciences. 2021; 22(1):419. https://doi.org/10.3390/ijms22010419
Chicago/Turabian StyleXie, Yifang, Dehui Xie, Bin Li, and Hang Zhao. 2021. "Gender Differences in Low-Molecular-Mass-Induced Acute Lung Inflammation in Mice" International Journal of Molecular Sciences 22, no. 1: 419. https://doi.org/10.3390/ijms22010419
APA StyleXie, Y., Xie, D., Li, B., & Zhao, H. (2021). Gender Differences in Low-Molecular-Mass-Induced Acute Lung Inflammation in Mice. International Journal of Molecular Sciences, 22(1), 419. https://doi.org/10.3390/ijms22010419




